Abstract
Introduction
High-density lipoproteins (HDL) are responsible for the efflux and transport of cholesterol from peripheral tissues to the liver. In addition, HDL can modulate various immunological mechanisms, including the inflammatory response. Inflammasomes are multiprotein complexes that have been reported to be activated during human immunodeficiency virus type 1 (HIV-1) infection, thus contributing to immune hyperactivation, which is the main pathogenic mechanism of HIV-1 progression. However, the relationship between HDL and inflammasomes in the context of HIV-1 infection is unclear. Therefore, this research aims to explore the association between HDL and the components of the inflammatory response during HIV-1 infection.
Methodology
A cross-sectional study, including 36 HIV-1-infected individuals without antiretroviral treatment and 36 healthy controls matched by sex and age, was conducted. Viral load, CD4+ T-cell counts, serum HDL, and C-reactive protein (CRP) were quantified. Serum cytokine levels, including IL-1β, IL-6, and IL-18, were assessed by ELISA. The inflammasome-related genes in peripheral blood mononuclear cells were determined by quantitative real-time PCR.
Results
HIV-1-infected individuals showed a significant decrease in HDL levels, particularly those subjects with higher viral load and lower CD4+ T-cell counts. Moreover, upregulation of inflammasome-related genes (NLRP3, AIM2, ASC, IL-1β, and IL-18) was observed, notably in those HIV-1-infected individuals with higher viral loads (above 5,000 copies/mL). Serum levels of IL-6 and CRP were also elevated in HIV-1-infected individuals. Significant negative correlations between HDL and the mRNA of NLRP3, AIM2, ASC, IL-1β, and IL-18, as well as viral load and CRP were observed in HIV-1-infected individuals. Likewise, a significant positive correlation between HDL and CD4+ T-cell counts was found.
Conclusion
In summary, our results indicate that HDL might modulate the expression of several key components of the inflammasomes during HIV-1 infection, suggesting a novel role of HDL in modifying the inflammatory state and consequently, the progression of HIV-1 infection.
Keywords: human immunodeficiency virus type 1, high-density lipoproteins, inflammation, inflammasomes, C-reactive protein, NLRP3, CD4+ T-cell count, acquired immunodeficiency syndrome
Introduction
Human immunodeficiency virus type 1 (HIV-1), etiological agent of the acquired immunodeficiency syndrome (AIDS), was identified during the early 1980s (1). Despite the implementation of preventive measures, it is currently estimated that 36.7 million people are infected with HIV-1 in the world, so this infection continues to be considered one of the major health problems worldwide (2). The HIV-1 infection is characterized by a gradual increase in viral load, loss of mucosal integrity, increased microbial translocation, and immunologic hyperactivation (3), which is one the main pathogenic mechanism of disease progression (4, 5). It has been reported that several inflammatory markers are increased during HIV-1 infection, including IL-1β, IL-6, IL-8, IL-18, and TNF-α (6–9), as well as others serum markers such as C-reactive protein (CRP), D-dimer, and soluble CD14, among others (10). In fact, in vitro studies have reported that IL-1β and IL-18 correlate directly with increased viral replication, which highlights the importance of these pro-inflammatory cytokines in the progression of this infection (11, 12).
In the last years, the importance of the host innate immunity in the initial response to HIV-1 has been recognized (13, 14). Candidate mechanisms responsible for the innate immune responses include stimulation of pattern recognition receptors and the activation of inflammasomes. The inflammasomes are multicomponent complex commonly composed of an NOD-like receptor (NLR), the adaptor protein ASC, and caspase-1, which is required for processing and secretion the active forms of IL-1β and IL-18 (15, 16). The inflammasome activation is a two-step process that requires priming, usually by TLR activation (signal I), which triggers NF-κB pathway, inducing the transcription of pro-IL-1β and pro-IL-18; the second signal, induced by distinct stimuli (16), promotes the assembly of inflammasomes, that in turn endorse the proteolytic activation of these two cytokines (17, 18), contributing to the inflammatory process. Indeed, we have previously shown that during HIV-1 infection, the virus might act as the first signal to activate the NLRP3 inflammasome in macrophages (19) and also that HIV is associated with inflammasome activation (20). In addition, it has been reported that HIV-1 infected dendritic cells, from healthy individuals, exhibit enhanced transcriptional expression of NLRP3, caspase-1, and IL-1β (21), suggesting that HIV-1 has the ability to induce an inflammatory response through these complexes.
Although antiretroviral therapy successfully controls viral load in most patients, it has a minimal effects on the inflammatory status of them (8), underlying the importance of exploring new therapeutic strategies to complement current antiretroviral therapy and improve the quality of life of patients, by decreasing the incidence of comorbidities. In this sense, the immunomodulatory properties of endogenous molecules such as the high-density lipoproteins (HDL) were recently described. Their main protein component is the apoprotein AI (apo-AI), and the lipid fraction is composed of free cholesterol, cholesterol esters, phospholipids, and triglycerides (22, 23). While the main function of HDL is to transport cholesterol from the peripheral tissues to the liver (24), they also have pleiotropic effects in the reduction of inflammation, apoptosis and oxidation of low density lipoprotein (LDL) (25–27). It has been found that HDL neutralize LPS (28), negatively regulate the expression of adhesion molecules such as V-CAM, I-CAM, and E-selectin (29), and modulate the composition of the lipid rafts, important for cell signaling (30) Previously, it was described that HDL can negatively regulate the expression of pro-inflammatory cytokines induced by TLRs triggering, through the transcriptional regulator ATF3 (31). One of the pathways by which HDL mediates their anti-inflammatory effects is the regulation of the inflammasomes activation in response to cholesterol crystals, due to (i) negative regulation of the mRNA expression of NLRP3 and IL-1β, (ii) greater stability of the lysosomal membrane, and (iii) less mitochondrial damage (32). Most studies exploring the immunomodulatory effects of HDL have focused on cardiovascular diseases (33–36); however, the effects on inflammation may be promising in the context of viral infections such as HIV-1, which has an important inflammatory component that defines its progression to AIDS. Other authors have reported metabolic alterations, including lower HDL level in HIV-1-infected patients, when compared with healthy donors (37). Furthermore, the HDL deficiency is a factor that contributes to the high cardiovascular risk observed in patients with HIV-1 (38) and which is associated with the inflammatory process (39). Therefore, the aim of this study was to explore the association between HDL levels and the expression of inflammasome components and other inflammatory markers during HIV-1 infection.
Materials and Methods
Population of Study
This is a cross-sectional study, including 36 HIV-1-infected individuals without antiretroviral therapy or active co-infections. Patients with a history of anemia, diabetes, cancer, or thyroid disease, consumption of statins, as well as pregnant women were excluded. As a control group, 36 healthy individuals, matched by age and sex, from the same geographic area were included. The clinical and demographic characteristics of the study participants are presented in Table 1.
Table 1.
Demographic features of enrolled individuals.
| HIV-1-infected individuals (n = 36) | Healthy donors (n = 36) | |
|---|---|---|
| Women:men | 17:19 | 17:19 |
| Age in years: median (IQR) | 28 (22.3–37) | 30 (24.3–42.5) |
| CD4+ T-cells/mm3: median (IQR) | 629 (401–748) | 791 (654–1,032) |
| Viral load copies/mL: median (IQR) | 12,552 (1,240–48,368) | N/A |
| Months since HIV-1 diagnosis: median (IQR) | 7 (2–40) | N/A |
IQR, interquartile range, N/A, not applicable; HIV-1, human immunodeficiency virus type 1.
