Abstract
Non-small cell lung cancer (NSCLC) patients harboring EGFR-activating mutations initially respond to EGFR tyrosine kinase inhibitors (EGFR-TKIs) and have shown favorable outcomes. However, acquired drug resistance to EGFR-TKIs develops in almost all patients mainly due to the EGFR T790M mutation. Here, we show that treatment with low-dose EGFR-TKI results in the emergence of the EGFR T790M mutation and in the reduction of HSP70 protein levels in HCC827 cells. Erlotinib treatment inhibits HSP70 phosphorylation at tyrosine 41 and increases HSP70 ubiquitination, resulting in HSP70 degradation. We show that EGFR-TKI treatment causes increased DNA damage and enhanced gene mutation rates, which are secondary to the EGFR-TKI-induced reduction of HSP70 protein. Importantly, HSP70 overexpression delays the occurrence of Erlotinib-induced EGFR T790M mutation. We further demonstrate that HSP70 interacts with multiple enzymes in the base excision repair (BER) pathway and promotes not only the efficiency but also the fidelity of BER. Collectively, our findings show that EGFR-TKI treatment facilitates gene mutation and the emergence of EGFR T790M secondary mutation by the attenuation of BER via induction of HSP70 protein degradation.
Keywords: EGFR-TKI, HSP70 phosphorylation and degradation, EGFR T790M mutation, Base excision repair, Lung cancer
1. Introduction
Lung carcinoma is the leading cause of cancer-related deaths worldwide [1]. Chemotherapy for non-small cell lung cancer (NSCLC), which accounts for approximately 85% of lung cancer cases, has remained marginally effective [2]. Considerable progress has been achieved in the treatment of NSCLC with the development of molecular targeted therapies against epidermal growth factor receptor (EGFR). EGFR is the oncogenic tyrosine kinase (TK) that triggers a cascade of phosphorylation and activation of signaling pathways that increase tumor cell proliferation, angiogenesis, invasion and metastasis as well as inhibit apoptosis [3]. Two EGFR TK inhibitors (EGFR-TKIs), Gefitinib (Iressa) and Erlotinib (Tarceva), have been FDA approved for the treatment of advanced or metastatic NSCLC [4–6]. Growing evidence has demonstrated that most advanced NSCLCs with EGFR-activating mutations occurring in the TK domain (mostly exon 19 deletions and L858R exon 21 mutations in EGFR gene) initially respond to Gefitinib or Erlotinib with hypersensitivity. However, over time (9–12 months of treatment), almost all these patients eventually develop acquired resistance to EGFR-TKIs, thus limiting the improvement in patient outcomes [7]. Recent efforts in developing strategies to overcome the acquired EGFR-TKI resistance have revealed several resistance mechanisms, as recently reviewed [7–9]. The most common mechanism that confers drug resistance involves a secondary T790M point mutation on exon 20 of the EGFR gene, which is associated to 50–65% of resistance cases [10, 11]. This mutation, which occurs at nucleotide position 2,369 resulting in a C-T transversion and a substitution of methionine for threonine at position 790 (T790M) within the EGFR TK domain, causes an increased affinity of EGFR for ATP rather than for EGFR-TKI [12]. Recently, Arcila et al. utilized a highly sensitive sequencing approach and identified the EGFR T790M mutation in lung tumors from 68% of the patients who acquired resistance to EGFR-TKIs in their study [13]. In fact, a new category of TKIs have been designed to directly target T790M-mutant NSCLC cells [14–17]; however, most of them are still either in early development or far from clinical applications because of the severe toxicities, except for AZD9291, the first and only FDA-approved third-generation EGFR-TKI. That said, resistance to AZD9291 has also been reported to arise after 9–13 months of therapy mainly due to an acquired C797S mutation in EGFR [18, 19]. Hence, an unmet need exists for unveiling the mechanisms underlying the occurrence of EGFR resistant mutations and developing alternative strategies in preventing the onset of resistance to enhance the clinical effectiveness of EGFR-TKIs.
Heat shock protein 70 (HSP70), also known as HSP72, functions as an ATP-dependent molecular chaperone that assists in folding newly synthesized polypeptides, the assembly of multiprotein complexes, the transport of proteins across cellular membranes, and targeting proteins for lysosomal degradation [20, 21]. HSP70 has also been documented to be associated with radio-resistance involving the promotion of base excision repair (BER) in human leukemic cells [22]. The BER pathway is considered as the main guardian in mammalian cells for eliminating small DNA lesions generated either endogenously or exogenously at DNA bases [23]. It has been supposed that HSP70 promotes the BER pathway to reduce DNA damage by stimulating the activities of the repair enzymes APE1 and Pol β in vitro [24, 25]. However, the links between HSP70-mediated BER and acquired drug resistance remains poorly understood.
In this study, we sought to investigate the mechanism by which EGFR-TKI induces the emergence of EGFR secondary mutations such as T790M. We observe that EGFR blockade by low-dose EGFR-TKI results in the degradation of HSP70 proteins in HCC827 and PC9 cells harboring EGFR-activating mutations. We identify the phosphorylation of HSP70 at tyrosine 41 (Y41) as a novel regulator of HSP70 protein stability. We also demonstrate that the resultant HSP70 reduction is highly associated with EGFR-TKI-elevated gene mutation rates and the occurrence of EGFR T790M mutation due to the involvement of HSP70 in the efficiency and fidelity of the BER pathway. Our study indicates, for the first time, that administration of EGFR-TKI to patients harboring EGFR-activating mutations promotes the gene mutation frequency via the induction of HSP70 degradation and, consequently, the suppression of HSP70-mediated BER, causing an accelerated occurrence of EGFR T790M mutation.
2. Materials and Methods
Cell culture and transfection
A549, HCC827, NCI-H1975 and HEK293T cells were obtained from the Institute of Biochemistry and Cell Biology (Chinese Academy of Sciences, Shanghai, China). These cell lines were passaged for fewer than 6 months after resuscitation. HCC827 and NCI-H1975 cells were maintained in RPMI 1640 (Invitrogen) with 10% (v/v) fetal bovine serum (HyClone). A549 and HEK293T cells were maintained in DMEM (Invitrogen) supplemented with 10% fetal bovine serum. Plasmid transfections were performed with Fugene HD reagent (Roche) according to the manufacturer’s instructions. In all cases, the total amount of transfected DNA was normalized by empty control plasmid.
Antibodies and reagents
Rabbit monoclonal antibodies against HSP27, HSP90, FEN1, phosphor-H2AX (Ser139), EGFR, phospho-EGFR (Y1068), Akt, phospho-Akt (Ser473) and mouse monoclonal antibody against ubiquitin (Ub) were purchased from Cell Signaling Technology (Beverly, MA, USA). Mouse monoclonal antibody against HSP70 was obtained from Stressgen Bioreagents (Victoria, BC, Canada). Pol β polyclonal antibody was obtained from Abcam (Cambridge, United Kingdom). Rabbit polyclonal antibodies against GAPDH and Flag-tag were obtained from Bioworld Biotechnology (Minneapolis, MN, USA). Rabbit polyclonal antibody against HA-tag was obtained from Santa Cruz Biotechnology (Wembley, Middlesex, UK). Secondary antibodies coupled to IRDye800 fluorophore were purchased from Rockland (Gilbertsville, PA, USA). Erlotinib HCL (CAS: 183319-69-9) and Gefitinib (CAS: 184475-35-2) were from Selleckchem (Houston, TX, USA). Methyl methanesulfonate (MMS), MG132, NH4Cl, 6-Thioguanine (6-TG) and adenosine triphosphate (ATP) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Cycloheximide (CHX) was obtained from Calbiochem (San Diego, Calif., USA). Hydrogen peroxide (H2O2) was obtained from Merck (Darmstadt, Germany).
Detection of EGFR T790M mutation
Cells were cultured in medium with 0.1 µM of Erlotinib. After 3, 4, 5 months, these cells were seeded into 96-well plate and allowed to grow for 2 days. Total genomic DNA from the cells in each well were extracted and subjected to PCR to amplify the EGFR exon 20 fragment using a Direct Cell PCR assay kit (Genebank Biosciences Inc., China). Primers used: forward primer, 5’-CATTCATGCGTCTTCACCTG-3’; reverse primer, 5’-TCTGCACACACCAGTTGAGC-3’. PCR was performed for 30 cycles in 25 µl of reaction mixture at 94°C (30 s), 58°C (30 s) and 72°C (30 s), followed by a 10 min-extension at 72°C. Final PCR products were all sequenced (Sangon Biotechnology, China).
Determination of BER efficiency
The efficiency of the BER pathway was determined using an in vitro BER assay as described previously [26]. The reactions were carried out in 20 µl of reaction buffer [40 mM HEPES-KOH (pH 7.8), 7 mM KCl, 7 mM MgCl2, 1 mM dithiothreitol, 0.5 mM EDTA, 2mM ATP, 50 µM each of dATP, dTTP, and dGTP, and 8 µM 2 µCi (α-32P)-dCTP] with the addition of total protein extracts (1–5 µg) and the DNA substrate containing uracil (Supplementary Table 2). The radio-labeled dCTP can be incorporated in the site of uracil after repair. Reactions (25 min, 37°C) were then stopped by adding an equal volume of gel loading buffer. The radio-labeled oligonucleotides were separated on 15% denaturing polyacrylamide gel and visualized by autoradiography.
Reconstituted BER assay
The BER assay was performed as described previously [27–29]. Complete repair reactions were carried out in 20 µl of reaction buffer (40 mM HEPES-KOH [pH 7.8], 70 mM KCI, 7 mM MgCl2, 1 mM dithiothreitol, 0.5 mM EDTA, 2 mM ATP, 50 µM each of dATP, dTTP, and dGTP, and 8 µM 2 µCi [α-32P]-dCTP). For reconstitution with purified proteins, Uracil-DNA Glycosylase (UDG, 8 ng), APE1 (2 ng), Ligase III (20 ng), Pol β (2 ng), and 5 ng of HSP70 or HSC70 or BSA were mixed and incubated with the BER substrate (Supplementary Table 2). Reactions (30 min, 37°C) were then stopped by adding an equal volume of the gel loading buffer and visualized by autoradiography.
