Abstract
Graves ophthalmopathy (GO) is an autoimmune disorder and the most frequent extrathyroidal manifestation of Graves’ disease. GO is an inflammatory process leading to an increased volume of the extraocular muscles and orbital connective and adipose tissues associated with multiple histopathological changes. Despite recent progress in the understanding of its pathogenesis, GO often remains a major diagnostic and therapeutic challenge. It has become increasingly important to classify patients into categories based on disease severity and activity. Low doses of radiotherapy (RT) have demonstrated a benefit in the treatment of moderate-to-severe GO with very few side effects. New RT techniques deliver a more conformal dose distribution to the target and decrease the dose to normal healthy tissue minimizing the risk of side effects. In this review we briefly analyzed the pathogenesis of GO and discussed the most relevant therapeutic approaches, with particular emphasis in the new RT technics. Appropriately designed and powered clinical studies are necessary to determine the most effective treatment with the lowest risk of side effects.
Keywords: Graves’ opthalmopathy, Radiotherapy, Toxicity
Introduction
Graves' disease (GD) is an autoimmune disorder involving the thyroid gland. Graves ophthalmopathy (GO) is an autoimmune condition of the orbit that occurs in 25–50% of patients with GD. GO is the most prevalent extra-thyroidal manifestation and may develop regardless of the presence of hyperthyroidism. In 3–5% of patients, GO evolves into a severe form, significantly impairing the quality of life of the patients 1.
GO is associated with multiple histopathological changes which induce inflammatory process leading to an increased volume of the extraocular muscles and orbital connective and adipose tissues 2. Pathogenesis is related to the activation of T lymphocytes (mostly CD4+) that invade the orbit and release cytokines, usually as a response to the presence of circulating autoantibodies that bind to and stimulate the thyroid hormone receptor (TSHR). These cytokines act in a paracrine manner and induce the activation of fibroblasts due to an increase in the production of the hydrophilic glycosaminoglycans (GAGs) in the orbital tissue. This excessive secretion of GAGs together with the lymphocyte infiltration results in an osmotic pressure increase, significant tissue oedema, and the clinical ophthalmopathy 3, 4.
Classification is based on severity and activity and determines the therapeutic approach 5, but activity and severity are not synonymous.
Severity is defined by the degree of functional deficit at any stage of the disease. The European Group of Graves’ Orbitopathy (EUGOGO) suggests classifying severity into three categories, based on the subjective symptoms and objective signs 6:
-
1.
Mild: minor impact on daily life insufficient to justify immunosuppressive or surgical treatment. Patients have one or more of the following signs/symptoms: minor lid retraction (<2 mm), mild soft tissue involvement, exophthalmos > 3 mm above normal for race and gender, transient or no diplopia, corneal exposure responsive to lubricants.
-
2.
Moderate-to-severe: Not sight-threatening but sufficient impact on daily life to justify the risks of immunosuppression (if active) or surgical intervention (if inactive). Patients have one or more of the following signs: lid retraction ≥ 2 mm, moderate or severe soft tissue involvement, exophthalmos ≥ 3 mm above normal for race and gender, inconstant or constant diplopia.
-
3.
Sight-threatening: patients with dysthyroid optic neuropathy and/or corneal breakdown. This category warrants immediate intervention.
Activity refers to the presence of inflammatory signs and is measured using the Clinical Activity Score (CAS) based on the classical features of inflammation. To be classified as active, at least 6 of 10 items (Table 1) should be present 6.
Table 1.
Items of the clinical activity score.
| Clinical activity score items |
|---|
| Painful, oppressive feeling on or behind the globe, during the last 4 weeks |
| Pain on attempted up, side or down gaze, during the last 4 weeks |
| Redness of the eyelid |
| Diffuse redness of the conjunctiva, covering at least one quadrant |
| Swelling of the eyelid |
| Chemosis |
| Swollen caruncle |
| Increase of proptosis of ≥2 mm during a period of 1–3 months |
| Decrease in eye movements in any direction ≥5o during a period of 1–3 months |
| Decrease of visual acuity of ≥1 line on the Snellen chart during a period of 1–3 months |
Treatment
Treatment is based on the severity and activity of GO. The approach is suggested by the EUGOGO consensus statement 5.
