Abstract
Moderate-to-vigorous physical activity (MVPA) in old age is an important indicator of good health and functional capacity enabling independent living. In our prospective twin cohort study with 616 individuals we investigated whether long-term physical activity assessed three times, in 1975, 1982 and 1990 (mean age 48 years in 1990), and other self-reported health habits predict objectively measured MVPA measured with a hip-worn triaxial accelerometer (at least 10 hours per day for at least 4 days) 25 years later (mean age of 73 years). Low leisure-time physical activity at younger age, higher relative weight, smoking, low socioeconomic status, and health problems predicted low MVPA in old age in individual-based analyses (altogether explaining 20.3% of the variation in MVPA). However, quantitative trait modeling indicated that shared genetic factors explained 82% of the correlation between baseline and follow-up physical activity. Pairwise analyses within monozygotic twin pairs showed that only baseline smoking was a statistically significant predictor of later-life MVPA. The results imply that younger-age physical activity is associated with later-life MVPA, but shared genetic factors underlies this association. Of the other predictors mid-life smoking predicted less physical activity at older age independent of genetic factors.
Introduction
Reduced physical activity in old age predisposes strongly to disability while exercise-based rehabilitation improves measured and self-rated function among individuals with various chronic diseases1, and prevents disability at older ages2. High participation in moderate-to-vigorous physical activity (MVPA) at older ages is an indicator of good physical fitness and health, and consequently predicts reduced risk of disability and death in the older population3,4.
Some observations suggest that midlife low physical activity, obesity, and poor health status predict sedentary lifestyle in old age5. Low physical activity6,7 and other lifestyle factors, such as smoking and use of alcohol8–10 predict or are associated with later disability and impaired mobility. However, no data exist describing whether long-term leisure-time physical activity during adulthood predicts objectively measured physical activity/mobility in old age. Non-communicable diseases and performance and activity limitations develop slowly, so it is important to investigate the long-term predictors of later-life physical activity levels.
Twin, family, and molecular genetic studies provide evidence for a role of genetic factors in obesity, many non-communicable diseases, fitness, and participation in physical activity, but the identity of specific genes for physical activity remains largely unknown11. Thus, both genetic factors, including the possibility of genetic pleiotropy, and childhood environment-related factors may predispose to different clusters of risk factors and associated diseases3,12,13. By studying outcomes in twin pairs discordant for exposure to different health habits and health outcomes, the possible confounding role of genetic and shared early childhood experiences can be considered. Twin pairs almost always share the same childhood family environment. Dizygotic (DZ) pairs share, on average, half of their segregating genes (like non-twin siblings), while monozygotic (MZ) pairs are genetically identical at the sequence level. Co-twin control analyses among discordant MZ twin pairs allow for stronger estimates of causal influences compared to associations seen in unrelated individuals.
In this study, we show how prospectively collected self-reported long-term leisure-time physical activity and other health habits from ages 31 to 48 years predict objectively measured physical activity and sedentary behavior a quarter of a century later at a mean age of 73. This prospective twin cohort study in Finland comprised 616 individuals (197 complete same-sex twin pairs, including 91 monozygotic pairs, born 1940–1944), who responded to baseline questionnaires in 1975, 1981, and 1990, and participated in accelerometer monitoring at follow-up (mean age, 73 years). Primary exposure was long-term leisure-time physical activity; the mean MET index expressed as the mean sum-score of leisure-time physical activity MET-hours per day in 1975, 1981 and 1990 (LT-mMET index). Covariates were body mass index (BMI), work-related physical activity, smoking, heavy alcohol use and health status in 1990, and socioeconomic status. Follow-up physical activity was measured with a hip-worn triaxial accelerometer (at least 10 hours per day for at least 4 days) to obtain daily mean MVPA, daily steps and other characteristics of physical activity or sedentary behaviour. Our data demonstrate how the younger-age physical activity is associated with later-life physical activity, but shared genetic factors underlie the association of mid-life physical activity with later-life MVPA over a 25 year period.
Results
Participant characteristics and selection
Mean age of the participants was 48.3 years (range 45.9–51.4) at the time of response to the 1990 questionnaire and 72.9 years (range 71.1–75.0) for objective physical activity monitoring. Among those who responded to all the baseline LT-mMET questions in 1975, 1982 and 1990 (1 646 individuals in this age group), the LT-mMET index including leisure-time and commuting activity was similar in those who participated in the follow-up accelerometry study (n = 616) and those who did not participate for various reasons (n = 1 030) (LT-mMET index in MET-h/day 2.65 ± 2.0 vs. 2.69 ± 2.6; men 2.97 ± 2.4 vs. 2.98 ± 3.1; women 2.38 ± 1.6 vs. 2.45 ± 2.0). However, among all cohort members having data on baseline predictors, there was a statistically non-significant tendency towards lower LT-mMET index among both men and women who died before the follow-up examination compared to those participating in the follow-up measurement of physical activity. Current smoking and socioeconomic status (not a white collar worker) at baseline statistically significantly associated with death during follow up among both men and women. Respectively, high body-mass index, heavy use of alcohol, non-sedentary work and health status (not healthy) at baseline statistically significantly associated with death during follow-up among men. Baseline participant characteristics by LT-mMET index tertiles are shown in Table 1. Among women, lower LT-mMET index was associated with reduced health, while among men, white collar work was more common in the highest LT-mMET index tertile.
Table 1.
Baseline participant characteristics in 1990 by LT-mMET (1975, 1981, 1990) tertiles.
