Abstract
AIM
To evaluate the efficacy of Goji berry supplementation on improving macular pigment, serum zeaxanthin levels and visual acuity in patients with early age-related macular degeneration (AMD).
METHODS
A total of 114 patients (aged from 51 to 92y, mean age 69.53±8.41y) with early AMD were enrolled in our prospective, randomized controlled study. The included patients were assigned randomly to the Goji group (n=57) with 25 g of Goji berries supplementation per day for 90d and the control group (n=57) with their normal diet for 90d. Macular pigment optical density (MPOD) was measured using heterochromatic flicker photometry (HFP). The levels of serum lutein (L)/zeaxanthin (Z) were analyzed using high-performance liquid chromatography (HPLC). MPOD, serum L/Z levels and best corrected visual acuity (BCVA) were recorded at baseline and 90d.
RESULTS
In the Goji group, there were no statistically significant differences in the serum L levels between the baseline (0.199±0.149 µmol/mL) and 90d (0.203±0.181 µmol/mL) (t=-0.186, P=0.850); however the serum Z levels were increased at 90d (0.101±0.087 µmol/mL) compared with those at the baseline (0.029±0.032 µmol/mL) (t=6.412, P<0.001). Patients treated with Goji berry for 90d showed an elevated MPOD (0.877±0.202 DU) from the baseline (0.731±0.205 DU) (t=-4.741, P=0.000). In contrast to the control group, the serum Z levels and MPOD were higher in the Goji group at 90d (both P<0.05). At 90d, patients with Goji berry supplementation had a relative decrease in BCVA (0.21±0.18 logMAR) compared with the baseline (0.27±0.20) (t=2.397, P=0.020).
CONCLUSION
Overall, daily supplementation with Goji berry for 90d improves MPOD by increasing serum Z levels rather than serum L levels in early AMD patients. Goji berry may be an effective therapeutic intervention for preventing the progression of early AMD.
Keywords: Goji berry, zeaxanthin, early age-related macular degeneration
INTRODUCTION
Age-related macular degeneration (AMD) is a progressive and degenerative disease at the central area of the retina, which results in severe visual impairment[1]. It is the leading cause of irreversible vision loss among people over 50 years of age, especially in developed countries[2]. As the average age of the population increases, the number of AMD patients is estimated to triple to 60 to 75 million worldwide in the next 30 to 40y[3]. Although new therapies with anti-vascular endothelial growth factor agents have been shown to slow down the progressive visual loss effectively in certain types of neovascular AMD, there are no effective treatments for early AMD at present[4]. Proper intervention at an earlier stage might slow down the progression of early AMD before it causes irreversible visual impairment, which would be more effective in enhancing or maintaining visual function. Therefore, it is urgent to find feasible interventions to prevent or delay the progress of early AMD.
The exact pathogenic mechanism of AMD has not been identified. The light-initiated oxidative damage and the reduction of macular pigment (MP) are hypothesized to be the putative mechanisms involved in this disease[5]. MP have a unique distribution in the retina. Concentrations of lutein (L), zeaxanthin (Z) and meso-zeaxanthin (MZ) are highest in the macula, especially in the center of the macula (the fovea)[6]. L and Z have a peak absorbance near 460 nm. In the inner retina, they serve as a filter for high energy, like short wavelength blue light[7]. Li et al[8] showed that the 1:1:1 mixture of L, Z and MZ more efficiently quenched singlet oxygen than any one of the three individually. Meanwhile, a Meta-analysis showed that supplementation of L, Z, and MZ could improve macular pigment optical density (MPOD) in early AMD patients[9]. Therefore, daily intake of supplementary foods rich in carotenoids may reduce the risk of early AMD.
Lycium barbarum L is a deciduous shrub with a height of 1 to 3 m, belonging to the Solanaceae family. The fruit of Lycium barbarum and the closely related species L. Chinese are known as ‘Goji berries’ or ‘Chinese wolfberries’. Goji berry is the richest natural source of antioxidants, such as carotenoids and Z[10] and was used for improving eyesight in ancient China. It contains a variety of carotenes and has a stronger antioxidant effect than L or Z alone. Hempel et al[11] showed the digestion in vitro revealed more enhanced liberation and bioavailability of Z from these tubular aggregates in Goji berries than protein-complexed L from spinach. Therefore, Goji berries might be a more potent source of MP than green leafy vegetables like spinach. Bucheli et al[12] found supplementation of Goji berries in the daily diet for 90d increased plasma Z and antioxidant levels by 26% and 57%, respectively, in healthy elderly individuals. However, there is no available analysis of the relationship between changes in serum Z and MPOD after Goji berry supplementation. Therefore, we conducted a prospective, randomized study to evaluate serum L/Z levels, MPOD, and best corrected visual acuity (BCVA) in early AMD patients with daily supplementation of Goji berries for 90d.
