Abstract
In non-small cell lung cancer (NSCLC) with an epidermal growth factor receptor (EGFR) mutation, 50%–65% of cases acquire resistance after treatment with EGFR-tyrosine kinase inhibitors (EGFR-TKIs) because of an EGFR T790M point mutation and 3%–14% of these cases transformed to small cell lung cancer (SCLC). Generally, the EGFR T790M secondary mutation develops with ongoing ATP competitive inhibition. We present a case of a 76-year-old woman with lung adenocarcinoma harboring an EGFR-L858R mutation who received first-line gefitinib and developed SCLC transformation. She was administered several chemotherapy agents, including a platinum doublet. The primary lesion that showed SCLC transformation had reconverted to adenocarcinoma with EGFR L858R and T790M mutations at the time of a second re-biopsy. Therefore, she was administered osimertinib, which resulted in clinical remission. This case suggested that serial biopsies are necessary even after SCLC transformation.
Keywords: NSCLC, EGFR mutation, SCLC transformation, T790M, Osimertinib
1. Introduction
Lung cancer remains a principal cause of death worldwide. However, there has been remarkable progress in the development of treatment strategies for lung cancer, particularly adenocarcinoma, over the past 15 years. Although epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) are key drugs for EGFR mutation-positive non-small cell lung cancers (NSCLCs), most patients become resistant to the drugs within 1 year, which has become an important issue. Several resistance mechanisms were recently clarified, including an EGFR T790M secondary mutation, small cell lung cancer (SCLC) transformation, and activation of bypass signaling [1,2]. Here, we report a case with an EGFR T790M mutation detected during chemotherapy for SCLC transformation of gefitinib resistance that responded to osimertinib.
2. Case
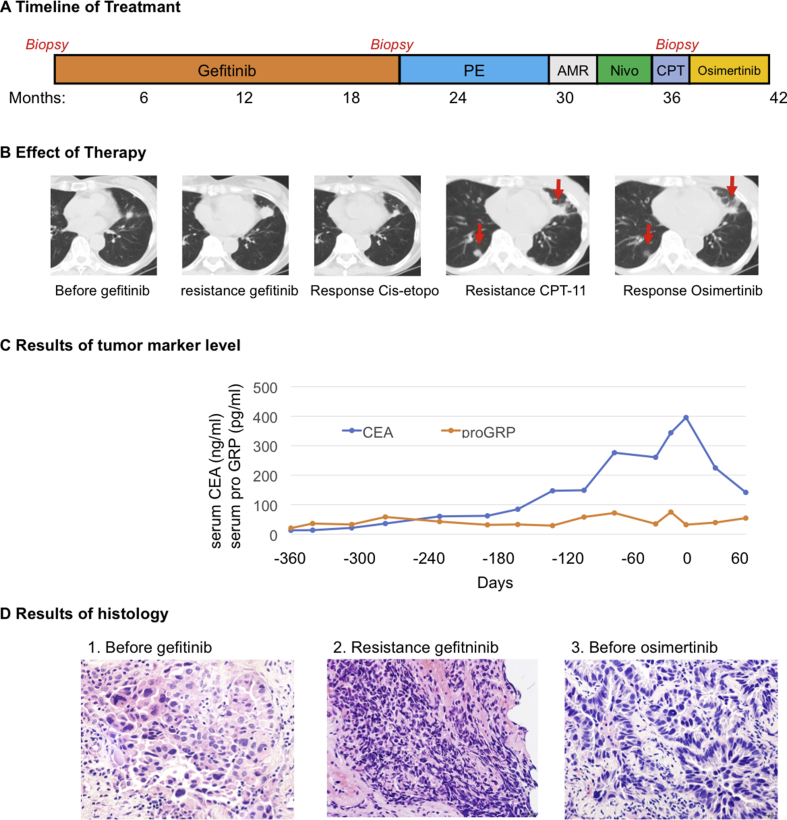
A 76-year-old woman visited The Cancer Institute Hospital of Japanese Foundation for Cancer Research because of left-side lateral chest pain. Computed tomography (CT) showed a tumor shadow in her left lung. After several examinations and a tumor biopsy, progressive lung adenocarcinoma harboring an EGFR-L858R mutation was diagnosed. Gefitinib was administered as a first-line treatment, which had a clinically significant response lasting for 20 months. CT performed again revealed re-progression of the primary lesion in the left upper lobe. A re-biopsy of the primary lesion during gefitinib treatment showed SCLC transformation, which was supported by positive immunohistochemical (IHC) staining of the neural cell adhesion molecule (NCAM) and synaptophysin, with an EGFR L858R mutation, but not T790M (Table 1). After discontinuation of gefitinib, four cycles of cisplatin and etoposide were started as a second-line therapy, which resulted in a response that lasted for 5 months. Then, because re-progression was observed during the second-line treatment, chemotherapy