Pol β polymerase fidelity assay
Measurement of Pol β fidelity was carried out in 20 µl of reaction buffer [50mM Tris-HCl (pH 8.0), 10 mM MgCl2, 2 mM dithiothreitol, 20 mM NaCl, 10% glycerol] with the addition of recombinant Pol β and incomplete DNA substrate labeling with 32P at the 5’-end (Supplementary Table 2). The individual reaction contained either dCTP, dATP, dGTP or dTTP (50 mM). After polymerase reaction (25 min, 37°C), the radio-labeled oligonucleotides were separated on 15% denaturing polyacrylamide gel and visualized by autoradiography.
FEN1 activity assay
The determination of enzyme activity of purified recombinant FEN1 was conducted using fluorogenic assay developed by Dorjsuren et al. [30]. The double-stranded DNA substrate containing a double flap region used in the fluorogenic assay was prepared from three oligodeoxynucleotides: quencher (5’-CAC GTT GAC TAC CGC TCA ATC CTG ACG AAC ACA TC-BHQ-2), flap (5’-TAMRA-GA TGT CAA GCA GTC CTA ACT TTG AGG CAG AGT CCG C) and template (5’-GC GGA CTC TGC CTC AAG ACG GTA GTC AAC GTG-30) strands by a standard annealing procedure. The FEN1 fluorogenic assay was performed in 40 µl of reaction mixture containing DNA substrate and 50 mM Tris-HCl pH 8.0, 10 mM MgCl2, 1 mM DTT and 0.01% Tween-20. The reaction was run at room temperature over the first 50 min of incubation and the generated fluorescence intensity was monitored on a SpectraMax M2 microplate reader (Molecular Devices, USA). The reaction curves and tangential slopes were generated by SoftMaxPro6.3 built-in software. Three replicates were carried out, and a histogram was plotted using the values of the slopes.
Statistical Analysis
Statistical analysis was carried out using SPSS software (version 17.0). Data were expressed as the mean ± SD, and one-way ANOVA was used to determine the significance of the difference between two groups. A P value of < 0.05 was considered statistically significant.
Methods for other experiments are detailed in Supplementary Materials and Methods
3. Results
3.1 EGFR-TKI treatment induces the emergence of EGFR T790M mutation and HSP70 reduction
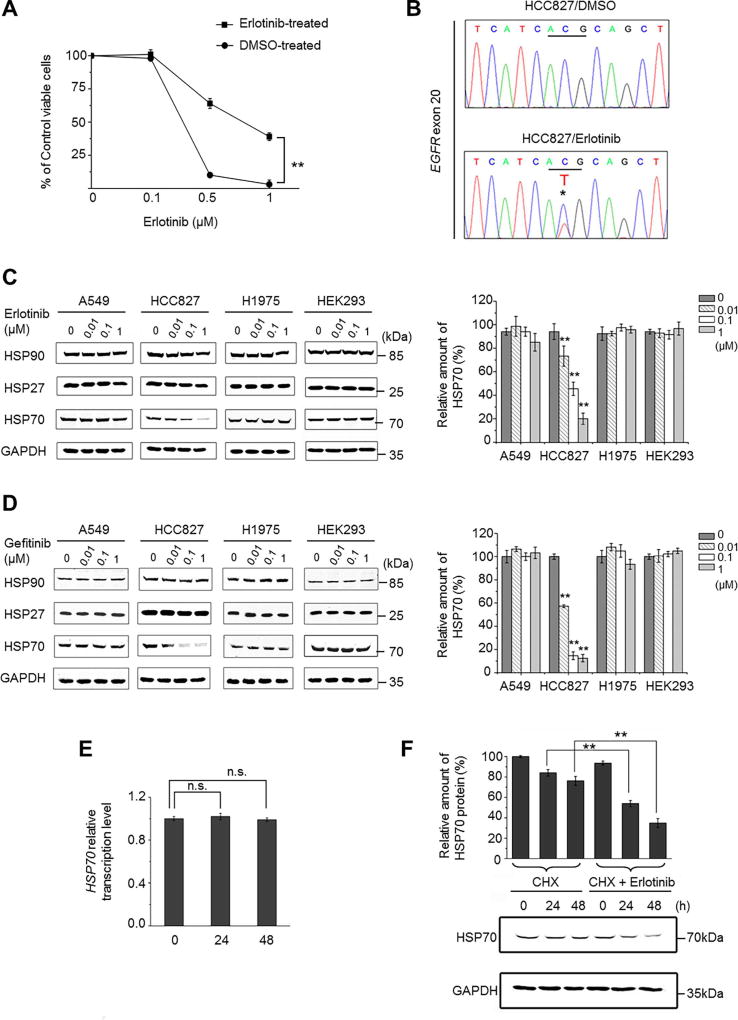
To investigate the molecular mechanism of how EGFR resistant mutations develop in the treated patients harboring the EGFR-activating mutation, we exposed the clinically-relevant NSCLC cell line, HCC827 cells (E19del, EGFR-TKI sensitive), to a constant low dose of Erlotinib (0.1 µΜ) for the following aims: i) to favor cell proliferation because no evident apoptosis induced by 0.1 µM of Erlotinib was observed (Supplementary Fig. S1A); and ii) to mimic the evolution of lung cancer cells in the presence of inadequate drug treatment. After long-term exposure (5 months) of Erlotinib and vehicle control DMSO, respectively, cell viabilities of these treated cells upon high-dose Erlotinib challenge were detected to investigate any changes in drug sensitivity. As shown in Figure 1A, the sensitivities of Erlotinib-treated HCC827 cells to higher concentrations of Erlotinib (0.5 µM and 1 µM) were significantly lower than those in the DMSO control. Sanger sequencing analyses of the exon 20 of the EGFR gene in the long-term Erlotinib-treated cells revealed an emergence of T790M mutation in EGFR (C-T transversion, Fig. 1B). These observations indicate that long-term treatment with low-dose Erlotinib induces the occurrence of the EGFR T790M mutation, which could contribute, at least in part, to the reduced Erlotinib-sensitivity in HCC827 cells.
Fig. 1. Erlotinib treatment induces the occurrence of EGFR T790M mutation and the degradation of HSP70 protein in HCC827 cells.
(A) HCC827 cells were treated continuously with 0.1 µM of Erlotinib or DMS. After 5 months, the treated cells were further treated with high doses of Erlotinib (0.5 µM and 1 µM) for 24 h and the cell viabilities were detected by CCK-8 assay. Values represent mean ± SD of at least six replications, **P<0.01. (B) After treating cells for 5 months with DMSO or Erlotinib, total genomic DNA from treated HCC827 cells were extracted and subjected to PCR and DNA sequencing to detect the existence of the EGFR T790M mutation. Sequencing Chromatograms show that treatment with 0.1 µM Erlotinib induced the emergence of the T790M mutation on the EGFR exon 20 fragment (C-T transverse indicated by asterisk). (C, D) A549, HCC827, H1975, and HEK293 cell lines were treated with the indicated concentrations of Erlotinib (C) or Gefitinib (D) for 48 h. Cell lysates were prepared and subjected to immunoblotting to measure the protein levels of HSP90, HSP27, and HSP70, respectively. GAPDH was used as an internal control. The relative amount of protein levels of HSP70 to GAPDH in each cell line upon different treatments were compared and are shown in histograms. (E) HCC827 cells were treated with 0.1 µM of Erlotinib for the indicated times. Total RNA was isolated and reverse transcribed. Real-time PCR was performed to detect HSP70 transcription levels. GAPDH was used as an internal control. (F) HCC827 cells were treated with CHX (1 µg/mL) alone or combined with Erlotinib (0.1 µM) for the indicated times. HSP70 and GAPDH protein levels were assessed by immunoblotting. Values represent mean ± SD of three independent experiments, **P < 0.01, n.s., non-significance.
Recent evidence supports that HSPs are actively associated with the sensitivities to chemotherapeutic agents in cancer cells [31, 32]. We therefore examined the expression levels of HSP90, HSP70, and HSP27 in three cell lines clinically relevant to NSCLC: HCC827, A549, and NCI-H1975 cells. A549 cells harbor wild-type (WT) EGFR and are resistant to EGFR-TKIs; whereas NCI-H1975 cells express both the EGFR activating L858R and resistant T790M mutations and show high resistance to EGFR-TKIs. These cell lines were treated with different doses of Erlotinib and Gefitinib, respectively. The concentrations of Erlotinib and Gefitinib that we chose were based on the range of drug levels achieved in the plasma of treated patients (0.01–1 µM) [33]. Western blotting was performed to determine the EGFR signaling in response to the drugs (Supplementary Fig. S1B). The obvious inhibitions of EGFR and its downstream AKT and ERK were observed in HCC827 cells upon treatment with Erlotinib; by contrast, similar inhibition was not evident in the insensitive A549 or in the resistant NCI-H1975 cells. Interestingly, we found that the protein levels of HSP70, but not HSP90 and HSP27, were evidently reduced only in HCC827 cells after 48 h of treatment with Erlotinib or Gefitinib (Fig. 1C, D). In support, we confirmed this observation in another EGFR-TKI-sensitive NSCLC cell line, PC9 cells (Supplementary Fig. S2A). These observations suggest that HSP70 reduction is highly related to EGFR mutation states and EGFR-TKI sensitivity. We then investigated how EGFR-TKI affects the HSP70 protein levels. Real-time PCR analyses showed no significant reduction of HSP70 transcripts within 48 h of Erlotinib treatment (0.1 µM) (Fig. 1E). If HCC827 cells were pretreated with cycloheximide (CHX) to inhibit protein synthesis, followed by Erlotinb treatment (0.1 µM), the protein levels of HSP70 were obviously lower than that CHX treatment alone, suggesting that Erlotinib induced the degradation of HSP70 protein (Fig. 1F).