SUBJECTS AND METHODS
Subjects
A total of 114 subjects with early AMD (aged 51-92y, mean age 69.53±8.41y) were recruited in Beijing Bo'ai Hospital in China. Inclusion criteria included a clinical diagnosis of early AMD, which was defined as the presence of soft distinct drusen and/or soft indistinct drusen and/or reticular drusen and/or pigmentary abnormalities. Soft distinct and indistinct drusen were larger than 125 µm in diameter and were concomitant with uniform density and sharp edges or decreasing density from the center outwards and fuzzy edges, respectively[13]. Pigmentary abnormalities were defined as areas of hyperpigmentation and/or hypopigmentation (without visibility of choroidal vessels). Patients who had other ocular disorders or unstable systemic or chronic illness or those who had consumed dietary supplements containing antioxidants or carotenoids within the past 6mo were excluded. This study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Medical Ethics Committee of Beijing Bo'ai Hospital. Written consent was obtained from all subjects.
Study Design
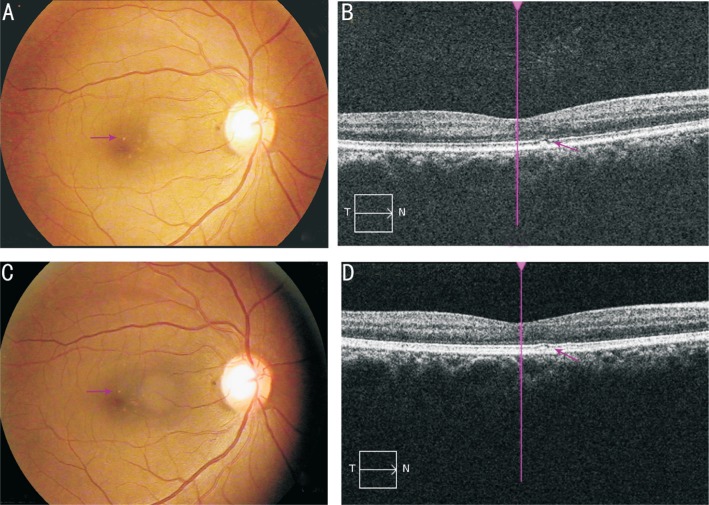
All subjects were screened for eligibility based on the protocol criteria. Diagnosis of early AMD was confirmed by 2 ophthalmologists by using fundoscopy and fundus photographs. The characteristics and demographic information of each included subject was collected using questionnaires and examinations. Physical and ophthalmologic examinations were conducted at baseline and 90d, including slit-lamp examination, BCVA assessment, color fundus photography, optical coherence tomography (OCT), blood pressure measurement, body weight and height. Representative fundus and OCT images were shown in Figure 1.
Figure 1. Representative of color fundus photography and OCT photographs in early AMD patients.
A, B: Fundus and OCT photographs showed a soft distinct drusen at baseline; C, D: Fundus and OCT photographs showed that the drusen gradually diminished at the same position as arrowed after 90d of Goji berry supplementation.
In this 90-day prospective, randomized controlled trial, all subjects were randomized by a 1:1 ratio to the Goji group and the control group. Goji berries were provided by the county of Zhongning in the Ningxia Hui Autonomous Region of China. The contents of carotene, L, Z, vitamin C, brass and zinc in each hectogram of wolfberry were 7.38, 10.05, 60.32, 18.4, 540.25 and 2.1 mg, individually. Goji berries also contain various vitamins, including vitamins E and C. Of the 57 randomized subjects in the Goji group, the mean age was 68.89±9.04y which included 38 (66.7%) women and 19 (33.3%) men. For the 57 subjects in the control group, the mean age was 70.19±7.79y including 34 (59.6%) women and 23 (40.4%) men, all the subjects were asked to continue their normal diet for 90d. Serum L/Z concentrations, MPOD and BCVA were measured at baseline and 90d. All clinical examinations were performed by two qualified ophthalmic technicians in Beijing Bo'ai Hospital.