For statistical analysis, HIV-1-infected individuals were grouped based on: (i) the CD4+ T-cells counts (<200 cells/μL, 200–500 cells/mm3, and <500 cells/mm3) according to the CDC classification (40); (ii) the viral load (low: <2,000 copies/mL and high: >5,000 copies/mL). The group was also divided according to the CRP levels (normal: <0.5 mg/dL and high: >0.5 mg/dL). Finally, they were classified according to HDL levels (low: <40 mg/dL and normal: >40 mg/dL).
According to ethical guidelines, all subjects signed a written informed consent, previously reviewed and approved by the research ethics committee of the Universidad Cooperativa de Colombia. The protocols were carried out in accordance with the principles of the Declaration of Helsinki. All the participants have completed (i) the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST V 3.0) of the World Health Organization to evaluate smoking, alcohol, and other psychoactive substances consumption, (ii) the International Physical Activity Questionnaire, and (iii) a survey of socio-demographic data.
Sample Taking and Processing
Approximately 20 mL of peripheral blood was obtained by venipuncture in vacutainer tubes. Plasma and serum were isolated by centrifugation, and stored at −70°C until use. Peripheral blood mononuclear cells (PBMCs) were isolated using the Ficoll-Histopaque density gradient method (Sigma-Aldrich Chemical Co., St. Louis, MO, USA).
Viral Load and CD4+ T-Cells Count in Peripheral Blood
Human immunodeficiency virus type 1 viral load was performed in plasma from peripheral blood samples, by a certified clinical laboratory. CD4+ T-cells counts were determined for all participants to assess their immune status; by flow cytometry (FACS BD Biosciences, San Jose, CA, USA) and using specific monoclonal antibodies (anti-CD3-FITC, clone: UCHT1, anti-CD4-APC; clone: RPA-T4, and anti-CD8-PE, clone: RPA-T8) (eBioscience, San Diego, CA, USA).
Quantification of HDL and CRP
Serum HDL levels were quantified with a colorimetric assay, using the commercial kit (Biosystems, Costa Brava 30.08030, Barcelona, Spain), the results were reported in milligrams per deciliter. CRP was quantified by a clinical laboratory certified using the immunoturbidimetry.
Cytokines Quantification by ELISA
Serum levels of IL-1β, IL-6 (BD Biosciences, Franklin Lakes, NJ, USA) and IL-18 (Human IL-18 Matched Antibody Pairs BMS267/2MST, eBioscience, Austria) were evaluated by ELISA using commercial kits, following the manufacturer’s instructions. The samples were processed in duplicate, and the concentration of the cytokines was calculated from a calibration curve.
Extraction of RNA and cDNA Synthesis
Total RNA was extracted from PBMCs using the commercial RNeasy Mini Kit (QIAGEN Inc., Valencia, CA, USA), following the manufacturer’s recommendations. The RNA concentration/purity was determined by spectrophotometry at 260–280 nm. The cDNA synthesis was performed with 230 ng of total RNA and using the Revertaid™ H Minus First Strand cDNA Synthesis Kit (Fermentas, Glen Burnie, USA), following the manufacturer’s recommendations.
Quantitative RT-PCR for the Inflammasome Components
The mRNA of NLRP1, NLRP3, NLRC4, AIM2, ASC, caspase-1, IL-1β, and IL-18 was quantified by real-time RT-PCR using Maxima SYBR Green qPCR master mix kit (Fermentas, Glen Burnie, MD, USA). The mRNA β-actin was used as the housekeeping gene to normalize the RNA content. The amplification protocols were 39 cycles and standardized for each gene (41–43). For real-time RT-PCR analysis, the CFX Manager Version: 1.5.534.0511 software (Bio-Rad, Hercules, CA, USA) was used.
Statistical Analysis
For data analysis, GraphPad Prism® 5.0 (San Diego, CA, USA) software was used. Normality and homoscedasticity were evaluated using Shapiro–Wilk test and Levene test, respectively. Student’s t-test or Mann–Whitney test was used for the comparison between groups for parametric and non-parametric data, respectively. Parametric ANOVA or Kruskal–Wallis tests were used for the comparison between three or more groups; in case of statistical association post hoc tests (or multiple benchmarks) HDS of Tukey and Dunn, respectively, were carried out. For the correlations, Pearson or Spearman tests were performed, for parametric or non-parametric data, respectively. p Values lower than 0.05 were considered statistically significant.
Results
Clinical and Demographic Features
The demographic information is presented in Table 1. As expected, HIV-1-infected individuals have a lower CD4+ T-cells count compared with healthy donors (p < 0.0001). Regarding physical activity, 23% (HIV-1-infected individuals) and 22% (healthy controls) have low physical activity. Less than 5% of the individuals in both groups have reported moderate levels of alcohol and psychoactive substances consumption.
HIV-1-Infected Individuals Have Decreased HDL Levels
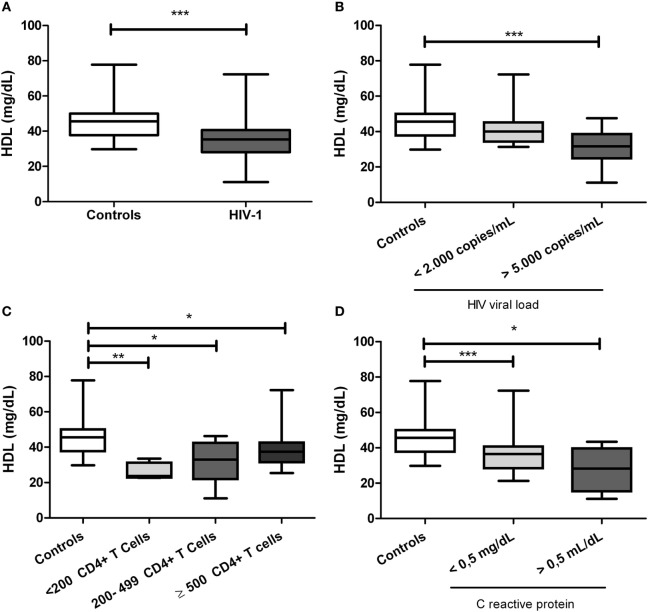
Since several metabolic alterations have been reported during HIV-1 infections (37), the lipid profile was quantified. It was found that HIV-1-infected individuals had significant lower HDL levels compared with healthy controls (p < 0.0001, Figure 1A). However, there were not alterations in other lipid profile parameters, including triglycerides, VLDL, LDL, and total cholesterol (data not shown). Moreover, the lower values in HDL were observed in those individuals with viral load over 5,000 copies/mL (p < 0.0001, Figure 1B) and also in those patients with CD4+ T-cells counts lower than 200 cells/mm3 (p < 0.0001; Figure 1C; Figure S1 in Supplementary Material). The decrease in HDL levels is conserved among HIV-1-infected individuals, independently of their CRP levels (Figure 1D). As previously reported (44), women showed higher HDL levels than men, but only within the healthy control group (data no shown). In addition, alterations in HDL levels were no affected by normal or overweight conditions of patients (data no shown).