Mild forms of GO may improve spontaneously and simple follow up and symptom management is usually sufficient. Lubricants and ointments are recommended to help symptoms. Glucocorticoids and radiotherapy (RT) are usually not recommended. In a multicenter, randomized, double blind, placebo-controlled trial performed by EUGOGO, it was reported that a 6-month course of selenium (sodium selenite 100 mg twice daily) is associated with a significant improvement in quality of life and GO symptoms, and a lower rate of progression to more severe forms without adverse effects 4.
The therapeutic approach in patients with moderate-to-severe GO depends on whether the disease is “active” or “inactive”. In patients with active disease an immunosuppressive or anti-inflammatory treatment, either systemic therapy and/or RT should be offered. In contrast, in patients with inactive GO rehabilitative surgery should be considered.
Finally, in patients with sight threatening GO, first line treatment is based on immunosuppressive or anti-inflammatory therapy but if there is a poor response or the disease is inactive, immediate surgical intervention is warranted.
Surgery
There are two surgical options, orbital decompression and corrective surgery for eyelid retraction and restrictive myopathy. Actual indications for surgical treatment are optic neuropathy, persistent inflammation or congestion refractory to steroid treatment, desire to reduce excess proptosis and cosmetic discomfort. Surgeons must decide whether or not to remove orbital bony walls and fat, as well as the amount and location to be removed 4.
Systemic therapy
First line treatment of active moderate to severe GO is systemic glucocorticoids, based on their anti-inflammatory and immunosuppressive effects. Intravenous glucocorticoids (ivGC) have a higher response rate and are better tolerated than oral. Glucocorticoids, however, are not devoid of adverse events (Cushingoid features, weight gain, hypertension, diabetes, etc) and in patients with planned glucocorticoids treatment longer than three months, antiosteoporotic therapy should be considered.
Other medical options that have a proven benefit include Cyclosporine, the most widely immunosuppressant drug used, that combined with oral prednisone was more effective than oral GC alone in a clinical trial conducted by Kahaly 4. Also other drugs such as Etanercept, a TNF-a inhibitor, used in the treatment of autoinmune diseases, based on the capacity to regulate the immune-inflammatory response of many organ systems, has shown some activity in a small-uncontrolled study 1. Rituximab, a chimeric monoclonal antibody against the CD 20 antigen, is under evaluation but in some studies shows a similar benefit to ivGC4. Other agents such as Somatostatin, a peptide hormone that regulates the endocrine system and inhibits numerous secondary hormones, had no proven benefit in randomized clinical trials 1.
Radiotherapy
Rationale: anti-Inflammatory and Inmunosupresor effect of RT
The rationale for using RT in GO is based on its modulating role of inflammatory response in irradiated tissues. Therefore this could be considering an alternative to systemic anti-inflammatory therapies 7. Although RT induces the production of pro-inflammatory cytokines and leads to an inflammatory response in irradiated tissues, however, RT administrated at low doses (LD-RT) modulates the inflammatory response, producing an anti-inflammatory effect. The inflammatory response of LD-RT is a tightly regulated process that involves a sequence of leukocyte-endothelium interactions; called rolling, adhesion, and migration to the interstitial space. In a first step, leukocytes are activated through the action of local inflammatory mediators. This activation allows the attachment to the endothelial cells and the migration into the interstitial space. The next step is the accumulation of a variety of immunocompetent cells such as lymphocytes, granulocytes, and monocytes/macrophages. Whereas some cytokines, such as interleukin (IL)-1, tumour necrosis factor-á (TNF-á), IL-6, IL-8, and IL-12, have pro-inflammatory effects, others have anti-inflammatory effects, as it occurs with transforming growth factor (TGF)- â1, IL-10, and IL-4.
