Abstract
HIV-1 infection of the central nervous system causes HIV-associated neurocognitive disorders, even in aviremic patients. Although astrocyte malfunction was associated to these disorders, their implication is overshadowed by contributions of microglia and macrophages. Astrocytes are infected with HIV-1 in vivo and express a relevant amount of viral protein Nef. Nef was shown to stimulate its own release in exosomes from diverse cell types, which in turn have damaging effects on neighboring cells. Using immunoblotting and electron microscopy, we showed that human astrocytes expressing Nef.GFP similarly release Nef in exosomes. Importantly, Nef.GFP expression increases the secretion of exosomes from human astrocytes up to 5.5-fold, as determined by total protein content and nanoparticle tracking analysis. Protein analysis of exosomes and viruses separated on iodixanol gradient further showed that native or pseudotyped HIV-1-infected human astrocytes release exosomes, which contain Nef. Our results provide the basis for future studies of the damaging role of Nef-exosomes produced by HIV-infected astrocytes on the central nervous system.
Keywords: Astrocytes, HIV-1, Nef, Extracellular vesicles, Exosomes, Central nervous system
Introduction
Astrocytes are glial cells found throughout the central nervous system (CNS) as the most abundant cell type. They supply energy substrates and neuroactive substances to neurons, protect them from reactive oxygen species, recycle neurotransmitters, and regulate ion and pH balance. Additionally, they regulate local blood flow in the CNS and induce blood-brain barrier (BBB) properties (Sofroniew and Vinters 2010). Furthermore, astrocytes are targets and responders to a variety of infectious agents, including human immunodeficiency virus type 1 (HIV-1) (Donati et al. 2005; Drogemuller et al. 2008; Semmler et al. 2005).
HIV-1 is a lymphotropic and neurotropic virus, which is able to enter the CNS, where it mostly infects perivascular/ parenchymal macrophages and microglia (Hult et al. 2008). The infection of astrocytes has also been documented in HIV-1-infected pediatric and adult patients, particularly in advanced brain disease (Churchill et al. 2006; Churchill et al. 2009; Epstein et al. 1984; Takahashi et al. 1996; Tornatore et al. 1994; Trillo-Pazos et al. 2003). Earlier in vitro studies support non-productive infection of astrocytes with restrictions at HIV-1 entry due to an absence of classical CD4-receptor and at the level of viral structural gene expression (Canki et al. 2001; Chauhan et al. 2014; Churchill et al. 2006; Churchill et al. 2009; Di Rienzo et al. 1998; Gorry et al. 1998; Li et al. 2002; Neumann et al. 2001). Recent studies however indicate restriction at the level of HIV-1 entry into astrocytes, which can be overridden to some extent by endocytosis or by pseudotyping the virus (Carroll-Anzinger and Al-Harthi 2006; Gray et al. 2014). Importantly, infection of astrocytes is connected to HIV associated dementia (HAD) (Churchill et al. 2009). HIV-1 infection of the brain provokes inflammatory and neurotoxic responses (Anthony et al. 2005), and despite the administration of the combined antiretroviral therapy, 40–60% of infected individuals eventually develop HIV-associated neurocognitive disorders. These range from asymptomatic neurocognitive impairment to mild neurocognitive disorder in majority of patients, but sometimes lead to more severe HAD (McArthur and Brew 2010).
HIV-1 accessory protein Nef (27–35 kDa depending on the isolate type) is one of the early viral transcripts expressed abundantly in all stages of infection. Nef contributes to viral pathogenesis by maintaining high viral loads and maximal pathogenic potential (Kestler et al. 1991). It was detected in up to 20% of astrocytes in post mortem pediatric CNS tissues (Saito et al. 1994) and in 40% of astrocytes in post mortem brain samples of infected adults (Ranki et al. 1995). In cultured astrocytes, Nef induces the production of IP-10 (van Marle et al. 2004), CCL2 (Lehmann et al. 2006), CCL5 (Liu et al. 2014), IL-6, and IL-8 (Liu and Kumar 2015) and impairs autophagy (Sardo et al. 2015), whereas, in rats implanted with astrocytes expressing Nef, it impairs spatial and recognition memory (Chompre et al. 2013). Additionally, Nef expression alters the endosomal system in HeLa cells (Madrid et al. 2005), increases the number of multivesicular bodies (MVB) in macrophages (Costa et al. 2006) and HeLa.CIITA cells (Stumptner-Cuvelette et al. 2003), and induces extracellular vesicle (EV) secretion from various cells (Lenassi et al. 2010; Muratori et al. 2009).
EVs are small membrane-bound particles released from cells in vitro and in vivo, which serve as a form of intercellular communication in physiological and pathological conditions (Raposo and Stoorvogel 2013). They are heterogeneous in size, subcellular origin, and molecular composition. Exosomes (30–100 nm in diameter) are formed as intraluminal vesicles in MVBs and released through MVB fusion with the plasma membrane, while microvesicles (100–1000 nm) bud directly from the plasma membrane. Importantly, Nef was detected in exosomes released from Hela.CIITA cells (Lenassi et al. 2010), HEK293 cells (Ali et al. 2010), T cells (Ali et al. 2010; Lenassi et al. 2010; Muratori et al. 2009), U937 monocytes (Aqil et al. 2015), microglia (Raymond et al. 2016), and conditionally from astrocytes (Raymond et al. 2016; Sami Saribas et al. 2017); and Nef-containing EVs were also detected in the plasma of HIV-1-infected patients (Khan et al. 2016; Lee et al. 2016; Raymond et al. 2011). Still, the study by Luo et al. raised some concerns about the EV-bound nature of extracellular Nef (Luo et al. 2015). These EVs trigger apoptosis in bystander T cells (Lenassi et al. 2010; Muratori et al. 2009), contribute to virus infectivity (Arenaccio et al. 2014), inflammation (Lee et al. 2016), and neurotoxicity (Khan et al. 2016; Sami Saribas et al. 2017; van Marle et al. 2004), and impair the BBB (Raymond et al. 2016).
Since astrocytes are long-lived and the most abundant brain cell type, those infected with HIV-1 could represent a significant source of extracellular Nef in the CNS. In this study, we examined whether Nef, expressed from the Nef.GFP plasmid or the HIV-1 provirus, induces the secretion of EVs (more specifically exosomes) and thereby its own release (Nefexosomes) from human astrocytes in culture (h-astrocyte). Using microscopic and biochemical methods, we demonstrate that Nef is released in exosomes from Nef.GFP-expressing astrocytes and show for the first time that Nef is also released in exosomes from native or pseudotyped HIV-1-infected h-astrocytes. Additionally, nanoparticle tracking and protein analyses support at least 5.5-fold increment of exosome release from Nef.GFP-expressing h-astrocytes.
Materials and methods
Cell lines
Immortalized (E6/E7/hTERT) normal human astrocytes (h-astrocytes; NHA; Lonza, Switzerland), isolated from the human fetal brain tissue (Sonoda et al. 2001) were a gift from Dr. Russell O. Pieper from UCSF, USA. Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Sigma-Aldrich, MO, USA), supplemented with 1 × GlutaMAX (Gibco, MA, USA), 10% heat-inactivated and sterile-filtered Fetal Bovine Serum (FBS; Sigma-Aldrich), 100 IU/ml penicillin, and 100 μg/ml streptomycin (Gibco). For EV isolation experiments, EV-depleted FBS was prepared by overnight ultracentrifugation at 100,000g and 4 °C and was additionally filtered through 0.22 μm-pore-size filter (Corning, NY, USA). HEK293T and TZM-bl cell lines used for virus production and infectivity tests, respectively, were cultured in supplemented DMEM. All cells were grown at 37 °C and 5% CO2.
Plasmids
For cellular expression of Nef, h-astrocytes were transfected with pEGFP-N1 plasmid (Takara Bio USA) coding for green fluorescent protein (GFP; pGFP) or HIV-1 SF2 Nef C-terminally tagged with GFP (pNef.GFP; NIH AIDS Reagent Program). For production of pseudotyped HIV-1 viral stocks, HEK293T were transfected with plasmids coding for HIV-1 isolates NL4-3, NL4-3 Δnef, or YU-2 (obtained through NIH AIDS Reagent Program), and a plasmid coding for vesicular stomatitis virus envelope protein (VSV-G; pCMV-VSV-G; Addgene).