Figure 1.
Serum high-density lipoprotein (HDL) levels in human immunodeficiency virus type 1 (HIV-1)-infected individuals and healthy controls. (A) HIV-1-infected individuals and healthy controls. In addition, HIV-1-infected individuals were classified according to (B) viral load, (C) CD4+ T-cells counts, and (D) C-reactive protein levels. The statistical comparison was made using Mann–Whitney U and Kruskal–Wallis tests with a 95% confidence level; and post hoc tests (or multiple benchmarks) HDS of Dunn was made. Significant differences are represented in the upper part of this figure (*p < 0.05; **p < 0, 01; and ***p < 0.0001).
The mRNA Expression of Inflammasome-Related Genes Is Altered in HIV-1-Infected Individuals
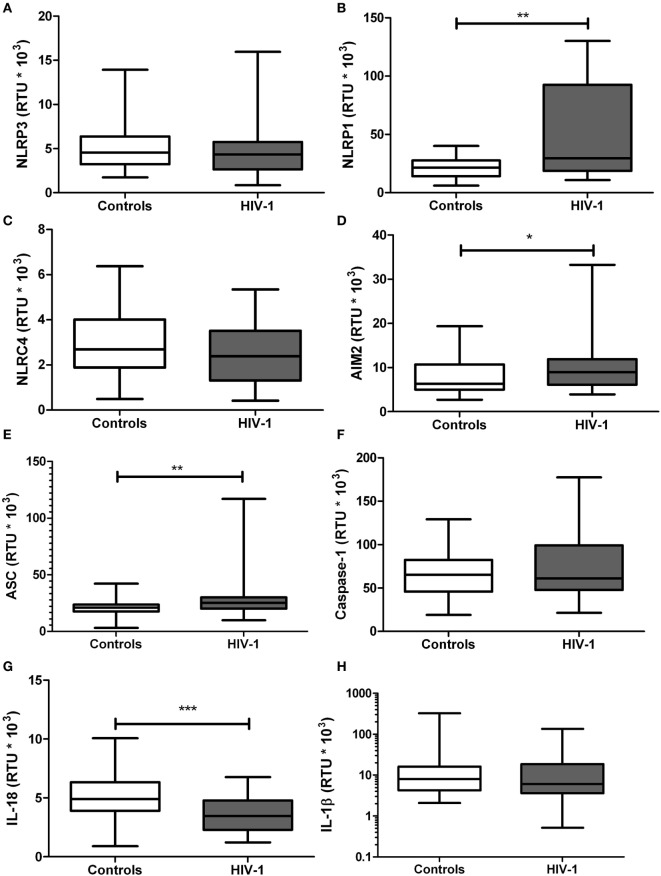
Since the exacerbated inflammatory response is one of the main pathogenic mechanisms of HIV-1 infection, mRNA expression of the inflammasome-related genes was quantified. A significant increase in gene expression of NLRP1 (p = 0.0073; Figure 2B), AIM2 (p = 0.0455; Figure 2D), and ASC (p = 0.0147; Figure 2E) was observed in HIV-1-infected individuals, compared with healthy controls. In addition, a significant decrease in IL-18 gene transcription was seen in HIV-1-infected patients compared with healthy controls (p < 0.001; Figure 2G). No significant changes in the mRNA expression were observed for the other evaluated genes, including NLRP3, NLRC4, caspase-1 and IL-1β (Figures 2A,C,F,H, respectively).
Figure 2.
mRNA expression of inflammasome-related genes in human immunodeficiency virus type 1 (HIV-1)-infected individuals and healthy controls. RNA samples obtained from HIV-1-infected individuals and controls were quantified by real-time PCR: (A) NLRP3, (B) NLRP1, (C) NLRC4, (D) AIM2, (E) ASC, (F) caspase-1, (G) IL-18, and (H) IL-1β. β-Actin was used as the constitutive gene to normalize the RNA content. The statistical comparison was made using the Mann–Whitney U test with a 95% confidence level. Significant differences are represented in the upper part of this figure (*p < 0.05; **p < 0, 01; and ***p < 0.0001).
Viral Load Is Related With mRNA Expression of NLRP3 and IL-1β in HIV-1-Infected Individuals
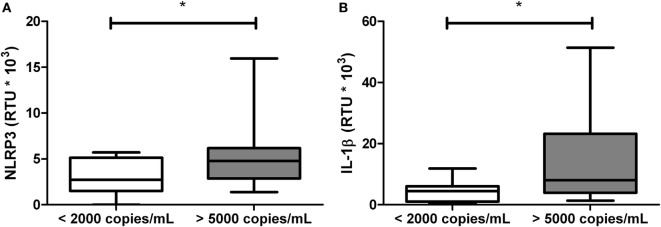
Since viral load, CD4+ T-cells counts, and CRP are related to the inflammatory status and progression in HIV-1-infected individuals, we analyzed whether these variables are related with the gene expression of inflammasome components. Patients with viral loads higher than 5,000 copies/mL were found to have increased expression of NLRP3 and IL-1β compared with those patients with viral loads below 2,000 copies/mL (Figure 3).
Figure 3.
Increased mRNA levels of NLRP3 and IL-1β in patients with high viral loads. Total RNA was obtained from human immunodeficiency virus type 1-infected individuals, classified according to the viral load and quantified by real-time PCR: (A) NLRP3 and (B) IL-1β. β-Actin was used as the constitutive gene to normalize the RNA content. The statistical comparison was made using the Mann–Whitney U with a 95% confidence level. Significant differences are represented in the upper part of this figure (*p < 0.05).
HIV-1-Infected Individuals Show Increases Level of IL-6 and CRP
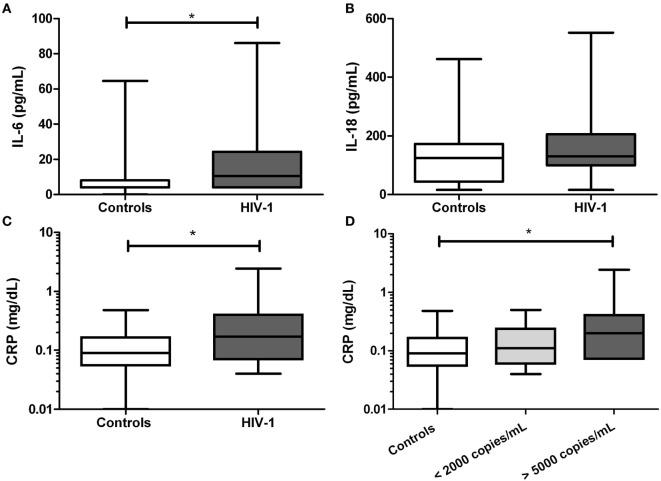
Next, inflammatory markers including IL-1β, IL-18, IL-6, and CRP were evaluated in serum. We found that the IL-6 level was significantly increased in HIV-1-infected individuals compared with healthy controls (p = 0.0175, Figure 4A), but no for IL-18 (Figure 4B). The IL-1β was undetectable in all tested subjects. In addition, a significant increase in CRP level (p = 0.0125) was observed in HIV-1-infected individuals compared with healthy controls (Figure 4C), and the increase was more evident in those patients with viral load over 5,000 copies/mL (Figure 4D).