The hypotheses that explain the anti-inflammatory mechanisms of LD-RT, include decreased adhesion leukocyte-endothelial cells, induction of apoptosis in the cells that comprise the inflammatory infiltrate, decreased expression of adhesion molecules (P-, L-, E-selectins, ICAM, VCAM), decreased iNOS that results in a decrease in nitric oxide NO and reactive oxygen species ROS, increased activation of nuclear factor-kappa B (NF-êB) and increased expression of anti-inflammatory cytokines (IL-10, TGF-â1) 8.
LD-RT also induces decreased levels of pro-inflammatory cytokines such as TNF-á, interleukin-1 beta (IL-1â), and iNOS at 24 and 48 h and increased levels of HO-1 and inducible heat shock protein 70 (HSP70). Therefore, clinical practice would likely require LD-RT treatments with fractions every 48–72 h.
In vitro studies suggest that the potent anti-inflammatory effect of LD-RT is produced through inhibition of leukocyte–endothelium interactions at doses < 0.7 Gy. This effect was observed in the first 48 h after irradiation and shows an inverse correlation with concentrations of TGF-â1 and activation of NF-KB.
In vivo animal studies show LD-RT led to an improvement of clinical symptoms and insigns of inflammation and pain. The efficacy of LD-RT has been demonstrated in the treatment of inflammatory diseases, such as osteoarthritis, humeral epicondylitis and scapular–humeral periarthritis. The optimal doses of LD-RT ranged from 0.5–1.5 Gy 8.
Radiation dose and fractionation -What do we know?
The most common fractionation is a cumulative dose of 20 Gy, in 10 daily doses over a two week period. Nevertheless there are some studies that demonstrate that lower doses of 10 Gy fractionated in 1 Gy once a week over 10 weeks have shown to be equally effective 9. In addition other protocols have also been employed including 10 Gy in 10 daily doses over a two week period, 20 Gy fractionated in 1 Gy once a week over 20 weeks and16-18 Gy in 8–10 daily doses over a two week period 10, 11. For re-irradiation some centers use a dose of 10 Gy in 5 fractions 12 (Table 2).
Table 2.
Comparison of the principal fractionations for GO.
| Author (year) | N | Doses | Fractionation | Response | Follow-up | Toxicity |
|---|---|---|---|---|---|---|
| Cardoso (2012) 9 | 18 | 10 Gy | 1 Gy/d-w 10 weeks | 100% | 18 months | 0% |
| Schaefer (2002) 13 | 250 | 16.8–24 Gy | 2–1.6 Gy/d 2 weeks |
43.6% CR 41.5% PR |
31 years | No reported |
| Matthiesen (2012) 14 | 211 | 20 Gy | 2 Gy/d 2 weeks | 44.5% CR 39.7% PR |
11 months | 12% |
| Kahaly (2000) 15 | 22 | 20 Gy | 2 Gy/d 2 weeks | 55% | 6 months | 36% |
| Kahaly (2000) 15 | 18 | 20 Gy | 1 Gy/d-w 20 weeks | 67% | 6 months | 0% |
| Kahaly (2000)15 | 22 | 10 Gy | 1 Gy/d 2 weeks | 59% | 6 months | 18% |
d-w: One day per week; CR: complete response; PR: Partial response.
When we look carefully to the results reported on Table 2, we observe that one of the biggest difficulties to compare the response rate is the different criteria use to evaluate this rate. The two biggest studies 13, 14, both with more than 200 patients, showed a similar rate of responses in both the classification of the response was based on the patient’s subjective symptoms. The two other studies 9, 15 were based on the response rate to objective symptoms. The results observed in the study of Cardoso et al. are in line with the expectations because, as mentioned above, GO is not a tumour but an inflammatory disease and, therefore, needs anti-inflammatory doses, not high doses of RT as is the case with tumour tissue. Low doses of RT rarely cause side effects, especially with this fractionation.