Visual Performance
After diopter correction, BCVA was measured according to the early treatment diabetic retinopathy study (ETDRS) protocol, and the results were converted to the logarithm of the minimum angle of resolution (logMAR)[14].
Macular Pigment Optical Density Measurement
The MPODs of the fovea and parafovea were measured using heterochromatic flicker photometry (HFP, Macular Metrics II™) by the same technician who was blinded to the patients' information[15]. The MPODs of the fovea and the parafovea were measured separately three times, and then a mean value of MPOD was calculated. Before testing, the visual acuity was corrected in each subject, and then they were allowed to fixate at the central target and the parafoveal fixation target, which was located 6° nasally and horizontally monocular for each eye. First, the subjects were asked to select the best flicker frequency for the stimuli as the normal variation in flicker sensitivity. Then, they were asked to view the test field superimposed on a blue background using one test eye. The test field alternated between a wavelength (blue or blue-green) that is absorbed by the MP and a reference wavelength (green to yellow-green) that is outside the absorption band of MP. When making measurements, the patients were instructed as the flicker stopped and the technician adjusted the energy of the bluish test light. The amount of bluish test light that is required to nullify the flicker provides a measure of the absorption of MP at the retinal location of the test light. Finally, the technician noted the data when the luminance became green, providing a measure of the absorption of MP.
Serum Lutein/Zeaxanthin Concentrations
Frozen serum samples were collected from all participants and stored in a -80°C freezer. Before using, the samples were kept at room temperature for 30min, and then vortexed adequately. A total of 500 µL of serum was transferred into a 2.0 mL centrifuge tube, adding 300 µL of ethanol. The mobile phase consisted of n-hexane (A) and isopropanol (B). A gradient program was used as follows: initial 0-45min, linear change from A-B (95:5, v/v) to A-B (88.5:11.5, v/v); 45-48min, linear change to A-B (80:20, v/v); 48-55min, linear change to A-B (95:5, v/v). The mobile phase flow rate was monitored at 1.0 mL/min. All separations were carried out on a CHIRALPAK AD-14 column (250 mm×4.6 mm i.d, 5.0 µm particle size) from Daicel Chiral Technologies under the temperature of 35°C, then were detected at 453 nm. The 20 µL volume was injected into the high-performance liquid chromatography (HPLC) system (Agilent Technologies, Waldbronn, Germany) equipped with the ChemStation software (Agilent Technologies) and a quaternary pump including an online vacuum degasser, an auto-sampler, a thermostated column compartment and a UV detector used for the chromatographic analysis[16]. All operations were under dim light. Then, all test samples were compared with the standard sample (Figure 2), and the peak values of (3R, 3′R)-zeaxanthin and (3R, 3′R, 6′R)-lutein were measured as shown in Figure 3.
Figure 2. The standard sample was used as a reference.
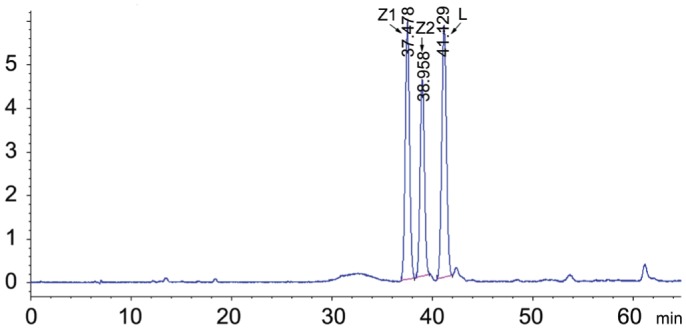
Z1 (3R, 3′S)-zeaxanthin (0.522 µg/mL), Z2 (3R, 3′R)-zeaxanthin (0.398 µg/mL) and L (3R, 3′R, 6′R)-lutein (0.582 µg/mL) are arrowed.
Figure 3. Chromatogram of extracted serum from a single volunteer.
