Abstract
Alpha-1 antitrypsin deficiency is an autosomal, codominant disorder caused by mutations of the SERPINA1 gene. This genetic disorder is mainly associated with development of pulmonary emphysema and/or chronic liver disease and cirrhosis.
Here we report a very rare alpha-1 antitrypsin Null Q0cairo homozygous mutation characterized by a complete absence of alpha-1 antitrypsin in the plasma, in a non-consanguineous Moroccan family. This mutation has been previously described in heterozygosis in only three cases worldwide: an Italian/Egyptian family and two Italian families (Zorzetto et al., 2005). The main clinical features in two members of this Moroccan family were the severity and precocity of bronchiectasis, quickly spreading and seriously limiting respiratory function and physical activity by the second decade of age. Moreover, the index case presented with many episodes of pulmonary infections concomitant with severe neutropenia. The third member of the family presented with ankylosing spondyloarthritis and developed panniculitis later but had no respiratory symptoms.
The presence of this alpha-1-antitrypsin Q0cairo homozygous mutation could explain the severity of clinical manifestations. Moreover, our observations highlight a great variability of clinical expression for the same mutation: early severe bronchiectasis, panniculitis, rheumatologic manifestations. This study further underlines the importance of genotyping by whole SERPINA1 gene sequencing in addition to serum alpha-1 antitrypsin determination, to enable detection of alpha-1 antitrypsin deficiency due to rare genotypes.
Keywords: Alpha-1 antitrypsin deficiency, Bronchiectasis, Panniculitis, Null mutation, SERPINA1 genotyping
Abbreviations
- AAT
alpha-1 antitrypsin
- AATD
alpha-1 antitrypsin deficiency
- ASA
ankylosing spondyloarthritis
- COPD
chronic obstructive pulmonary disease
- CT
computed tomography
- ERAD
Endoplasmic-reticulum-associated protein degradation
- FEV1
Forced expiratory volume in 1 second
- FVC
forced vital capacity
- FRA
functional respiratory assessment
- EIA
Elastase Inhibitory Activity
- IEF
IsoElectroFocusing
- IPJ
interphalangeal joint
1. Background
Human alpha-1 antitrypsin (AAT), also called alpha-1 protease inhibitor (alpha-1 Pi), is a 52 kDa circulating glycoprotein mainly synthetized by the liver. It operates as a serine protease inhibitor, mainly inhibiting elastase from neutrophils, and is a positive acute phase inflammatory protein. It diffuses into the lung interstitium and alveolar lining fluid, where it inactivates neutrophilic elastase, thereby protecting the lung tissue from protease-mediated damage [1,2].
AAT deficiency (AATD) is a rather common genetic disorder in the Caucasian population which remains significantly under-recognized. The AAT protein is encoded by the SERPINA1 gene, which is highly polymorphic and located on 14q31–32.3 locus of the chromosome 14. The most common allele, known as PI*M, is associated with normal AAT circulating levels and the most common deficient alleles are PI*S and PI*Z. Prevalence of AATD is about 1:2000–1:5000 individuals in Europe and 1:5000–1:10000 in North America [3]. A small number of studies supports the rarity of AATD induced by the PI*Z allele in the North African populations [[4], [5], [6]]. Null alleles, called Q0, can arise due to mutations that produce premature stop codons in the SERPINA1 gene resulting in very severe AATD.
Serum AAT concentration is generally measured by an immunoturbidimetric or immunonephelemetric method [7] with reference values ranging from 0.9 to 2.0 g/L (17–39 μmoL/L) in healthy adults. AATD is defined as severe for blood concentrations of AAT <0.57 g/L (11μmol/L) and as moderate when AAT levels range from 0.6 to 0.8 g/L (12–15 μmoL/L). Reduced blood and tissue concentrations of AAT, insufficient to protect tissues against proteases, mainly lead to lung tissue damage and emphysema in adults and to liver disease in children by way of cholestasis and cirrhosis [8,9]. Patients with AATD might also present with other inflammatory diseases such as vasculitis and necrotizing panniculitis [1,10].
The absence of an alpha-1 globulin peak in serum protein electrophoresis could suggest AATD [11]. This must be confirmed via serum AAT quantification and characterization by phenotyping using isoelectrofocusing electrophoresis (IEF) or genotyping if necessary. These analyses can now be performed on dried blood spot specimens [12].
2. Case presentation
2.1. Patient 1: index case
A 13-year-old girl was referred to hospital for respiratory distress and mild hemoptysis.