Human astrocyte transfection
Cells were transfected using Amaxa Cell Line Nucleofector Kit V (Lonza; #VCA-1003) and Nucleofector 2b Device (Lonza) according to the manufacturer’s instructions. Briefly, 2.5 × 106 cells per reaction were transfected with 4 μg of pDNA using nucleofection program A-033. Immediately after transfection, cells were transferred into prewarmed calcium-free DMEM (Sigma-Aldrich) supplemented with 10% FBS in cell culture plates, covered with poly-L-lysine (Sigma-Aldrich). After 4 h, attached cells were washed with Dulbecco’s phosphate-buffered saline (DPBS, Sigma-Aldrich) and cultured in complete medium, supplemented with EV-depleted FBS. After 48 h of incubation, released EVs were isolated from cell culture media. Additionally, cell lysates were prepared every 24 h for 4 days after transfection.
Human astrocyte infection with native or pseudotyped HIV-1
Native or pseudotyped HIV-1 viral stocks were isolated from 72 h old cultures of HEK293T, transfected with HIV-1 or HIV-1 and pCMV-VSV-G coding plasmids, respectively. Transfections were performed using X-tremeGENE HP DNA Transfection Reagent (Roche, Germany), according to manufacturer’s instructions. Precleared culture media of transfected cells was filtered through a 0.22-μm-pore-size filter; viral particles were concentrated using Amicon Ultra-15 filters (Millipore, MA, USA), aliquoted, and stored at −80 °C. To analyze the virion composition by immunoblotting, 200 μl of the viral stock was diluted with DPBS to 10 ml and carefully overlaid on 20% sucrose cushion, ultracentrifuged at 100,000g for 2 h at 4 °C (SW-41 Ti rotor, Beckman Coulter, CA, USA), and the pellet was resuspended in 1× loading buffer. The p24 content for each virus stock was determined using HIV-1 p24 quantitative enzyme-linked immunosorbent assay (ELISA; PerkinElmer, MA, USA). The infectivity of each virus was tested on TZM-bl cell line as previously published (Sarzotti-Kelsoe et al. 2014). Briefly, cells were sown into 24-well dishes and incubated overnight with viral volumes equivalent to 1–5 ng of HIV-1 p24 antigen. Three-days post infection, viral infectivity was demonstrated by measuring luciferase activity using a microplate luminometer.
For h-astrocyte infection with native or pseudotyped HIV-1, volume of viral stock equivalent to 500 ng of HIV-1 p24 was added to 1 × 107 cells resuspended in 15 ml of supplemented DMEM, and incubated overnight at 37 °C. Next day, the excess virus was removed; cells were washed with DPBS and cultured in complete medium depleted of EVs for the indicated time.
Isolation of EVs (more specifically exosomes) and HIV-1 virions from h-astrocyte culture
EVs were isolated as described previously (Thery et al. 2001). Briefly, transfected cells were removed from culture media by centrifugation at 2000g for 5 min followed by passage of the media through a 0.22-μm-pore-size filter for isolation of exosomes. The filtration step was omitted for isolation of the complete EV population. Cell culture media was then ultracentrifuged at 100,000g for 1 h, at 4 °C (MLA-50, BC); the pellet was collected and resuspended in DPBS. Further isolation depended on the sample type and the method of analysis: (i) for transmission electron microscopy (TEM), nanoparticle trafficking analysis (NTA), determination of protein content, and immunoblotting; exosome pellet was additionally washed at 100,000g for 1 h at 4 °C (TLA-55, BC) and resuspended in either DPBS, lysis buffer (1% IPEGAL CA-630 (Sigma-Aldrich), 0.1% sodium dodecyl sulfate (SDS), and 0.5% sodium deoxycholate in PBS) supplemented with protein inhibitors (PIs), or 1× loading buffer; (ii) for isolation of the complete EV population, pellet was ultracentrifuged on 20–60% sucrose gradient at 100,000g for 18 h, at 4 °C (MLS-50, BC); twelve 400 μL fractions were collected from the gradient, diluted to final volume of 1 ml with DPBS; proteins were precipitated using trichloroacetic acid and sodium deoxycholate, and resuspended in 1 × loading buffer for immunoblot analysis.
Similar procedure was used for the separation of Nefexosomes and HIV-1 virions, with the following modifications: after filtration, cell culture media was ultracentrifuged at 100,000g for 2 h at 4 °C (SW-28 rotor, BC). Pellets containing exosomes and/or virions were resuspened in 500 μl of DPBS and ultracentrifuged on 6–18% iodixanol velocity gradient (Optiprep, Sigma-Aldrich) at 250,000g for 2 h, at 4 °C (SW-41 Ti, BC). Each sample was individually analyzed with acetylcholinesterase (AChE) enzyme activity assay, HIV-1 p24 ELISA (PerkinElmer), and immunoblot analysis after trichloroacetic acid and sodium deoxycholate protein precipitation.
Immunoblotting
Cells were lysed in lysis buffer supplemented with PIs, for 15 min at 4 °C, centrifuged at 12000g for 15 min at 4 °C and the supernatant collected and stored at −20 °C until further use. EV (exosomes) and cell lysate protein concentration was measured using Pierce BCA Protein Assay Kit (Thermo Scientific MA, USA).
For immunoblotting, total EV, exosomal, and viral proteins or 30 μg of cell lysates were separated by 12% SDS-PAGE and transferred to PVDF membrane (Millipore). Goat polyclonal antibodies against Hsc70 (sc-1059, Santa Cruz Biotechnology, CA, USA), actin (sc-1615), rabbit polyclonal against calnexin (sc-11,397), CD63 (sc-15,363), alpha-tubulin (ab4074, Abcam, UK), VSV-G (ab1874, Abcam), HIV-1 Nef (NIH AIDS Reagent Program, 2949), and HIV-1 gp120 (NBP1–76371, Novus Biologicals, CO, USA); or mouse monoclonal against acetylcholinesterase (AChE, MAB303, Millipore), flotillin, (610820, BD Bioscience, NJ, USA), GFP (sc-9996), p24/Gag (ab9071, Abcam), cytochrome c (556432, BD Biosciences), and Alix (2171S, Cell Signaling, MA, USA) were used as the primary antibodies; and appropriate HRP-conjugated anti-goat, anti-rabbit, or anti-mouse (Jackson Immunoresearch, PA, USA) were used as the secondary antibodies. Membranes were developed by SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific).
Acetylcholinesterase activity
Acetylcholinesterase activity was determined as described previously (Savina et al. 2002). Briefly, 100 μl of each fraction collected from the iodixanol density gradient was diluted with DPBS to 180 μl and incubated with 20 μl mixture of 1.25 mM acetylthiocholine (Sigma-Aldrich; A5751) and 0.1 mM 5,5′-dithiobis(2-nitrobenzoic acid) (Sigma-Aldrich, D8130) for 30 min at 37 °C. The absorbance was measured at 412 nm with spectrophotometer Synergy 2 Multi-Mode Reader (BioTek Inc., Germany) and the activity presented as pmol/ul*min.
HIV-1 p24 capture ELISA
The HIV-1 p24 concentration was determined in 100 μl of undiluted samples using HIV-1 p24 capture ELISA assay (PerkinElmer) following manufacturer’s instructions. Absorbance of each sample was measured at 450 nm, and HIV-1 p24 concentration (pg/ml) was calculated based on standard curve of positive samples.
Microscopy
To image exosomes by TEM, pellets obtained after 100,000g ultracentrifugation were fixed in 2% PFA and negatively stained by uranyl formate following the established protocol (Ohi et al. 2004). All samples were imaged at room temperature in a Tecnai T12 microscope (FEI Company, USA), and images were recorded on a 4 K × 4 K UltraScan CCD camera (Gatan Inc., USA).
