Abstract
This study investigated the changes in interhemispheric functional connectivity (FC) of the whole brain in open globe injury (OGI) patients, using voxel-mirrored homotopic connectivity (VMHC), and their relationships with clinical features. Totally, 16 male and 2 female acute OGI patients and 18 sex, age, and education-matched healthy volunteers were enrolled in the study. All subjects were scanned through functional magnetic resonance imaging (fMRI). Receiver operating characteristic (ROC) curves analyses had been used to identify the VMHC in these brain areas could be used as biomarkers to distinguish OGI and from healthy control (HC). The mean VMHC values in multiple brain areas and clinical OGI manifestations were evaluated with a Pearson correlation analysis. OGI patients had significantly decreased VMHC in the bilateral calcarine/lingual/cuneus (BA18, 19, 30) and middle occipital gyrus (BA18, 19). The OGI patients had abnormal interhemispheric FC in the dorsal visual pathway, which may represent the pathophysiological mechanism that underlies acute vision loss after OGI.
Keywords: unilateral acute open globe injury, voxel-mirrored homotopic connectivity, resting state, functional magnetic resonance imaging
INTRODUCTION
Ocular trauma is responsible for a large proportion of vision loss, with a prevalence in the adult population in Beijing of 1.6%±0.2% according to one survey[1]. Ocular trauma caused open globe injury (OGI) is an important cause of the blindness[2]. These OGIs can lead to corneal rupture[3] or scleral rupture[4]. At present, surgery is the definitive treatment for OGI[5]. However, OGI can still result in various serious complications including retinal detachment[6], glaucoma[7], and endophthalmitis[8]. The successful application of functional magnetic resonance imaging (fMRI) in OGI patients to assess the neurophysiological changes has been reported previously. In our own study, acute OGI patients were found to present with abnormal amplitudes of the low-frequency fluctuation (ALFF) in the precuneus, left cuneus, and left middle cingulate cortex[9]. Moreover, the OGI patients were also characterize by altered regional homogeneity in many brain regions[10]. However, it remains to be investigated if these patients also have alterations of interhemispheric functional synchronization. However, it is known that visual experience is closely related to interhemispheric synchrony[11]–[12]. The resting state-fMRI technology termed voxel-mirrored homotopic connectivity (VMHC) was used to quantify the functional connectivity (FC) of the time series of mirrored pairs of corresponding voxels in opposite hemispheres[13]. Its main advantage lies in its ability to reflect interhemispheric disconnection, revealing specific patterns. This method has also been used to investigate the underlying physiological mechanisms of a number of dysfunctions, such as early blindness[14], strabismic amblyopia[15], and postherpetic neuralgia[16]. Here, we assessed alternations of interhemispheric FC in OGI patients using the VMHC method.
METHODS
This research was conducted in accordance with the Declaration of Helsinki, with approval by the medical ethics committee of the Department of Ophthalmology, the First Affiliated Hospital of Nanchang University, and all participants signed informed consent forms. A total of 16 male and 2 female patients with acute OGI from the Department of Ophthalmology, the First Affiliated Hospital of Nanchang University in Jiangxi Province, China, were enrolled in this study. Patients were selected for unilateral acute OGI, with inclusion criteria as follows: 1) proven history of severe ocular trauma; 2) acute loss of vision following the trauma; 3) incomplete eyeball wall as shown by orbital computed tomography (CT) or MRI; 4) corneal or scleral rupture; 5) a value from 1.0 to 1.5 for best-corrected visual acuity (BCVA) in the contralateral eye. The exclusion criteria were eye disease (cataract, corneal ulcer, glaucoma, or optic nerve disease) affecting vision before the ocular trauma; other diseases, trauma or surgery of the eyes; psychiatric disorders (depressive disorder or delusions), cerebral disease (cerebral hemorrhage, vascular malformation or infarction), cardiovascular disease, or diabetes. The final cohort encompassed 16 male and 2 female healthy volunteers, matched for sex, age, and educational status. The requirements for healthy controls (HCs) were: normal brain parenchyma as shown by cranial MRI; no ocular or psychiatric disease as defined above; visual acuity (VA) from 1.0 to 1.5; and no contraindications for MRI (implanted metal devices, e.g. cardiac pacemaker).