and immunotherapy were initiated with two cycles of amrubicin, two cycles of nivolumab, and two cycles of CPT-11 administered over a 6-month period (Fig. 1A). A follow-up CT showed worsening of the primary lesion (Fig. 1B) accompanied by an increase in plasma carcinoembryonic antigen (CEA), which is a known tumor marker of adenocarcinoma. A second re-biopsy via bronchoscopy of the primary lesion showed adenocarcinoma, which was supported by negative IHC staining of NCAM, synaptophysin, and chromogranin A. At this point, mutation analysis revealed the presence of EGFR L858R and T790M mutations. Thus, osimertinib was started at a dose of 80 mg per day, which resulted in rapid and dramatic clinical improvement with a decrease in serum CEA levels (Fig. 1C). Serial CT after initiation of osimertinib showed a partial response that lasted almost 3 months (Fig. 1B).
Table 1.
Tumor biopsy tissue finding of Histology, IHC stain and EGFR mutation status.
| Before Gefitinib | Resistance Gefitinib | Before Osimertinib | |
|---|---|---|---|
| Histology | Adenocarcinoma | Small cell carcinoma | Adenocarcinoma |
| IHC stain | |||
| TTF-1 (clone 8G7G3/1, DAKO) | + | + | + |
| NCAM (clone 1B6, Leica) | – | + | – |
| SYN (clone 27G12, Leica) | – | + | – |
| CGA (clone DAK-A3, DAKO) | NE | NE | – |
| EGFR mutation status | |||
| L858R | + | + | + |
| T790M | – | – | + |
IHC Immunohistochemical, TTF-1 Thyroid Transcription Factor-1, NCAM Neural cell adhesion molecule, SYN Synaptophysin, CGA Chromogranin A, EGFR epidermal growth factor receptor, NE not evaluated.
Fig. 1.
Panel A shows the series of treatments the patient received for metastatic non-small cell lung cancer as well as the duration of each treatment. Panel B shows computed tomographic images of the primary left upper lung tumor before gefitinib administration, when the disease subsequently relapsed at 20 months, and during the response to combination chemotherapy of cisplatin and etoposide. After treatment with several chemotherapy agents and immunotherapy sequentially, the primary lesion enlarged and a metastatic lesion appeared in right lower lung after CPT-11 therapy (marked with arrows). A radiologic response to osimertinib was noted after 4 weeks of treatment. Panel C shows serial monitoring of CEA levels and pro-GRP levels before and after retreatment with osimertinib, which was initiated at a dose of 80 mg per day on day 1. The CEA level decreased with tumor shrinkage. Panel D shows the results of histology. (1) A tissue section from pleural dissemination (hematoxylin and eosin stained, magnification ×400) at first diagnosis shows metastatic adenocarcinoma. (2) Tissue section of re-biopsy from the left upper lung after resistance to gefitinib (hematoxylin and eosin stained, magnification ×400) shows small cell carcinoma. (3) Tissue section of second re-biopsy from the same lesion at resistance to sequential chemotherapy for SCLC (hematoxylin and eosin stained, magnification ×400) shows adenocarcinoma.