Although RT is well tolerated and safe 13 its use is rather limited in the management of benign disease due to the fear of toxicity and the risk of radiation induced tumours. The most frequent toxicity is chronic dry eyes (12%) 16. The risk of other side effects, such as cataract is small (1%) with doses of 20 Gy and is reduced further with fractionation. Radiation retinopathy is extremely rare. RT should not be used in patients younger than 35 years (potential long term carcinogenic risk), and in patients with severe hypertension or diabetic retinopathy (possible worsening of pre-existing retinal damage) 14, 15. Calculation using risk factors presently known reveal a theoretical risk of radiation-induced cancer of 1–4 % 17. However in a long term follow-up study of 205 patients with GO treated with RT 18 there are no differences in the risk of developing cancer after a median follow-up of 31 years, compared with a cohort of patients that didn′t receive RT. All this studies was done with the standard fractionation of 20 Gy in 10 daily doses over a two week period.
Clinical results
RT has demonstrated benefit in the management of GO in two randomized clinical trials which compared orbital RT versus placebo (sham irradiation of the orbit). A better response was found in irradiated patients, mainly in those with impaired eye motility. In one of these studies, there was an improvement in the grade of diplopia in 60% of irradiated patients versus 31% in the placebo group 19. The second study shows a benefit in improving both eye muscle motility and decreasing the severity of diplopia in 52% of irradiated patients vs. 27% in the sham-irradiated group 13.
In patients with active disease the efficacy of RT is higher when it is given within the first year of developing signs and symptoms 20. This is probably because in patients with long-standing GO, fibrosis has already occurred.
A double-blind randomized clinical trial was designed to compare glucocorticoids as the standard of care versus RT (a 3-month course of oral prednisone and sham irradiation vs. retrobulbar irradiation (20 Gy) and placebo capsules). This study proved equal effectiveness in both groups. However the RT group had less side effects, both in severity and frequency 8. Another randomized clinical trial plus two other studies showed that the combination of RT and oral glucocorticoids was more effective than either treatment alone 21, 22, 23, 24 (Table 3). In future studies standardize and meticulous report of the toxicity is necessary in order to know the real incidence of side effects and which one of them are consequence of the RT treatment.
Table 3.
Principal studies that demonstrate the benefit of radiotherapy.
| Author (year) | N | Study (patients) | Treatment response | Side effects | Follow up |
|---|---|---|---|---|---|
| Mourits (2000) 10 | 60 | RT (30) No RT (30) |
60% 31% |
Not reported | 6 months |
| Prummel (2004) 11 | 88 | RT (44) No RT (44) |
52 % 27% |
Not reported | 12 months |
| Bartalena (1983) 21 | 48 | RT + GC (36) GC (12) |
72% 33% |
Not reported | 26 months |
| Marcocci (1991) 22 | 30 | RT + GC (15) RT (15) |
69% 38% |
Not reported | 6–9 months |
| Prummel (1993) 23 | 28 | GC (14) RT (14) |
48% 50% |
78.57% 7.14% |
6 months |
| Marcocci (2001) 24 | 82 | RT + ivGC (41) RT + oralGC (41) |
87.8% 63.4% |
56.1% 85.4% |
12 months |
RT: Radiotherapy; GC: Glucocorticoids.