| LT-mMET tertile* | P value† | ||||
|---|---|---|---|---|---|
| Low | Moderate | High | |||
| All, No. | 197 | 221 | 198 | ||
| Men, No. | 91 | 93 | 106 | ||
| Women, No. | 106 | 128 | 92 | ||
| LT-mMET, median (IQR), MET-h/day | |||||
| All | 0.97 (0.56) | 2.12 (0.76) | 4.11 (2.24) | ||
| Men | 0.98 (0.54) | 2.14 (0.71) | 4.52 (3.35) | ||
| Women | 0.95 (0.57) | 2.10 (0.76) | 3.79 (1.75) | ||
| Body mass index, median (IQR), kg/m2 | |||||
| All | 24.8 (3.99) | 24.7 (4.01) | 23.7 (3.28) | 0.005‡ | |
| Men | 25.2 (3.33) | 25.5 (3.10) | 24.3 (3.26) | 0.034 | |
| Women | 24.2 (4.35) | 23.6 (4.15) | 23.1 (3.46) | 0.060 | |
| Work-related loading, No. (%) | |||||
| All | Sedentary | 87 (44.6) | 96 (44.0) | 95 (48.7) | 0.595 |
| Non-sedentary | 108 (55.4) | 122 (56.0) | 100 (51.3) | ||
| Men | Sedentary | 40 (44.0) | 45 (48.9) | 52 (49.5) | 0.722 |
| Non-sedentary | 51 (56.0) | 47 (51.1) | 53 (50.5) | ||
| Women | Sedentary | 47 (45.2) | 51 (40.5) | 43 (47.8) | 0.529 |
| Non-sedentary | 57 (54.8) | 75 (59.5) | 47 (52.2) | ||
| Socioeconomic status, No. (%) | |||||
| All | White collar | 24 (12.5) | 31 (14.4) | 45 (22.8) | 0.012 |
| Others | 168 (87.5) | 185 (85.6) | 152 (77.2) | ||
| Men | White collar | 10 (11.2) | 12 (13.2) | 26 (24.8) | 0.019 |
| Others | 79 (88.8) | 79 (86.8) | 79 (75.2) | ||
| Women | White collar | 14 (13.6) | 19 (15.2) | 19 (20.7) | 0.368 |
| Others | 89 (86.4) | 106 (84.8) | 73 (79.3) | ||
| Cigarette smoking, No. (%) | |||||
| All | No current smoking | 160 (81.6) | 184 (83.6) | 169 (86.2) | 0.479 |
| Current smoker | 36 (18.4) | 36 (16.4) | 27 (13.8) | ||
| Men | No current smoking | 76 (84.4) | 77 (83.7) | 87 (82.9) | 0.953 |
| Current | 14 (15.6) | 15 (16.3) | 18 (17.1) | ||
| Women | No current smoking | 84 (79.2) | 107 (83.6) | 82 (90.1) | 0.137 |
| Current | 22 (20.8) | 21 (16.4) | 9 (9.9) | ||
| Heavy (high-density drinking occasions) alcohol users, No. (%) | |||||
| All | No | 151 (77.0) | 177 (81.2) | 153 (77.3) | 0.500 |
| Yes | 45 (23.0) | 41 (18.8) | 45 (22.7) | ||
| Men | No | 59 (65.6) | 59 (64.8) | 69 (65.1) | 0.995 |
| Yes | 31 (34.4) | 32 (35.2) | 37 (34.9) | ||
| Women | No | 92 (86.8) | 118 (92.9) | 84 (91.3) | 0.259 |
| Yes | 14 (13.2) | 9 (7.1) | 8 (8.7) | ||
| Health status, No. (%) | |||||
| All | Not healthy | 123 (64.1) | 137 (63.4) | 100 (50.8) | 0.010 |
| Healthy | 69 (35.9) | 79 (36.6) | 97 (49.2) | ||
| Men | Not healthy | 54 (60.7) | 48 (52.7) | 50 (47.6) | 0.205 |
| Healthy | 35 (39.3) | 43 (47.3) | 55 (52.4) | ||
| Women | Not healthy | 69 (67.0) | 89 (71.2) | 50 (54.3) | 0.029 |
| Healthy | 34 (33.0) | 36 (28.8) | 42 (45.7) | ||
*All descriptive analyses with bootstrapping (1000 samples unless otherwise noted). †Rao & Scott Chi-Square test except for body-mass index differences. ‡Linear regression cluster for family, with LT-mMET and BMI both used as continuous variables.
Predictors of later life objectively measured physical activity and sedentary behavior: individual-based analyses
High baseline LT-mMET index predicted less sedentary behavior (additional R2 2.0% after age- and sex adjustment, P = 0.002), more MVPA (R2 6.9, P < 0.001), more daily steps (R2 5.6%, P < 0.001) and also higher intensity of 10 minute continuous physical activity (Peak-10 min MET) during the monitoring week (R2 7.5%, P < 0.001) (Table 2, with results also by sex). The LT-mMET index was a stronger predictor of follow-up MVPA than any of the MET values from individual baseline time-points.
Table 2.
Follow-up objective physical activity measurements by mean baseline LT-mMET index (1975, 1981, 1990) tertiles.
| Activity/inactivity variable* | LT-mMET index tertile† | R2 (%)‡ | P value§ | ||
|---|---|---|---|---|---|
| Low | Moderate | High | |||
| Mean sedentary time/day, median (95% CI), h:min:sec | |||||
| All | 9:10:22 (9:00:59 to 9:18:18) |
8:38:03 (8:26:27 to 8:52:01) |
8:38:58 (8:20:09 to 9:00:49) |
2.0 | 0.002 |
| Men | 9:11:19 (9:03:35 to 9:43:39) |
8:52:01 (8:38:03 to 9:12:51) |
8:43:49 (8:23:46 to 9:03:39) |
3.4 | 0.012 |
| Women | 9:05:05 (8:45:39 to 9:22:43) |
8:25:34 (8:07:18 to 8:46:19) |
8:23:52 (7:58:15 to 9:04:13) |
1.1 | 0.041 |
| Mean standing time/day, median (95% CI), h:min:sec | |||||
| All | 1:19:09 (1:07:45 to 1:27:03) |
1:28:05 (1:21:59 to 1:33:49) |
1:22:10 (1:16:32 to 1:29:57) |
0.4 | 0.110 |
| Men | 1:17:23 (1:01:59 to 1:25:40) |
1:21:43 (1:12:49 to 1:32:57) |
1:18:18 (1:11:52 to 1:29:00) |
1.0 | 0.090 |
| Women | 1:21:46 (1:07:22 to 1:37:36) |
1:30:07 (1:24:27 to 1:37:34) |
1:25:45 (1:17:15 to 1:35:49) |
0.1 | 0.509 |
| Mean time of light physical activity/day, median (95% CI), h:min:sec | |||||
| All | 2:43:53 (2:35:57 to 2:54:55) |
2:55:05 (2:37:26 to 3:01:21) |
3:01:18 (2:46:53 to 3:13:56) |
0.7 | 0.040 |
| Men | 2:50:12 (2:25:46 to 3:01:44) |
2:57:13 (2:38:06 to 3:09:59) |
2:52:12 (2:31:10 to 3:11:30) |
0.1 | 0.696 |
| Women | 2:41:43 (2:34:47 to 2:55:56) |
2:47:05 (2:33:10 to 3:01:21) |
3:09:24 (2:55:19 to 3:27:10) |
2.3 | 0.011 |
| Mean time of moderate-to-vigorous physical activity/day, median (95% CI), h:min:sec | |||||
| All | 0:26:43 (0:22:06 to 0:29:52) |
0:36:31 (0:32:16 to 0:40:35) |
0:42:43 (0:36:37 to 0:50:49) |
6.9 | <0.001 |
| Men | 0:31:31 (0:24:52 to 0:38:21) |
0:40:43 (0:32:01 to 0:45:47) |
0:47:00 (0:34:25 to 0:56:57) |
7.5 | <0.001 |
| Women | 0:22:05 (0:18:05 to 0:26:57) |
0:34:40 (0:28:27 to 0:38:48) |
0:41:00 (0:34:18 to 0:49:39) |
6.5 | <0.001 |
| Mean daily step count, median (95% CI), No. | |||||
| All | 5099 (4744 to 5706) |
6114 (5610 to 6656) |
7072 (6245 to 7788) |
5.6 | <0.001 |
| Men | 5838 (5126 to 6531) |
6519 (5958 to 6788) |
7272 (6132 to 8340) |
6.7 | <0.001 |
| Women | 4753 (3964 to 5099) |
5612 (5231 to 6656) |
6800 (6236 to 7844) |
4.8 | <0.001 |
| Peak-10min MET, median (95% CI), MET | |||||
| All | 3.26 (3.17 to 3.38) |
3.57 (3.39 to 3.68) |
3.81 (3.55 to 3.96) |
7.5 | <0.001 |
| Men | 3.35 (3.17 to 3.45) |
3.67 (3.39 to 3.83) |
3.81 (3.43 to 4.18) |
9.9 | <0.001 |
| Women | 3.20 (2.97 to 3.33) |
3.54 (3.30 to 3.66) |
3.81 (3.51 to 3.99) |
5.0 | <0.001 |
*Activity variables calculated based on 1 minute epochs. †Descriptive analyses with bootstrapping (1000 samples). ‡R2 for LT-mMET index calculated as a difference (∆R2) from age and sex model compared to model with LT-mMET + age and sex, indicating the true R2 of LT-mMET. §P value calculated with continuous LT-mMET variable from linear regression adjusted for sex and age and cluster for family.