Exosome quantification
To quantify exosomes, the total amount of exosomal proteins (μg) per 1 × 106 transfected cells was determined by Pierce BCA protein Assay Kit (Thermo Scientific).
Alternatively, Nanoparticle Tracking Analysis (NTA) measurements were performed with a NanoSight LM10 apparatus (Amesbury, UK) with a 488-nm Blue Laser Module. For the NTA measurements, 10 μl of exosome sample was diluted to 1 ml with particle-free DPBS buffer and transferred into the NTA sample chamber through the syringe pump. The settings for the shutter and the camera gain were 800 and 350, respectively. During the measurements, seven videos within 60 s were captured by the software. The number of particles per millilitre was normalized to particle number per 1 × 106 transfected and viable cells.
Results
Nef.GFP is released in exosomes from Nef.GFP-expressing h-astrocytes
Nef was previously shown to promote its own secretion in exosomes from various cells (Ali et al. 2010; Aqil et al. 2015; Lenassi et al. 2010; Muratori et al. 2009; Raymond et al. 2016), but there are contradictory reports regarding its release from astrocytes (Raymond et al. 2016; Sami Saribas et al. 2017). Therefore, we wanted to explore if Nef-exosomes are released from Nef.GFP-expressing astrocytes.
First, we transiently expressed Nef.GFP in h-astrocytes and analyzed cell lysates and secreted EVs. Nef.GFP expression was not toxic for astrocytes, since the number of dead cells in the population never exceeded 20%, as shown by propidium iodide staining and flow cytometric analysis, and cells had typical h-astrocyte morphology, as observed by fluorescent m icroscopy (data not shown). Immunoblotting of h-astrocyte cell lysates showed that Nef.GFP is expressed in cells up to 72 h post transfection, with stronger signal for the first 48 h (Fig. 1a). Therefore, we isolated the whole population of EVs from cultures expressing Nef.GFP for 48 h, removing only possible apoptotic bodies. We further separated EVs on 20–60% sucrose gradient and tested the collected fractions for the presence of Nef.GFP by immunoblotting, using antibody against GFP. The strongest signal for Nef.GFP was detected in fractions 3–6, while weaker signal was detected in fractions 2 and 7–12 (Fig. 1b). Fractions 3–6 mostly overlap with fractions immunopositive for proteins typically enriched in exosomes; Hsc70 is enriched in fractions 5–12, AChE in fractions 2–7, flotillin in fractions 3–7, and CD63 in fractions 9–12. The purity of the EV isolate was confirmed by the absence of calnexin, a marker for endoplasmic reticulum. Nef.GFP immunopositive fractions have a buoyant density from 1.12 to 1.20 g/ml (measured by refractometer, data not shown), which correspond to the reported buoyant flotation density of exosomes on sucrose gradients (1.08–1.22 g/ml) (Tauro et al. 2012). To further support the identity of the Nef-containing EVs, we isolated exosomes from Nef.GFP-expressing h-astrocyte culture by additional filtration of EVs through 0.22-μm filter and analyzed them for Nef.GFP presence with immunoblotting (Fig. 1c) and their morphology with TEM (Fig. 1d). Isolated exosomes were immunopositive for Nef.GFP and typical exosomal protein AChE and were devoid of contaminating cellular debris, as there was no signal for calnexin (Fig. 1c). Micrographs of the purified pellet confirmed the isolation of small vesicles with a cup-shaped structure typical for exosomes (Fevrier and Raposo 2004) (Fig. 1d). Interestingly, GFP was also released in exosomes from GFP-expressing h-astrocytes (Fig. 1c). We conclude that Nef.GFP is mostly secreted in exosomes, when expressed in h-astrocytes, and have thus focused on these vesicles in all further experiments.
Fig. 1.
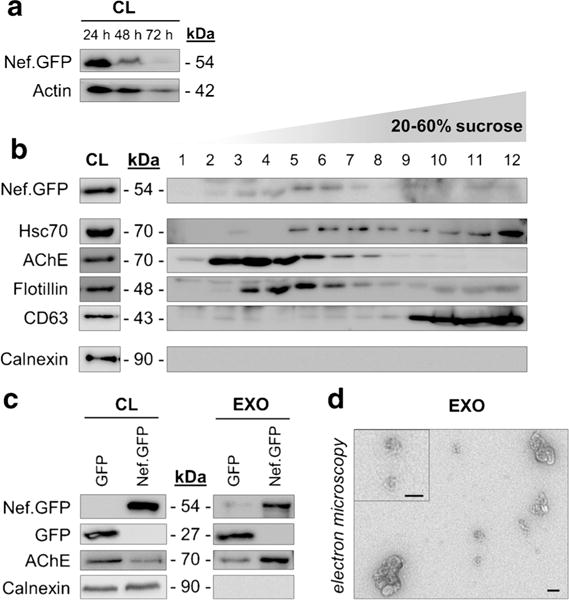
Nef.GFP-expressing h-astrocytes secrete exosomes containing Nef.GFP. a Immunoblot of cell lysates from Nef.GFP-expressing h-astrocytes collected every 24 h for 3 days post transfection. Antibodies directed against GFP and actin (loading control) were used. CL, cell lysate b, c Protein composition of EVs from Nef.GFP-expressing h-astrocytes cultures, separated on 20–60% sucrose gradient (b), and exosomes (c) isolated from GFP and Nef.GFP-expressing h-astrocytes. Immunoblotting was performed with antibodies directed against GFP, proteins enriched in exosomes (Hsc70, AChE, flotillin, CD63), and against marker for endoplasmic reticulum (calnexin). d TEM image showing exosomes isolated from Nef.GFP-expressing h-astrocytes. The selected exosomes are displayed magnified in the top left corner. Scale bars 100 nm
Nef.GFP expression increases the secretion of exosomes from h-astrocytes
We previously showed that in addition to its incorporation into exosomes, Nef also induces the secretion of exosomes from Nef.GFP-expressing Jurkat and SupT1 cells (Lenassi et al. 2010). We wanted to explore if Nef.GFP has a similar effect in h-astrocytes. For this purpose, we isolated exosomes from Nef.GFP-expressing h-astrocyte culture by additional filtration of EVs through 0.22-μm filter and quantified them by normalizing the total protein content (Fig. 2a) or number of vesicles counted by NTA (Fig. 2b) to the number of transfected cells. We compared this to the exosome numbers isolated from GFP-expressing h-astrocytes. We observed up to 5.5-fold and up to 4.5-fold increase in the release of exosomes from Nef.GFP-expressing h-astrocytes when quantified by total protein content (Fig. 2a) and NTA (Fig. 2b), respectively. Average diameter of exosomes released from Nef.GFP- and GFP-expressing h-astrocytes, as determined by NTA, is 164 ± 2 and 173 ± 4 nm, respectively (Fig. 2c, d). Taken together, Nef.GFP expression increases the secretion of smaller exosomes from h-astrocytes, when compared to GFP-expressing culture.
Fig. 2.