3.2 EGFR blockade affects HSP70 protein stability by suppression of HSP70 phosphorylation at Y41
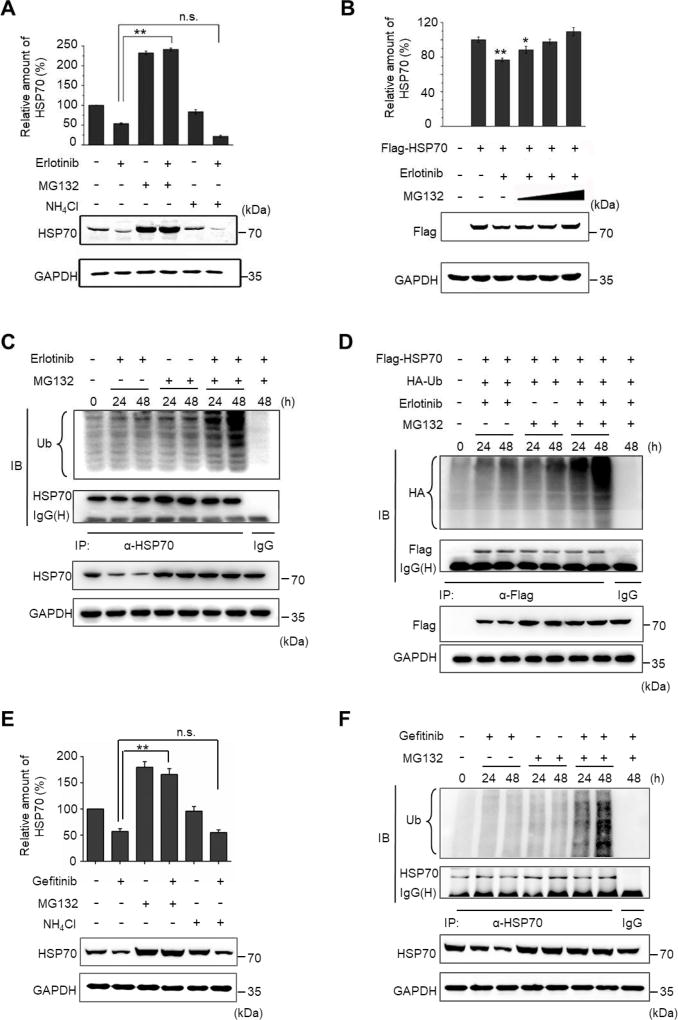
Next, we utilized MG132 and NH4Cl, two chemical inhibitors of the known protein degradation apparatus in the cell, to discriminate the way HSP70 was degraded. NH4Cl inhibits lysosomal protein degradation, and the addition of NH4Cl failed to rescue the Erlotinib-induced HSP70 reduction. By contrast, MG132, a proteasome inhibitor, evidently caused the accumulation of HSP70 (Fig. 2A). Indeed, Erlotinib also caused the reduction of ectopically overexpressed Flag-tagged HSP70, and MG132 significantly compromised HSP70 degradation (Fig. 2B). Although the Erlotinib-induced reduction of Flag-HSP70 shows statistical significance compared to the untreated control, the overexpressed Flag-HSP70 did not degrade as obvious as the endogenous HSP70 did upon Erlotinib treatment. This might be due to the saturated protein level resulted from HSP70 overexpression. In addition, we also noticed that the addition of MG132 has differential effects on the endogenous and the exogenous HSP70. We thought that this discrepancy might be due to the reported effect of MG132 on HSP70 transcription through the activation of HSF1 [34]. Moreover, we observed that, in the presence of MG132, Erlotinib treatment resulted in an obvious ubiquitination of both endogenous and exogenous HSP70 at 48 h (Fig. 2C, D), when an obvious reduction in HSP70 protein levels was observed. Gefitinib treatment gave the same results (Fig. 2E, F).
Fig. 2. Erlotinib or Gefitinib treatment causes HSP70 degradation through the ubiquitination pathway in HCC827 cells.
(A) Cells were treated with 0.1 µM Erlotinib combined with NH4Cl (10 mM) or MG132 (5 µM) for 48 h. HSP70 and GAPDH protein levels were determined by immunoblotting. (B) Cells were transfected with pcDNA-Flag-HSP70 for HSP70 overexpression. After the cells were transfected for 24 h, they were treated with or without Erlotinib in the presence or absence of MG132 (5 µM, 10 µM, and 15 µM). Exogenous HSP70 and GAPDH protein levels were determined by immunoblotting. The relative protein levels of HSP70 to GAPDH upon different treatments are shown in each histogram. (C) Cells were treated with Erlotinib (0.1 µM) alone, MG132 (5 µM) alone, or Erlotinib combined with MG132 for 24 h or 48 h. The lysates were immunoprecipitated using anti-HSP70 antibody, followed by immunoblotting using antibodies against Ubiquitin (Ub) and HSP70, respectively, to show the effect of Erlotinib on the ubiquitination levels of HSP70. (D) Cells were co-transfected with expression vectors encoding HA-Ubiquitin and Flag-HSP70. After the cells were transfected for 24 h, they were treated with Erlotinib (0.1 µM) alone, MG132 (5 µM) alone, or Erlotinib combined with MG132 for 24 h or 48 h. The lysates were immunoprecipitated using anti-Flag antibody, followed by immunoblotting using anti-HA and anti-Flag antibodies, respectively, to show the effect of Erlotinib on the ubiquitination levels of exogenous HSP70. (E) Cells were treated with 0.1 µM Gefitinib combined with NH4Cl (10 mM) or MG132 (5 µM) for 48 h. HSP70 and GAPDH protein levels were determined by immunoblotting. (F) Cells were treated with Gefitinib (0.1 µM) alone, MG132 (5 µM) alone, or Erlotinib combined with MG132 for 24 h or 48 h. The lysates were immunoprecipitated using anti-HSP70 antibody, followed by immunoblotting using antibodies against Ubiquitin (Ub) and HSP70, respectively. All values represent mean ± SD of three independent assays, *P < 0.05, **P < 0.01, n.s., non-significance.
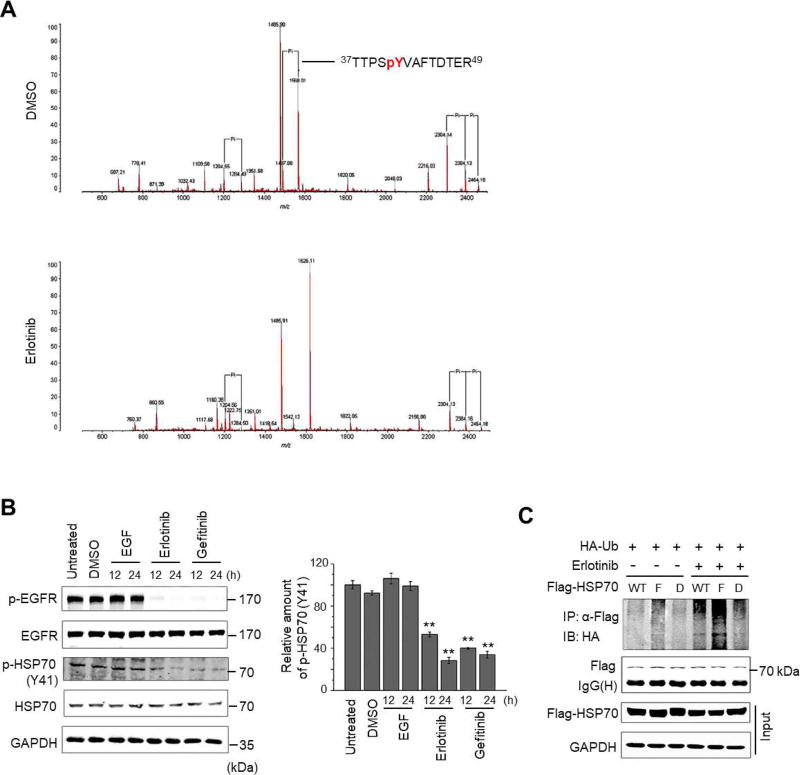
Phosphorylation has emerged as an important post-translational regulatory mechanism for HSP70 functions [35, 36]; however, the impact of phosphorylation on the HSP70 protein stability is still elusive. We, therefore, profiled the phosphorylation status of HSP70 in the presence and absence of Erlotinib. HCC827 cells transiently overexpressing Flag-tagged HSP70 were treated with Erlotinib or DMSO. The Flag-HSP70 was immunoprecipitated followed by MALDI-QIT-TOF-MS analyses. In DMSO-treated HCC827 cells, four phosphorylated residues in HSP70, i.e., Y41, S362, Y371, and S631 were detected; whereas three phosphorylated residues S362, Y371, and S631 were detected in the Erlotinib-treated cells (see Fig. 3A for MS spectra and Supplementary Table 1). This observation led us to speculate that the significantly-inhibited phosphorylation at Y41 of HSP70 is implicated in HSP70 protein degradation induced by Erlotinib. To test this hypothesis, we generated a phospho-specific antibody against the HSP70 Y41 motif that detects a specific band at the expected molecular weight of HSP70 (~72 kDa). The phospho-HSP70 signal was obviously reduced when EGFR was inhibited by Erlotinib or Gefitinib (Fig. 3B). Moreover, the observed suppression of HSP70 phosphorylation at Y41 was also confirmed in EGFR-TKI-sensitive PC9 cells (Supplementary Fig. S2B). It is noteworthy that EGF did not substantially increase the phosphorylation of HSP70 at Y41, and we reasoned that the activating-mutation of EGFR leads to constant activation of EGFR, which causes it to fail to respond further to EGF stimulation in HCC827 cells. To test this possibility, we detected the phosphorylation of HSP70 at Y41 in A549 cells harboring WT EGFR. In the untreated A549 cells, the basal level of HSP70 phosphorylation at Y41 can be detected as a band at the molecular weight approximately 72 kDa. After stimulating cells with EGF at different doses, the phosphorylation levels of HSP70 at Y41 were obviously increased, indicating that phosphorylation of HSP70 at Y41 can be regulated by EGFR signaling (Supplementary Fig. S3).