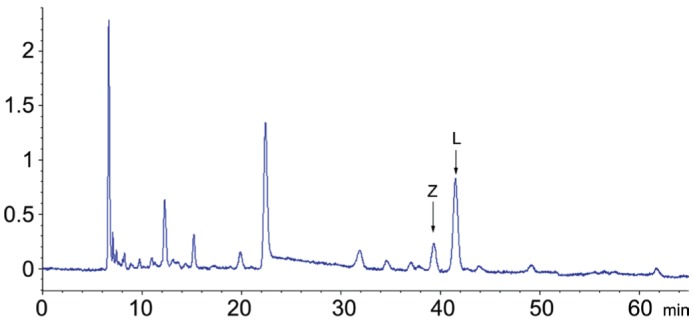
Peaks of zeaxanthin and lutein (identified by comparison with injection of pure zeaxanthin and lutein) are arrowed.
Statistical Analysis
Continuous variables were summarized as means or medians with appropriate measures of dispersion. Categorical variables were summarized as frequencies and percentages. Baseline characteristics of the two study groups were compared and evaluated using independent-sample t-tests or Chi-squared tests. Statistical significance for changes from baseline in each group was tested using the paired t-test. The linear correlation between MPOD and serum Z levels was assessed by the Pearson test. All analyses were performed using SPSS software version 21.0 (SPSS, Inc., IBM, USA). A 2-tailed P value of less than 0.05 was considered statistically significant.
RESULTS
Participant Characteristics
The baseline characteristics of the study population were shown in Table 1. Totally 114 participants met all the criteria in this study, and half of them were assigned randomly to receive Goji berry treatment. Of the 114 patients, 72 (63.2%) were women and 42 (36.8%) were men. There were no statistically significant differences in risk factors, including age, gender, educational background, smoking status, hypertension, hyperlipidemia, diabetes mellitus and body mass index (BMI) between two groups or during the intervention (P>0.05). No significant differences were observed in BCVA (P=0.186) and MPOD (P=0.837) before intervention between two groups.
Table 1. Baseline characteristics of study participants.
| Characteristics | Goji group (n=57) | Controls (n=57) | P |
| Age (y) | 68.89±9.04 | 70.19±7.79 | 0.412 |
| Gender | 0.437 | ||
| M | 19 (33.3) | 23 (40.4) | |
| F | 38 (66.7) | 34 (59.6) | |
| Educational level | 0.255 | ||
| Primary school | 10 (17.5) | 14 (24.6) | |
| Middle school | 27 (47.4) | 29 (50.9) | |
| High school | 9 (15.8) | 10 (17.5) | |
| College | 11 (19.3) | 4 (7.0) | |
| Hypertension | 33 (57.9) | 31 (54.4) | 0.850 |
| Diabetes mellitus | 13 (22.8) | 17 (29.8) | 0.542 |
| Hyperlipidemia | 29 (50.9) | 19 (33.3) | 0.088 |
| Smoking status | 0.799 | ||
| Never | 42 (73.7) | 40 (70.2) | |
| Former | 4 (7.0) | 6 (10.5) | |
| Current | 11 (19.3) | 11 (19.3) | |
| Body mass index, kg/m2 | 24.81±3.01 | 24.86±2.09 | 0.928 |
| BCVA, logMAR | 0.27±0.20 | 0.22±0.22 | 0.186 |
| MPOD, DU | 0.731±0.205 | 0.725±0.187 | 0.837 |
BCVA: Best corrected visual acuity; DU: Density unit; logMAR: Logarithm of minimum angle of resolution; MPOD: Macular pigment optical density; Values are expressed as mean±standard deviation unless otherwise noted; P values for any difference in groups derived from analysis of the independent sample t-test or Wilcoxon Rank Sum test for categorical variables; Body mass index was calculated as weight in kilograms divided by height in square meters.
n (%)
Best Corrected Visual Acuity
BCVA decreased from the baseline 0.27±0.20 (logMAR) to 0.21±0.18 after 90d of Goji berry supplementation, the difference is statistically significant (t=2.397, P=0.020). In the control group, BCVA was 0.22±0.22 and 0.22±0.19 at the baseline and 90d, respectively, with no statistically significant difference (t=0.136, P=0.892).
Serum Lutein/Zeaxanthin Levels and Macular Pigment Optical Density
At baseline, serum L levels were 0.199± 0.149 µmol/mL in the Goji group and 0.216±0.205 µmol/mL in the control group, and the Z levels were 0.029±0.032 µmol/mL and 0.030±0.049 µmol/mL, individually. There were no differences in serum L and Z levels between the two groups at baseline (P>0.05). Serum Z levels were 0.101±0.087 µmol/mL after 90d of Goji berry supplementation, which is significantly higher than the baseline level (t=6.412, P<0.001) and shows a statistically significant difference compared with in the control group (0.032±0.057 µmol/mL) (t=4.622, P<0.001). However, serum L levels at 90d were 0.203±0.181 µmol/mL in the Goji group, which shows no statistically significant difference in both the baseline level and that in the control group (t=-0.186, P=0.853; t=0.182, P=0.856).