Table 3 shows the association between other baseline predictors from 1990 and MVPA at follow-up. High BMI had the strongest association with an additional R2 of 10.7% (for details on analyses of daily steps see Supplementary Table S1).
Table 3.
Moderate-to-vigorous physical activity by 1990 baseline covariates.
| Mean time of moderate-to-vigorous physical activity/day* hours:minutes:seconds |
R2 (%)† | P value‡ | ||||
|---|---|---|---|---|---|---|
| Body mass index | Normal weight (BMI < 25.0) |
Overweight (BMI = 25.0–29.99) |
Obese (BMI > 30.00) |
|||
| All | No. = 653 median (95% CI) |
378 0:40:00 (0:36:57 to 0:44:34) |
235 0:28:03 (0:25:33 to 0:31:30) |
40 0:10:46 (0:03:54 to 0:23:49) |
10.7 | <0.001 |
| Men | No. = 303 median (95% CI) |
149 0:47:02 (0:40:43 to 0:51:39) |
136 0:31:49 (0:28:13 to 0:37:54) |
18 0:15:16 (0:03:54 to 0:51:58) |
6.5 | <0.001 |
| Women | No. = 350 median (95% CI) |
229 0:36:58 (0:34:09 to 0:40:21) |
99 0:24:28 (0:18:14 to 0:27:18) |
22 0:07:49 (0:01:47 to 0:25:06) |
15.0 | <0.001 |
| Work-related loading | Sedentary | Non-sedentary | ||||
| All | No. = 650 median (95% CI) |
288 0:36:36 (0:33:22 to 0:42:14) |
362 0:32:33 (0:29:07 to 0:36:28) |
0.1 | 0.468 | |
| Men | No. = 304 median (95% CI) |
141 0:42:57 (0:36:33 to 0:47:01) |
163 0:34:42 (0:28:13 to 0:43:02) |
0.8 | 0.133 | |
| Women | No. = 346 median (95% CI) |
147 0:29:37 (0:24:49 to 0:36:25) |
199 0:31:09 (0:27:39 to 0:36:03) |
0.1 | 0.682 | |
| Socioeconomic status | White collar | Others | ||||
| All | No. = 605 median (95% CI) |
100 0:43:29 (0:38:03 to 0:51:33) |
505 0:32:01 (0:28:50 to 0:35:26) |
3.0 | <0.001 | |
| Men | No. = 285 median (95% CI) |
48 0:50:38 (0:42:57 to 1:05:02) |
237 0:33:20 (0:28:59 to 0:39:39) |
4.8 | <0.001 | |
| Women | No. = 320 median (95% CI) |
52 0:36:49 (0:29:21 to 0:45:16) |
268 0:30:30 (0:26:36 to 0:35:14) |
1.6 | 0.014 | |
| Cigarette smoking | No current smoking | Current | ||||
| All | No. = 654 median (95% CI) |
551 0:36:21 (0:34:08 to 0:38:43) |
103 0:25:41 (0:21:38 to 0:30:38) |
1.7 | 0.001 | |
| Men | No. = 304 median (95% CI) |
254 0:42:21 (0:36:26 to 0:44:59) |
50 0:28:06 (0:21:04 to 0:35:18) |
3.0 | 0.002 | |
| Women | No. = 350 median (95% CI) |
297 0:34:00 (0:28:50 to 0:36:35) |
53 0:24:05 (0:18:21 to 0:28:00) |
0.8 | 0.110 | |
| Heavy alcohol use | No | Yes | ||||
| All | No. = 651 median (95% CI) |
511 0:35:21 (0:32:36 to 0:37:07) |
140 0:33:49 (0:27:08 to 0:42:30) |
0.6 | 0.065 | |
| Men | No. = 303 median (95% CI) |
196 0:39:35 (0:35:14 to 0:45:24) |
107 0:39:08 (0:28:25 to 0:44:34) |
0.3 | 0.397 | |
| Women | No. = 348 median (95% CI) |
315 0:33:05 (0:28:40 to 0:35:56) |
33 0:17:22 (0:11:47 to 0:37:25) |
1.6 | 0.034 | |
| Health status | Healthy | Not healthy | ||||
| All | No. = 605 median (95% CI) |
245 0:42:27 (0:35:41 to 0:46:06) |
360 0:30:28 (0:27:49 to 0:34:18) |
1.9 | 0.001 | |
| Men | No. = 285 median (95% CI) |
133 0:43:51 (0:36:26 to 0:48:33) |
152 0:31:49 (0:28:00 to 0:39:08) |
2.1 | 0.020 | |
| Women | No. = 320 median (95% CI) |
112 0:39:05 (0:30:13 to 0:47:25) |
208 0:28:45 (0:25:54 to 0:34:18) |
1.8 | 0.023 | |
*All descriptive analyses with bootstrapping (1000 samples unless otherwise noted). †R2 for each baseline variable calculated as a difference (∆R2) from age and sex model compared to model with variable (e.g., bmi90) + age and sex, indicating the true R2 of the studied variable. ‡P value from linear regression adjusted for sex and age and cluster for family; continuous variables used for BMI and moderate-to-vigorous physical activity.