Fig. 3. Erlotinib treatment suppresses the phosphorylation of HSP70 at the site of Y41.
(A) MALDI-QIT-TOF-MS spectra show the phospho-peptides of HSP70 treated with DMSO vehicle (upper panel) or Erlotinib (lower panel). (B) Cells were stimulated with EGF (100 ng/mL), Erlotinib (0.1 µM), or Gefitinib (0.1 µM), respectively. At 12 h and 24 h, lysates were prepared and subjected to Western blotting using indicated antibodies. Untreated or DMSO treated cells were used as control. The relative amounts of phosphorylated HSP70 at Y41 to total HSP70 under different treatments were shown in histogram. (C) Cells were co-transfected with HA-Ub and wild-type HSP70 (WT), HSP70 (Y41F) (F), or HSP70 (Y41D) (D) for 24 h, followed by 48 h of treatment with or without Erlotinib in the presence of MG132. The ubiquitination levels of HSP70 and its mutants were determined by immunoprecipitation and immunoblotting using the indicated antibodies. All values represent mean ± SD of three independent assays, *P < 0.05.
To assess the impact of Y41 phosphorylation of HSP70, we constructed two HSP70 mutants: HSP70 (Y41F) (tyrosine-41 was mutated to phenylalanine, a Y41 non-phosphorylatable mutant) and HSP70 (Y41D) (tyrosine-41 was mutated to aspartate, a Y41 constant-phosphomimetic mutant). We separately overexpressed WT HSP70, HSP70 (Y41F), and HSP70 (Y41D) in HCC827 cells to determine the differential effects of Y41 phosphorylation status on HSP70 ubiquitination. As shown in Figure 3C, in the presence of MG132, the ubiquitination level of HSP70 (Y41F) was higher than those of WT HSP70 and HSP70 (Y41D) in untreated cells; 0.1 µM of Erlotinib treatment obviously enhanced the ubiquitination levels of all constructs, and consistently, HSP70 (Y41F) exhibited the highest ubiquitination level. Taken together, these results indicate that HSP70 phosphorylation at Y41 functions as a novel regulator in HSP70 ubiquitination and possibly in the degradation of HSP70 through the ubiquitin-proteasome pathway.
3.3 The protein levels of HSP70 are correlated with gene mutation rates and EGFR T790M mutation in HCC827 cells
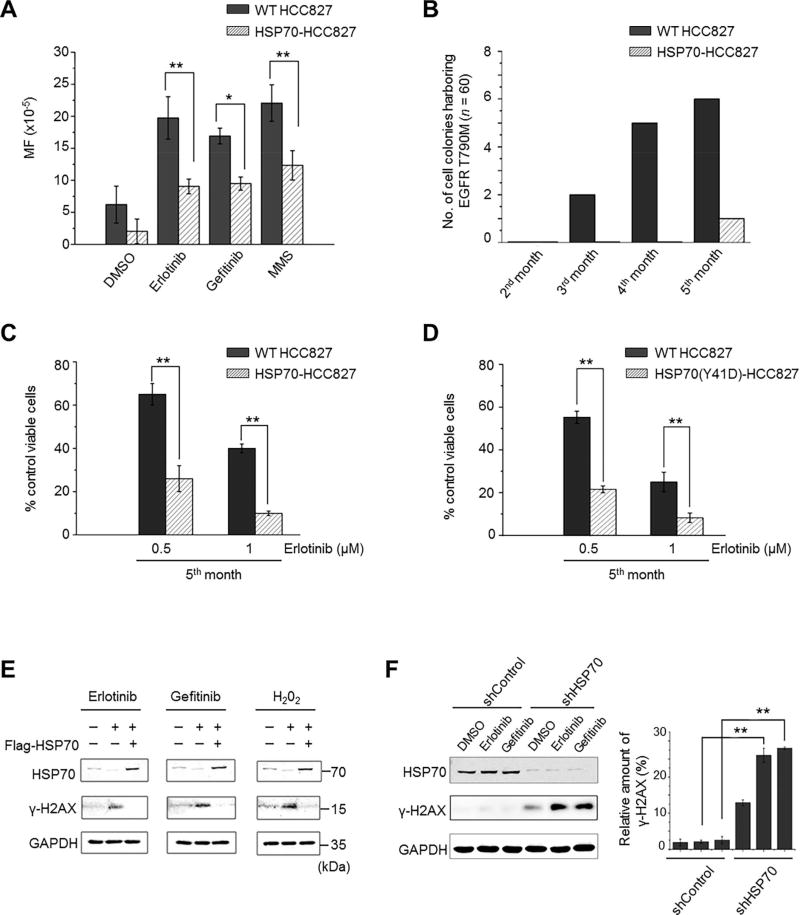
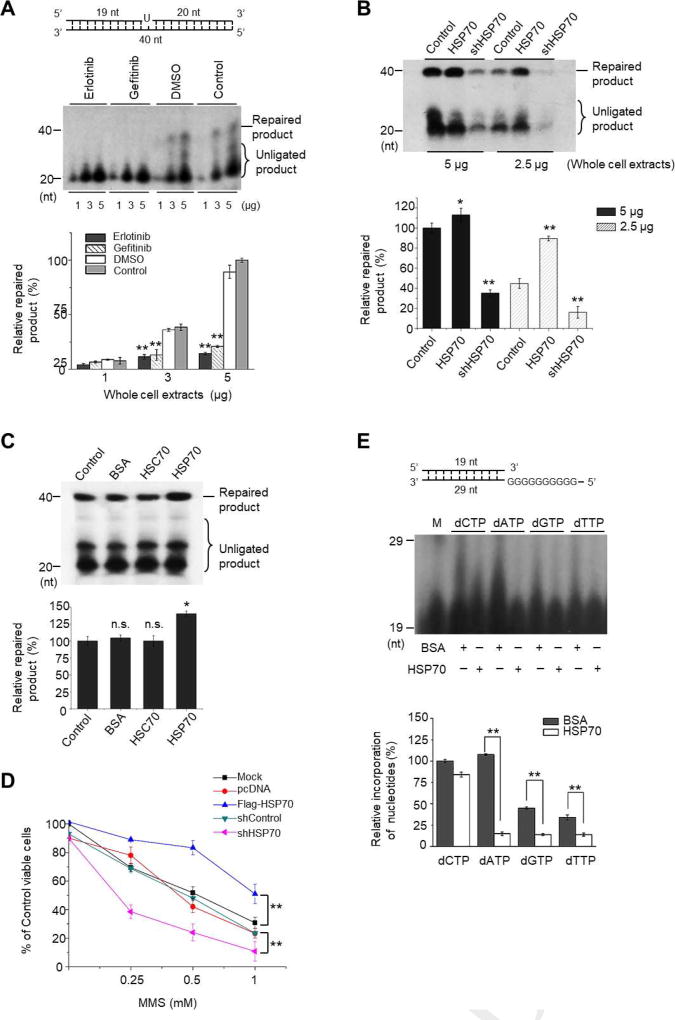
The observation that Erlotinib treatment coincidentally induces HSP70 reduction and the occurrence of the EGFR T790M mutation led us to investigate the relationship between HSP70 protein levels and gene mutation rates. We established an HCC827 cell line with HSP70 stably overexpressed (for details in Supplementary Fig. S4A) and employed the hypoxanthine phosphorybosyl-transferase (HPRT) mutation assay, which is widely used to detect gene mutation frequency in mammalian cell lines upon mutagens [37]. As shown in Table 1, the exposure of WT HCC827 cells to Erlotinib or Gefitinib (0.1 µM for 28 days) resulted in a striking increase in the gene mutation frequency compared to that of DMSO-treated controls, while HSP70 overexpression significantly ameliorated the TKI-enhanced gene mutation rates. As a positive control, DNA damaging agent methyl methanesulfonate (MMS)-treated WT HCC827 cells exhibited a higher mutation frequency than MMS-treated and HSP70-overexpressed HCC827 cells (Fig. 4A). Moreover, we compared the occurrence of EGFR T790M mutation between WT and HSP70-overexpressing HCC827 cells upon Erlotinib treatment. Indeed, at 2, 3, 4, and 5 months post Erlotinib exposure, the rates of EGFR T790M mutation in WT HCC827 cells were 0% (0 of 60), 3.33% (2 of 60), 8.33% (5 of 60), and 10% (6 of 60), respectively. By contrast, only one cell colony (1 of 60) harboring EGFR T790M mutation was observed in HSP70-overexpressing cells at the end of the 5-month treatment (Table 2 and Fig. 4B). The cell viabilities of these two cell lines upon the high doses of Erlotinib (0.5 µM and 1 µM) were also determined. We noticed that HSP70-overexpressing HCC827 cells were more sensitive to high doses of Erlotinib compared to WT HCC827 cells after the 5-month treatment (Fig. 4C).
Table 1.
Comparison of HPRT mutation frequencies in wild-type (WT) HCC827 and HCC827 cells stably overexpressed with HSP70 (HSP70-HCC827) under different treatments
| WT HCC827 | HSP70-HCC827 | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Total No. of wells |
No. of wells without colonies |
Mutation frequency (×10−5) |
Total No. of wells |
No. of wells without colonies |
Mutation frequency (×10−5) |
|
| DMSO | 300 | 205 | 6.212±2.879 | 300 | 255 | 2.043±1.916 |
| 300 | 247 | 300 | 297 | |||
| 300 | 253 | 300 | 279 | |||
| Erlotinib | 300 | 145 | 19.737±3.311 | 300 | 210 | 9.054±1.146** |
| 300 | 117 | 300 | 218 | |||
| 300 | 149 | 300 | 199 | |||
| Gefitinib | 300 | 160 | 16.908±1.232 | 300 | 215 | 9.493±1.025* |
| 300 | 145 | 300 | 199 | |||
| 300 | 153 | 300 | 202 | |||
| MMS | 300 | 130 | 22.060±2.855 | 300 | 165 | 12.336±2.294** |
| 300 | 109 | 300 | 196 | |||
| 300 | 135 | 300 | 190 | |||
Data shown are the average ± standard deviation from three independent experiments.