Figure 4.
Serum levels of inflammatory markers in human immunodeficiency virus type 1 (HIV-1)-infected individuals. In serum samples from HIV-1-infected individuals and healthy controls were quantified (A) IL-6, (B) IL-18, and (C) C-reactive protein (CRP). In HIV-1-infected individuals, the CRP was compared according to viral load (D). The statistical comparison was made using the Mann–Whitney U and Kruskal–Wallis tests with a 95% confidence level and post hoc tests (or multiple benchmarks) HDS of Dunn was made. Significant differences are represented in the upper part of this figure (*p < 0.05).
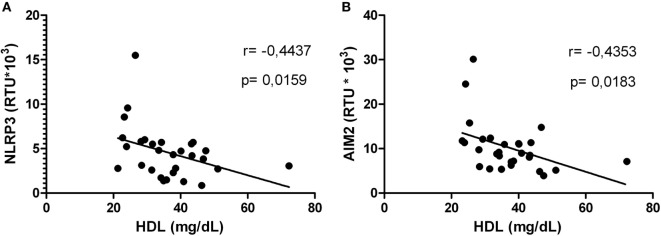
HDL Levels Correlated With mRNA Expression of Inflammasome-Related Genes in HIV-1-Infected Individuals
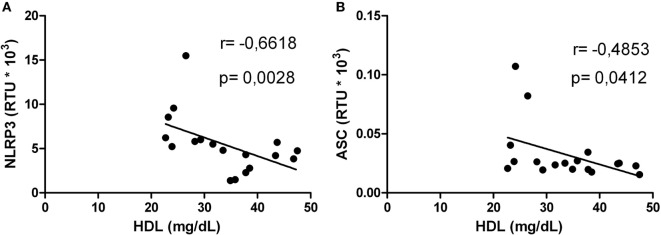
Correlations between HDL levels and the mRNA expression of inflammasome-related genes were explored. Negative correlations were found between HDL and NLRP3 (Figure 5A) or AIM2 (Figure 5B). In addition, HIV-1-infected individuals with viral loads above 5,000 copies/mL showed a negative correlation between HDL and NLRP3 or ASC (Figures 6A,B). Furthermore, it was found in HIV-1-infected individuals with CD4+ T-cells counts greater than 500 cells/mm3 a negative correlation between HDL and AIM2 or IL-18 (data no shown).
Figure 5.
High-density lipoprotein (HDL) level correlated with NLRP3 or AIM2 in human immunodeficiency virus type 1-infected individuals. Correlation between HDL and NLRP3 (A) or AIM2 (B) was performed using a Spearman test. The r and p values are indicated in each figure. A p value lower than 0.05 was considered as significant.
Figure 6.
Correlation analysis between high-density lipoprotein (HDL) and NLRP3 or ASC in human immunodeficiency virus type 1-infected individuals. Correlation between HDL and NLRP3 (A) or ASC (B) in patients with viral load greater than 5,000 copies/mL was performed using a Spearman test. The r and p values are indicated in each figure. A p value lower than 0.05 was considered as significant correlation.
HIV-1-Infected Individuals With Normal HDL Levels Exhibit Minor Clinical Progression to Diseases
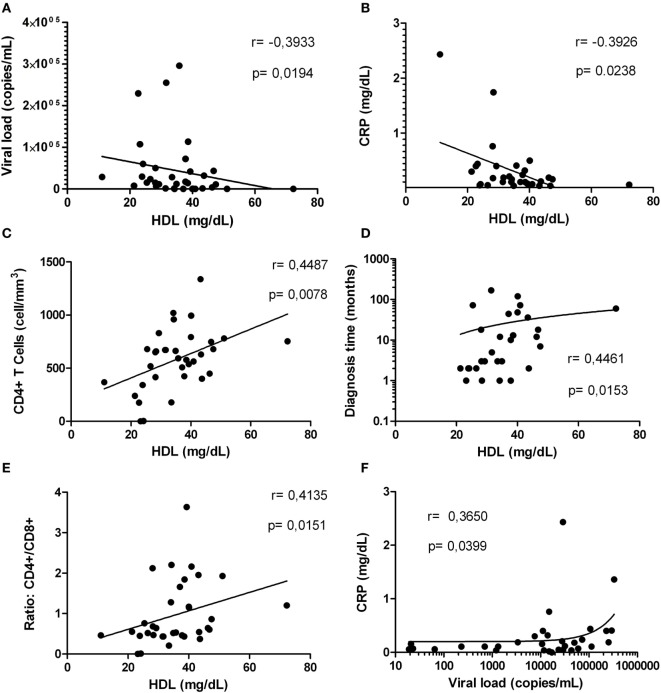
Based on our results indicating alterations in the inflammatory status and the HDL levels in HIV-1-infected individuals, we proceeded to determine whether HDL correlate with parameters associated with HIV-1 progression. A negative correlation between HDL level and viral load was found (Figure 7A); this observation was most evident in HIV-1-infected individuals with viral loads below 2,000 copies/mL (data no shown). Finally, a negative correlation between HDL levels and CRP levels was demonstrated (Figure 7B).
Figure 7.
Correlations between high-density lipoprotein (HDL) levels with factors associated with human immunodeficiency virus type 1 progression. Correlation analysis between HDL levels and: viral load (A), C-reactive protein (CRP) (B), CD4+ T-cells (C), diagnosis time (D), and ratio CD4+/CD8+ (E). In addition, correlation between viral load and CRP is presented (F). The correlation analyses were performed using a Spearman test. The r and p values are indicated in each figure. A p value lower than 0.05 was considered as significant correlation.
Finally, since other markers such as CD4+ T-cells count and diagnosis time are important predictors of the HIV-1 disease progression, we assessed whether there was a correlation between HDL levels and these two parameters. Interestingly, HDL levels were positively correlated with CD4+ T-cells count (Figure 7C) and diagnosis time (Figure 7D). These correlations were more evident in HIV-1-infected individuals with viral loads greater than 5,000 copies/mL (data not shown). In addition, a positive correlation between HDL and CD4+/CD8+ T-cells ratio, a predictor of disease progression (45) was found (Figure 7E). Interestingly, the viral load and CRP levels were positively correlated (Figure 7F), as an indicator of the relationship between viral replication and inflammation.
Discussion
Immune hyperactivation is the major pathogenic mechanism of HIV-1 infection and directly correlated with disease progression, since it leads to immune depletion, loss of viral control, and ultimately to development of AIDS (4, 5). Different inflammatory markers, such as CRP, D-dimer, and pro-inflammatory cytokines, are increased during the course of the infection (6–10). Although the implementation of antiretroviral therapy has led to an increase in the life expectancy of individuals with HIV-1, they continue to present chronic inflammation that is related to various pathological outcomes, such as cardiovascular diseases (46), cancer (47), and renal diseases (48). These conditions underline the importance of searching for immunomodulators that complement antiretroviral therapy, reducing inflammation and thus the comorbidities associated with infection.