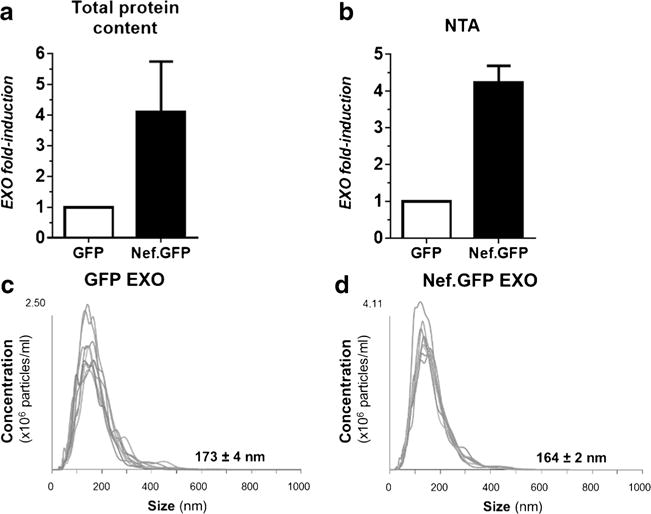
Nef.GFP expression increases the secretion of smaller exosomes from h-astrocytes. a, b Amounts of exosomes released from GFP and Nef.GFP-expressing h-astrocytes were assessed by normalizing the total protein content (a) or the total number of vesicles detected by NTA (b) to the number of transfected cells. c, d Size and concentration of exosomes isolated from GFP-expressing h-astrocytes (c) and Nef.GFP-expressing h-astrocytes (d) assessed by NTA. EXO exosomes
Pseudotyped HIV-1-infected h-astrocytes-release Nef extracellularly
Next we wanted to explore if Nef is released from HIV-1-infected h-astrocytes. Several recent reports suggested that pseudotyped HIV-1 overcomes the restriction of viral entry into the astrocytes (Canki et al. 2001; Carroll-Anzinger and Al-Harthi 2006; Chauhan et al. 2014; Churchill et al. 2006; Churchill et al. 2009; Gray et al. 2014), therefore we infected h-astrocytes with VSV-G pseudotyped HIV-1 isolates NL4-3, NL4-3 Δnef, or YU-2 and analyzed cell culture media for the presence of Nef, as well as typical exosomal and viral proteins. First, we characterized the pseudotyped HIV-1 virions composition and their effectiveness in h-astrocyte infection by immunoblotting (Fig. 3a, b). All virions tested positive for processed Gag proteins p55, p41, and p24 and for the envelope protein VSV-G (Fig. 3a). Both, the T cell tropic laboratory adapted strain NL4-3 and the brain isolate YU-2 contain Nef protein in the virions, but the molecular weight differs between the strains (24 and 27 kDa, respectively), as previously observed (Geyer and Peterlin 2001). Both strains also successfully infected h-astrocytes, as the corresponding cell lysates tested positive for Nef and p24 (Fig. 3b). NL4-3 Δnef-infected h-astrocytes showed weaker signal for p24, but the infectivity assay performed on the TMZ-bl cell lines demonstrated comparable infectivity with other strains (data not shown).
Fig. 3.
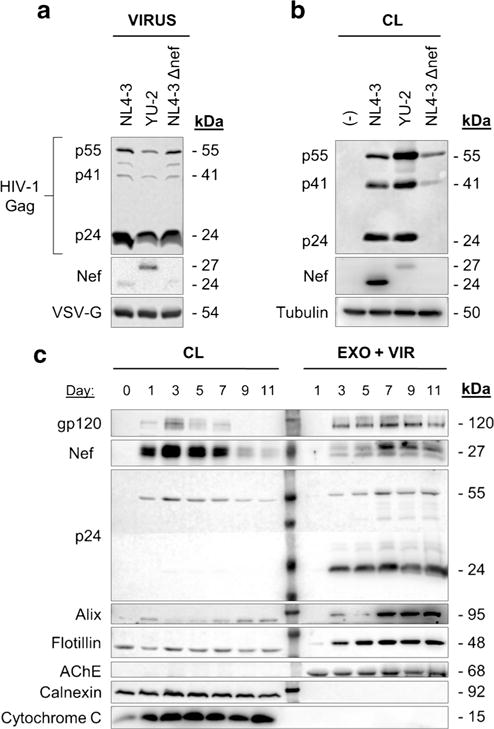
HIV-1-infected h-astrocytes release Nef extracellularly. a Immunoblot of pseudotyped NL4-3, YU-2, and NL4-3 Δnef virions. Antibodies directed against viral proteins Gag (p24, p41, and p55), VSV-G, and Nef were used. b Immunoblot of lysed pseudotyped HIV-1-infected astrocytes. Antibodies directed against viral proteins Nef and p24 were used, while tubulin was tested as loading control. c Immunoblot of lysed pseudotyped NL4–3-infected h-astrocytes and the particles they released into the media. Particles were pelleted by ultracentrifugation through sucrose cushion. Antibodies directed against viral proteins (gp120, Nef, p24), proteins enriched in exosomes (Alix, flotillin, AChE), and marker for endoplasmic reticulum (calnexin) and mitochondria (cytochrome c) were used. CL cell lysate, EXO exosomes, VIR virus
We then wanted to establish if and at what time-point after pseudotyped HIV-1 infection of h-astrocytes viral protein Nef is released extracellularly. Thus, we grew NL4-3-infected h-astrocytes for 11 days, collected the particles released into the culture by purification through sucrose cushion at indicated times, and tested the pellet for the presence of Nef and selected viral and exosomal proteins by immunoblotting (Fig. 3c). Corresponding HIV-1-infected h-astrocyte cell lysates were similarly analyzed. While Nef was expressed in high amounts in infected h-astrocytes from day 1 to 7, it was only released extracellularly from the third day onwards, with the peak in release on day 7. Viral proteins gp120 and p24 were detected in the media of infected h-astrocytes growing at least for 3 days, indicating that viruses are released constantly from the third day onwards (Fig. 3c). Alix, flotillin, and AChE, proteins typically enriched in exosomes, were detected in the media from the seventh, third, or first day onwards, respectively, indicating that different subpopulations of exosomes are released from infected h-astrocytes. AChE was not in the detection range in cell lysates, probably due to low level of this protein inside the cells, relative to its enrichment in exosomes. As the signal for extracellular Nef strongly intensified at day 7, but similar signal increase was not observed for p24, the increase in Nef signal might be due to release of Nef in exosomes carrying Alix, signal for which also intensified on day 7. The purified pellet tested negative for calnexin, a marker for endoplasmic reticulum, and cytochrome c, a marker for mitochondria. These results suggest that pseudotyped HIV-1-infected h-astrocytes release Nef extracellularly, possibly in viruses and exosomes.
Nef is released in exosomes from pseudotyped HIV-1-infected h-astrocytes
To further support the hypothesis that Nef is released from pseudotyped HIV-1-infected h-astrocytes in exosomes, we separated the released exosomes and viruses based on their sedimentation velocity and tested the particles for Nef and typical exosomal and viral proteins to discriminate between the virus-associated and exosome-associated Nef (Fig. 4a).
Fig. 4.
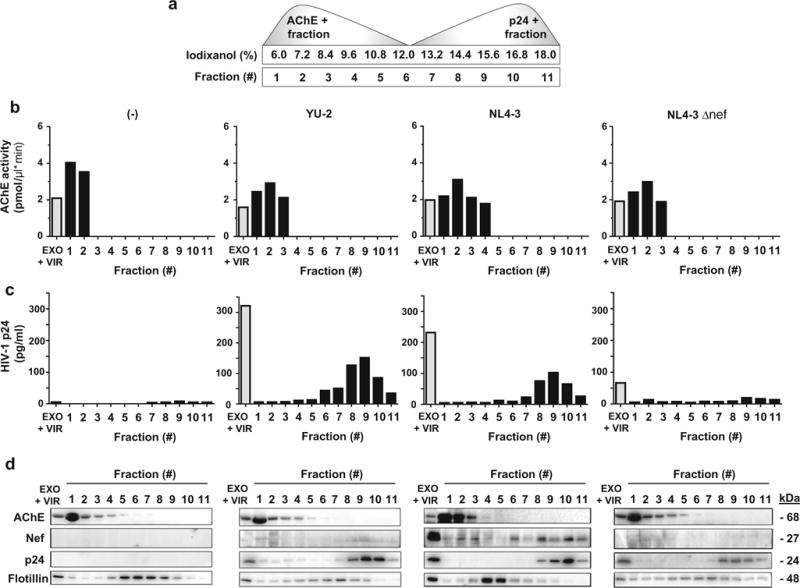
Pseudotyped HIV-1-infected h-astrocytes secrete exosomes containing Nef. a Schematic illustration of exosomal (AChE+) and viral (p24+) fractions after separation on iodixanol velocity gradient. b AChE activity was quantified by colorimetric assay in all fractions for the presence of exosomes. c The presence of p24 in fractions was quantified by HIV-1 p24 ELISA assay. d Immunoblot of proteins precipitated from 11 collected fractions. Antibodies directed against viral proteins, Nef and p24, and proteins enriched in exosomes, AChE and flotillin, were used. EXO exosomes, VIR virus, AChE acetylcholinesterase
We infected h-astrocytes with pseudotyped isolates YU-2 and NL4-3 and collected the cell culture media for 7 days, to maximize the number of released particles carrying Nef. We used pseudotyped NL4-3 Δnef infected and uninfected h-astrocytes as controls. To separate exosomes from viruses, we subjected them to velocity gradient centrifugation in 6–18% iodixanol, as described previously (Cantin et al. 2008; Lenassi et al. 2010). We collected 11 fractions from the top of the gradient and measured the AChE activity, which is typically enriched in exosomes (Savina et al. 2002) and p24 concentration (with ELISA), which is abundantly present in virions, for each fraction. AChE activity was detected mostly in first four fractions in all tested samples (Fig. 4b), while viral protein p24 was present in fractions 6–11 in YU-2 and NL4-3 samples (Fig. 4c). Concentration of p24 was low in NL4-3 Δnef sample and absent in uninfected control. Altogether, these data support the efficient separation of exosomes and viruses on the gradient.