In the multivariate MVPA prediction regression model, with the addition of BMI after age, sex, and LT-mMET index, the R2 value increased from 8.4% to 17.2%, and up to 20.3% with smoking, socioeconomic status, and health status also in the model (Supplementary Table S2). Use of alcohol and work-related physical activity were not significant contributors when added to this model. Similar models for daily step count showed rather similar results with a slightly lower proportion of variance accounted for.
Predictors of later-life objectively measured physical activity: pairwise analyses
Although there were some trends in the same direction in pairwise analyses among same-sex twin pairs discordant for different predictors at baseline, only twin pairs who were discordant for smoking (n = 40 discordant pairs; median follow-up MVPA volumes of 25 minutes for current smokers at baseline and 35 minutes for non-smokers; P = 0.037) or for health status (n = 69 discordant pairs, 30 vs. 44 minutes, P = 0.014) differed in their follow-up MVPA volumes (Table 4). For smoking, the difference also was seen for MZ pairs, but for health status, it was seen only for DZ pairs. In the smaller number of socioeconomic status–discordant MZ pairs, lower socioeconomic status predicted less MVPA at follow-up. The findings were similar for daily step count (Supplementary Table S3).
Table 4.
Moderate-to-vigorous physical activity in twin pairs discordant for different baseline characteristics*.
| No. of discordant pairs | Mean time of moderate-to-vigorous physical activity/day† hours:minutes:seconds |
Z and P value‡ |
|||
|---|---|---|---|---|---|
| LT-mMET index | Lower Mean MET | Higher Mean MET | |||
| All twin pairs | 23 | median (IQR) (95% CI) |
0:27:59 (0:46:39) (0:19:30 to 0:45:00) |
0:32:38 (0:49:13) (0:22:14 to 0:58:09) |
Z = 0.517 P = 0.605 |
| DZ twin pairs | 13 | median (IQR) (95% CI) |
0:22:34 (0:52:28) (0:16:33 to 1:04:19) |
0:34:42 (0:48:34) (0:25:33 to 1:11:26) |
Z = 1.293 P = 0.196 |
| MZ twin pairs | 10 | median (IQR) (95% CI) |
0:33:10 (0:32:06) (0:20:37 to 0:54:21) |
0:25:11 (0:49:09) (0:10:55 to 1:01:17) |
Z = 0.663 P = 0.508 |
| Body mass index § | Lower BMI | Higher BMI | |||
| All twin pairs | 55 | median (IQR) (95% CI) |
0:26:54 (0:42:41) (0:24:58 to 0:37:06) |
0:25:06 (0:37:34) (0:17:24 to 0:34:23) |
Z = 0.997 P = 0.319 |
| DZ twin pairs | 37 | median (IQR) (95% CI) |
0:36:00 (0:42:24) (0:26:17 to 0:54:04) |
0:18:32 (0:48:39) (0:08:42 to 0:40:10) |
Z = 1.577 P = 0.115 |
| MZ twin pairs | 15 | median (IQR) (95% CI) |
0:23:48 (0:26:08) (0:08:00 to 0:34:09) |
0:26:59 (0:28:29) (0:14:38 to 0:36:30) |
Z = 0.568 P = 0.570 |
| Work-related loading | Sedentary | Non-sedentary | |||
| All twin pairs | 77 | median (IQR) (95% CI) |
0:45:28 (0:36:21) (0:39:16 to 0:49:59) |
0:31:09 (0:34:30) (0:25:48 to 0:38:20) |
Z = 1.891 P = 0.059 |
| DZ twin pairs | 45 | median (IQR) (95% CI) |
0:44:28 (0:43:00) (0:35:13 to 0:53:24) |
0:29:07 (0:35:38) (0:18:34 to 0:43:14) |
Z = 1.699 P = 0.089 |
| MZ twin pairs | 29 | median (IQR) (95% CI) |
0:49:12 (0:34:42) (0:28:58 to 0:50:48) |
0:32:36 (0:28:33) (0:28:50 to 0:40:28) |
Z = 0.811 P = 0.417 |
| Socioeconomic status | White collar | Others | |||
| All twin pairs | 24 | median (IQR) (95% CI) |
0:43:47 (0:50:04) (0:31:57 to 1:06:58) |
0:36:32 (0:43:42) (0:24:18 to 0:53:28) |
Z = 1.557 P = 0.119 |
| DZ twin pairs | 17 | median (IQR) (95% CI) |
0:41:09 (0:48:37) (0:20:09 to 1:06:51) |
0:32:55 (0:43:53) (0:18:37 to 0:59:17) |
Z = 0.450 P = 0.653 |
| MZ twin pairs | 7 | median (IQR) (95% CI) |
1:14:43 (0:45:19) (0:38:48 to 1:28:26)† |
0:44:01 (0:47:30) (0:25:11 to 1:12:42)† |
Z = 2.366 P = 0.018 |
| Cigarette smoking | No current smoking | Current | |||
| All twin pairs | 40 | median (IQR) (95% CI) |
0:35:03 (0:37:59) (0:27:21 to 0:42:22) |
0:25:10 (0:31:38) (0:19:03 to 0:33:27) |
Z = 2.083 P = 0.037 |
| DZ twin pairs | 21 | median (IQR) (95% CI) |
0:33:05 (0:42:08) (0:17:12 to 0:47:36) |
0:22:34 (0:39:19) (0:15:30 to 0:45:42) |
Z = 1.060 P = 0.289 |
| MZ twin pairs | 15 | median (IQR) (95% CI) |
0:42:01 (0:39:27) (0:28:20 to 1:03:03) |
0:27:46 (0:29:21) (0:18:27 to 0:43:07) |
Z = 2.272 P = 0.023 |
| Heavy alcohol use | No | Yes | |||
| All twin pairs | 36 | median (IQR) (95% CI) |
0:32:23 (0:35:37) (0:26:15 to 0:48:10) |
0:39:23 (0:32:59) (0:27:07 to 0:51:56) |
Z = 0.047 P = 0.962 |
| DZ twin pairs | 22 | median (IQR) (95% CI) |
0:30:59 (0:42:44) (0:20:36 to 0:57:24) |
0:43:32 (0:47:50) (0:23:28 to 0:56:56) |
Z = 0.438 P = 0.661 |
| MZ twin pairs | 13 | median (IQR) (95% CI) |
0:37:51 (0:26:15) (0:26:10 to 0:51:14) |
0:36:56 (0:30:22) (0:24:18 to 0:53:28) |
Z = 0.874 P = 0.382 |
| Health status | Healthy | Not healthy | |||
| All twin pairs | 69 | median (IQR) (95% CI) |
0:43:55 (0:41:01) (0:35:13 to 0:51:12) |
0:29:32 (0:30:18) (0:26:54 to 0:37:51) |
Z = 2.466 P = 0.014 |
| DZ twin pairs | 37 | median (IQR) (95% CI) |
0:42:30 (0:40:28) (0:31:49 to 0:51:18) |
0:27:36 (0:38:15) (0:16:33 to 0:39:16) |
Z = 1.984 P = 0.047 |
| MZ twin pairs | 26 | median (IQR) (95% CI) |
0:42:12 (0:40:46) (0:28:06 to 0:55:39) |
0:37:58 (0:22:18) (0:29:07 to 0:47:06) |
Z = 0.013 P = 0.990 |
*Descriptive analyses with bootstrapping (1000 samples unless otherwise noted).