A 3T MRI system (Siemens, Munich, Germany) was used for imaging, as described previously, and functional data were analyzed according to a published protocol[14]. A study-specific symmetric Montreal Neurological Institute (MNI) template was used to register reprocessed images of each subject, which were used to calculate the VMHC. Fisher's z-transformation in REST software (http://resting-fmri.sourceforge.net) was used to convert the individual VMHC maps to z values to improve the normality. A voxel-based random-effects two-sample t-test [false discovery rate (FDR) corrected, P<0.05 and cluster >20] was conducted using the individual z-maps in conjunction with the global VMHC as covariate to identify the differences of VMHC in the two groups. A detailed description is available in a previous paper[14].
Finally, independent-sample t-tests used to evaluate the onset, duration of OGI and other cumulative clinical measurements using SPSS version 16.0 (IBM Corp., USA). Differences with P<0.05 were considered statistically significant. The differences in the z-maps between the OGI groups and the HCs were examined using two-sample t-tests using Gaussian Random Field (GRF) theory (cluster >20 voxels, FDR corrected, z>2.3, P<0.01). The mean differences of VMHC values between pairs of groups in corresponding brain regions were analyzed using receiver operating characteristic (ROC) curves, and Pearson correlation analysis was used to analyze the relationships between the mean VMHC values. Differences with P<0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Demographic Characteristics and Visual Acuity
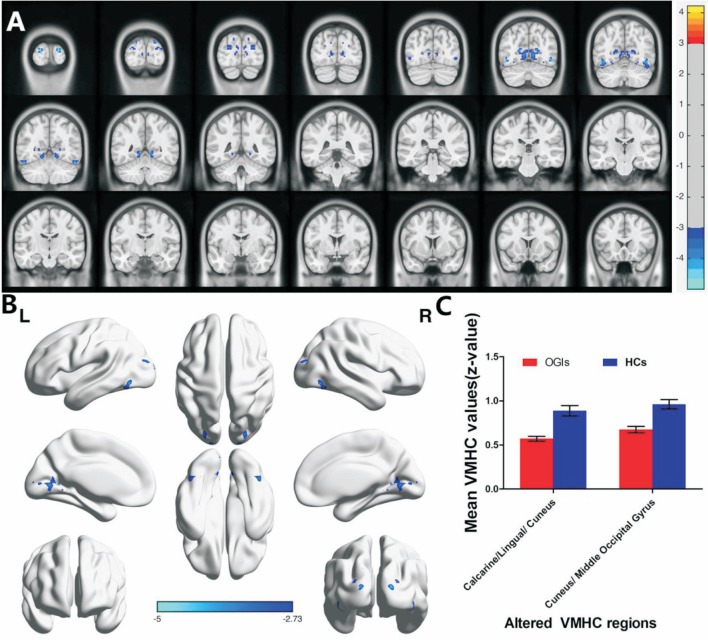
There were no statistically significant differences between the study and control groups in age and weight (P=0.856 and 0.908, respectively; Table 1). However, there were significantly lower VMHC values in the OGIs of bilateral lingual/calcarine/cunei (BA18, 19, 30) and the cunei/middle occipital gyri [BA19; Figure 1A and 1B (blue); Table 2]. The mean values of the changed VMHC between the two groups are shown in Figure 1C (cluster >20 voxels, FDR corrected; z>2.3, P<0.01). There was no significant correlation between the clinical manifestations and the mean VMHC values in the OGI group (P>0.05). We hypothesized that differences in the VMHC of different brain regions between the OGI and HC groups have diagnostic potential as markers. To test this, we used receiver operating characteristic (ROC) curves to analyze the mean VMHC values of different brain regions. The VMHC values had areas under the ROC curve (AUC) of 0.861 for calcarine/lingual/cuneus and 0.870 for cuneus/middle occipital gyrus (OGIs<HCs; Figure 2).
Table 1. Demographics and clinical measurements by group.
| Condition | OGI | HC | t | aP |
| Male/Female | 16/2 | 16/2 | N/A | >0.99 |
| Age (y) | 43.05±8.79 | 42.50±9.41 | 0.183 | 0.856 |
| Weight (kg) | 61.61±5.79 | 61.39±5.69 | 0.116 | 0.908 |
| Handedness | 18R | 18R | N/A | >0.99 |
| The side of injured eye | 10 left/8 right | |||
| Duration of OGI (d) | 6.56±3.43 | N/A | N/A | N/A |
| BCVA-right injured eye (n=8) | 0.23±0.07 | 1.21±0.16 | -12.342 | <0.001 |
| BCVA-left injured eye (n=10) | 0.11±0.05 | 1.25±0.29 | -19.852 | <0.001 |
A comparison of the patients and HCs via independent t-tests. OGI: Open globe injury; HC: Healthy control; N/A: Not applicable; BCVA: Best-corrected visual acuity; aP<0.05.