Statistical analysis (t-test) is indicated as shown in the table, (*P<0.05, **P<0.01 versus DMSO group).
Fig. 4. The EGFR-TKI-induced HSP70 reduction causes elevated gene mutation frequency and DNA damage in HCC827 cells.
(A) The gene mutation frequencies determined by HPRT assay in wild type (WT) and HSP70-stably overexpressing (HSP70-)HCC827 cells after a long-term treatment with DMSO, Erlotinib (0.1 µM), Gefitinib (0.1 µM) or MMS. (B) The values in Table 2 were plotted to show the comparison of numbers of cell colonies having the EGFR T790M mutation between WT and HSP70-HCC827 cells after long-term treatment of Erlotinib (0.1 µM) (n = 60). (C) After the cells were treated for 5 months with 0.1 µM Erlotinib, the drug sensitivities of WT and HSP70-HCC827 cells to the high doses of Erlotinib were determined by cell viability assay and compared. (D) After the cells were treated for 5 months with 0.1 Μm Erlotinib, the drug sensitivities of WT HCC827 cells and the HSP70 (Y41D)-stably overexpressing HCC827 cells to the indicated doses of Erlotinib were determined by cell viability assay and compared. (E) HCC827 cells were transfected with pcDNA-Flag-HSP70 (+) or empty vector (−) for 24 h and then treated with or without the 0.1 µM of Erlotinib or Gefitinib for another 48 h, or stimulated with or without H2O2 (60 µM) for 10 minutes. Western blots show the expression levels of HSP70 and γ-H2AX. (F) NCI-H1975 cells were transfected with HSP70-specific shRNA (shHSP70) or non-targeting control shRNA (shControl) for 48 h. Then cells were treated with DMSO, 0.1 µM of Erlotinib and Gefitinib, respectively, for another 48 h. Western blots show the expression levels of HSP70 and γ-H2AX. The relative amounts of γ-H2AX to GAPDH in each treatment were compared and are shown in a histogram. All values represent mean ± SD of three independent assays, *P < 0.05, **P < 0.01.
Table 2.
Stable overexpression of HSP70 delays the occurrence of EGFR T790M mutation in HCC827 cells upon long-term Elrotinib treatment
| WT HCC827 | HSP70-HCC827 | |||
|---|---|---|---|---|
|
|
|
|||
| Time | Total No. | No. of T790M | Total No. | No. of T790M |
| 2nd month | 60 | 0 | 60 | 0 |
| 3rd month | 60 | 2 | 60 | 0 |
| 4th month | 60 | 5 | 60 | 0 |
| 5th month | 60 | 6 | 60 | 1 |
| Total | 240 | 13 | 240 | 1 |
Additionally, to evaluate the correlation between HSP70 protein stability and gene mutation, we established an HSP70 (Y41D) mutant stably overexpressing HCC827 cell line (for details in Supplementary Fig. S4B, C). After treatment with 0.1 µM of Erlotinib for 5 months, HSP70 (Y41D)-overexpressing cells were more sensitive to high doses of Erlotinib than WT cells (Fig. 4D), and the occurrence of EGFR T790M mutation was also determined. In fact, at the end of the 5-month treatment, 6 of 90 (6.67%) colonies harboring the EGFR T790M mutation were detectable in WT HCC827 cells, while none of the colonies having the T790M mutation (0 of 90) were observed in HSP70 (Y41D)-overexpressing cells.
Notably, we found that both the treatment of Erlotinib and Gefitinib (0.1 µM) induced the reduction of HSP70 along with the enhanced phosphorylation of histone H2AX (γ-H2AX), a marker for DNA damage (Fig. 4E). By contrast, if HSP70 was ectopically expressed in cells prior to the treatment of EGFR-TKI or H2O2, the amount of γ-H2AX was evidently reduced. As we showed above, the reduced HSP70 protein levels by the treatment of Erlotinib or Gefitinib (0.1 µM) were only observed in EGFR-TKI-sensitive HCC827 cells. We questioned whether the effect of EGFR-TKI on DNA damage was a consequence of EGFR-TKI-induced HSP70 reduction. Therefore, we investigated this phenomenon in EGFR-TKI-resistant NCI-H1975 cells. As shown in Figure 4F, the treatment of Erlotinib or Gefitinib (0.1 µM) had no effects on the levels of γ-H2AX and HSP70 in NCI-H1975 cells transfected with non-targeting control shRNA; however, when HSP70 was knocked down by HSP70-specific shRNA, the γ-H2AX upregulation was readily observed after the Erlotinib or Gefitinib treatment. These findings led us to speculate that the DNA damage generated by EGFR-TKI treatment in HCC827 cells is secondary to the EGFR-TKI-induced HSP70 reduction.
3.4 The protein levels of HSP70 are implicated in the efficiency and fidelity of BER
The elevated gene mutation rates and DNA damage induced by EGFR-TKI reflects the impaired DNA repair in cells. HSP70 has been shown to be associated with radio-resistance involving the promotion of BER in human leukemic cells [22]. We then assessed the impacts of EGFR-TKI and HSP70 on BER function in NSCLC cells. First, HCC827 cells were treated with or without the reagent (Erlotinib, Gefitinib or DMSO) for 48 h, and the whole cell extracts were prepared and subjected to in vitro determination of BER efficiency using uracil-containing DNA substrate (Supplementary Table 2). The cleavage of the uracil and the incorporation of 32P-dCTP and other deoxynucleotides produced 20–39 nt non-ligated intermediates. Complete processing of the intermediates generated a fully repaired 40 nt product. We observed that the uracil lesions were efficiently repaired with the protein extracts from untreated control or DMSO-treated cells. By contrast, very few repaired products were detected using the protein extracts from Erlotinib- or Gefitinib-treated cells (Fig. 5A), suggesting that the efficiency of BER was suppressed in the EGFR-TKI-treated HCC827 cells. Then, we compared the expression patterns of BER-associated proteins in HCC827 cells in the presence or absence of EGFR-TKI treatment. Western blot analyses showed that the protein levels of HSP70, but not other repair enzymes, were substantially reduced after Erlotinib or Gefitinib treatment (Supplementary Fig. S5). In addition, using protein extracts from HSP70-overexpressing cells markedly promoted BER efficiency, whereas those from the cells with HSP70 knockdown suppressed BER activity (Fig. 5B). Moreover, in vitro reconstitute BER assays were employed to determine the direct effect of HSP70 on BER efficiency. Obviously, the addition of HSP70 recombinant protein, but not BSA or HSC70, increased the amount of repaired product (Fig. 5C). Consistently, HSP70 overexpression protected HCC827 cells against MMS, a DNA alkylating agent that specifically triggers BER reaction [38], -induced growth inhibition, whereas HSP70 knockdown potentiated MMS toxicity compared to the controls (Fig. 5D). In support of these findings, HSP70 (Y41D)-overexpressing cells exhibited higher viabilities upon MMS stimulation than WT cells (Supplementary Fig. S6).
Fig. 5. EGFR-TKI treatment suppresses the efficiency and fidelity of BER due to HSP70 reduction.
(A) HCC827 cells were treated with or without drug (Erlotinib or Gefitinib) for 48 h, and the whole cell extracts were prepared and used to conduct in vitro BER assays. Top panel, the schematic structure of DNA substrate; middle panel, PAGE-separated radio-labeled BER products in each indicated treatment; bottom panel, the percentages of relative repaired products obtained with the indicated amounts of protein extracts (1 µg, 3 µg, and 5 µg) were quantified and compared. The amount of repaired product produced using 5 µg of untreated cell extracts (Control) was considered as 100%. (B) HCC827 cells were transfected with pcDNA-Flag-HSP70 or pRNA-U6.1/shHSP70 to induce HSP70 overexpression or knockdown, respectively. Mock transfection was used as a control. Cell lysates were prepared and used to conduct in vitro BER assays. The PAGE-separated radio-labelled BER products in each indicated treatment were shown (Upper), and the percentages of relative repaired products obtained with the indicated amounts of whole cell extracts (2.5 µg and 5 µg) were quantified (Lower). The amount of repaired product produced using 5 µg of mock-transfected cell extracts was considered as 100%. (C) In the reconstitute BER assays, purified HSC70, HSP70 or BSA was separately added into the reaction mixture. The PAGE-separated radio-labeled BER products in each reaction were shown (Upper), and the percentages of relative repaired product obtained with the addition of BSA, HSC70, or HSP70 were quantified and compared (Lower). The amount of repaired product produced in the control reaction was considered as 100%. All values represent mean ± SD of three independent assays, *P < 0.05, **P < 0.01, n.s., non-significance. (D) HSP70 overexpression or knockdown was conducted in HCC827 cells by transfection with pcDNA-Flag-HSP70 or pRNA-U6.1/shHSP70. After 48 h, the cells were treated with the indicated concentrations of MMS for 1 h. Cellular sensitivities to MMS were determined as described in SI Materials and methods. Values represent mean ± SD of at least six replications, **P<0.01. (E) In vitro measurements of Pol β fidelity. Top panel, the schematic structure of DNA substrate for polymerase reaction with Pol β; middle panel, PAGE-separated radio-labelled products in each reaction containing either dCTP, dATP, dGTP or dTTP in the presence of BSA or HSP70; lower panel, the relative amounts of elongated products obtained from each reaction were quantified according to the Grey value and compared. The amount of product in the presence of BSA was considered as 100%. All values represent mean ± SD of three independent assays, *P<0.05, **P<0.01.
We further tested the possibility that HSP70 could promote the BER fidelity to suppress gene mutation. Pol β has no known proofreading activity and has a significant impact on repair fidelity [39, 40]. We, therefore, adopted the primer extension assay to test the effect of HSP70 on the fidelity of Pol β, and we observed that the incubation of HSP70, but not BSA, in the reaction mixtures substantially reduced the number of G-N mispairs on the DNA substrates (Fig. 5E), suggesting that HSP70 promotes the fidelity of Pol β to incorporate dNTP into DNA. Taken together, our data demonstrate that HSP70 promotes not only the efficiency but also the fidelity of BER, while the treatment of EGFR-TKI induces HSP70 reduction and suppresses HSP70-mediated BER, elevating the gene mutation rates in HCC827 cells.