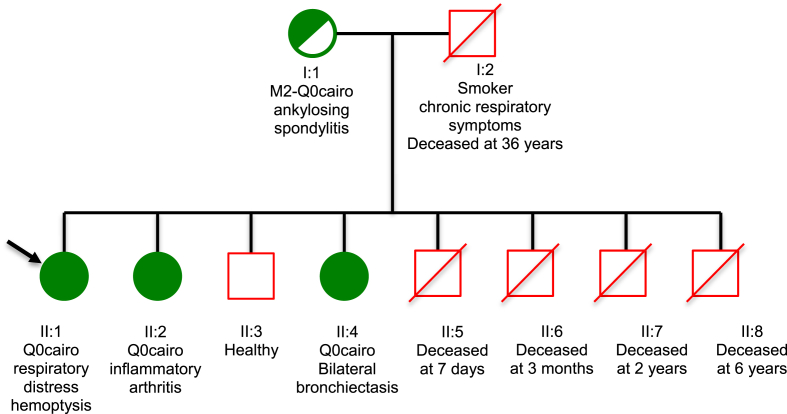
Her family history revealed that she was born from non-consanguineous parents. Her father, a smoker affected with a chronic cough and bronchorrhea with chronic bouts of bronchitis and sibilance, died at the age of 36 from respiratory distress. Four male siblings also died from respiratory distress at the age of 7 days, 3 months, 2 years and 6 years, respectively. A 10-year-old brother was healthy, while a 5-year-old sister was affected with chronic respiratory symptoms (patient 2) and an 11-year-old sister presented with joint and skin symptoms (patient 3). Her mother was suffering from ankylosing spondyloarthritis (ASA). The genealogical tree is shown in Fig. 1.
Fig. 1.
Genealogic tree of the Moroccan family with AATD (Q0cairo mutation). The arrow signifies the proband. Dark circles indicate the affected individuals.
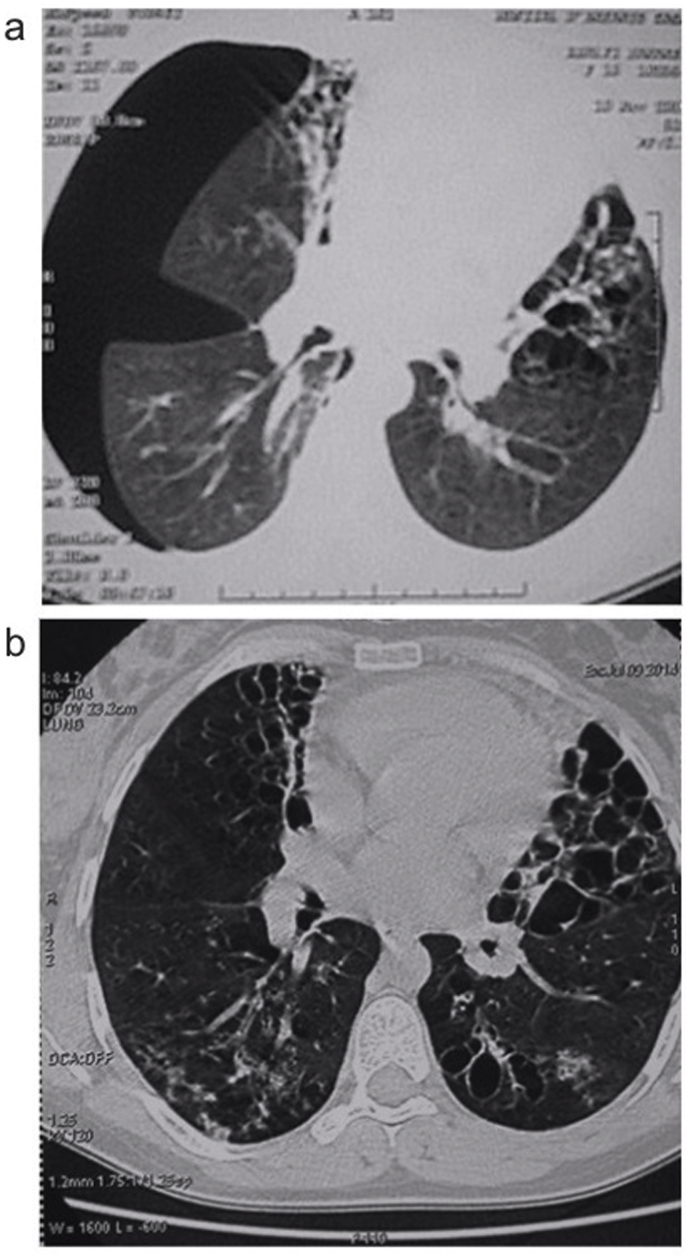
At admission, physical examination of the index case described a 151-cm-tall girl weighing 49 kg, 37 °C of body temperature, 92 heart beats/min, 100/60 mm Hg of blood pressure, 38 breaths/min of respiratory rate, absence of cyanosis, no nail clubbing, a cambered and tympanic chest to percussion, wheezing in the tops, and crackles at lung bases. Chest X-ray showed bronchiectasis. Thoracic computed tomography (CT) confirmed cystic right bronchiectasis of the middle and lingular lobes (Fig. 2a). A functional respiratory assessment (FRA) showed a mixed and diffuse ventilation defect with moderate obstruction, more severe in the small airways.