Since HDL have immunomodulatory properties (49–52), their study and subsequent use as complementary to antiretroviral therapy would be considered (i.e., pharmacological strategies to increase HDL levels). In the current study, and in agreement with previous reports (37, 53), HIV-1-infected individuals exhibited lower HDL levels than healthy controls (Figure 1A), suggesting that HIV-1 infection leads to an alteration in the metabolism of this lipoprotein. Although no differences were observed in other parameters of the lipid profile, other authors have reported higher levels of triglycerides and a decrease in both, total and LDL cholesterol (49, 50, 54). The apparent discrepancy in the results could be explained by differences among the studied group, in particular related to stages of progression; in addition, the differences show the importance of studies on specific populations, given the heterogeneity in the metabolic and inflammatory profiles.
Pro-inflammatory cytokines have been reported to induce hepatic cholesterol synthesis (55). Based on our results and also in previous data, some questions are raised: (i) Are those lower HDL levels due to sustained inflammatory response in HIV-1-infected individuals? or (ii) Are the lower HDL levels prior HIV-1 infection, a factor that promotes the persistent inflammatory response? These questions underline the importance of further studies to elucidate the causal mechanisms. In accordance to this, it has been reported in HIV-1-infected individuals a negative correlation between HDL levels and inflammatory biomarkers such as neopterin and sTNFR-75, suggesting a relationship between this lipoprotein and immune activation during the infection (56). In fact, according with our previous results (manuscript in preparation) as well as those reported by other authors (25, 26, 57), have shown that HDL have regulatory effects on several components of innate immunity, including the inflammasomes (32), which also have an important role in the pathogenesis of HIV-1 infection. In fact, it has been reported that HIV-1 acts as the primary signal for the activation of the NLRP3 inflammasome in macrophages (19). In addition, polymorphisms in inflammasome-related genes have been associated, not only with increased susceptibility to HIV-1 infection (58, 59) but also with progression to AIDS (59), demonstrating the participation of inflammasomes in the course of HIV-1 infection. Our results indicate alterations in mRNA expression of inflammasome-related genes during HIV-1 infection. In particular, increased mRNA expression of NLRP1, AIM2 and ASC, and decreased expression of IL-18, was observed (Figure 2). Other authors have reported similar results on dendritic cells in vitro infected with HIV-1 (21) and ex vivo evaluations in microglia and podocytes from HIV-1-infected individuals (60, 61). It is noteworthy that HIV-1 Tat and Vpr proteins have the ability to induce the expression of caspase-1 and IL-1β (60, 62). It should be noted that the patients with higher viral replication display stronger alterations in the inflammasome components; together, these results suggest that HIIV-1 infection promotes the activation of these complexes (19).
The inflammasome activation ends with the active forms of IL-1β and IL-18 that are increased during HIV-1 infection, promoting disease progression (11, 12). Even though the HIV-1 has the ability to induce the expression of IL-1β through TLR8 signaling and inflammasome activation (63), here we were unable to detect IL-1β in sera from HIV-1-infected individuals. This can be explained by the sensitivity of the technique used, since other study reported that serum concentration of IL-1β is very low (8), suggesting the need to use ultrasensitive techniques. In addition, the presence of high levels of IL-1β receptor antagonists such as IL-1RII, previously reported during HIV-1 infection (64), could be responsible for this result, a hypothesis that requires further studies.
Other inflammatory markers reported during HIV-1 infection are IL-6 and the acute-phase reactant CRP; both have been previously correlated with mortality in HIV-1 individuals (65). Taking into account that acute inflammatory responses induce alterations in HDL composition and function (66), and that we found a positive correlation between HIV RNA load and CRP, we postulated that high CRP levels could be related with the decrease of HDL, favoring an inflammatory state. In addition, our results indicate that the increase in CRP occurs mainly in HIV-1-infected individuals with high viral load, suggesting a higher viral replication in the presence of inflammatory response and lower HDL levels. Supporting our results, Thacker et al. reported that HDL mediate anti-inflammatory effects through the regulation of inflammasomes activity (32), among the findings reported, there is a decrease in the gene expression of NLRP3 and IL-1β, as well as high expression of the transcriptional regulator ATF3. This HDL-induced factor has been described as a negative regulator of TLRs signaling and cytokines production (31). In accordance with this, negative correlations between HDL and NLRP3 or AIM2 were observed. The decrease in HDL levels could lead to a reduction in the total cholesterol efflux, resulting in accumulation of cholesterol and the development of cholesterol crystals, which activate the NLRP3 inflammasome (67). In this context, the response mediated by the cholesterol crystals could contribute to chronic inflammation during HIV-1 infection, and hence to disease progression. In fact, our previous results show that HDL decreases the IL-1β production in vitro, in response to cholesterol crystals and in fact it was seen a decrease in the size of the crystals of cholesterol in a dose-dependent manner, which could be one of the anti-inflammatory mechanisms exerted by this lipoprotein (manuscript in preparation). These results support our hypothesis that control of HIV-1 progression could be, at least in part, associated with the regulation of inflammasomes by the HDL.
The negative correlations between HDL and the inflammasome components, especially those observed in patients with active viral replication, may indicate that these interactions occur in advanced stages of infection. This could be associated with the immune collapse and progression to AIDS, evidenced by the relationship found between HDL and CD4+ T-cells count or HIV RNA load. Similarly, according to reported by Shen et al. (54), patients with lower CD4+ T-cells counts have lower HDL levels, reinforcing the hypothesis that patients with lower HDL levels could have a higher risk of disease progression.
In addition, it is important to keep in mind that the activity of caspase-1-dependent inflammasomes results in pyroptosis (a type of cell death with release of IL-1β) (68–70), contributing with the elimination of CD4+ T-cells during HIV-1 infection (71). In this context, lower HDL levels could result in increased inflammasomes activation, leading to an increased CD4+ T-cells death, according to the positive correlation between HDL and CD4+ T-cells count observed in this study. In fact, HDL levels have been proposed as a predictor parameter in patients receiving non-nucleoside reverse transcriptase inhibitors (52, 72). On other hand, HDL has antioxidant activity on LDL, which once oxidized (oxLDL) induce the expression of IL-1β, via inflammasomes (67, 73). It has been reported that HDL inhibit the inflammatory response induced by the complement system in response to cholesterol crystals (74, 75), contributing with the regulation of the inflammatory process in HIV-1-infected individuals.
Taken together, our results indicate that during HIV-1 infection there is a decrease in HDL levels along with a persistent inflammatory response, in particular in those individuals with evidence of disease progression (increased HIV RNA load and lower CD4+ T-cells counts). Our results suggest that there is a relationship between HDL and inflammasomes activation during HIV-1 infection, pointing toward new therapeutic strategies based on the ability of this lipoprotein for regulate the activation of these complexes that haven been involved in multi-systemic diseases associated with HIV-1 infection. However, given the complexity of the immune response during HIV-1 infection, more studies are needed to elucidate the precise mechanisms behind these processes, since chronic inflammation may in turn lead to an alteration in HDL composition and hence function, as previously reported in patients with chronic renal dysfunction (76). Moreover, it is needed to conduct new studies in patients under antiretroviral therapy (HAART), since it has been reported that HAART has an impact in lipid levels (77) and in some inflammatory markers (78); to know how this interaction can affect the relation between HDL and inflammasomes and how it could be taking in account in the search of new therapeutic targets.
Ethics Statement
According to ethical guidelines, all subjects signed a written informed consent, previously reviewed and approved by the research ethics committee of the Universidad Cooperativa de Colombia. The protocols were carried out in accordance with the principles of the Declaration of Helsinki.