New radiation techniques
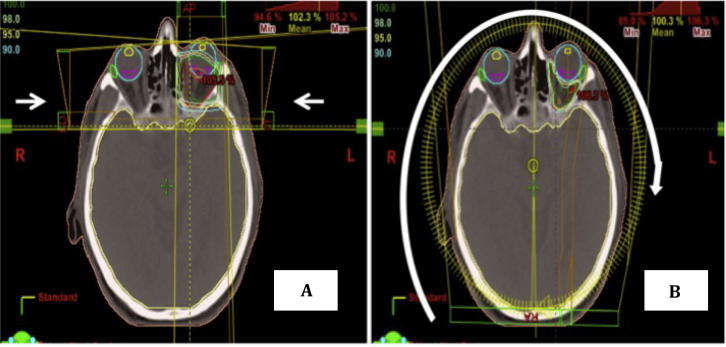
The treatment volume in the GO is the retrobulbar area. The structures at risk are the retina, lacrimal gland and lens. Until now current RT treatment consists of three-dimensional conformal planning using parallel-opposed lateral beams. The anterior border of the field is at the lateral canthus of the eye, and the posterior border at the edge of the sphenoid sinus. Superiorly the border is at the roof of the orbit, and inferiorly it includes the floor of the orbit. The normal structures are protected using shielding. Fields are usually angled 5° to avoid dose to the lens (Fig. 1). With these techniques all the structures encompassed inside the field receive the same amount of radiation.
Fig. 1.
Dosimetry for Graves’ disease showing the fields are usually angled 50° to avoid dose to the lens with three dimensional radiotherapy (Fig. 1A) versus volumetric modulated arc therapy (Fig. 1B).
New techniques of RT, such as volumetric modulated arc therapy (VMAT) or Intensity-modulated radiation therapy (IMRT), give radiation therapists the ability to “sculpt” the edges of the target, minimizing the damage to adjacent healthy tissue. Radiation targets are irregularly shaped and conventional radiation treatments deliver radiation in straight lines. This means that, while the whole target is treated, any healthy tissue close to the target may receive radiation as well. VMAT technology allows delivery of radiation to a target with more precision and accuracy, potentially resulting in fewer side effects while achieving the same high response rates. With VMAT the treatment delivery time is also faster than with conventional RT or IMRT. Shortened treatment times improve tolerability of the treatments and reduce the chance of error due to intra-fraction motion.
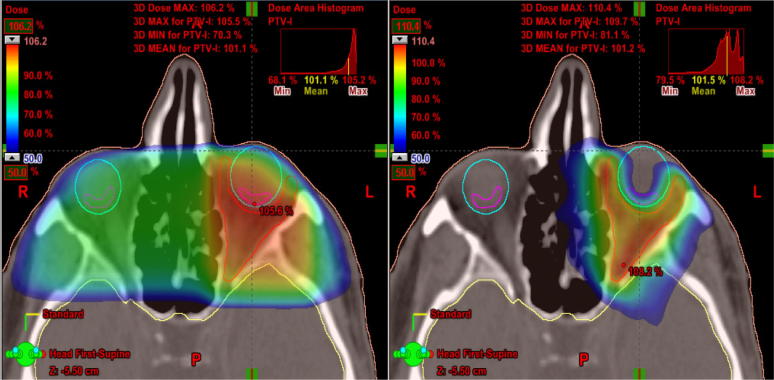
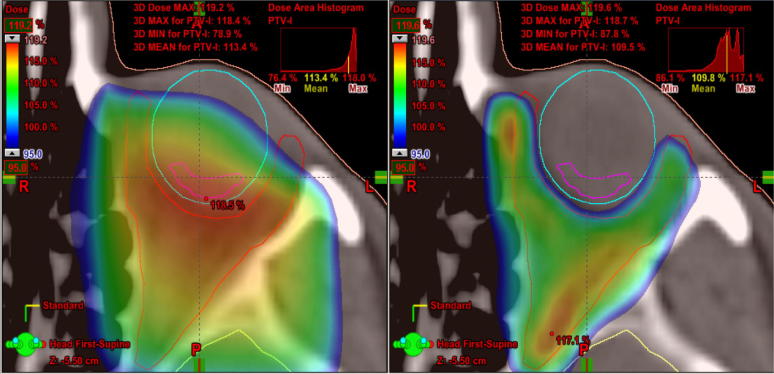
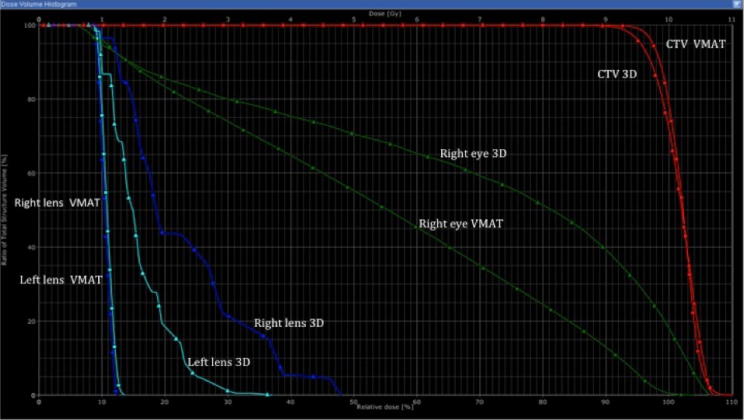
On Fig. 2, Fig. 3 we can appreciate the capacity of VMAT to adjust the doses of radiation to the target preserving normal tissue. Fig. 2 shows how low doses of radiation are adapted to fit the target with greater sparing of the opposite globe and the lens of the GO-affected eye so that neither receives radiation. Fig. 3 only shows the high doses that provide a better sparing of the globe, including the retina close to the target. Fig. 4 shows a dose volume histogram that demonstrates how, with the new techniques, we can reduce the