We next tested the fractions of all samples for Nef, virion protein p24, and typical exosomal proteins AChE and flotillin by immunoblotting (Fig. 4d). Nef was detected in most fractions in YU-2 and NL4-3 samples, but the signal for YU-2 was very weak. Interestingly, NL4-3 sample showed varying signal intensities between the fractions, with most Nef localizing to fractions 2, 3, 6, 8, and 10. AChE was detected in the first three to four fractions in YU-2 and NL4-3 samples, which mostly coincided with the measured AChE activity. Conversely, p24 was detected in fractions 8–11, consistent with the p24 ELISA measurements. Flotillin was detected in all fractions, but the signal was more intense in the middle of the iodixanol velocity gradient. Thus, upper Nef immunopositive fractions are also immunopositive for exosomal proteins AChE and/or flotillin, while lower Nef immunopositive fractions are also immunopositive for viral protein p24 (Fig. 4d). The NL4-3 Δnef sample and the uninfected control showed similar patterns to YU-2 and NL4-3, except Nef or/and p24 were absent in control samples, respectively. These results confirm that in addition to viruses, pseudotyped HIV-1-infected h-astrocytes secrete exosomes, which contain Nef.
Nef is released in exosomes from native HIV-1-infected h-astrocytes
Recently, a low level native infection of astrocytes by receptor-mediated endocytosis of HIV-1 was demonstrated in vitro (Chauhan et al. 2014; Gray et al. 2014; Liu et al. 2004). To test the effect of direct HIV-1 infection of astrocytes on Nef-exosome release, we infected h-astrocytes with native NL4-3 and tested the released exosomes for Nef with immunoblotting.
First, we characterized the NL4-3 virions composition and effectiveness of h-astrocyte infection by immunoblotting (Fig. 5a, b). The virions tested positive for Gag proteins p55, p41, and p24, for envelope protein gp120 and for Nef (27 kDa; Fig. 5a); and demonstrated comparable infectivity of TMZ-bl cell lines to pseudotyped strains (data not shown). Compared to pseudotyped HIV-1 infection (Fig. 3b), native infection of h-astrocytes with NL4-3 was weaker, with cell lysates immunopositive only for p55 Gag precursor (Fig. 5b). Nevertheless, we could observe similar expression of Nef in native NL4-3 (Fig. 5b) compared to pseudotyped NL4-3 infection (Fig. 3b).
Fig. 5.
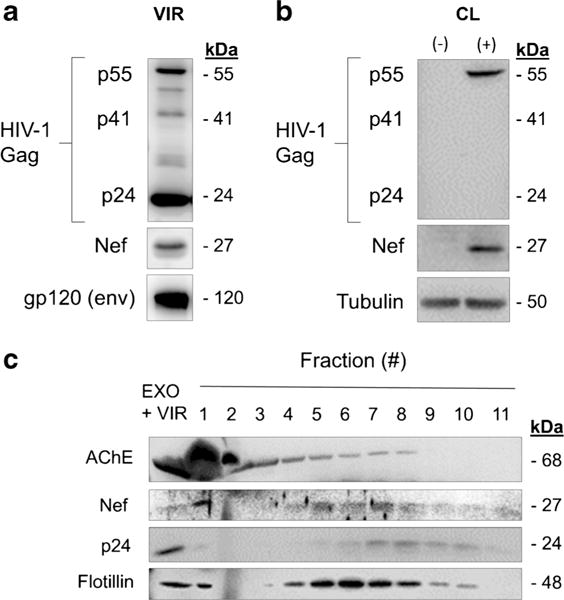
HIV-1-infected h-astrocytes secrete exosomes containing Nef. a Immunoblot of native NL4–3 virions. Antibodies directed against viral proteins Gag (p24, p41, and p55), gp120, and Nef were used. b Immunoblot of lysed native NL4-3-infected h-astrocytes. Antibodies directed against viral proteins Nef and Gag were used, while tubulin was tested as loading control. c Immunoblot of proteins precipitated from 11 fractions collected from iodixanol velocity gradient. Antibodies directed against viral proteins, Nef and p24, and proteins enriched in exosomes, AChE and flotillin, were used. EXO exosomes, VIR virus, AChE acetylcholinesterase
We next isolated the particles from the media of a 7-day old culture of h-astrocytes infected with native NL4-3, separated the exosome and viral populations with velocity gradient centrifugation, and tested the collected fractions for Nef, virion protein p24, and typical exosomal proteins AChE and flotillin by immunoblotting (Fig. 5c), as described for Fig. 4. Exosomal proteins AChE and flotillin were detected in fractions 1–5 (weaker signal in fractions 6–8) and fractions 1 and 4–10, respectively; whereas, viral protein p24 was detected in fractions 7–10. Importantly, fractions immunopositive for exosomal proteins AChE and/or flotillin were overlapping with upper Nef immunopositive fractions (1, 4–6), while lower Nef immunopositive fractions (7–8) were overlapping with fractions immunopositive for viral protein p24 (7–10; Fig. 5c). Altogether, these data support the release of Nef-exosomes from h-astrocytes infected with native HIV-1.
Discussion
In this study, we showed that Nef.GFP-expressing or HIV-1-infected h-astrocytes release Nef in exosomes. Importantly, Nef.GFP expression induces the secretion of exosomes from h-astrocytes up to 5.5-fold in comparison to control.
In addition to macrophages and microglia, astrocytes are also infected with HIV-1 in vivo, as was demonstrated in the brain tissue of individuals who died with HAD (Churchill et al., 2006) or were in the pre-symptomatic stage of HIV-1 infection (Thompson et al. 2011). The percentage of infected astrocytes in vivo was first believed to be relatively low (mean 2.6%) (Bagasra et al. 1996), but newer studies show that 16–19% of astrocytes are infected with HIV-1 in individuals with HAD (Churchill et al. 2009). It is believed that infection of astrocytes is followed by an acute phase, where astrocytes produce low levels of virus (Gorry et al. 1999) and later by a dormant phase with low levels of viral expression (Shahabuddin et al. 1992) or even latency (Brack-Werner 1999; Churchill et al. 2009). Nevertheless, HIV-1 infection of astrocytes leads to abundant expression of HIV-1 early gene products like Nef, Tat, and Rev (Ranki et al. 1995; Saito et al. 1994). As astrocytes are the most abundant cell type in the brain (approximately 0.4–2.0 × 1012 cells), they may represent a significant source of viral proteins. In the present study, we used normal human astrocytes with over-expressed telomerase and alternations in tumor suppressors (h-astrocytes) (Sonoda et al. 2001) as a model for brain astrocytes. We appreciate the tendency for experiments to be performed on primary cells, but large culture volumes are necessary to isolate enough EVs (exosomes) for biochemical (and other) analysis, which is only possible when growing immortalized cells.