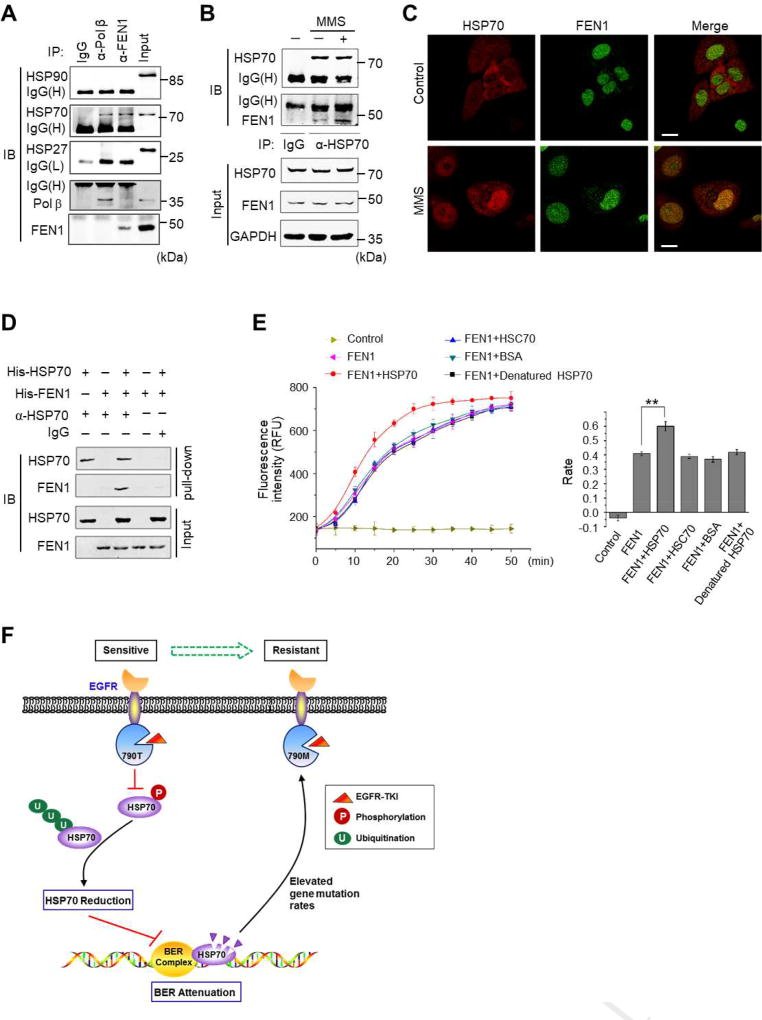
3.5 HSP70 directly interacts with FEN1 and promotes the activity of FEN1
To further determine the role of HSP70 in BER, we examined the proteins interacting with HSP70 in the nuclei of HCC827 cells after the stimulation of MMS. Using immunoprecipitation with HSP70 antibody coupled to mass spectrometry, we identified known partners of HSP70, including APE1 and Pol β, and novel binding proteins such as FEN1 (Supplementary Table 3). FEN1 (Flap endonuclease 1) plays a critical role in DNA replication and in the BER pathway for maintaining genome stability and integrity [41]. By immunoprecipitation, we confirmed that Pol β and FEN1 both physically associated with HSP70, but not with HSP90 and HSP27 (Fig. 6A). Indeed, the association between HSP70 and FEN1 was enhanced upon MMS stimulation (Fig. 6B). Confocal microscopy imaging also illustrated that MMS stimulation enhanced the amount of HSP70 in the nuclei, as evidenced by the increased immunostaining of HSP70 superposed with that of FEN1 (Fig. 6C). To confirm the HSP70-FEN1 interaction, His-tagged HSP70 and His-tagged FEN1 recombinant proteins were expressed and purified separately and were subjected to in vitro pull-down assays. HSP70 antibody could precipitate HSP70 accompanied by FEN1, demonstrating that HSP70 directly binds to FEN1 (Fig. 6D). Next, we used an in vitro fluorogenic assay to measure the enzymatic activity of FEN1 in processing the DNA substrate containing a double-flap structure. Clearly, the addition of HSP70 significantly enhanced the enzymatic reaction rate compared to the addition of HSC70, BSA, or the denatured HSP70, suggesting the specificity of HSP70 in accelerating FEN1 activity (Fig. 6E).
Fig. 6. HSP70 directly interacts with FEN1 and promotes the enzymatic activity of FEN1.
(A) HCC827 cell lysates were immunoprecipitated using antibodies against Pol β and FEN1, respectively, followed by immunoblotting using antibodies as indicated. (B) Cells were treated with or without MMS for 1 h, and the cell lysates were immunoprecipitated using HSP70 antibody or IgG, followed by immunoblotting using antibodies as indicated. (C) Cells were treated with or without MMS for 1 h and fixed. Immunostaining was performed using antibodies against FEN1 and HSP70, respectively. Confocal images were captured (red: HSP70, green: FEN1, Scale bar: 10 µm). (D) In vitro binding between recombinant HSP70 and FEN1 proteins. Bacterial overexpressed His-HSP70 (500 ng) and His-FEN1 (321 ng) were incubated in the binding buffer containing HSP70 antibody or IgG. The pre-cleared protein A/G plus-agarose beads were added, precipitated, and resolved on SDS-PAGE followed by probing with antibodies against FEN1 and HSP70, respectively. (E) In vitro FEN1 activity assays. The curves of FEN1 enzymatic reactions in the buffer with the addition of HSP70, HSC70, BSA, or heat-denatured HSP70. The reaction rates of FEN1 are shown in a histogram. All values represent mean ± SD of three independent assays, **P<0.01. (F) Schematic diagram of the model of HSP70 reduction upon EGFR-TKI treatment and the consequent attenuation of BER capacity and the promotion of gene mutation. We propose that EGFR blockade by EGFR-TKI inhibits the phosphorylation of HSP70 at residue Y41, resulting in the enhanced ubiquitination level of HSP70 and subsequent protein degradation. Due to HSP70 reduction, the efficiency and the fidelity of the BER pathway are attenuated and the gene mutation rates are elevated, thereby facilitating the emergence of EGFR resistant T790M mutation upon EGFR-TKI treatment.
4. Discussion
In the present study, we demonstrate that, in the EGFR-TKI-sensitive lung cancer cells, EGFR inhibition by Gefitinib or Erlotinib suppresses the phosphorylation of HSP70 at residue Y41, resulting in the ubiquitination and degradation of HSP70 proteins. The consequence of EGFR-TKI-induced HSP70 reduction is highly associated with EGFR-TKI-enhanced DNA damage and gene mutation rates. We reveal that HSP70 interacts with multiple enzymes in the BER pathway and promotes not only the repair efficiency but also the fidelity of BER. Importantly, we show that ectopic expression of HSP70 or HSP70 Y41 phospho-mimicry mutant delays the Erlotinib-induced emergence of EGFR T790M mutation. We speculate that Gefitinib or Erlotinib induces HSP70 reduction, which in turn results in the attenuation of BER capacity, thereby causing an elevated DNA mutation frequency and facilitating the occurrence of the EGFR T790M mutation (as illustrated in Figure 6F). Consistently, after long-term treatment with low-dose Erlotinib, HSP70- or HSP70 (Y41D)-stably expressing HCC827 cells were more sensitive to high doses of Erlotinib in comparison to WT HCC827 cells. Therefore, our findings highlight an important role of HSP70 in DNA repair and reveal a novel link between HSP70 and the EGFR T790M mutation-mediated drug resistance in NSCLC.
The lung cancer cells were treated with Gefitinib or Erlotinib at pharmacologically achievable levels (0.01–1 µM), resulting in the reduction of HSP70 protein levels, specifically in the EGFR-TKI-sensitive HCC827 and PC9 cells (Fig. 1C, D, and Supplementary Fig. S2). Recently, Namba et al. reported that 1 µM of Gefitinib inhibits the translation of HSP70 and 10 µM of Gefitinib stimulates the degradation of HSP70 in A549 cells [42]. Since EGFR-TKIs are typically administered to the patients with EGFR-activating mutations, we mainly examined the effects of Gefitinib and Erlotinib in HCC827 cells due to clinical relevance. In fact, we did not observe that 1 µM of Gefitinib could induce the reduction of HSP70 protein in A549 cells, but 10 µM of Gefitinib did. However, 10 µM is much higher than the drug concentrations achieved in the plasma of treated patients (about 1 µM for Gefitinib and 2.5 µM for Erlotinib [33]). Of note, we intentionally tested dosages less than 1 µM to examine the physiological consequence of HSP70 reduction in surviving HCC827 cells, and we also aimed to mimic the evolution of cancer cells in the presence of inadequate drug treatment in solid tumor.
HSP70 is a well-known molecular chaperone that functions in the folding of newly synthesized polypeptides and in the targeting of denatured proteins for lysosomal degradation. However, how HSP70 protein levels are regulated to maintain its homeostasis remains largely unknown. Our data show that Erlotinib-treatment evidently suppressed the phosphorylation of HSP70 at the site of Y41, which is clearly associated with the ubiquitination level of HSP70 (Fig. 3). It has been previously suggested that the ubiquitin-dependent degradation of HSP70 is mediated by an E3 ligase CHIP during the stress recovery process [43]; we, therefore, reason that HSP70 phosphorylation at Y41 serves as a novel regulator in HSP70 ubiquitination and its stability. In addition, the observed inhibition of HSP70 phosphorylation raised the possibility that the blockade of EGFR and its downstream protein kinases may influence HSP70 stability. We detected the effects of U0126, LY294002, and SP600125, inhibitors to ERK, PI3K/AKT, and JNK, respectively, on the protein levels of HSP70. The treatment with Erlotinib or U0126, but not with LY294002 or SP600125, induced an obvious reduction of HSP70 protein levels in HCC827 cells. When LY294002 or SP600125 was combined with U0126, the reduced HSP70 was observed (Supplementary Fig. S7). These observations suggest that the inhibition of ERK signaling contributes greatly to the Erlotinib-induced HSP70 reduction. We also determined and compared the HSP70 interaction partners in HCC827 cells in the presence or absence of Erlotinib using immunoprecipitation and mass spectrometry (Supplementary Table 4). We observed that HSP70 interacted with EGFR before the Erlotinib treatment (Supplementary Table 4A) and that HSP70 bound to CHIP (STUB1) after the Erlotinib treatment (Supplementary Table 4B). We cannot exclude the possibility that EGFR itself phosphorylates HSP70 at site of Y41. Further in-depth investigation is needed to determine the upstream kinase(s) responsible for the differential phosphorylation status of HSP70.