After 90d of Goji supplementation, MPOD values were 0.877±0.202 DU, which is significantly higher than the baseline value (0.731±0.205) (t=-4.741, P=0.000), and the difference is statistically significant compared with that in the control group (t=-2.871, P=0.007). The MPOD values in the control group were 0.725±0.187 DU and 0.762±0.185 DU at baseline and at 90d, respectively, and the change is non-significant (P>0.05).
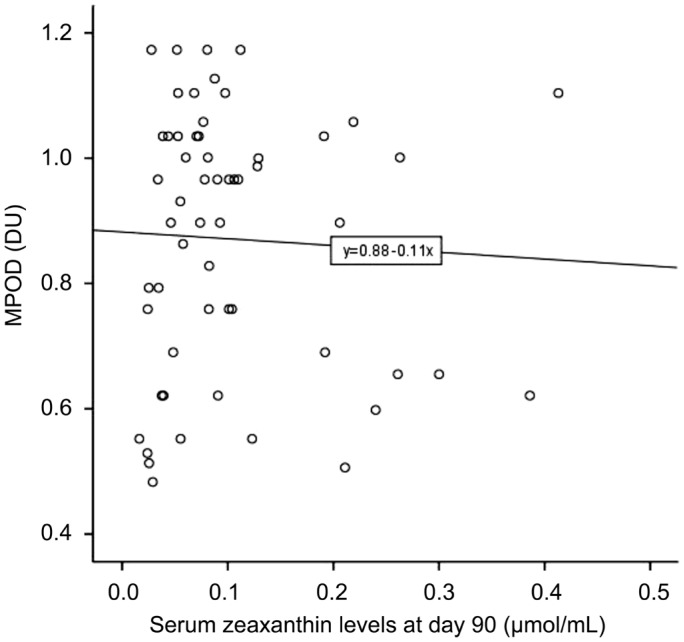
Furthermore, we analyzed the correlation between serum Z levels and MPOD. There was no statistically significant difference between the two variables at 90d (r=-0.046, P=0.733). The linear regression equation is shown in Figure 4.
Figure 4. Associations between serum Z levels and MPOD in the Goji group at 90d.
The linear regression equation is Y(MPOD)=0.882-0.108X(serum Z levels).
DISCUSSION
This prospective, randomized controlled trial demonstrated that 90d of Goji berry supplementation could increase serum Z concentrations, MPOD, and visual function in patients with early AMD, without causing any detectable adverse effects. Another important finding of this study was that improvements in MPOD were associated with increased serum Z levels, rather than serum L levels, which suggested that increasing serum Z concentrations is important for improving the visual function of patients with early AMD. These findings provide evidence that Goji berry supplementation may have some benefits for the macular function of patients with early AMD by increasing MPOD value.
Our result is consistent with Bucheli et al[12]. Moreover, we are the first to report the effect of oral Goji berry supplementation in early AMD patients. We found that serum Z levels were increased by Goji berry supplementation for 90d, but serum L levels did not change. This may be related to the higher concentration of Z in Goji berries and that approximately 90% of Z is in the form of the ester and zeaxanthin dipalmitate[17]. We found that serum Z levels increased three-fold after 90d of treatment with 25 g of Goji berry supplementation per day. Another human study showed a three-fold increase of blood Z levels after 28d of treatment with 15 g of Goji berry supplementation per day[17]. Several factors were found to affect the nutrient absorption and bioavailability, including age, smoking, lipoproteins, and BMI. The average age of subjects was 28y for the study conducted by Cheng et al[17]. In contrast, the average age of subjects was 70y in our study. Renzi et al[18] found that there is a significant and positive association between serum L and Z, MP, and high-density lipoproteins (HDLs). L and Z within the liver are incorporated into lipoprotein molecules that are transported to the retina through the HDLs[19]. Additionally, adipose tissue is a major storage organ for carotenoids, and higher concentrations of adipose tissues may trap L/Z and make it less available to other organs, such as the macula[20]. In our study, the average BMI was 24.81±3.01 kg/m2, which may be another factor that makes Goji berries less effective. Smokers have lower serum L and Z concentrations than those in nonsmokers independently of dietary L/Z intake[21]. In our study, serum Z concentrations increased in both smokers and nonsmokers, the reason might be that a modest antioxidant effect of Goji berries could be overwhelmed by the strong oxidative stress due to smoking.