Figure 1. Group comparison of interhemispheric FC between OGI patients and HCs.
There were significant differences of FC in the bilateral calcarine/lingual/cunei (BA18, 19, 30) and cuneus/middle occipital gyri (BA19). Lower VMHC values are shown in blue. P<0.01; multiple comparisons via Gaussian Random Field (GRF) theory (cluster >20 voxels, FDR corrected; z>2.3, P<0.01; A and B, C: The mean values of the altered regional homogeneity were shown with a histogram between two groups. FC: functional connectivity; OGI: Open globe injury; HCs: Healthy controls; BA: Brodmann area; VMHC: Voxel-mirrored homotopic connectivity; FDR: False discovery rate.
Table 2. Brain areas with significant differences of VMHC between the patients and the control group.
| Brain areas | BA | MNI |
Cluster size | t | ||
| x | y | z | ||||
| OGI<HCs | ||||||
| Calcarine/Lingual/ Cuneus | 18, 19, 30 | ±51 | -57 | -21 | 223 | -4.484 |
| Cuneus/ Middle Occipital Gyrus | 19 | ±18 | -99 | 12 | 68 | -4.996 |
The voxel-level statistical threshold for multiple comparisons using Gaussian random field (GRF) theory was P<0.05 (cluster >20 voxels, FDR corrected; z>2.3, P<0.01). OGI: Open globe injury; HCs: Healthy controls; VMHC: Voxel-mirrored homotopic connectivity; BA: Brodmann area; MNI: Montreal Neurological Institute.
Figure 2. Analysis of the mean VMHC values for altered brain regions using ROC curve methodology.
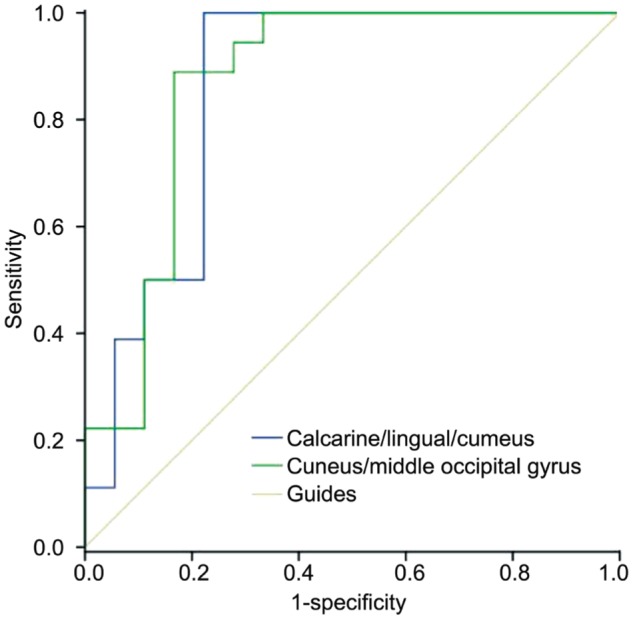
The area under the ROC curve was 0.861 for the calcarine/lingual/cuneus (P<0.001; 95% CI: 0.728-0.994), and 0.870 for the cuneus/middle occipital gyrus (P<0.001; 95%CI: 0.743-0.998; BA19: OGIs <HCs). VMHC: Voxel-mirrored homotopic connectivity; CI: Confidence interval.
The VMHC method utilizes reliable and non-invasive resting state-fMRI to reveal interhemispheric FC. Compared with the HC, the OGI patients in our study had significantly lower VMHC values in the bilateral calcarine/lingual/cunei (BA18, 19, 30) and middle occipital gyri (BA18, 19), which are important parts of the dorsal visual pathways.