3. Discussion
Previous reports have identified several mechanisms of acquired resistance to EGFR-TKIs. The most frequent acquired resistance is the EGFR T790M point mutation, which occurs in 50%–65% of resistant cases, and SCLC transformation in 3%–14% [3,4]. Generally, a T790M secondary mutation develops with ongoing ATP competitive inhibition or T790M, which can be detected in a small proportion of tumors before treatment with EGFR-TKIs, with increasing frequency post-treatment, consistent with ongoing selection of T790M clones upon EGFR-TKI [5]. Third-generation TKIs, such as osimertinib, demonstrated efficacy for patients who developed resistance to first or second generation EGFR-TKIs due to T790M mutation [6].
Several case reports have described SCLC transformation from adenocarcinoma with an EGFR mutation after resistance to EGFR-TKI. However, the mechanism of SCLC transformation remains unclear. Oser et al. proposed two hypotheses of SCLC transformation [2]. The first is that type II alveolar cells, the origin of some EGFR-mutant adenocarcinomas, also have the potential to become SCLC, which was discovered in a pre-clinical study. The second is a combined SCLC and NSCLC histology at first diagnosis, where each histology becomes dominant alternately in response to anti-cancer drugs. SCLC with an EGFR mutation may be sensitive to standard chemotherapy [4].
Our patient had an EGFR L858R mutation and received multiple treatments, including first generation EGFR-TKIs, chemotherapy, and third generation EGFR-TKIs. Several types of EGFR resistance developed during treatment. The first resistance was SCLC transformation with an L858R mutation during gefitinib treatment and the second resistance was an unexpected EGFR-T790M mutation identified during chemotherapy. This acquired resistance conferred sensitivity to osimertinib, however its short response duration might have been cause by a dominant histological change like a pendulum. Over the course of the treatment, serial repeated biopsies provided clinically relevant and invaluable insights. Although the developing mechanism of EGFR T790M mutation without last-minute EGFR-TKI exposure is unclear, we considered that EGFR T790M mutated cells might have been involved in SCLC transformation at first resistance, but this mutation could not be detected because of the small biopsy sample and was detected after chemotherapy. Indeed, the phenotype of the second re-biopsy sample again changed to adenocarcinoma. However, it remains unclear why the proportion of cells with the EGFR T790M mutation had increased without ongoing selection of T790M clones upon EGFR-TKI.
This case suggested that serial biopsies are necessary to choose an effective treatment strategy and testing for the T790M mutation should be performed even after SCLC transformation.
Conflict of interest
MN received research funding from Novartis, ONO Pharmaceutical, Chugai Pharmaceutical, Bristol-Myers Squibb, TAIHO Pharmaceutical, Eli Lilly, Pfizer, Astellas Pharma, and AstraZeneca and received honorariums from Pfizer, Bristol-Myers Squibb, ONO Pharmaceutical, Chugai Pharmaceutical, Eli Lilly, TAIHO Pharmaceutical, and AstraZeneca.
AH received research funding from Chugai Pharma, Quintiles, MSD, and Daiichi Sankyo and received honorariums from Pfizer, Chugai Pharma, Eli Lilly, and AstraZeneca.
NY is a consultant of Chugai Pharmaceutical.
All other authors declare no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Dorantes-Heredia R. Histopathological transformation to small-cell lung carcinoma in non-small cell lung carcinoma tumors. Transl. Lung Cancer Res. 2016;5:401–412. doi: 10.21037/tlcr.2016.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oser M.G. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16:e165–172. doi: 10.1016/S1470-2045(14)71180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sequist L.V. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3002003. 75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu H.A. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camidge D.R. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat. Rev. Clin. Oncol. 2014;11:473–481. doi: 10.1038/nrclinonc.2014.104. [DOI] [PubMed] [Google Scholar]
- 6.Janne P.A. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N. Engl. J. Med. 2015;372 doi: 10.1056/NEJMoa1411817. 1689–1689. [DOI] [PubMed] [Google Scholar]