Unlike the commonly applied chemotherapeutic drugs targeting DNA or DNA topoisomerase, EGFR-TKIs have not yet been postulated to directly generate DNA damage. However, there have been indications in the literature that inhibition of EGFR signaling mediates the radiation-triggered DNA damage response [44–47]. Indeed, both Gefitinib [48] and Erlotinib [49] have been shown to suppress the cellular DNA repair capacity; however, the involvement of HSP70 and HSP70-mediated BER in this phenomenon is unclear. Herein, we demonstrate that Gefitinib and Erlotinib cause DNA damage concurrently with HSP70 reduction in HCC827 cells, and, consistently, HSP70 overexpression attenuates the Gefitinib- and Erlotinib-induced DNA damage (Fig. 4E). Since we did not observe that Gefitinib or Erlotinib at the same concentration could induce DNA damage in EGFR-TKI-resistant NCI-H1975 cells unless HSP70 had been knocked down (Fig. 4F), we, therefore, propose that EGFR-TKI-induced DNA damage is secondary to HSP70 reduction. Thus, the remaining question is by which mechanism EGFR-TKI-induced HSP70 reduction causes DNA damage. Based on the studies by others [22, 24, 25] and our data herein, it is clear that HSP70 interacts with multiple enzymes and proteins in the BER pathway (at least APE1, Pol β, FEN1, XRCC1 [50], and candidates shown in Supplementary Table 3), indicating that HSP70 participates in the sequential coordination of the BER pathway. Using in vitro reconstitute BER assays, we directly show that the incubation of HSP70, but not HSC70 or BSA, increased the complete repair of the uracil-containing DNA substrate (Fig. 5C). Thus, HSP70 overexpression reduces DNA damage through promoting BER capacity. Previously, Li et al. reported that Erlotinib attenuates homologous recombinational repair in human breast cancer cells [49], it therefore should be noted that HSP70 may also have effects on other DNA repair pathways. Our preliminary studies show that Erlotinib inhibited the nuclear translocation of BRCA1 in HCC827 cells as observed in human breast cancer cells, but HSP70 overexpression did not rescue the decrease of BRCA1 in the nuclear fraction (Supplementary Fig. S8). Nonetheless, the identification of HSP70 binding partners in cell nuclei will provide useful information for the investigation of the comprehensive role of HSP70 in the DNA damage response and in DNA repair pathways (Supplementary Table 3), though most of them have yet to be confirmed in vivo.
Cells are confronted with tens of thousands of DNA lesions each day by endogenous and exogenous DNA-damaging agents. A significant portion of the extensive daily DNA damage occurring in each cell of the body is caused by reactive oxygen species (ROS) [51]. It is known that the reactions of pyrimidines and purines with ROS may result in DNA base modifications. If not properly repaired, such damage potentially leads to mutagenesis. For example, deamination of cytosine to uracil is an important pro-mutagenic event in DNA with the potential to produce G:C to T:A transition mutations [51]. In mammalian cells, the BER pathway is the major mechanism for repair of ROS-generated lesions, such as base modifications and single strand breaks. We observed that HSP70 overexpression in HCC827 cells evidently protected cells from H2O2-induced damage (Fig. 3E), reasonably, by promoting the capacity of BER. Although the BER pathway removes damaged bases and protects the genome from mutagenesis, it is one of the sources of mutation because of misincorporation during repair. Given that the EGFR T790M mutation is a C-T transversion at nucleotide position 2,369 of the EGFR gene, one can envision that the fidelity of BER could be of critical importance in preventing the emergence of EGFR secondary mutations. Our Pol β fidelity assay allows us to visualize the dNTP misincorporation produced by Pol β during DNA polymerase reaction. We show that the addition of HSP70 protein in the reaction mixture significantly reduced the incorrect incorporation of dNTP into DNA substrates (Fig. 4E), demonstrating that HSP70 promotes the fidelity of Pol β and suppresses gene mutation. We thus provide a more reasonable explanation for how HSP70 affects the gene mutation rates and the incidence of Erlotinib-induced EGFR T790M mutation in lung cancer cells.
Our study provides a potential explanation for the clinical observation that lung cancers bearing EGFR-activating mutation have shown favorable outcomes after the administration of EGFR-TKI; however, EGFR blockade may paradoxically enhance the gene mutation rates by induction of HSP70 degradation and attenuation of HSP70-mediated BER, thus facilitating the emergence of the EGFR T790M resistance mutation. The understanding of the mechanism underlying the effects of EGFR-TKI-induced HSP70 protein reduction will help develop novel strategies in governing DNA stability to prevent gene mutation-induced acquired drug resistance during the EGFR-TKI treatment of NSCLC.
Supplementary Material
Highlights.
-
◆
Low-dose EGFR-TKI-treatment causes HSP70 degradation
-
◆
Phosphorylation of HSP70 at residue Y41 regulates HSP70 protein stability
-
◆
EGFR-TKI-treatment elevates DNA damage and gene mutation rates via HSP70 reduction
-
◆
HSP70 promotes the efficiency and the fidelity of base excision repair
Acknowledgments
We are grateful to Dr. Ningwei Zhao for his help in MALDI-QIT-TOF-MS. This work was supported by grants from the National Nature Science Foundation (No. 31500628) to L. Lan, National Basic Research Program of China (973 Program, 2013CB911600) to Z.G. and L. Lan, the National Nature Science Foundation (No. 81471557 and No. 81671565) to Z.Y., the National Nature Science Foundation (No. 31571166) to L. Luo, the Natural Science Foundation of Jiangsu Province (No. BK20161555) to L. Lan, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PADD) to the Department of Life Science, Nanjing Normal University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
B.S., L. Lan, L. Luo, Z.G. and Z.Y. conceived and designed the experiments; X.C., Y.Z., H.S., M.X. and X.B. performed the experiments; Z.Z., B.S., F.W., Z.H., L. Lan, L. Luo, Z.G., and Z.Y. analyzed the data; L. Lan, Z.G., F.W., and L. Luo wrote the paper.
Conflicts of Interest
The authors declare no potential conflicts of interest.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Breathnach OS, Freidlin B, Conley B, Green MR, Johnson DH, Gandara DR, O'Connell M, Shepherd FA, Johnson BE. Twenty-two years of phase III trials for patients with advanced non-small-cell lung cancer: sobering results. J Clin Oncol. 2001;19:1734–1742. doi: 10.1200/JCO.2001.19.6.1734. [DOI] [PubMed] [Google Scholar]
- 3.Rosa DD, Ismael G, Lago LD, Awada A. Molecular-targeted therapies: lessons from years of clinical development. Cancer Treat Rev. 2008;34:61–80. doi: 10.1016/j.ctrv.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Comis RL. The current situation: erlotinib (Tarceva) and gefitinib (Iressa) in non-small cell lung cancer. Oncologist. 2005;10:467–470. doi: 10.1634/theoncologist.10-7-467. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 6.Ramalingam S, Sandler AB. Salvage therapy for advanced non-small cell lung cancer: factors influencing treatment selection. Oncologist. 2006;11:655–665. doi: 10.1634/theoncologist.11-6-655. [DOI] [PubMed] [Google Scholar]
- 7.Suda K, Mizuuchi H, Maehara Y, Mitsudomi T. Acquired resistance mechanisms to tyrosine kinase inhibitors in lung cancer with activating epidermal growth factor receptor mutation--diversity, ductility, and destiny. Cancer Metastasis Rev. 2012;31:807–814. doi: 10.1007/s10555-012-9391-7. [DOI] [PubMed] [Google Scholar]
- 8.Tartarone A, Lazzari C, Lerose R, Conteduca V, Improta G, Zupa A, Bulotta A, Aieta M, Gregorc V. Mechanisms of resistance to EGFR tyrosine kinase inhibitors gefitinib/erlotinib and to ALK inhibitor crizotinib. Lung Cancer. 2013;81:328–336. doi: 10.1016/j.lungcan.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol. 2014;11:473–481. doi: 10.1038/nrclinonc.2014.104. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 11.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, Wong KK, Meyerson M, Eck MJ. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105:2070–2075. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arcila ME, Oxnard GR, Nafa K, Riely GJ, Solomon SB, Zakowski MF, Kris MG, Pao W, Miller VA, Ladanyi M. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res. 2011;17:1169–1180. doi: 10.1158/1078-0432.CCR-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelman JA, Zejnullahu K, Gale CM, Lifshits E, Gonzales AJ, Shimamura T, Zhao F, Vincent PW, Naumov GN, Bradner JE, Althaus IW, Gandhi L, Shapiro GI, Nelson JM, Heymach JV, Meyerson M, Wong KK, Janne PA. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67:11924–11932. doi: 10.1158/0008-5472.CAN-07-1885. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, Padera RF, Shapiro GI, Baum A, Himmelsbach F, Rettig WJ, Meyerson M, Solca F, Greulich H, Wong KK. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cha MY, Lee KO, Kim M, Song JY, Lee KH, Park J, Chae YJ, Kim YH, Suh KH, Lee GS, Park SB, Kim MS. Antitumor activity of HM781-36B, a highly effective pan-HER inhibitor in erlotinib-resistant NSCLC and other EGFR-dependent cancer models. Int J Cancer. 2012;130:2445–2454. doi: 10.1002/ijc.26276. [DOI] [PubMed] [Google Scholar]