The cuneus is a crucial part for visual processing in the occipital lobe. The anteromedial cuneus interacts with the primary visual cortex (V1) to encode visual information for the extrastriate cortex[17]. The cuneus is the part of the visual pathway involved in spatial location[18]. In patients with late monocular blindness, we already demonstrated a decreased amplitude of low-frequency fluctuation values in the right cunei in an earlier study[19]. Our present results also indicate that unilateral acute OGI significantly reduced the VMHC in the cuneus, an indication of interhemispheric FC impairment in the cuneus. We therefore speculated that acute OGI might cause the cuneal dysfunction.
BA18, the lingual gyrus, is part of the occipital cortex that performs visual processing[20]. It has a critical function in reading[21] and spatial memory[22]. At the same time, the lingual gyrus is an important part of the dorsal visual pathway. Previously we demonstrated that the regional homogeneity in the right lingual gyrus was increased in OGI patients[10]. The patients with acute OGI in this study had lower VMHC values in the lingual gyrus, which reflected dysfunction of their interhemispheric FC. Thus, we thought that acute OGI might cause functional impairment of the lingual gyri.
The middle occipital gyrus, known as BA19, is the part of the visual cortex, which involves spatial processing[23] and category-selective attention[24]. The middle occipital gyrus is the dorsal visual pathway. In our study, acute OGI led to a reduction of the VMHC in the middle occipital gyrus, which reflected interhemispheric FC impairment in the middle occipital gyrus. We therefore speculated that the middle occipital gyrus of acute OGI patients might be dysfunctional. Our results demonstrated that acute OGI was associated with interhemispheric FC impairment in the dorsal visual pathway. This provides new knowledge on the physiological mechanisms underlying sharp loss of visual acuity in unilateral acute OGI patients.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81660158; No.81400372); Natural Science Key Project of Jiangxi Province (No.20161ACB21017); Health Development Planning Commission Science Foundation of Jiangxi Province (No.20175116).
Conflicts of Interest: Ye L, None; Wei R, None; Huang X, None; Shi WQ, None; Yang QC, None; Yuan Q, None; Zhu PW, None; Jiang N, None; Li B, None; Zhou Q, None; Zhou FQ, None; Shao Y, None.
REFERENCES
- 1.Wang JD, Xu L, Wang YX, You QS, Zhang JS, Jonas JB. Prevalence and incidence of ocular trauma in North China: the Beijing Eye Study. Acta Ophthalmol. 2012;90(1):e61–e67. doi: 10.1111/j.1755-3768.2011.02230.x. [DOI] [PubMed] [Google Scholar]
- 2.Yildiz M, Kıvanç SA, Akova-Budak B, Ozmen AT, Çevik SG. An important cause of blindness in children: open globe injuries. J Ophthalmol. 2016;2016:7173515. doi: 10.1155/2016/7173515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nawani N, Vazirani J, Ojha H, Sangwan VS. Conjunctival pedicle flap in management of open globe injury with corneal tissue loss. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2015-213703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yucel OE, Demir S, Niyaz L, Sayin O, Gul A, Ariturk N. Clinical characteristics and prognostic factors of scleral rupture due to blunt ocular trauma. Eye (Lond) 2016;30(12):1606–1613. doi: 10.1038/eye.2016.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salehi-Had H, Andreoli CM, Andreoli MT, Kloek CE, Mukai S. Visual outcomes of vitreoretinal surgery in eyes with severe open-globe injury presenting with no-light-perception vision. Graefes Arch Clin Exp Ophthalmol. 2009;247(4):477–483. doi: 10.1007/s00417-009-1035-4. [DOI] [PubMed] [Google Scholar]
- 6.Stryjewski TP, Andreoli CM, Eliott D. Retinal detachment after open globe injury. Ophthalmology. 2014;121(1):327–333. doi: 10.1016/j.ophtha.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osman EA. Glaucoma after open globe injury. Saudi J Ophthalmol. 2015;29(3):222–224. doi: 10.1016/j.sjopt.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Zhang MN, Jiang CH, Yao Y, Zhang K. Endophthalmitis following open globe injury. Br J Ophthalmol. 2010;94(1):111–114. doi: 10.1136/bjo.2009.164913. [DOI] [PubMed] [Google Scholar]