†Bootstrap based on 995 samples.
‡Z as absolute value and P by Wilcoxon matched-pairs signed-rank test.
§BMI difference ≥ 3 between twin pairs when at least one twin is overweight (BMI ≥ 25).
Mediation analysis by quantitative trait modeling
Based on quantitative trait models (for more details see Supplementary Results and Supplementary Tables S4–S8, and Supplementary Fig. S1), joint genetic effects mediated the association from baseline MET factor (calculated from 1975, 1981 and 1990 questionnaires; using also cases with some incomplete data unlike in the calculation of LT-mMET index) on daily MVPA time and Peak-10min MET at follow-up. The MET factor was observed to be a direct risk factor for number of daily steps and sedentary behavior (lying and sitting). No relationship was observed of MET factor with standing and light physical activity. In more detail, the broad sense heritability for MVPA was 60% (Supplementary Table S8). When cross-trait correlation between baseline MET factor and follow-up MVPA was decomposed into genetic and residual parts based on the model where we estimated both the genetic and environmental correlations the estimated cross-trait correlation was 0.35 (95% CI 0.25–0.43) with 82% (53–100%) contribution from genetic factors.
Discussion
Younger-age self-reported leisure-time physical activity (including commuting activity) and other covariates explained one fifth (20.3%) of the variation in objectively measured moderate-to-vigorous activity in older age in this prospective twin cohort study. According to pairwise analyses, much of the association was driven by shared genes underlying mid-life physical activity and later objectively measured activity. Smoking contributed independent of genes.
Comparison to other studies
In line with our findings, high physical activity is associated in cross-sectional or longitudinal designs with high previous physical activity, low BMI, low work-related physical loading, and good health status14–16. In cross-sectional and shorter-term follow-up studies, low physical activity is associated with lower fitness, more frailty, higher disability, and poor health17–20. No long-term randomized trials have addressed whether changes in health behavior in middle age lead to late-life differences in physical activity. Also, observational follow-ups on this topic are rare, and we are not aware of other studies relating long-term leisure-time physical activity differences in younger adulthood to objectively measured physical activity/inactivity in later years21.
In our individual-based analyses, we found significant predictors for later-life physical activity, but could not replicate all of the results in pairwise analyses among the predictor-discordant MZ twin pairs. The outcome is a reminder that genetic or other familial factors may explain why associations are often seen between younger-age physical activity and later-age health-related factors and, consequently, mobility.
Smoking at baseline also predicted less MVPA at follow-up in pairwise analysis among MZ twin pairs, which is evidence for an association not explained by genetic factors. These results are in line with our earlier finding that MZ twin pairs discordant for smoking show a clear difference in overall mortality while pairs discordant for physical activity participation do not22,23. Our quantitative trait modelling was in agreement with the results of the pairwise analyses. Smoking affects both pulmonary and cardiovascular health and increases systemic inflammation, all of which may decrease the ability to exercise. We cannot exclude the possibility that smoking is also a marker for other lifestyle factors that predict less physical activity.
The strengths of our study include that we had physical activity data from three different baseline time-points, a nationally representative large twin cohort, very long-term prospective data, and novel valid analysis of the follow-up physical activity and sedentary behavior profile24.
Limitations
Our study has also limitations. Our baseline predictor assessments relied on self-reported questionnaire data. We lack comprehensive data on dietary factors or clinical examinations at baseline. Although our study was large enough for the individual-based analyses, the number of MZ twin pairs discordant for some of the predictors was quite low providing only moderate statistical power for some analyses. At follow-up, most twins were community dwelling, so there were very few individuals with severe mobility limitations. Individuals who have survived to the mean age of 73 years represent a selected group, which may weaken the statistical power to detect the influence of some predictors of the capacity for being physically active.
Clinical implications
The public health community has been trying for years to get people more active at the population level, but with limited to moderate success. Our study increases understanding on this challenge. As participation in physical activity and its tracking has a familial component (including genetic and childhood environmental factors) it is understandable that it is challenging to activate middle-aged and older adults having been previously physically inactive. As the genetic components contributing to the correlations between mid-life LTPA and later life MVPA or Peak-10min MET were high unlike that for daily steps, recommending less vigorous (walking-type) activities for older individuals may be a successful strategy. Be it noted that objective accelerometry-based monitoring records absolute physical activity intensities and previously inactive low-fit older individuals may not be able to participate in MVPA in terms of absolute intensity although they may be able to participate in physical activity which is moderate-to-vigorous relative to their fitness level25. Also, paying attention to body weight control and physical activity since early childhood may be a good strategy to increase later life activity levels. In addition, promoting other health habits such as not smoking and avoidance of heavy drinking may influence later life physical activity capacity. Finally, it is good to keep in mind that randomized controlled intervention studies have shown that among individuals whose physical functioning is reduced due to chronic diseases appropriately tailored exercise therapy improves their physical function and mobility1,2.
Conclusions
Our follow-up study among twins showed that middle-age low leisure-time physical activity, obesity, smoking, low socioeconomic status, and health problems predicted low objectively measured MVPA at older age in individual-based analyses. According to pairwise analyses, smoking seemed to causally predict less physical activity in later years while other associations were more likely attributable to shared genetic factors and childhood environment.
Materials and Methods
This MOBILETWIN study is an ancillary to the older Finnish Twin Cohort Study26. Written informed consent was obtained from all participants, and the study was approved by the Ethics Committee of the Hospital District of Southwest Finland on 20 May 2014. All study methods were performed in accordance with the Helsinki Declaration and good clinical and scientific practice.
Participant inclusion
The study is based on a nationwide sample of all same-sex twin pairs born before 1958 with both co-twins alive in 197526. A baseline questionnaire was sent to all twin candidates in 1975. Among those whose home addresses could be identified (93.5%) in 1975, the response rate for twins was 87.6%. A subsequent questionnaire was mailed in 1981 to all of the verified twins. The corresponding response rate among those responding in 1975 and alive in 1981 was 90.7%. A third questionnaire was sent out in 1990 to all twin individuals aged 33–60 (birth cohorts 1930–1957) years who had responded to at least one of the earlier questionnaires (response rate was 77.3% of all surviving cohort members)27.