dose received by normal tissue such as the lens. A recent study proves how, with these new techniques of RT, there is more efficient target coverage, conformity, homogeneity, and dose sparing to surrounding structures, despite a slightly higher, but clinically negligible, dose to other structures 25. The reduction in dose to normal tissue potentially decreases the risk of developing side effects such as cataracts or radiation retinopathy.
Fig. 2.
Low doses of radiation are adapted to fit the target with greater sparing of the opposite globe and the lens of the Graves’ disease-affected eye with three dimensional radiotherapy (Fig. 2A) versus volumetric modulated arc therapy (Fig. 2B).
Fig. 3.
High doses that provide a better sparing of the globe, including the retina close to the target volume with three dimensional radiotherapy (Fig. 3A) versus volumetric modulated arc therapy (Fig. 3B).
Fig. 4.
Dose volume histogram that demonstrates the benefit with new techniques (volumetric modulated arc therapy (VMAT)) versus three dimensional radiotherapy in reduction the doses received by normal tissue such as the lens and eye.
Conclusion
RT has a demonstrated advantage in the management of GO particularly in the first stages of the disease. However, treatment with RT for benign disease continues to be infrequent due to the risk of toxicity and radiation induced tumours. The benefit of low doses of radiation and fractionation has been proven due to the anti-inflammatory effect on the disease course.
New radiation techniques and modalities are associated with both higher efficacy and lower toxicity. Moreover, the capacity of RT to induce anti-inflammatory response represent an attractive therapeutic opportunity for patients with moderate-to- severe GO, in order to avoid the toxicity of systemic therapies. Appropriately designed and implemented clinical studies are necessary to determine the most efficacious treatment with the lowest risk of side effects in GO. Also is necessary to create a homogeneous classification of the side effects to compare the studies in a suitable manner.
Conflicts of Interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
Contributor Information
Iñigo San Miguel, Email: isanmiguela@gmail.com.
Meritxell Arenas, Email: marenas@grupsagessa.com, meritxell.arenas@gmail.com.
Ruth Carmona, Email: rcarmona@ulpgc.es.
Joaquin Rutllan, Email: jrutllan@ulpgc.es.
Francisco Medina-Rivero, Email: fmedina@ulpgc.es.
Pedro Lara, Email: pedrocarlos.lara@ulpgc.es.