Author Contributions
Funding acquisition and project administration: JH. Conceived and designed the experiments; writing original draft: DM-P, SU-I, and JH. Investigation: DM-P, GC, and JH. Formal analysis: DM-P, JC-A, and JH. Writing review and editing: JC-A, SU-I, and JH. Approval of manuscript for publication: DM-P, GC, JC-A, SU-I, and JH.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the donors who participated in this study and the collaboration of the personnel from the institutions where the patients were recruited. We acknowledge the help of Maria T. Rugeles for critically reading and reviewing of the manuscript. Universidad de Antioquia UdeA and Universidad Cooperativa de Colombia.
Footnotes
Funding. This study was funded by Colciencias Grant 141565741029, Universidad Cooperativa de Colombia, and Universidad de Antioquia UdeA. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fimmu.2018.01350/full#supplementary-material.
Viral load and CD4+ T-cells counts according to high-density lipoprotein (HDL) levels in human immunodeficiency virus type 1 (HIV-1) (HIV-1)-infected individuals. HIV-1-infected individuals were divided according to HDL levels (normal: >40 mg/dL and low: <40 mg/dL) and the viral load (A) and the CD4+ T-cells counts (B) were analyzed. The statistical comparison was made using the Mann–Whitney U tests with a 95% confidence level. Significant differences are represented in the upper part of this figure (*p < 0.05 and **p < 0.01).
References
- 1.Salemi M. The intra-host evolutionary and population dynamics of human immunodeficiency virus type 1: a phylogenetic perspective. Infect Dis Rep (2013) 5(Suppl 1):e3. 10.4081/idr.2013.s1.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS. Fact sheet – latest statistics on the status of the AIDS epidemic 2016. Available from: http://www.unaids.org/en/resources/fact-sheet. (Accessed: December 20, 2017).
- 3.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med (2006) 12(3):289–95. 10.1038/nm1380 [DOI] [PubMed] [Google Scholar]
- 4.Boasso A, Shearer GM. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin Immunol (2008) 126(3):235–42. 10.1016/j.clim.2007.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hazenberg MD, Otto SA, van Benthem BH, Roos MT, Coutinho RA, Lange JM, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS (2003) 17(13):1881–8. 10.1097/01.aids.0000076311.76477.6e [DOI] [PubMed] [Google Scholar]
- 6.Thea DM, Porat R, Nagimbi K, Baangi M, St Louis ME, Kaplan G, et al. Plasma cytokines, cytokine antagonists, and disease progression in African women infected with HIV-1. Ann Intern Med (1996) 124(8):757–62. 10.7326/0003-4819-124-8-199604150-00009 [DOI] [PubMed] [Google Scholar]
- 7.Grunfeld C, Kotler DP, Shigenaga JK, Doerrler W, Tierney A, Wang J, et al. Circulating interferon-alpha levels and hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med (1991) 90(2):154–62. 10.1016/0002-9343(91)80154-E [DOI] [PubMed] [Google Scholar]
- 8.Espindola MS, Lima LJ, Soares LS, Cacemiro MC, Zambuzi FA, de Souza Gomes M, et al. Dysregulated immune activation in second-line HAART HIV+ patients is similar to that of untreated patients. PLoS One (2015) 10(12):e0145261. 10.1371/journal.pone.0145261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad R, Sindhu ST, Toma E, Morisset R, Ahmad A. Elevated levels of circulating interleukin-18 in human immunodeficiency virus-infected individuals: role of peripheral blood mononuclear cells and implications for AIDS pathogenesis. J Virol (2002) 76(24):12448–56. 10.1128/JVI.76.24.12448-12456.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuhaus J, Jacobs DR, Jr, Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis (2010) 201(12):1788–95. 10.1086/652749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granowitz EV, Saget BM, Wang MZ, Dinarello CA, Skolnik PR. Interleukin 1 induces HIV-1 expression in chronically infected U1 cells: blockade by interleukin 1 receptor antagonist and tumor necrosis factor binding protein type 1. Mol Med (1995) 1(6):667–77. [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro L, Puren AJ, Barton HA, Novick D, Peskind RL, Shenkar R, et al. Interleukin 18 stimulates HIV type 1 in monocytic cells. Proc Natl Acad Sci U S A (1998) 95(21):12550–5. 10.1073/pnas.95.21.12550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol (2006) 7(2):131–7. 10.1038/ni1303 [DOI] [PubMed] [Google Scholar]
- 14.Antonucci JM, St Gelais C, Wu L. The dynamic interplay between HIV-1, SAMHD1, and the innate antiviral response. Front Immunol (2017) 8:1541. 10.3389/fimmu.2017.01541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci (2014) 1319:82–95. 10.1111/nyas.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol (2013) 13(6):397–411. 10.1038/nri3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol (2016) 16(7):407–20. 10.1038/nri.2016.58 [DOI] [PubMed] [Google Scholar]
- 18.Hernandez J, Sirois C, Latz E. “Activation and regulation of the NLRP3 inflammasome” inflammasomes. In: Couillin I, Pétrilli V, Martinon F, editors. The Inflammasomes. Progress in Inflammation Research. Basel: Springer; (2011). p. 197–208. [Google Scholar]
- 19.Hernandez JC, Latz E, Urcuqui-Inchima S. HIV-1 induces the first signal to activate the NLRP3 inflammasome in monocyte-derived macrophages. Intervirology (2014) 57(1):36–42. 10.1159/000353902 [DOI] [PubMed] [Google Scholar]
- 20.Feria MG, Taborda NA, Hernandez JC, Rugeles MT. HIV replication is associated to inflammasomes activation, IL-1beta, IL-18 and caspase-1 expression in GALT and peripheral blood. PLoS One (2018) 13(4):e0192845. 10.1371/journal.pone.0192845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pontillo A, Silva LT, Oshiro TM, Finazzo C, Crovella S, Duarte AJ. HIV-1 induces NALP3-inflammasome expression and interleukin-1beta secretion in dendritic cells from healthy individuals but not from HIV-positive patients. AIDS (2012) 26(1):11–8. 10.1097/QAD.0b013e32834d697f [DOI] [PubMed] [Google Scholar]
- 22.Feingold KR, Grunfeld C. In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, et al., editors. Introduction to Lipids and Lipoproteins. South Dartmouth, MA: Endotext; (2000). [Google Scholar]
- 23.Marín-Palma D, Taborda N, Urucuqui-Inchima S, Hernandez JC. Inflamación y respuesta inmune innata: Participación de las lipoproteínas de alta densidad. Iatreia (2017) 30(4):423–35. 10.17533/udea.iatreia.v30n4a06 [DOI] [Google Scholar]
- 24.Asztalos BF, de la Llera-Moya M, Dallal GE, Horvath KV, Schaefer EJ, Rothblat GH. Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J Lipid Res (2005) 46(10):2246–53. 10.1194/jlr.M500187-JLR200 [DOI] [PubMed] [Google Scholar]