Nef is secreted in exosomes from Nef-expressing Hela.CIITA cells (Lenassi et al. 2010), HEK293 cells (Ali et al. 2010), T cells (Ali et al. 2010; Lenassi et al. 2010; Muratori et al. 2009), U937 monocytes (Aqil et al. 2015), and microglia (Raymond et al. 2016). A recent report supports the release of Nef-exosomes from astrocytes transduced with adenovirus construct expressing Nef, but only a trace amount (Sami Saribas et al. 2017) or no Nef.GFP (Raymond et al. 2016) was detected in exosomes released from Nef.GFP-expressing astrocytes. To resolve this conundrum, we reevaluated EVs released from Nef.GFP-expressing h-astrocytes using a method previously employed by us for EVs released from HeLa.CIITA, Jurkat, SupT1, primary T cells and microglia. Here, we show that Nef.GFP-expressing h-astrocytes secrete Nef in EVs. Based on separation of Nef.GFP in fractions with densities typical for exosomes (Raposo et al. 1996), colocalization of Nef.GFP with distinctive exosome proteins (AChE, flotillin) in the density gradient fractions, detection of Nef.GFP in purified exosomes, and the characteristic cup-shaped morphology of these vesicles under TEM (Fevrier and Raposo 2004), we identified these vesicles as exosomes. Using protein and NTA analysis, we additionally showed that h-astrocytes expressing Nef.GFP released up to 5.5-fold more and smaller exosomes compared to GFP-expressing controls, similarly to what we observed in Nef.GFP-expressing Jurkat and SupT1 T cells (Lenassi et al. 2010).
We can only speculate about the reasons for observed differences in the effects of Nef on vesicle release from astrocytes. The expression of different Nef alleles in these studies was connected to conserved effect of Nef SF2 allele on Nefexosome release and the lack of this effect in the case of Nef NL4-3 allele (Raymond et al. 2016; Sami Saribas et al. 2017). Similar functional differences between SF2 and NL4-3 Nef alleles were also observed for downregulation of diverse receptors, regulation of signaling pathways, virus particle infectivity, and inducement of pathogenicity in transgenic mice (Foster et al. 2001; Priceputu et al. 2007). Additionally, the method used for transient ectopic expression of Nef in astrocytes (various transfection protocols vs transduction) can affect the percentage of Nef-expressing astrocytes in the population, thereby affecting the concentration of EVs in the culture. Interestingly, GFP was detected in exosomes released from GFP-expressing h-astrocytes in our study, which could imply that GFP drives the incorporation of Nef.GFP into exosomes. This was also one of the concerns raised in the study by Luo et al., where low level of GFP was detected in exosomes purified from HEK293T and Jurkat cultures, which could be interpreted as some non-specific artifact (Luo et al. 2015). We cannot exclude some contribution of GFP to incorporation of Nef.GFP into exosomes, but we have shown here that Nef-exosomes are also released from h-astrocytes infected with native or pseudotyped HIV-1, which supports a more direct role of Nef in the process. Previous studies also detected Nef-containing EVs in the plasma of HIV-1-infected patients (Khan et al. 2016; Lee et al. 2016; Raymond et al. 2011). All this is in alignment with the study by Ali et al. that identified basic cluster of four arginine residues, the phosphofurin acidic cluster sequence, and the secretion modification region of Nef as critical for Nef-induced vesicle binding and vesicle secretion (Ali et al. 2010). It would be interesting to learn if these Nef regions are also critical for the documented effect of Nef expression or HIV-1 infection on the increase in the number of MVBs (Costa et al. 2006; Stumptner-Cuvelette et al. 2003), the organelle involved in exosome biogenesis.
Previous studies support restriction of astrocyte infection at the level of HIV-1 entry due to absence of classical CD4-receptor (Di Rienzo et al. 1998; Gorry et al. 1998; Neumann et al. 2001) and further show that HIV-1 entry into astrocytes is CD4, CXCR4, and CCR5-independent (Sabri et al. Schweighardt and Atwood 2001). This restriction can be overridden to some extent by pseudotyping the virus (Canki et al. 2001; Carroll-Anzinger and Al-Harthi 2006; Chauhan et al. 2014; Churchill et al. 2006; Churchill et al. 2009; Gray et al. 2014; Wang et al. 2008); thus, we used VSV-G pseudotyped prototypic blood-derived (NL4–3), brain-derived (YU-2), and nef-deleted (NL4-3 Δnef) HIV-1 strains to infect astrocytes in our studies. Importantly, low level natural infection of astrocytes through HIV-1 endocytosis (Chauhan et al. 2014) or through interaction with alternative mannose receptor (Gray et al. 2014; Liu et al. 2004) or DC-SIGN receptor (Deiva et al. 2006) was also demonstrated. Similarly, we showed low level infection of h-astrocytes with native HIV-1, although additional restrictions seem to be present intracellularly as we could only detect unprocessed Gag in the cell lysate and observed only limited release of virus bound p24, corroborating previous reports of additional intracellular restrictions on HIV replication in astrocytes (Brack-Werner 1999; Li et al. 2002). When analyzing the infected h-astrocytes culture media, we used iodixanol gradient to separate exosomes from viruses, according to their sedimentation velocity (Cantin et al. 2008; Lenassi et al. 2010). We showed by immunoblotting that Nef is secreted with exosomes and viruses from h-astrocytes infected with native or pseudotyped HIV-1. Signal for Nef namely appeared in fractions, immunopositive for proteins enriched in exosomes, and with measurable AChE activity typically detected in exosomes (Savina et al. 2002), as well as in separate fractions immunopositive for the viral capsid protein p24. Nef signal in general was much weaker when h-astrocytes were infected with pseudotyped YU-2 strain. This coincides with the weak signal for Nef in cell lysates of HIV-1 YU-2-infected h-astrocytes (Fig. 3b). Interestingly, Nef signal from lysed virions of HIV-1 YU-2-infected HEK293T cells was strong, suggesting that Nef is efficiently packed in virions in HEK293T cells but is weakly expressed in YU-2-infected h-astrocytes. Yet Nef was expressed to similar levels in h-astrocytes infected with native compared to pseudotyped HIV-1 NL4-3, although only unprocessed Gag was detected in the cell lysate, and low level of virus bound p24 was released extracellularly. Nevertheless, regardless of the HIV-1 strain used, Nef was released in exosomes from HIV-1-infected h-astrocytes, implying that these cells could contribute to the pool of biologically active Nef-exosomes in the brain.
Biological activity of Nef-exosomes on the surrounding cellular environment has been demonstrated numerous times. Nef-exosomes from HIV-1-infected cells were shown to render resting human primary CD4+ T cells permissive to HIV-1 infection (Arenaccio et al. 2014) and can activate latent HIV-1 in primary human CD4+ T lymphocytes (Arenaccio et al. 2014) and U937 cells (Arenaccio et al. 2015). In human monocytes, Nef expression modulates cellular and exosomal miRNA profiles (Aqil et al. 2015). Nef-exosomes from Nef-expressing cells trigger apoptosis in bystander T cells (Lenassi et al. 2010) and impair the BBB (Raymond et al. 2016), while Nef-exosomes isolated from the plasma of individuals with HAD induce the secretion of Aβ from neuroblastoma cell line SH-SY5Y (Khan et al. 2016). Recently, it was also shown that EVs, released from Nef-expressing astrocyte cultures, are taken up by neurons, where they induce oxidative stress (Sami Saribas et al. 2017). Furthermore, elevated levels of EVs carrying viral proteins, including Nef, in the blood of aviremic HIV-infected patients, were shown to have pro-inflammatory function (Lee et al. 2016). All these biological functions could affect homeostasis in neurons, which are not infected by HIV-1 themselves, but are damaged by soluble factors (including viral proteins) released from infected non-neural cells, and by the inflammatory response in the central nervous system. Neuron injury eventually manifests as cognitive, motor, and behavioral impairments in HIV-1-infected individuals, collectively called HIV-associated neurocognitive disorders (Gonzalez-Scarano and Martin-Garcia 2005; Kaul et al. 2005). Our results provide the basis for future studies of the damaging role of Nef-exosomes and infected astrocytes producing them, on the central nervous system.
Acknowledgments
We thank Prof. Robert Zorec, PhD, for the access to the Nucleofector 2b, Prof. Yifan Cheng, PhD, for help with TEM imaging and Anja Kejžar, PhD, for helping us with the NTA measurements. This work was supported by research grants J3-5499 and P1-0170 funded by the Slovenian Research Agency (ARRS).