Fig. 2.
Computed tomography of the chest in the index case at admission (a) showing bilateral basal bronchiectasis and pneumothorax and, at the age of 24 (b) showing diffuse bronchiectasis.
Blood count detailed 6.6 G/L leucocytes, 3.66 G/L neutrophils, 116 g/L hemoglobin and 362 G/L platelets. A normal sweat test (<50 mmoL/L) excluded the diagnosis of cystic fibrosis. HIV serology was negative, immunoglobulin quantitation was within the normal ranges (IgM: 0.75 g/L, IgA: 3.19 g/L, IgG: 15.32 g/L). Tuberculosis and aspergillar superinfection were also excluded.
Serum protein electrophoresis, performed as a first-line of investigation, showed no alpha-1 globulin peak. Serum AAT quantitative analysis was performed by an immunoturbidimetric test adapted on a multiparametric analyser Konelab 60* (Thermo Fisher Scientific, Cergy Pontoise, France) [7] and revealed undetectable levels (AAT <0.1 g/L). AAT elastase inhibitory activity (EIA), assessed through a kinetic spectrophotometric method [7,13], was drastically decreased (3849 Inhibitory Units/L, with 17,500–31,500 IU/L as reference values), in accordance with the very low level of serum AAT.
AAT phenotyping, carried out by isoelectrofocusing electrophoresis (IEF) using ready-to-use agarose gels with immunological detection, revealed a profile without any band, consistent with a very low circulating level of AAT [14]. PI*S and PI*Z-allele-screening by dot blot PCR was negative so a further search for rare variants, by genotyping of the whole coding sequence of SERPINA1 gene by Next Generation Sequencing (NGS), was advised [7]. Genotyping results, according to usual nomenclature, were as follows: a c.775A>T homozygous mutation in exon III of SERPINA1 was characterized. The expected consequence is the generation of a truncated protein with premature stop codon p.(Lys259*), leading to an inactive protein. This Null mutation was previously identified by Zorzetto et al. and recorded as Q0cairo [15]. An additional homozygous c.638T>C sequence variation was also identified, corresponding to a frequently observed variant p.(Val213Ala); this variant was associated with the Q0cairo described by Zorzetto et al.
The same homozygous genotype was identified for patients 2 and 3. The Q0cairo mutation was also detected, in heterozygosis, for the maternal DNA, along with the heterozygous point mutations characterizing the PI*M2 allele, which is generally considered to be without physiological or clinical impact. Nevertheless, an increase in PI*M2 allele frequency has been reported in Tunisian asthmatic patients [16].
The biochemical and genetic results of the four family members are summarized in Table 1.
Table 1.
Biological results (ND: not determined).
| Family member | SERPINA1 genotyping (usual nomenclature) | SERPINA1 (NM_000295.4) genotyping (HGVS nomenclature) | AAT quantification | Elastase inhibitory capacity |
|---|---|---|---|---|
| I:1 (mother) |
M2 Q0cairo c.[302G>A; 1128A>C]; [638T>C; 775A>T] p.[(Arg101His); (Glu376Asp)]; ([Val213Ala); (Lys259*)] |
M2 Q0cairo c.[374G>A; 1200A>C]; [710T>C; 847A>T] p.[(Arg125His); (Glu400Asp)]; [(Val237Ala; Lys283*)] |
ND | ND |
| II:1 (Patient 1) |
Q0cairo homozygous c.[638T>C; 775A>T]; [638T>C; 775A>T] p.[(Val213Ala); (Lys259*)]; [(Val213Ala); (Lys259*)] |
Q0cairo homozygous c.[710T>C; 847A>T]; [710T>C; 847A>T] p.[(Val237Ala; Lys283*)]; [(Val237Ala); (Lys283*)] |
<0,1 g/L | 3849 UI/l |
| II:4 (Patient 2) |
Q0cairo homozygous c.[638T>C; 775A>T]; [638T>C; 775A>T] p.[(Val213Ala); (Lys259*)]; [(Val213Ala); (Lys259*)] |
Q0cairo homozygous c.[710T>C; 847A>T]; [710T>C; 847A>T] p.[(Val237Ala; Lys283*)]; [(Val237Ala); (Lys283*)] |
<0,1 g/L | 3850 UI/l |
| II:2 (Patient 3) |
Q0cairo homozygous c.[638T>C; 775A>T]; [638T>C; 775A>T] p.[(Val213Ala); (Lys259*)]; [(Val213Ala); (Lys259*)] |
Q0cairo homozygous c.[710T>C; 847A>T]; [710T>C; 847A>T] p.[(Val237Ala; Lys283*)]; [(Val237Ala); (Lys283*)] |
<0,1 g/L | <2000 UI/l |
The clinical history of the index case revealed recurrent episodes of cough, bronchial congestion and dyspnea from the age of 3 months. At the age of 8, she developed a bronchorrhea with chronic wheezing and bronchial exacerbations.