Puell et al[22] indicated there were no significant differences in foveal MPOD between the control eyes and eyes with early AMD. However, some researchers believe that MPOD is reduced in patients with AMD compared with healthy people, and MPOD declines with age in AMD patients[23]. Nevertheless, they all support the view that low MP levels were related to worse visual function in both healthy eyes and eyes with early AMD. Therefore, increasing the MP level is helpful to protect macular function in early AMD. Murray et al[24] reported that MPOD was increased in early AMD patients (mean age 70.5±8.7) by taking a lutein capsule daily for 12mo, but there was no change in visual acuity. Gale et al[25] examined the relationship between AMD and plasma L/Z levels in 380 AMD patients and found that the risk of AMD (early or late) was significantly higher in individuals with lower plasma Z levels. Subjects with the third lowest Z levels had twice the risks of AMD compared to those in the highest third. Risks of AMD are also associated with plasma L levels. However, the relationship between L and AMD was not significant[25]. This maybe because the distribution of the carotenoids in the macula is quite different. Z predominates in the central part of the macula and L predominates in the periphery[26]. The ratio of L to Z in the fovea is approximately 1:2.4. Z is a much more effective antioxidant than L[27]. Therefore, we cannot supplement L only. It is necessary to supplement the materials rich in Z. After the Goji berry supplementation, our result is consistent with that of Huang et al[28], who also focused on L and Z supplementation. A total of 112 early AMD patients supplemented with L (10 mg) and/or Z (10 mg), MPOD, and mean retinal sensitivity (MRS) increased after 12mo. Most of the studies were observed for at least 6 to 12mo. Hammond et al[15] investigated a total of 322 healthy subjects aged 16 to 50y intaking L and Z for 6mo. Serum L and Z levels were raised from baseline following 3mo of supplementation, but there was no increase in MPOD. Until 6mo, a small increase in MPOD was seen. Ma et al[29] reported that supplementation with L and Z improved MPOD, BCVA, and contrast sensitirity in patients with early AMD after 12mo. In our study, MPOD and BCVA improvements were observed after 3mo of Goji berry supplementation. We assume this is related to the oral dose of Goji berry 25 g/d compared to the oral dose of L or Z 10 to 20 mg/d. Although BCVA had a statistically significant improvement in the Goji group, the significant difference in the results may be caused by a substantial discrepancy (but not statistically significant) of the baseline BCVA between the Goji group and the control group at baseline, which left little room for further improvement in the control group. Therefore, the increase of MPOD in the macular region was the main measure for the improvement of retinal function at the early AMD stage and may have a positive impact on the progress of the disease.
The present study also has several limitations to be considered. First, the relatively small sample size in our study would reduce the statistical power to assess the association between supplementation of the Goji berries and MPOD. Second, the majority of the studies intervened for more than 6mo. However, our observation time is 90d, and it remains unclear whether a modest dosing strategy over time may be associated with greater benefit. Third, it is possible that populations with specific genetic backgrounds or nutritional status may affect the analysis of the relationship. Therefore, a larger-scale and longer-term study should be undertaken to focus on the effects of Goji berry on early AMD, and more sensitive measurements should be used in the future.
Acknowledgments
The authors would like to acknowledge Yan-Ping Liu, Jin Xu, Yan-Lin Zhao, Shu-Jin Ruan, Lin Liang (Department of Ophthalmology, China Rehabilitation Research Center) for their assistance with the research process and Xue-Yin Li (University of Beijing) for her excellent statistical assistance.
Foundation: Supported by Special Foundation for Public Welfare Research of China (No.2013CZ-9).
Conflicts of Interest: Li S, None; Liu N, None; Lin L, None; Sun ED, None; Li JD, None; Li PK, None.