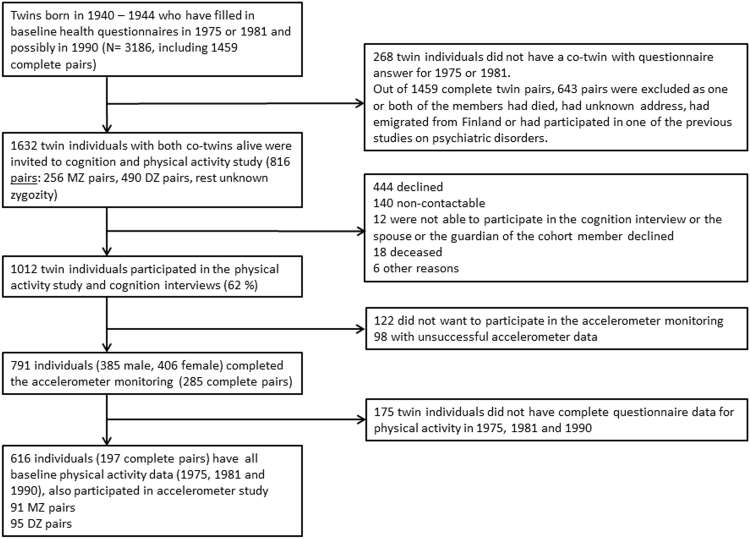
For the current physical activity study (MOBILETWIN), twins from the 1940–1944 birth cohorts were selected (Fig. 1). Altogether, 3186 twin individuals belonged to these birth cohorts and had responded to at least one of the first two questionnaires (1975 or 1981). A total of 145 twin individuals were excluded because they had participated in one of the previous studies on psychiatric disorders (schizophrenia and bipolar studies). All remaining 816 complete twin pairs, i.e., both alive and contactable, were invited to participate in the present study for a total of; 256 MZ, 490 DZ and 70 with unknown zygosity. The twins were sent an invitation letter in which they chose whether to participate in a health and cognition telephone interview and/or accelerometer study complemented with physical functioning questionnaire. Altogether, 1012 (61.9%) twin individuals participated in the telephone interview, 791 twin individuals wore the accelerometer for the required time, and 817 individuals filled in the whole questionnaire on physical functioning. A total of 616 participants (197 complete pairs, including 91 MZ and 95 DZ pairs with known zygosity) in the accelerometer study also had baseline physical activity data for all the baseline time points (1975, 1981, and 1990). For other baseline health variables, we maximized the statistical power of the analyses by including all possible twin individuals and discordant twin pairs who had data for these other health habits; therefore, the number of participants in different analyses may have varied according to variable under investigation.
Figure 1.
Participant flow diagram.
Baseline predictor assessment
The postal questionnaires in 1975 and 1981 were very similar, but the questionnaire in 1990 was slightly different in some parts; however, they all included questions on physical activity, occupation, work-related physical activity, smoking, use of alcohol, and physician-diagnosed diseases (available on the Twin Study website: www.twinstudy.helsinki.fi).
Physical activity habits were assessed by identical questions in 1975 and 1981 and with slightly different questions in 1990. All three questionnaires enabled calculation of the MET index. On the bases of earlier studies, the physical activity questionnaire data can be considered valid28–31. Assessment of the MET index was based on a series of structured questions28,32 on leisure-time physical activity (monthly frequency, mean duration, and mean intensity of sessions) and physical activity during commuting. The index was calculated by assigning a MET score to each activity and by calculating the product of that activity: intensity × duration × frequency28. The MET index was expressed as the sum-score of leisure-time physical activity MET-hours per day. To estimate the mean volume of physical activity during the three baseline survey years, the average of the MET index values obtained in 1975, 1981, and 1990 was computed. This new leisure-time mean MET value (LT-mMET index) was then divided into three activity tertiles labelled low (LT-mMET index 0–1.54 MET h/day), medium (1.54–2.92 MET h/day), and high (2.92–26.13 MET h/day) using the same tertiles as in an earlier study31. Twin pairs were classified as discordant for physical activity if one co-twin was in the low-activity tertile and the other co-twin was in the high-activity tertile.
As other predictors and covariates, body mass index (BMI), self-reported work-related physical activity, smoking status, use of alcohol and health status in 1990, and socioeconomic status were used. After preliminary analyses, to maximize statistical power for pairwise twin and multivariate analyses, covariates were dichotomized by merging classes not differing for baseline and follow-up physical activity levels.
BMI was calculated based on self-reported height and weight. In 1990 work-related physical activity of their current or latest work was classified by the respondents using a categorical variable evaluated with a four-point ordinal scale22 ignoring the type of employment. A response to the first option “mainly sedentary work, which requires very little physical activity” was classified as sedentary work, while all other responses (“work that involves standing and walking, but no other physical activity” and more strenuous) were classified as non-sedentary work. Majority of the participants who were sedentary workers worked outside home (94.9%) or at home (2.1%) while in the non-sedentary category there were more individuals who worked at home (8.6%), were on disability pension (5.5.%) or were unemployed (2.8%). Three socioeconomic status categories (white collar, intermediate, and blue collar) were defined by years of education and amount of physical activity at work27. The blue collar and intermediate groups were combined in the analyses because their baseline and follow-up physical activity was similar. Great majority of the white collar workers worked outside home (97.0%) while in the others category there were more individuals who worked at home (6.5%), were on disability pension (3.8%) or were unemployed (2.2%). Smoking status, originally coded into four categories33, was dichotomized (current daily vs. others) for the main analyses. Alcohol use was expressed as a dichotomous variable of heavy drinking occasions (i.e., consumption of at least six drinks on one occasion) at least monthly34,35. Somatic health status (healthy/not) was defined as having/not having a disease diagnosed by a physician, serious injury/illness, or permanent work disability, according to self-report items in 199027.
Accelerometer data collection and analysis
Physical activity was measured with a hip-worn, light triaxial accelerometer (Hookie AM20, Traxmeet Ltd, Espoo). The device and instructions for use were mailed to the participants, who were asked to use the accelerometer during waking hours for 7 consecutive days. Participants mailed the device back to UKK Institute for data analysis, and they were later provided with their own results. The analysis of raw acceleration data was based on novel algorithms that employ the mean amplitude deviation (MAD) of the resultant acceleration during a 6 s epoch and the angle for posture estimation (APE) of the body, metrics that provide a consistent assessment of the intensity of physical activity and separate accurately sedentary and stationary behaviors from any physical activity36–38.