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- Ali SA, Huang MB, Campbell PE, Roth WW, Campbell T, Khan M, Newman G, Villinger F, Powell MD, Bond VC. Genetic characterization of HIV type 1 Nef-induced vesicle secretion. AIDS Res Hum Retrovir. 2010;26:173–192. doi: 10.1089/aid.2009.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. 2005;64:529–536. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- Aqil M, Mallik S, Bandyopadhyay S, Maulik U, Jameel S. Transcriptomic analysis of mRNAs in human monocytic cells expressing the HIV-1 Nef protein and their exosomes. Biomed Res Int. 2015;2015:492395. doi: 10.1155/2015/492395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenaccio C, Anticoli S, Manfredi F, Chiozzini C, Olivetta E, Federico M. Latent HIV-1 is activated by exosomes from cells infected with either replication-competent or defective HIV-1. Retrovirology. 2015;12:87. doi: 10.1186/s12977-015-0216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenaccio C, Chiozzini C, Columba-Cabezas S, Manfredi F, Affabris E, Baur A, Federico M. Exosomes from human immunodeficiency virus type 1 (HIV-1)-infected cells license quiescent CD4+ T lymphocytes to replicate HIV-1 through a Nef- and ADAM17-dependent mechanism. J Virol. 2014;88:11529–11539. doi: 10.1128/JVI.01712-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner JP, Tawadros R, Pomerantz RJ. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10:573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- Canki M, Thai JN, Chao W, Ghorpade A, Potash MJ, Volsky DJ. Highly productive infection with pseudotyped human immunodeficiency virus type 1 (HIV-1) indicates no intracellular restrictions to HIV-1 replication in primary human astrocytes. J Virol. 2001;75:7925–7933. doi: 10.1128/JVI.75.17.7925-7933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin R, Diou J, Belanger D, Tremblay AM, Gilbert C. Discrimination between exosomes and HIV-1: purification of both vesicles from cell-free supernatants. J Immunol Methods. 2008;338:21–30. doi: 10.1016/j.jim.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Carroll-Anzinger D, Al-Harthi L. Gamma interferon primes productive human immunodeficiency virus infection in astrocytes. J Virol. 2006;80:541–544. doi: 10.1128/JVI.80.1.541-544.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, Mehla R, Vijayakumar TS, Handy I. Endocytosis-mediated HIV-1 entry and its significance in the elusive behavior of the virus in astrocytes. Virology. 2014;457:1–19. doi: 10.1016/j.virol.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chompre G, Cruz E, Maldonado L, Rivera-Amill V, Porter JT, Noel RJ., Jr Astrocytic expression of HIV-1 Nef impairs spatial and recognition memory. Neurobiol Dis. 2013;49:128–136. doi: 10.1016/j.nbd.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill MJ, Gorry PR, Cowley D, Lal L, Sonza S, Purcell DF, Thompson KA, Gabuzda D, McArthur JC, Pardo CA, Wesselingh SL. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neuro-Oncol. 2006;12:146–152. doi: 10.1080/13550280600748946. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol. 2009;66:253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- Costa LJ, Chen N, Lopes A, Aguiar RS, Tanuri A, Plemenitas A, Peterlin BM. Interactions between Nef and AIP1 proliferate multivesicular bodies and facilitate egress of HIV-1. Retrovirology. 2006;3:33. doi: 10.1186/1742-4690-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiva K, Khiati A, Hery C, Salim H, Leclerc P, Horellou P, Tardieu M. CCR5-, DC-SIGN-dependent endocytosis and delayed reverse transcription after human immunodeficiency virus type 1 infection in human astrocytes. AIDS Res Hum Retrovir. 2006;22:1152–1161. doi: 10.1089/aid.2006.22.1152. [DOI] [PubMed] [Google Scholar]
- Di Rienzo AM, Aloisi F, Santarcangelo AC, Palladino C, Olivetta E, Genovese D, Verani P, Levi G. Virological and molecular parameters of HIV-1 infection of human embryonic astrocytes. Arch Virol. 1998;143:1599–1615. doi: 10.1007/s007050050401. [DOI] [PubMed] [Google Scholar]
- Donati D, Martinelli E, Cassiani-Ingoni R, Ahlqvist J, Hou J, Major EO, Jacobson S. Variant-specific tropism of human herpesvirus 6 in human astrocytes. J Virol. 2005;79:9439–9448. doi: 10.1128/JVI.79.15.9439-9448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drogemuller K, Helmuth U, Brunn A, Sakowicz-Burkiewicz M, Gutmann DH, Mueller W, Deckert M, Schluter D. Astrocyte gp130 expression is critical for the control of toxoplasma encephalitis. J Immunol. 2008;181:2683–2693. doi: 10.4049/jimmunol.181.4.2683. [DOI] [PubMed] [Google Scholar]
- Epstein LG, Sharer LR, Cho ES, Myenhofer M, Navia B, Price RW. HTLV-III/LAV-like retrovirus particles in the brains of patients with AIDS encephalopathy. AIDS Res. 1984;1:447–454. doi: 10.1089/aid.1.1983.1.447. [DOI] [PubMed] [Google Scholar]
- Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Foster JL, Molina RP, Luo T, Arora VK, Huang Y, Ho DD, Garcia JV. Genetic and functional diversity of human immunodeficiency virus type 1 subtype B Nef primary isolates. J Virol. 2001;75:1672–1680. doi: 10.1128/JVI.75.4.1672-1680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer M, Peterlin BM. Domain assembly, surface accessibility and sequence conservation in full length HIV-1 Nef. FEBS Lett. 2001;496:91–95. doi: 10.1016/s0014-5793(01)02394-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Gorry P, Purcell D, Howard J, McPhee D. Restricted HIV-1 infection of human astrocytes: potential role of nef in the regulation of virus replication. J Neuro-Oncol. 1998;4:377–386. doi: 10.3109/13550289809114536. [DOI] [PubMed] [Google Scholar]
- Gorry PR, Howard JL, Churchill MJ, Anderson JL, Cunningham A, Adrian D, McPhee DA, Purcell DF. Diminished production of human immunodeficiency virus type 1 in astrocytes results from inefficient translation of Gag, Env, and Nef mRNAs despite efficient expression of Tat and Rev. J Virol. 1999;73:352–361. doi: 10.1128/jvi.73.1.352-361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LR, Turville SG, Hitchen TL, Cheng WJ, Ellett AM, Salimi H, Roche MJ, Wesselingh SL, Gorry PR, Churchill MJ. HIV-1 entry and trans-infection of astrocytes involves CD81 vesicles. PLoS One. 2014;9:e90620. doi: 10.1371/journal.pone.0090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hult B, Chana G, Masliah E, Everall I. Neurobiology of HIV. Int Rev Psychiatry. 2008;20:3–13. doi: 10.1080/09540260701862086. [DOI] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12:878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Kestler HW, 3rd, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Importance of the Nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Khan MB, Lang MJ, Huang MB, Raymond A, Bond VC, Shiramizu B, Powell MD. Nef exosomes isolated from the plasma of individuals with HIV-associated dementia (HAD) can induce Abeta(1-42) secretion in SH-SY5Y neural cells. J Neuro-Oncol. 2016;22:179–190. doi: 10.1007/s13365-015-0383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Schierer S, Blume K, Dindorf J, Wittki S, Xiang W, Ostalecki C, Koliha N, Wild S, Schuler G, Fackler OT, Saksela K, Harrer T, Baur AS. HIV-Nef and ADAM17-containing plasma extracellular vesicles induce and correlate with immune pathogenesis in chronic HIV infection. EBioMedicine. 2016;6:103–113. doi: 10.1016/j.ebiom.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann MH, Masanetz S, Kramer S, Erfle V. HIV-1 Nef upregulates CCL2/MCP-1 expression in astrocytes in a myristoylation- and calmodulin-dependent manner. J Cell Sci. 2006;119:4520–4530. doi: 10.1242/jcs.03231. [DOI] [PubMed] [Google Scholar]