REFERENCES
- 1.Chew EY, Klein ML, Clemons TE, Agrón E, Ratnapriya R, Edwards AO, Fritsche LG, Swaroop A, Abecasis GR. No clinically significant association between CFH and ARMS2 genotypes and response to nutritional supplements: AREDS report number 38. Ophthalmology. 2014;121(11):2173–2180. doi: 10.1016/j.ophtha.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elfervig LS. Age-related macular degeneration. Nurse Pract Forum. 1998;9(1):4–6. [PubMed] [Google Scholar]
- 3.Chopdar A. Age related macular degeneration. BMJ. 2003;326(7387):485–488. doi: 10.1136/bmj.326.7387.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subramanian ML, Ness S, Abedi G, Ahmed E, Daly M, Feinberg E, Bhatia S, Patel P, Nguyen M, Houranieh A. Bevacizumab vs ranibizumab for age-related macular degeneration: early results of a prospective double-masked, randomized clinical trial. Am J Ophthalmol. 2009;148(6):875–882.e1. doi: 10.1016/j.ajo.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Hollyfield JG. Age-related macular degeneration: the molecular link between oxidative damage, tissue-specific inflammation and outer retinal disease: the proctor lecture. Invest Ophthalmol Vis Sci. 2010;51(3):1275–1281. doi: 10.1167/iovs.09-4478. [DOI] [PubMed] [Google Scholar]
- 6.Scripsema NK, Hu DN, Rosen RB. Lutein, zeaxanthin, and meso-zeaxanthin in the clinical management of eye disease. J Ophthalmol. 2015;2015:1–13. doi: 10.1155/2015/865179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Invest Ophthalmol Vis Sci. 1984;25(6):674–685. [PubMed] [Google Scholar]
- 8.Li B, Ahmed F, Bernstein PS. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Arch Biochem Biophys. 2010;504(1):56–60. doi: 10.1016/j.abb.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma L, Liu R, Du JH, Liu T, Wu SS, Liu XH. Lutein, zeaxanthin and meso-zeaxanthin supplementation associated with macular pigment optical density. Nutrients. 2016;8(7):426. doi: 10.3390/nu8070426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potterat O. Goji (Lycium barbarum and L. Chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010;76(1):7–19. doi: 10.1055/s-0029-1186218. [DOI] [PubMed] [Google Scholar]
- 11.Hempel J, Schädle CN, Sprenger J, Heller A, Carle R, Schweiggert RM. Ultrastructural deposition forms and bioaccessibility of carotenoids and carotenoid esters from goji berries (Lycium barbarum L.) Food Chem. 2017;218:525–533. doi: 10.1016/j.foodchem.2016.09.065. [DOI] [PubMed] [Google Scholar]
- 12.Bucheli P, Vidal K, Shen L, Gu Z, Zhang C, Miller LE, Wang J. Goji berry effects on macular characteristics and plasma antioxidant levels. Optom Vis Sci. 2011;88(2):257–262. doi: 10.1097/OPX.0b013e318205a18f. [DOI] [PubMed] [Google Scholar]
- 13.Merle BM, Maubaret C, Korobelnik JF, Delyfer MN, Rougier MB, Lambert JC, Amouyel P, Malet F, Le Goff M, Dartigues JF, Barberger-Gateau P, Delcourt C. Association of HDL-related loci with age-related macular degeneration and plasma lutein and zeaxanthin: The Alienor study. PLoS One. 2013;8(11):e79848. doi: 10.1371/journal.pone.0079848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo HK, Kuo MT, Tiong IS, Wu PC, Chen YJ, Chen CH. Visual acuity as measured with Landolt C chart and early treatment of diabetic retinopathy study (ETDRS) chart. Graefes Arch Clin Exp Ophthalmol. 2011;249(4):601–605. doi: 10.1007/s00417-010-1461-3. [DOI] [PubMed] [Google Scholar]
- 15.Hammond CJ, Liew SH, Van Kuijk FJ, Beatty S, Nolan JM, Spector TD, Gilbert CE. The heritability of macular response to supplemental lutein and zeaxanthin: a classic twin study. Invest Ophthalmol Vis Sci. 2012;53(8):4963–4968. doi: 10.1167/iovs.12-9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang YM, Yan SF, Ma L, Zou ZY, Xu XR, Dou HL, Lin XM. Serum and macular responses to multiple xanthophyll supplements in patients with early age-related macular degeneration. Nutrition. 2013;29(2):387–392. doi: 10.1016/j.nut.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Cheng CY, Chung WY, Szeto YT, Benzie IF. Fasting plasma zeaxanthin response to Fructus barbarum L. (Wolfberry; Kei Tze) in a food-based human supplementation trial. Br J Nutr. 2005;93(1):123–130. doi: 10.1079/bjn20041284. [DOI] [PubMed] [Google Scholar]