MAD was also validated through directly measured incident VO2 during walking or running on an indoor track36. This strong association allowed for conversion of MAD values to incident energy consumption (MET). The MET values for each minute were calculated as the one-minute exponential moving average of epoch-wise MAD values. According to standard use24, cut-off points for different activities were set as 1.5–3 MET for light activities, 3–6 MET for moderate activities, and over 6 MET for vigorous activities, and corresponding mean daily total times were determined. Mean daily sedentary time was defined as MET under 1.5 during lying down or sitting. Mean daily standing time was analyzed separately. Average daily step count and the most intensive continuous 10-minute period of physical activity (Peak-10min MET) during the monitoring week were also documented.
Altogether, 791 twin individuals wore the accelerometer for at least 10 hours per day for 4 days. On average, they wore the device 6.73 days (95% confidence interval [CI] 6.69–6.77) and 14:01:44 h:min:sec/day (95% CI 13:56:31–14:04:37). A total of 616 had complete data for calculating MET indices from all of the 1975, 1981, and 1990 questionnaires. No significant differences in MVPA (40.2 min vs. 37.7 min, P = 0.30) and daily steps (6440 vs. 6120, P = 0.23) were seen between these 616 individuals and the 175 individuals who did not have complete physical activity data for the calculation of baseline LT-mMET but participated in the accelerometer study.
Statistical methods
Descriptive statistics were calculated with bootstrapping (1000 samples unless otherwise noted) and are given as medians and interquartile ranges (IQRs) or 95% confidence intervals (CIs). We used linear regression analyses to define R squared (R2) as a measure of variance accounted for. The analyses were done with twins treated as individuals; however, because the observations obtained from twin pairs may be correlated, robust estimators of variance (the cluster option in Stata) were used39. All basic analyses yielding R2 values were adjusted for age and sex. To obtain R2 only for the studied variable, the variable was entered the model after the basic model and then the difference in R2 (∆R2) was calculated. Multivariate models were adjusted for BMI, smoking, alcohol, work-related physical activity, health status, and socioeconomic status. Square root-transformation for MVPA, logarithm-transformation for Peak-10min MET, and cubic root transformation for LT-mMET were used for regression analyses because these variables were not normally distributed.
Pairwise analyses among twin pairs (all pairs, DZ pairs, and MZ pairs separately) were done using Wilcoxon matched-pairs signed-rank test for whether pairs discordant for specific baseline characteristics or health habits differed in the objectively measured physical activity variables at follow-up.
Quantitative trait modeling was done using the MET variables from 1975, 1981, and 1990 to analyze whether they were direct risk factors or whether the association with the follow-up physical activity variables was mediated by genetic or other environmental factors. The quantitative trait modeling is described in Supplementary Methods and Results, and only the main results are given above.
Data availability
All the MOBILETWIN Study data is not allowed to be placed in open archives due to data protection issues of possibly identifiable twins. Researchers are encouraged to contact the last author (U.M.K.) and we will make every effort to accommodate additional analyses.
Electronic supplementary material
Acknowledgements
The MOBILETWIN Study was supported by the Finnish Ministry of Education and Culture (grant OKM/56/626/2013 to UMK). Sample collection and JK were supported by the Academy of Finland (grants 265240 & 263278). TT was funded by the Academy of Finland (grant no. 286536).
Author Contributions
K.W., J.K., H.S. and U.M.K. conception and design of the research; all authors contributed to acquisition, analysis, or interpretation of data; K.W. and U.M.K. drafted the manuscript; all authors contributed to the critical revision of the manuscript for important intellectual content; K,W., H.V.-Y., T.T. (quantitative trait modeling), P.H. and U.M.K. analyzed data; All authors approved the final version of the manuscript.
Competing Interests
Dr Kaprio consulted for Pfizer Inc on nicotine dependence in 2014–2015. Other authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-27704-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pasanen T, Tolvanen S, Heinonen A, Kujala UM. Exercise therapy for functional capacity in chronic diseases: an overview of meta-analyses of randomized controlled trials. Br. J. Sports Med. 2017;51:1459–1465. doi: 10.1136/bjsports-2016-097132. [DOI] [PubMed] [Google Scholar]
- 2.Pahor M, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kujala UM. Physical activity, genes, and lifetime predisposition to chronic diseases. Eur. Rev. Aging Phys. Act. 2011;8:31–36. doi: 10.1007/s11556-010-0077-2. [DOI] [Google Scholar]
- 4.Ross R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: A case for fitness as a clinical vital sign. A scientific statement from the American Heart Association. Circulation. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 5.Van der Berg JD, et al. Midlife determinants associated with sedentary behavior in old age. Med. Sci. Sports. Exerc. 2014;46:1359–1365. doi: 10.1249/MSS.0000000000000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gretebeck RJ, Ferraro KF, Black DR, Holland K, Gretebeck KA. Longitudinal change in physical activity and disability in adults. Am. J. Health. Behav. 2012;36:385–394. doi: 10.5993/AJHB.36.3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sternfeld B, et al. The effect of a healthy lifestyle on future physical functioning in midlife women. Med. Sci. Sports. Exerc. 2017;49:274–282. doi: 10.1249/MSS.0000000000001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostbye T, Taylor DH, Jr., Krause KM, Van Scoyoc L. The role of smoking and other modifiable lifestyle risk factors in maintaining and restoring lower body mobility in middle-aged and older Americans: results from the HRS and AHEAD. Health and Retirement Study. Asset and Health Dynamics Among the Oldest Old. J. Am. Geriatr. Soc. 2002;50:691–699. doi: 10.1046/j.1532-5415.2002.50164.x. [DOI] [PubMed] [Google Scholar]
- 9.Houston DK, et al. The association between weight history and physical performance in the Health, Aging and Body Composition study. Int. J. Obes. (Lond.) 2007;31:1680–1687. doi: 10.1038/sj.ijo.0803652. [DOI] [PubMed] [Google Scholar]
- 10.Koster A, et al. Lifestyle factors and incident mobility limitations in obese and non-obese older adults. Obesity (Silver Spring) 2007;15:3122–3132. doi: 10.1038/oby.2007.372. [DOI] [PubMed] [Google Scholar]
- 11.Sarzynski MA, et al. Advances in exercise, fitness, and performance genomics. Med. Sci. Sports Exerc. 2016;48:1906–1916. doi: 10.1249/MSS.0000000000000982. [DOI] [PubMed] [Google Scholar]