References
- 1.Stiebel-kalish H., Robenshtok E., Hasanreisoglu M., Ezrachi D., Shimon I., Leibovici L. Treatment modalities for Graves’ ophthalmopathy: systematic review and metaanalysis. J Clin Endocrinol Metab. 2009;94(8):2708–2716. doi: 10.1210/jc.2009-0376. [DOI] [PubMed] [Google Scholar]
- 2.Bahn R.S., Heufelder A.E. Pathogenesis of Graves' ophthalmopathy. N Engl J Med. 1993;329(20):1468–1475. doi: 10.1056/NEJM199311113292007. 11. [DOI] [PubMed] [Google Scholar]
- 3.Menconi, Marcocci C., Marinò M. Diagnosis and classification of Graves' disease. Autoimmun Rev. 2014;13(4–5):398–402. doi: 10.1016/j.autrev.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Marcocci C., Marinò M. Treatment of mild, moderate-to-severe and very severe Graves’ orbitopathy. Best Pract Res Clin Endocrinol Metab. 2012;26:325–337. doi: 10.1016/j.beem.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Micke O., Seegenschmiedt M.H. German working group on radiotherapy in germany. Consensus guidelines for radiation therapy of benign diseases: Int J Radiat Oncol Biol Phys. 2002;52(2):496–513. doi: 10.1016/s0360-3016(01)01814-4. [DOI] [PubMed] [Google Scholar]
- 6.Bartalena L., Baldeschi L., Dickinson A., Eckstein A., Kendall-Taylor P., Marcocci C. Consensus statement of the European Group on Graves’ Orbitopathy (EUGOGO) on management of Graves’ Orbitopathy. Eur J Endocrinol. 2008;158:273–285. doi: 10.1530/EJE-07-0666. [DOI] [PubMed] [Google Scholar]
- 7.Arenas M., Sabater S., Lara P.C., Rovirosa A., Biete A., Linares V. Radiotherapy for Grave’s disease. The possible role of low-dose radiotherapy. Rep Pract Oncol Radiother. 2016;21(3):213–218. doi: 10.1016/j.rpor.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arenas M., Sabater S., Hernández V., Rovirosa A., Lara P.C., Biete A. Anti-inflammatory effects of low-dose radiotherapy. Indications, dose, and radiobiological mechanisms involved. Strahlenther Onkol. 2012;88(11):975–8137. doi: 10.1007/s00066-012-0170-8. [DOI] [PubMed] [Google Scholar]
- 9.Cardoso C.C., Giordani A.J., Wolosker A.M., Souhami L., Manso P.G., Dias R.S. Protracted hypofractionated radiotherapy for Graves' ophthalmopathy: a pilot study of clinical and radiologic response. Int J Radiat Oncol Biol Phys. 2012;82(3):1285–1291. doi: 10.1016/j.ijrobp.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Mourits M.P., van Kempen-Harteveld M.L., García M.B., Koppeschaar H.P., Tick L., Terwee C.B. Radiotherapy for Graves' orbitopathy: randomised placebo-controlled study. Lancet. 2000;355(9214):1505–1509. doi: 10.1016/S0140-6736(00)02165-6. [DOI] [PubMed] [Google Scholar]
- 11.Prummel M.F., Terwee C.B., Gerding M.N., Baldeschi L., Mourits M.P., Blank L. A randomized controlled trial of orbital radiotherapy versus sham irradiation in patients with mild Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2004;89:15–20. doi: 10.1210/jc.2003-030809. [DOI] [PubMed] [Google Scholar]
- 12.Wiersinga W., Prummel M.F. Therapeutic controversies. Retrobulbar radiation in Graves' ophthalmopathy. J Clin Endocrinol Metab. 1995;80(2):345–347. doi: 10.1210/jcem.80.2.7852487. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer U., Hesselmann S., Micke O., Schueller P., Bruns F., Palma C. A long-term follow-up study after retro-orbital irradiation for Graves' ophthalmopathy. Int J Radiat Oncol Biol Phys. 2002;52(1):192–197. doi: 10.1016/s0360-3016(01)01754-0. [DOI] [PubMed] [Google Scholar]