- 18.Renzi LM, Hammond BJ, Jr, Dengler M, Roberts R. The relation between serum lipids and lutein and zeaxanthin in the serum and retina: results from cross-sectional, case-control and case study designs. Lipids Health Di. 2012;11:33. doi: 10.1186/1476-511X-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connor WE, Duell PB, Kean R, Wang Y. The prime role of HDL to transport lutein into the retina: Evidence from HDL-deficient WHAM chicks having a mutant ABCA1 transporter. Invest Ophthalmol Vis Sci. 2007;48(9):4226–4231. doi: 10.1167/iovs.06-1275. [DOI] [PubMed] [Google Scholar]
- 20.Johnson EJ. Obesity, lutein metabolism, and age-related macular degeneration: a web of connections. Nutr Rev. 2005;63(1):9–15. doi: 10.1111/j.1753-4887.2005.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 21.Gabriel HE, Liu Z, Crott JW, Choi SW, Song BC, Mason JB, Johnson EJ. A comparison of carotenoids, retinoids, and tocopherols in the serum and buccal mucosa of chronic cigarette smokers versus nonsmokers. Cancer Epidemiol Biomarkers Prev. 2006;15(5):993–999. doi: 10.1158/1055-9965.EPI-05-0664. [DOI] [PubMed] [Google Scholar]
- 22.Puell MC, Palomo-Alvarez C, Barrio AR, Gómez-Sanz FJ, Pérez-Carrasco MJ. Relationship between macular pigment and visual acuity in eyes with early age-related macular degeneration. Acta Ophthalmol. 2013;91(4):e298–e303. doi: 10.1111/aos.12067. [DOI] [PubMed] [Google Scholar]
- 23.Kaya S, Weigert G, Pemp B, Sacu S, Werkmeister RM, Dragostinoff N, Garhöfer G, Schmidt-Erfurth U, Schmetterer L. Comparison of macular pigment in patients with age-related macular degeneration and healthy control subjects - a study using spectral fundus reflectance. Acta Ophthalmol. 2012;90(5):e399–e403. doi: 10.1111/j.1755-3768.2012.02423.x. [DOI] [PubMed] [Google Scholar]
- 24.Murray IJ, Makridaki M, van der Veen RL, Carden D, Parry NR, Berendschot TT. Lutein supplementation over a one-year period in early AMD might have a mild beneficial effect on visual acuity: the CLEAR study. Invest Ophthalmol Vis Sci. 2013;54(3):1781–1788. doi: 10.1167/iovs.12-10715. [DOI] [PubMed] [Google Scholar]
- 25.Gale CR, Hall NF, Phillips DI, Martyn CN. Lutein and zeaxanthin status and risk of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2003;44(6):2461–2465. doi: 10.1167/iovs.02-0929. [DOI] [PubMed] [Google Scholar]
- 26.Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional manipulation of primate retinas, III: effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2005;46(2):692–702. doi: 10.1167/iovs.02-1192. [DOI] [PubMed] [Google Scholar]
- 27.Kim SR, Nakanishi K, Itagaki Y, Sparrow JR. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp Eye Res. 2006;82(5):828–839. doi: 10.1016/j.exer.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Huang YM, Dou HL, Huang FF, Xu XR, Zou ZY, Lu XR, Lin XM. Changes following supplementation with lutein and zeaxanthin in retinal function in eyes with early age-related macular degeneration: a randomised, double-blind, placebo-controlled trial. Br J Ophthalmol. 2015;99(3):371–375. doi: 10.1136/bjophthalmol-2014-305503. [DOI] [PubMed] [Google Scholar]
- 29.Ma L, Yan SF, Huang YM, Lu XR, Qian F, Pang HL, Xu XR, Zou ZY, Dong PC, Xiao X, Wang X, Sun TT, Dou HL, Lin XM. Effect of lutein and zeaxanthin on macular pigment and visual function in patients with early age-related macular degeneration. Ophthalmology. 2012;119(11):2290–2297. doi: 10.1016/j.ophtha.2012.06.014. [DOI] [PubMed] [Google Scholar]