- 12.Lee H, et al. Obesity-related genetic variants and their association with physical activity. Sports Medicine – Open. 2015;1:34. doi: 10.1186/s40798-015-0036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visscher PM, et al. 10 years of GWAS discovery: Biology, function, and translation. Am. J. Hum. Genet. 2017;101:5–22. doi: 10.1016/j.ajhg.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Stralen MM, De Vries H, Mudde AN, Bolman C, Lechner L. Determinants of initiation and maintenance of physical activity among older adults: a literature review. Health Psychol. Rev. 2009;3:147–207. doi: 10.1080/17437190903229462. [DOI] [Google Scholar]
- 15.Kirk MA, Rhodes RE. Occupational correlates of adults’ participation in leisure-time physical activity. A systematic review. Am. J. Prev. Med. 2011;40:476–485. doi: 10.1016/j.amepre.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Bauman AE, et al. Correlates of physical activity: why are some people physically active and others not? Lancet. 2012;380:258–271. doi: 10.1016/S0140-6736(12)60735-1. [DOI] [PubMed] [Google Scholar]
- 17.Boyle PA, Buchmann AS, Wilson RS, Bienias JL, Bennett DA. Physical activity is associated with incident disability in community-based older persons. J. Am. Geriatr. Soc. 2007;55:195–201. doi: 10.1111/j.1532-5415.2007.01038.x. [DOI] [PubMed] [Google Scholar]
- 18.Balzi D, et al. Risk factors for disability in older persons over 3-year follow-up. Age Ageing. 2010;39:92–98. doi: 10.1093/ageing/afp209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. The association between sedentary behaviour, moderate-vigorous physical activity and frailty in NHANES cohorts. Maturitas. 2015;80:187–191. doi: 10.1016/j.maturitas.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Sjölund B-M, Wimo A, Engström M, von Strauss E. Incidence of ADL disability in older persons, physical activities as a protective factor and the need for informal and formal care – Results from the SNAC-N project. PLoS One. 2015;10:e0138901. doi: 10.1371/journal.pone.0138901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koeneman MA, Verheijden MW, Chinapaw MJM, Hopman-Rock M. Determinants of physical activity and exercise in healthy older adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011;8:142. doi: 10.1186/1479-5868-8-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kujala UM, Kaprio J, Koskenvuo M. Modifiable risk factors as predictors of all-cause mortality: the roles of genetics and childhood environment. Am. J. Epidemiol. 2002;156:985–993. doi: 10.1093/aje/kwf151. [DOI] [PubMed] [Google Scholar]
- 23.Karvinen S, et al. Physical activity in adulthood: genes and mortality. Sci. Rep. 2015;5:18259. doi: 10.1038/srep18259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sievänen H, Kujala UM. Accelerometry – simple, but challenging (Editorial) Scand. J. Med. Sci. Sports. 2017;27:574–578. doi: 10.1111/sms.12887. [DOI] [PubMed] [Google Scholar]
- 25.Kujala UM, et al. Physical activity: Absolute intensity versus relative-to-fitness-level volumes. Med. Sci. Sports. Exerc. 2017;49:474–481. doi: 10.1249/MSS.0000000000001134. [DOI] [PubMed] [Google Scholar]
- 26.Kaprio J, Koskenvuo M. Genetic and environmental factors in complex diseases: the older Finnish Twin Cohort. Twin. Res. 2002;5:358–65. doi: 10.1375/136905202320906093. [DOI] [PubMed] [Google Scholar]
- 27.Romanov K, Varjonen J, Kaprio J, Koskenvuo M. Life events and depressiveness - the effect of adjustment for psychosocial factors, somatic health and genetic liability. Acta Psychiatr. Scand. 2003;107:25–33. doi: 10.1034/j.1600-0447.2003.01419.x. [DOI] [PubMed] [Google Scholar]
- 28.Kujala UM, Kaprio J, Sarna S, Koskenvuo M. Relationship of leisure-time physical activity and mortality: the Finnish twin cohort. JAMA. 1998;279:440–444. doi: 10.1001/jama.279.6.440. [DOI] [PubMed] [Google Scholar]
- 29.Leskinen T, et al. Effects of 32-year leisure time physical activity discordance in twin pairs on health (TWINACTIVE Study): Aims, design and results for physical fitness. Twin. Res. Hum. Genet. 2009;12:108–117. doi: 10.1375/twin.12.1.108. [DOI] [PubMed] [Google Scholar]
- 30.Waller K, Kaprio J, Kujala UM. Associations between long-term physical activity, waist circumference and weight gain: a 30-year longitudinal twin study. Int. J. Obes. (Lond.) 2008;32:353–361. doi: 10.1038/sj.ijo.0803692. [DOI] [PubMed] [Google Scholar]
- 31.Waller K, Kaprio J, Korhonen T, Tuulio-Henriksson A, Kujala UM. Persistent leisure-time physical activity in adulthood and use of antidepressants; a follow-up study among twins. J. Affect. Disord. 2016;200:172–177. doi: 10.1016/j.jad.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 32.Kaprio, J., Sarna, S., Koskenvuo, M. & Rantasalo, I. The Finnish Twin Registry: Baseline Characteristics. Section II. History of Symptoms and Illnesses, use of Drugs, Physical Characteristics, Smoking, Alcohol and Physical Activity. Helsinki: Public Health Publication M 37. Available From: University of Helsinki, Department ofPublic Health (1978).
- 33.Kaprio J, Koskenvuo M. A prospective study of psychological and socioeconomic characteristics, health behavior and morbidity in cigarette smokers prior to quitting compared to persistent smokers and non-smokers. J. Clin. Epidemiol. 1988;41:139–150. doi: 10.1016/0895-4356(88)90088-1. [DOI] [PubMed] [Google Scholar]
- 34.Kaprio J, et al. Genetic influences on use and abuse of alcohol: a study of 5638 adult Finnish twin brothers. Alcohol Clin. Exp. Res. 1987;11:349–356. doi: 10.1111/j.1530-0277.1987.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 35.Sipilä P, Rose RJ, Kaprio J. Drinking and mortality: long-term follow-up of drinking-discordant twin pairs. Addiction. 2016;111:245–254. doi: 10.1111/add.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vähä-Ypyä H, et al. Validation of cut-points for evaluating the intensity of physical activity with accelerometry-based mean amplitude deviation (MAD) PLoS One. 2015;10:e0134813. doi: 10.1371/journal.pone.0134813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vähä-Ypyä H, Vasankari T, Husu P, Suni J, Sievänen H. A universal, accurate intensity-based classification of different physical activities using raw data of accelerometer. Clin. Physiol. Funct. Imaging. 2015;35:64–70. doi: 10.1111/cpf.12127. [DOI] [PubMed] [Google Scholar]
- 38.Vähä-Ypyä, H., Husu, P., Suni, J., Vasankari, T. & Sievänen, H. Reliable recording of lying, sitting and standing with a hip-worn accelerometer. Scand. J. Med. Sci. Sports. epub ahead of print; 10.1111/sms.13017 (2017). [DOI] [PubMed]
- 39.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341X.2000.00645.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the MOBILETWIN Study data is not allowed to be placed in open archives due to data protection issues of possibly identifiable twins. Researchers are encouraged to contact the last author (U.M.K.) and we will make every effort to accommodate additional analyses.