- 25.Uittenbogaard A, Shaul PW, Yuhanna IS, Blair A, Smart EJ. High density lipoprotein prevents oxidized low density lipoprotein-induced inhibition of endothelial nitric-oxide synthase localization and activation in caveolae. J Biol Chem (2000) 275(15):11278–83. 10.1074/jbc.275.15.11278 [DOI] [PubMed] [Google Scholar]
- 26.Kameda T, Ohkawa R, Yano K, Usami Y, Miyazaki A, Matsuda K, et al. Effects of myeloperoxidase-induced oxidation on antiatherogenic functions of high-density lipoprotein. J Lipids (2015) 2015:592594. 10.1155/2015/592594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nofer JR, Levkau B, Wolinska I, Junker R, Fobker M, von Eckardstein A, et al. Suppression of endothelial cell apoptosis by high density lipoproteins (HDL) and HDL-associated lysosphingolipids. J Biol Chem (2001) 276(37):34480–5. 10.1074/jbc.M103782200 [DOI] [PubMed] [Google Scholar]
- 28.Henning MF, Herlax V, Bakas L. Contribution of the C-terminal end of apolipoprotein AI to neutralization of lipopolysaccharide endotoxic effect. Innate Immun (2011) 17(3):327–37. 10.1177/1753425910370709 [DOI] [PubMed] [Google Scholar]
- 29.Cockerill GW, Rye KA, Gamble JR, Vadas MA, Barter PJ. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler Thromb Vasc Biol (1995) 15(11):1987–94. 10.1161/01.ATV.15.11.1987 [DOI] [PubMed] [Google Scholar]
- 30.Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, et al. Macrophage ABCA1 reduces MyD88-dependent toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res (2010) 51(11):3196–206. 10.1194/jlr.M006486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Nardo D, Labzin LI, Kono H, Seki R, Schmidt SV, Beyer M, et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat Immunol (2014) 15(2):152–60. 10.1038/ni.2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thacker SG, Zarzour A, Chen Y, Alcicek MS, Freeman LA, Sviridov DO, et al. High-density lipoprotein reduces inflammation from cholesterol crystals by inhibiting inflammasome activation. Immunology (2016) 149(3):306–19. 10.1111/imm.12638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng F, Xing S, Gong Z, Mu W, Xing Q. Silence of NLRP3 suppresses atherosclerosis and stabilizes plaques in apolipoprotein E-deficient mice. Mediators Inflamm (2014) 2014:507208. 10.1155/2014/507208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med (1977) 62(5):707–14. 10.1016/0002-9343(77)90874-9 [DOI] [PubMed] [Google Scholar]
- 35.Harel M, Aharoni A, Gaidukov L, Brumshtein B, Khersonsky O, Meged R, et al. Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat Struct Mol Biol (2004) 11(5):412–9. 10.1038/nsmb767 [DOI] [PubMed] [Google Scholar]
- 36.Bounafaa A, Berrougui H, Ghalim N, Nasser B, Bagri A, Moujahid A, et al. Association between paraoxonase 1 (PON1) polymorphisms and the risk of acute coronary syndrome in a north African population. PLoS One (2015) 10(8):e0133719. 10.1371/journal.pone.0133719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estrada V, Martinez-Larrad MT, Gonzalez-Sanchez JL, de Villar NG, Zabena C, Fernandez C, et al. Lipodystrophy and metabolic syndrome in HIV-infected patients treated with antiretroviral therapy. Metabolism (2006) 55(7):940–5. 10.1016/j.metabol.2006.02.024 [DOI] [PubMed] [Google Scholar]
- 38.Marbaniang IP, Kadam D, Suman R, Gupte N, Salvi S, Patil S, et al. Cardiovascular risk in an HIV-infected population in India. Heart Asia (2017) 9(2):e010893. 10.1136/heartasia-2017-010893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross AC, Rizk N, O’Riordan MA, Dogra V, El-Bejjani D, Storer N, et al. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis (2009) 49(7):1119–27. 10.1086/605578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider E, Whitmore S, Glynn KM, Dominguez K, Mitsch A, McKenna MT. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years – United States. MMWR Recomm Rep (2008;) 57(RR–10):1–12. [PubMed] [Google Scholar]
- 41.Gomez DM, Urcuqui-Inchima S, Hernandez JC. Silica nanoparticles induce NLRP3 inflammasome activation in human primary immune cells. Innate Immun (2017) 23(8):697–708. 10.1177/1753425917738331 [DOI] [PubMed] [Google Scholar]
- 42.Hernandez JC, St Laurent G, III, Urcuqui-Inchima S. HIV-1-exposed seronegative individuals show alteration in TLR expression and pro-inflammatory cytokine production ex vivo: An innate immune quiescence status? Immunol Res (2016) 64(1):280–90. 10.1007/s12026-015-8748-8 [DOI] [PubMed] [Google Scholar]
- 43.Hernandez JC, Giraldo DM, Paul S, Urcuqui-Inchima S. Involvement of neutrophil hyporesponse and the role of Toll-like receptors in human immunodeficiency virus 1 protection. PLoS One (2015) 10(3):e0119844. 10.1371/journal.pone.0119844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Godsland IF, Wynn V, Crook D, Miller NE. Sex, plasma lipoproteins, and atherosclerosis: prevailing assumptions and outstanding questions. Am Heart J (1987) 114(6):1467–503. 10.1016/0002-8703(87)90552-7 [DOI] [PubMed] [Google Scholar]
- 45.Taylor JM, Fahey JL, Detels R, Giorgi JV. CD4 percentage, CD4 number, and CD4:CD8 ratio in HIV infection: which to choose and how to use. J Acquir Immune Defic Syndr (1989) 2(2):114–24. [PubMed] [Google Scholar]
- 46.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med (2013) 173(8):614–22. 10.1001/jamainternmed.2013.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borges AH, Silverberg MJ, Wentworth D, Grulich AE, Fatkenheuer G, Mitsuyasu R, et al. Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS (2013) 27(9):1433–41. 10.1097/QAD.0b013e32835f6b0c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta SK, Kitch D, Tierney C, Melbourne K, Ha B, McComsey GA, et al. Markers of renal disease and function are associated with systemic inflammation in HIV infection. HIV Med (2015) 16(10):591–8. 10.1111/hiv.12268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riddler SA, Smit E, Cole SR, Li R, Chmiel JS, Dobs A, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA (2003) 289(22):2978–82. 10.1001/jama.289.22.2978 [DOI] [PubMed] [Google Scholar]
- 50.Anastos K, Lu D, Shi Q, Tien PC, Kaplan RC, Hessol NA, et al. Association of serum lipid levels with HIV serostatus, specific antiretroviral agents, and treatment regimens. J Acquir Immune Defic Syndr (2007) 45(1):34–42. 10.1097/QAI.0b013e318042d5fe [DOI] [PubMed] [Google Scholar]
- 51.Buchacz K, Weidle PJ, Moore D, Were W, Mermin J, Downing R, et al. Changes in lipid profile over 24 months among adults on first-line highly active antiretroviral therapy in the home-based AIDS care program in rural Uganda. J Acquir Immune Defic Syndr (2008) 47(3):304–11. 10.1097/QAI.0b013e31815e7453 [DOI] [PubMed] [Google Scholar]
- 52.Rose H, Woolley I, Hoy J, Dart A, Bryant B, Mijch A, et al. HIV infection and high-density lipoprotein: the effect of the disease vs the effect of treatment. Metabolism (2006) 55(1):90–5. 10.1016/j.metabol.2005.07.012 [DOI] [PubMed] [Google Scholar]