- Lenassi M, Cagney G, Liao M, Vaupotic T, Bartholomeeusen K, Cheng Y, Krogan NJ, Plemenitas A, Peterlin BM. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11:110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu Y, Park IW, He JJ. Expression of exogenous Sam68, the 68-kilodalton SRC-associated protein in mitosis, is able to alleviate impaired Rev function in astrocytes. J Virol. 2002;76:4526–4535. doi: 10.1128/JVI.76.9.4526-4535.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kumar A. Differential signaling mechanism for HIV-1 Nef-mediated production of IL-6 and IL-8 in human astrocytes. Sci Rep. 2015;5:9867. doi: 10.1038/srep09867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Shah A, Gangwani MR, Silverstein PS, Fu M, Kumar A. HIV-1 Nef induces CCL5 production in astrocytes through p38-MAPK and PI3K/Akt pathway and utilizes NF-kB, CEBP and AP-1 transcription factors. Sci Rep. 2014;4:4450. doi: 10.1038/srep04450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu H, Kim BO, Gattone VH, Li J, Nath A, Blum J, He JJ. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. J Virol. 2004;78:4120–4133. doi: 10.1128/JVI.78.8.4120-4133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Fan Y, Park IW, He JJ. Exosomes are unlikely involved in intercellular Nef transfer. PLoS One. 2015;10:e0124436. doi: 10.1371/journal.pone.0124436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid R, Janvier K, Hitchin D, Day J, Coleman S, Noviello C, Bouchet J, Benmerah A, Guatelli J, Benichou S. Nef-induced alteration of the early/recycling endosomal compartment correlates with enhancement of HIV-1 infectivity. J Biol Chem. 2005;280:5032–5044. doi: 10.1074/jbc.M401202200. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Brew BJ. HIV-associated neurocognitive disorders: is there a hidden epidemic? AIDS. 2010;24:1367–1370. doi: 10.1097/QAD.0b013e3283391d56. [DOI] [PubMed] [Google Scholar]
- Muratori C, Cavallin LE, Kratzel K, Tinari A, De Milito A, Fais S, D'Aloja P, Federico M, Vullo V, Fomina A, Mesri EA, Superti F, Baur AS. Massive secretion by T cells is caused by HIV Nef in infected cells and by Nef transfer to bystander cells. Cell Host Microbe. 2009;6:218–230. doi: 10.1016/j.chom.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Neumann M, Afonina E, Ceccherini-Silberstein F, Schlicht S, Erfle V, Pavlakis GN, Brack-Werner R. Nucleocytoplasmic transport in human astrocytes: decreased nuclear uptake of the HIV Rev shuttle protein. J Cell Sci. 2001;114:1717–1729. doi: 10.1242/jcs.114.9.1717. [DOI] [PubMed] [Google Scholar]
- Ohi M, Li Y, Cheng Y, Walz T. Negative staining and image classification—powerful tools in modern electron microscopy. Biol Proced Online. 2004;6:23–34. doi: 10.1251/bpo70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priceputu E, Hanna Z, Hu C, Simard MC, Vincent P, Wildum S, Schindler M, Kirchhoff F, Jolicoeur P. Primary human immunodeficiency virus type 1 nef alleles show major differences in pathogenicity in transgenic mice. J Virol. 2007;81:4677–4693. doi: 10.1128/JVI.02691-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranki A, Nyberg M, Ovod V, Haltia M, Elovaara I, Raininko R, Haapasalo H, Krohn K. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS. 1995;9:1001–1008. doi: 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond AD, Campbell-Sims TC, Khan M, Lang M, Huang MB, Bond VC, Powell MD. HIV type 1 Nef is released from infected cells in CD45(+) microvesicles and is present in the plasma of HIV-infected individuals. AIDS Res Hum Retrovir. 2011;27:167–178. doi: 10.1089/aid.2009.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond AD, Diaz P, Chevelon S, Agudelo M, Yndart-Arias A, Ding H, Kaushik A, Jayant RD, Nikkhah-Moshaie R, Roy U, Pilakka-Kanthikeel S, Nair MP. Microglia-derived HIV Nef+ exosome impairment of the blood-brain barrier is treatable by nanomedicine-based delivery of Nef peptides. J Neuro-Oncol. 2016;22:129–139. doi: 10.1007/s13365-015-0397-0. [DOI] [PubMed] [Google Scholar]
- Saito Y, Sharer LR, Epstein LG, Michaels J, Mintz M, Louder M, Golding K, Cvetkovich TA, Blumberg BM. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994;44:474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- Sami Saribas A, Cicalese S, Ahooyi TM, Khalili K, Amini S, Sariyer IK. HIV-1 Nef is released in extracellular vesicles derived from astrocytes: evidence for Nef-mediated neurotoxicity. Cell Death Dis. 2017;8:e2542. doi: 10.1038/cddis.2016.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardo L, Iordanskiy S, Klase Z, Kashanchi F. HIV-1 Nef blocks autophagy in human astrocytes. Cell Cycle. 2015;14:3781–3782. doi: 10.1080/15384101.2015.1105700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarzotti-Kelsoe M, Bailer RT, Turk E, Lin CL, Bilska M, Greene KM, Gao H, Todd CA, Ozaki DA, Seaman MS, Mascola JR, Montefiori DC. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J Immunol Methods. 2014;409:131–146. doi: 10.1016/j.jim.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci. 2002;115:2505–2515. doi: 10.1242/jcs.115.12.2505. [DOI] [PubMed] [Google Scholar]
- Schweighardt B, Atwood WJ. HIV type 1 infection of human astrocytes is restricted by inefficient viral entry. AIDS Res Hum Retrovir. 2001;17:1133–1142. doi: 10.1089/088922201316912745. [DOI] [PubMed] [Google Scholar]
- Semmler A, Okulla T, Sastre M, Dumitrescu-Ozimek L, Heneka MT. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat. 2005;30:144–157. doi: 10.1016/j.jchemneu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Shahabuddin M, Volsky B, Kim H, Sakai K, Volsky DJ. Regulated expression of human immunodeficiency virus type 1 in human glial cells: induction of dormant virus. Pathobiology. 1992;60:195–205. doi: 10.1159/000163723. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda Y, Ozawa T, Hirose Y, Aldape KD, McMahon M, Berger MS, Pieper RO. Formation of intracranial tumors by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer Res. 2001;61:4956–4960. [PubMed] [Google Scholar]
- Stumptner-Cuvelette P, Jouve M, Helft J, Dugast M, Glouzman AS, Jooss K, Raposo G, Benaroch P. Human immunodeficiency virus-1 Nef expression induces intracellular accumulation of multivesicular bodies and major histocompatibility complex class II complexes: potential role of phosphatidylinositol 3-kinase. Mol Biol Cell. 2003;14:4857–4870. doi: 10.1091/mbc.E03-04-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Wesselingh SL, Griffin DE, McArthur JC, Johnson RT, Glass JD. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol. 1996;39:705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, Simpson RJ. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56:293–304. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- Thompson KA, Cherry CL, Bell JE, McLean CA. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am J Pathol. 2011;179:1623–1629. doi: 10.1016/j.ajpath.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatore C, Chandra R, Berger JR, Major EO. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994;44:481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- Trillo-Pazos G, Diamanturos A, Rislove L, Menza T, Chao W, Belem P, Sadiq S, Morgello S, Sharer L, Volsky DJ. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain Pathol. 2003;13:144–154. doi: 10.1111/j.1750-3639.2003.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle G, Henry S, Todoruk T, Sullivan A, Silva C, Rourke SB, Holden J, McArthur JC, Gill MJ, Power C. Human immunedeficiency virus type 1 Nef protein mediates neural cell death: a neurotoxic role for IP-10. Virology. 2004;329:302–318. doi: 10.1016/j.virol.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Wang T, Gong N, Liu J, Kadiu I, Kraft-Terry SD, Schlautman JD, Ciborowski P, Volsky DJ, Gendelman HE. HIV-1-infected astrocytes and the microglial proteome. J NeuroImmune Pharmacol. 2008;3:173–186. doi: 10.1007/s11481-008-9110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