- 9.Tan G, Huang X, Ye L, Wu AH, He LX, Zhong YL, Jiang N, Zhou FQ, Shao Y. Altered spontaneous brain activity patterns in patients with unilateral acute open globe injury using amplitude of low-frequency fluctuation: a functional magnetic resonance imaging study. Neuropsychiatr Dis Treat. 2016;12:2015–2020. doi: 10.2147/NDT.S110539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang X, Li HJ, Ye L, Zhang Y, Wei R, Zhong YL, Peng DC, Shao Y. Altered regional homogeneity in patients with unilateral acute open-globe injury: a resting-state functional MRI study. Neuropsychiatr Dis Treat. 2016;12:1901–1906. doi: 10.2147/NDT.S110541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foubert L, Bennequin D, Thomas MA, Droulez J, Milleret C. Interhemispheric synchrony in visual cortex and abnormal postnatal visual experience. Front Biosci (Landmark Ed) 2010;15:681–707. doi: 10.2741/3640. [DOI] [PubMed] [Google Scholar]
- 12.Mima T, Oluwatimilehin T, Hiraoka T, Hallett M. Transient interhemispheric neuronal synchrony correlates with object recognition. J Neurosci. 2001;21(11):3942–3948. doi: 10.1523/JNEUROSCI.21-11-03942.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, Grzadzinski R, Evans AC, Zang YF, Castellanos FX, Milham MP. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 2010;30(45):15034–15043. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou F, Liu X, Zhou Z, Zhou J, Li H. Reduction of interhemispheric functional brain connectivity in early blindness: a resting-state fMRI study. Biomed Res Int. 2017;2017:6756927. doi: 10.1155/2017/6756927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang M, Xie B, Yang H, Yin X, Wang H, Yu L, He S, Wang J. Altered interhemispheric functional connectivity in patients with anisometropic and strabismic amblyopia: a resting-state fMRI study. Neuroradiology. 2017;59(5):517–524. doi: 10.1007/s00234-017-1824-0. [DOI] [PubMed] [Google Scholar]
- 16.Jiang J, Gu L, Bao D, Hong S, He W, Tan Y, Zeng X, Gong H, Zhang D, Zhou F. Altered homotopic connectivity in postherpetic neuralgia: a resting state fMRI study. J Pain Res. 2016;9:877–886. doi: 10.2147/JPR.S117787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanni S, Tanskanen T, Seppä M, Uutela K, Hari R. Coinciding early activation of the human primary visual cortex and anteromedial cuneus. Proc Natl Acad Sci U S A. 2001;98(5):2776–2780. doi: 10.1073/pnas.041600898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao H, Zhou T, Zhuo Y, Fan S, Chen L. Spatiotemporal activation of the two visual pathways in form discrimination and spatial location: a brain mapping study. Hum Brain Mapp. 2003;18(2):79–89. doi: 10.1002/hbm.10076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Huang X, Ye L, Wei R, Zhang Y, Zhong YL, Jiang N, Shao Y. Altered spontaneous brain activity pattern in patients with late monocular blindness in middle-age using amplitude of low-frequency fluctuation: a resting-state functional MRI study. Clin Interv Aging. 2016;11:1773–1780. doi: 10.2147/CIA.S117292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogousslavsky J, Miklossy J, Deruaz JP, Assal G, Regli F. Lingual and fusiform gyri in visual processing: a clinico-pathologic study of superior altitudinal hemianopia. J Neurol Neurosurg Psychiatry. 1987;50(5):607–614. doi: 10.1136/jnnp.50.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mechelli A, Humphreys GW, Mayall K, Olson A, Price CJ. Differential effects of word length and visual contrast in the fusiform and lingual gyri during reading. Proc Biol Sci. 2000;267(1455):1909–1913. doi: 10.1098/rspb.2000.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sulpizio V, Committeri G, Lambrey S, Berthoz A, Galati G. Selective role of lingual/parahippocampal gyrus and retrosplenial complex in spatial memory across viewpoint changes relative to the environmental reference frame. Behav Brain Res. 2013;242:62–75. doi: 10.1016/j.bbr.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 23.Renier LA, Anurova I, De Volder AG, Carlson S, VanMeter J, Rauschecker JP. Preserved functional specialization for spatial processing in the middle occipital gyrus of the early blind. Neuron. 2010;68(1):138–148. doi: 10.1016/j.neuron.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tu S, Qiu J, Martens U, Zhang Q. Category-selective attention modulates unconscious processes in the middle occipital gyrus. Conscious Cogn. 2013;22(2):479–485. doi: 10.1016/j.concog.2013.02.007. [DOI] [PubMed] [Google Scholar]