- 53.Bernal E, Masia M, Padilla S, Gutierrez F. High-density lipoprotein cholesterol in HIV-infected patients: evidence for an association with HIV-1 viral load, antiretroviral therapy status, and regimen composition. AIDS Patient Care STDS (2008) 22(7):569–75. 10.1089/apc.2007.0186 [DOI] [PubMed] [Google Scholar]
- 54.Shen Y, Wang J, Wang Z, Qi T, Song W, Tang Y, et al. Prevalence of dyslipidemia among antiretroviral-naive HIV-infected individuals in China. Medicine (2015) 94(48):e2201. 10.1097/MD.0000000000002201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feingold KR, Soued M, Serio MK, Moser AH, Dinarello CA, Grunfeld C. Multiple cytokines stimulate hepatic lipid synthesis in vivo. Endocrinology (1989) 125(1):267–74. 10.1210/endo-125-1-267 [DOI] [PubMed] [Google Scholar]
- 56.Zangerle R, Sarcletti M, Gallati H, Reibnegger G, Wachter H, Fuchs D. Decreased plasma concentrations of HDL cholesterol in HIV-infected individuals are associated with immune activation. J Acquir Immune Defic Syndr (1994) 7(11):1149–56. [PubMed] [Google Scholar]
- 57.Li XA, Titlow WB, Jackson BA, Giltiay N, Nikolova-Karakashian M, Uittenbogaard A, et al. High density lipoprotein binding to scavenger receptor, Class B, type I activates endothelial nitric-oxide synthase in a ceramide-dependent manner. J Biol Chem (2002) 277(13):11058–63. 10.1074/jbc.M110985200 [DOI] [PubMed] [Google Scholar]
- 58.Pontillo A, Oshiro TM, Girardelli M, Kamada AJ, Crovella S, Duarte AJ. Polymorphisms in inflammasome’ genes and susceptibility to HIV-1 infection. J Acquir Immune Defic Syndr (2012) 59(2):121–5. 10.1097/QAI.0b013e3182392ebe [DOI] [PubMed] [Google Scholar]
- 59.Pontillo A, Carvalho MS, Kamada AJ, Moura R, Schindler HC, Duarte AJ, et al. Susceptibility to Mycobacterium tuberculosis infection in HIV-positive patients is associated with CARD8 genetic variant. J Acquir Immune Defic Syndr (2013) 63(2):147–51. 10.1097/QAI.0b013e31828f93bb [DOI] [PubMed] [Google Scholar]
- 60.Chivero ET, Guo ML, Periyasamy P, Liao K, Callen SE, Buch S. HIV-1 Tat primes and activates microglial NLRP3 inflammasome-mediated neuroinflammation. J Neurosci (2017) 37(13):3599–609. 10.1523/JNEUROSCI.3045-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haque S, Lan X, Wen H, Lederman R, Chawla A, Attia M, et al. HIV promotes NLRP3 inflammasome complex activation in murine HIV-associated nephropathy. Am J Pathol (2016) 186(2):347–58. 10.1016/j.ajpath.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mamik MK, Hui E, Branton WG, McKenzie BA, Chisholm J, Cohen EA, et al. HIV-1 viral protein R activates NLRP3 inflammasome in microglia: implications for HIV-1 associated neuroinflammation. J Neuroimmune Pharmacol (2016) 12(2):233–48. 10.1007/s11481-016-9708-3 [DOI] [PubMed] [Google Scholar]
- 63.Guo H, Gao J, Taxman DJ, Ting JP, Su L. HIV-1 infection induces interleukin-1beta production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytes. J Biol Chem (2014) 289(31):21716–26. 10.1074/jbc.M114.566620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kreuzer KA, Dayer JM, Rockstroh JK, Sauerbruch T, Spengler U. The IL-1 system in HIV infection: peripheral concentrations of IL-1beta, IL-1 receptor antagonist and soluble IL-1 receptor type II. Clin Exp Immunol (1997) 109(1):54–8. 10.1046/j.1365-2249.1997.4181315.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med (2008) 5(10):e203. 10.1371/journal.pmed.0050203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res (2004) 45(7):1169–96. 10.1194/jlr.R300019-JLR200 [DOI] [PubMed] [Google Scholar]
- 67.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature (2010) 464(7293):1357–61. 10.1038/nature08938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schroder K, Tschopp J. The inflammasomes. Cell (2010) 140(6):821–32. 10.1016/j.cell.2010.01.040 [DOI] [PubMed] [Google Scholar]
- 69.Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe (2010) 8(1):44–54. 10.1016/j.chom.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 70.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol (2009) 7(2):99–109. 10.1038/nrmicro2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature (2014) 505(7484):509–14. 10.1038/nature12940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rangaswamy KS. Correlation between High-density Lipoprotein Cholesterol Level and CD4 Cell Count in HIV Patients on NNRTI-Based ART Regimen at Tertiary Care Hospital in Mysuru. Int J Sci Stud (2017) 5(3):150–4. 10.17354/ijss/2017/286 [DOI] [Google Scholar]
- 73.Jiang Y, Wang M, Huang K, Zhang Z, Shao N, Zhang Y, et al. Oxidized low-density lipoprotein induces secretion of interleukin-1beta by macrophages via reactive oxygen species-dependent NLRP3 inflammasome activation. Biochem Biophys Res Commun (2012) 425(2):121–6. 10.1016/j.bbrc.2012.07.011 [DOI] [PubMed] [Google Scholar]
- 74.Samstad EO, Niyonzima N, Nymo S, Aune MH, Ryan L, Bakke SS, et al. Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release. J Immunol (2014) 192(6):2837–45. 10.4049/jimmunol.1302484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Niyonzima N, Samstad EO, Aune MH, Ryan L, Bakke SS, Rokstad AM, et al. Reconstituted high-density lipoprotein attenuates cholesterol crystal-induced inflammatory responses by reducing complement activation. J Immunol (2015) 195(1):257–64. 10.4049/jimmunol.1403044 [DOI] [PubMed] [Google Scholar]
- 76.Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Krankel N, et al. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of toll-like receptor-2. Immunity (2013) 38(4):754–68. 10.1016/j.immuni.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 77.Duro M, Sarmento-Castro R, Almeida C, Medeiros R, Rebelo I. Lipid profile changes by high activity anti-retroviral therapy. Clin Biochem (2013) 46(9):740–4. 10.1016/j.clinbiochem.2012.12.017 [DOI] [PubMed] [Google Scholar]
- 78.Zangerle R, Widner B, Quirchmair G, Neurauter G, Sarcletti M, Fuchs D. Effective antiretroviral therapy reduces degradation of tryptophan in patients with HIV-1 infection. Clin Immunol (2002) 104(3):242–7. 10.1006/clim.2002.5231 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Viral load and CD4+ T-cells counts according to high-density lipoprotein (HDL) levels in human immunodeficiency virus type 1 (HIV-1) (HIV-1)-infected individuals. HIV-1-infected individuals were divided according to HDL levels (normal: >40 mg/dL and low: <40 mg/dL) and the viral load (A) and the CD4+ T-cells counts (B) were analyzed. The statistical comparison was made using the Mann–Whitney U tests with a 95% confidence level. Significant differences are represented in the upper part of this figure (*p < 0.05 and **p < 0.01).