- 14.Matthiesen C., Thompson J.S., Thompson D., Farris B., Wilkes B., Ahmad S. The efficacy of radiation therapy in the treatment of Graves' orbitopathy. Int J Radiat Oncol Biol Phys. 2012;82(1):117–123. doi: 10.1016/j.ijrobp.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 15.Kahaly G.J., Rösler H.P., Pitz S., Hommel G. Low- versus high-dose radiotherapy for Graves' ophthalmopathy: a randomized, single blind trial. J Clin Endocrinol Metab Jan. 2000;85(1):102–108. doi: 10.1210/jcem.85.1.6257. [DOI] [PubMed] [Google Scholar]
- 16.Perros P., Krassas G.E. Orbital irradiation for thyroid-associated orbitopathy: conventional dose, low dose or no dose? Clin Endocrinol (Oxf) 2002;56(6):689–691. doi: 10.1046/j.1365-2265.2002.01525.x. [DOI] [PubMed] [Google Scholar]
- 17.Prabhu R.S., Liebman L., Wojno T., Hayek B., Hall W.A., Crocker I. Clinical outcomes of radiotherapy as initial local therapy for Graves’ ophthalmopathy and predictors of the need for post-radiotherapy decompressive surgery. Radiat Oncol. 2012;7:95. doi: 10.1186/1748-717X-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhandare N, Mendenhall WM. A Literature Review of Late Complications of Radiation Therapy for Head and Neck Cancers: Incidence and Dose Response J Nucl Med Radiat Ther 2012;S:2.
- 19.Zygulska A. Radiotherapy in the treatment of Graves ophthalmopathy—to do it or not? Ocul Biol Dis Infor. 2010;3(1):1–11. doi: 10.1007/s12177-009-9021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeGroot L.J., Gorman C.A., Pinchera A., Bartalena L., Marcocci C., Wiersinga W.M. Therapeutic controversies. Retro-orbital radiation and radioactive iodide ablation of the thyroid may be good for Graves' ophthalmopathy. J Clin Endocrinol Metab. 1995;80(2):339–340. doi: 10.1210/jcem.80.2.7677823. [DOI] [PubMed] [Google Scholar]
- 21.Bartalena L., Marcocci C., Chiovato L., Laddaga M., Lepri G., Andreani D. Orbital cobalt irradiation combined with systemic corticosteroids for Graves’ ophthalmopathy: comparison with systemic corticosteroids alone. J Clin Endocrinol Metab. 1983;56:1139–1144. doi: 10.1210/jcem-56-6-1139. [DOI] [PubMed] [Google Scholar]
- 22.Marcocci C., Bartalena L., Bogazzi F., Bruno-Bossio G., Lepri A., Pinchera A. Orbital radiotherapy combined with high dose systemic glucocorticosteroids for Graves’ ophthalmopathy is more effective than radiotherapy alone: results of a prospective randomized study. J Endocrinol Invest. 1991;14:853–860. doi: 10.1007/BF03347943. [DOI] [PubMed] [Google Scholar]
- 23.Prummel M.F., Mourits M.P., Blank L., Berghout A., Koornneef L., Wiersinga W.M. Randomised double-blind trial of prednisone versus radiotherapy in Graves' ophthalmopathy. Lancet. 1993;342:949–954. doi: 10.1016/0140-6736(93)92001-a. [DOI] [PubMed] [Google Scholar]
- 24.Marcocci C., Bartalena L., Tanda M.L., Manetti L., Dell'Unto E., Rocchi R. Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves’ ophthalmopathy: results of a prospective, single-blind, randomized study. J Clin Endocrinol Metab. 2001;86:3562–3567. doi: 10.1210/jcem.86.8.7737. [DOI] [PubMed] [Google Scholar]
- 25.Lee V.H., Ng S.C., Choi C.W., Luk M.Y., Leung T.W., Au G.K. Comparative analysis of dosimetric parameters of three different radiation techniques for patients with Graves' ophthalmopathy treated with retro-orbital irradiation. Radiat Oncol. 2012;7:199. doi: 10.1186/1748-717X-7-199. [DOI] [PMC free article] [PubMed] [Google Scholar]