- 17.Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ, Hughes G, Rahi A, Jacobs VN, Red Brewer M, Ichihara E, Sun J, Jin H, Ballard P, Al-Kadhimi K, Rowlinson R, Klinowska T, Richmond GH, Cantarini M, Kim DW, Ranson MR, Pao W. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014 doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Song Y, Yan F, Liu D. Mechanisms of resistance to third-generation EGFR tyrosine kinase inhibitors. Frontiers of medicine. 2016 doi: 10.1007/s11684-016-0488-1. [DOI] [PubMed] [Google Scholar]
- 19.Thress KS, Paweletz CP, Felip E, Cho BC, Stetson D, Dougherty B, Lai Z, Markovets A, Vivancos A, Kuang Y, Ercan D, Matthews SE, Cantarini M, Barrett JC, Janne PA, Oxnard GR. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nature medicine. 2015;21:560–562. doi: 10.1038/nm.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 21.Daugaard M, Kirkegaard-Sorensen T, Ostenfeld MS, Aaboe M, Hoyer-Hansen M, Orntoft TF, Rohde M, Jaattela M. Lens epithelium-derived growth factor is an Hsp70-2 regulated guardian of lysosomal stability in human cancer. Cancer Res. 2007;67:2559–2567. doi: 10.1158/0008-5472.CAN-06-4121. [DOI] [PubMed] [Google Scholar]
- 22.Bases R. Heat shock protein 70 enhanced deoxyribonucleic acid base excision repair in human leukemic cells after ionizing radiation. Cell stress & chaperones. 2006;11:240–249. doi: 10.1379/CSC-185R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobol RW, Kartalou M, Almeida KH, Joyce DF, Engelward BP, Horton JK, Prasad R, Samson LD, Wilson SH. Base excision repair intermediates induce p53-independent cytotoxic and genotoxic responses. J Biol Chem. 2003;278:39951–39959. doi: 10.1074/jbc.M306592200. [DOI] [PubMed] [Google Scholar]
- 24.Kenny MK, Mendez F, Sandigursky M, Kureekattil RP, Goldman JD, Franklin WA, Bases R. Heat shock protein 70 binds to human apurinic/apyrimidinic endonuclease and stimulates endonuclease activity at abasic sites. The Journal of biological chemistry. 2001;276:9532–9536. doi: 10.1074/jbc.M009297200. [DOI] [PubMed] [Google Scholar]
- 25.Mendez F, Kozin E, Bases R. Heat shock protein 70 stimulation of the deoxyribonucleic acid base excision repair enzyme polymerase beta. Cell stress & chaperones. 2003;8:153–161. doi: 10.1379/1466-1268(2003)008<0153:hspsot>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Z, Zheng L, Dai H, Zhou M, Xu H, Shen B. Human DNA polymerase beta polymorphism, Arg137Gln, impairs its polymerase activity and interaction with PCNA and the cellular base excision repair capacity. Nucleic Acids Res. 2009;37:3431–3441. doi: 10.1093/nar/gkp201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Podlutsky AJ, Dianova II, Podust VN, Bohr VA, Dianov GL. Human DNA polymerase beta initiates DNA synthesis during long-patch repair of reduced AP sites in DNA. EMBO J. 2001;20:1477–1482. doi: 10.1093/emboj/20.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kubota Y, Nash RA, Klungland A, Schar P, Barnes DE, Lindahl T. Reconstitution of DNA base excision-repair with purified human proteins: interaction between DNA polymerase beta and the XRCC1 protein. EMBO J. 1996;15:6662–6670. [PMC free article] [PubMed] [Google Scholar]
- 29.Biade S, Sobol RW, Wilson SH, Matsumoto Y. Impairment of proliferating cell nuclear antigen-dependent apurinic/apyrimidinic site repair on linear DNA. J Biol Chem. 1998;273:898–902. doi: 10.1074/jbc.273.2.898. [DOI] [PubMed] [Google Scholar]
- 30.Dorjsuren D, Kim D, Maloney DJ, Wilson DM, 3rd, Simeonov A. Complementary non-radioactive assays for investigation of human flap endonuclease 1 activity. Nucleic Acids Res. 2011;39:e11. doi: 10.1093/nar/gkq1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Chen M, Zhou J, Zhang X. HSP27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy (Review) Int J Oncol. 2014;45:18–30. doi: 10.3892/ijo.2014.2399. [DOI] [PubMed] [Google Scholar]
- 32.Lianos GD, Alexiou GA, Mangano A, Mangano A, Rausei S, Boni L, Dionigi G, Roukos DH. The role of heat shock proteins in cancer. Cancer Lett. 2015;360:114–118. doi: 10.1016/j.canlet.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 33.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nature reviews. Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 34.Kim D, Kim SH, Li GC. Proteasome inhibitors MG132 and lactacystin hyperphosphorylate HSF1 and induce hsp70 and hsp27 expression. Biochem Biophys Res Commun. 1999;254:264–268. doi: 10.1006/bbrc.1998.9840. [DOI] [PubMed] [Google Scholar]
- 35.Knowlton AA, Grenier M, Kirchhoff SR, Salfity M. Phosphorylation at tyrosine-524 influences nuclear accumulation of HSP72 with heat stress. Am J Physiol Heart Circ Physiol. 2000;278:H2143–2149. doi: 10.1152/ajpheart.2000.278.6.H2143. [DOI] [PubMed] [Google Scholar]
- 36.Muller P, Ruckova E, Halada P, Coates PJ, Hrstka R, Lane DP, Vojtesek B. C-terminal phosphorylation of Hsp70 and Hsp90 regulates alternate binding to co-chaperones CHIP and HOP to determine cellular protein folding/degradation balances. Oncogene. 2013;32:3101–3110. doi: 10.1038/onc.2012.314. [DOI] [PubMed] [Google Scholar]
- 37.Johnson GE. Mammalian cell HPRT gene mutation assay: test methods. Methods Mol Biol. 2012;817:55–67. doi: 10.1007/978-1-61779-421-6_4. [DOI] [PubMed] [Google Scholar]
- 38.Sobol RW, Watson DE, Nakamura J, Yakes FM, Hou E, Horton JK, Ladapo J, Van Houten B, Swenberg JA, Tindall KR, Samson LD, Wilson SH. Mutations associated with base excision repair deficiency and methylation-induced genotoxic stress. Proc Natl Acad Sci U S A. 2002;99:6860–6865. doi: 10.1073/pnas.092662499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zharkov DO. Base excision DNA repair. Cell Mol Life Sci. 2008;65:1544–1565. doi: 10.1007/s00018-008-7543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sweasy JB. Fidelity mechanisms of DNA polymerase beta. Prog Nucleic Acid Res Mol Biol. 2003;73:137–169. doi: 10.1016/s0079-6603(03)01005-5. [DOI] [PubMed] [Google Scholar]
- 41.Kleppa L, Mari PO, Larsen E, Lien GF, Godon C, Theil AF, Nesse GJ, Wiksen H, Vermeulen W, Giglia-Mari G, Klungland A. Kinetics of endogenous mouse FEN1 in base excision repair. Nucleic Acids Res. 2012;40:9044–9059. doi: 10.1093/nar/gks673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Namba T, Tanaka K, Hoshino T, Azuma A, Mizushima T. Suppression of expression of heat shock protein 70 by gefitinib and its contribution to pulmonary fibrosis. PloS one. 2011;6:e27296. doi: 10.1371/journal.pone.0027296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qian SB, McDonough H, Boellmann F, Cyr DM, Patterson C. CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature. 2006;440:551–555. doi: 10.1038/nature04600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2000;6:2166–2174. [PubMed] [Google Scholar]
- 45.Chinnaiyan P, Huang S, Vallabhaneni G, Armstrong E, Varambally S, Tomlins SA, Chinnaiyan AM, Harari PM. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva) Cancer research. 2005;65:3328–3335. doi: 10.1158/0008-5472.CAN-04-3547. [DOI] [PubMed] [Google Scholar]
- 46.Dittmann K, Mayer C, Fehrenbacher B, Schaller M, Raju U, Milas L, Chen DJ, Kehlbach R, Rodemann HP. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. The Journal of biological chemistry. 2005;280:31182–31189. doi: 10.1074/jbc.M506591200. [DOI] [PubMed] [Google Scholar]
- 47.Toulany M, Kasten-Pisula U, Brammer I, Wang S, Chen J, Dittmann K, Baumann M, Dikomey E, Rodemann HP. Blockage of epidermal growth factor receptor-phosphatidylinositol 3-kinase-AKT signaling increases radiosensitivity of K-RAS mutated human tumor cells in vitro by affecting DNA repair. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:4119–4126. doi: 10.1158/1078-0432.CCR-05-2454. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka T, Munshi A, Brooks C, Liu J, Hobbs ML, Meyn RE. Gefitinib radiosensitizes non-small cell lung cancer cells by suppressing cellular DNA repair capacity. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:1266–1273. doi: 10.1158/1078-0432.CCR-07-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L, Wang H, Yang ES, Arteaga CL, Xia F. Erlotinib attenuates homologous recombinational repair of chromosomal breaks in human breast cancer cells. Cancer research. 2008;68:9141–9146. doi: 10.1158/0008-5472.CAN-08-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kotoglou P, Kalaitzakis A, Vezyraki P, Tzavaras T, Michalis LK, Dantzer F, Jung JU, Angelidis C. Hsp70 translocates to the nuclei and nucleoli, binds to XRCC1 and PARP-1, and protects HeLa cells from single-strand DNA breaks. Cell Stress Chaperones. 2009;14:391–406. doi: 10.1007/s12192-008-0093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maynard S, Schurman SH, Harboe C, de Souza-Pinto NC, Bohr VA. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis. 2009;30:2–10. doi: 10.1093/carcin/bgn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.