Moreover, she presented with several episodes of bronchial super-infection that required hospitalization. Bronchoconstriction was chronic with severe exacerbations in autumn/winter that required salbutamol nebulization at home in addition to a bronchodilator, inhaled corticosteroid, leukotriene antagonists and annual influenza vaccination. The patient developed two episodes of right pneumothorax: the first was treated with two thoracic drains, the second was more severe and spread to bilateral sides respecting apexes after a broncho-pleural fistula and a cystic bronchiectasis complication. Intermittent neutropenia was revealed by blood counting.
The patient is currently 29 years-old; the latest CT showed more diffuse bronchiectasis (Fig. 2b), FRA revealed a severe restrictive syndrome with 46% of forced vital capacity (FVC) which was no longer sensitive to beta2 agonists. This patient had no history of jaundice.
2.2. Patient 2
The youngest sister, born in 1997, was hospitalized at 5 years of age for recurrent respiratory symptoms since birth. Physical examination showed: weight 24 kg, height 112 cm, T° 38.5 °C, pulse 85 beats/min, RR 20 breaths/min, a symmetrical chest, wheezing at the peaks and crackles at the bases. A chest X-ray showed retractile bronchiectasis of the basal right lobe. Sweat test and several blood counts were normal; however the AAT serum level was less than 0.1 g/L, revealing very severe AATD.
This sibling is now 21 years-old and has not been hospitalized in the past 3 years. She is slightly symptomatic with diffuse crackles and few sibilants revealed at auscultation. Her somatic development was not affected (weight 58 kg, height 165 cm) despite the diffuse and bilateral bronchial dilatation. The last FRA revealed a restrictive syndrome (FVC of 61%) with peripheral, moderate obstructive syndrome partially reversible under salbutamol.
2.3. Patient 3
The middle sister, born in 1991, was referred to hospital at the age of 11 for inflammatory arthritis. The year before, she had suffered from painful inflammatory arthritis of small and large joints with functional disability without orthopedic sequelae.
At admission, physical examination revealed significant swelling of the right ankle with limping, swelling of the proximal interphalangeal joint (IPJ) of the 4th left finger and of the 3rd right finger, and of the back of hands, without morning stiffness, no buttock or heel pain. There was no skin- or eye-associated symptoms. Laboratory tests disclosed a mild inflammation and a very severe AATD. Radiological assessment showed decalcification in strip form in the hands with swelling of the soft tissues next to the 3rd right and the 4th left fingers IPJ. Iliac scintigraphy revealed a hyperactivity at the sacroiliac joint, indicating a bilateral joint inflammation. HLA B27 investigation was negative. Diagnosis of ASA was retained. The child had been treated by non-steroidal anti-inflammatory drugs and sulfasalazine for 3.5 years, then by methotrexate (25 mg/week).
Since June 2008, worsening of skin symptoms has been observed with occurrence of dermo-hypodermic lesions affecting the limbs and evolving towards ulceration. The skin biopsy revealed fibromuscular-dermo-hypodermic lesions. Necrotizing panniculitis and ASA associated with a severe AATD were diagnosed. Since 2008, she has been under long-term treatment with 200 mg/day dapsone-iron oxalate, with a total remission of cutaneous signs, which reoccurred when treatment was stopped. Thoracic CT remains normal.
3. Discussion
Previous studies have shown that the SERPINA1 PI*Z deficient allele is very rare in North African populations [4,5,17,18]. The prevalence of the PI*S allele is also very low in these populations and a particular PI*S variant (Sberber) was described in Tunisian Berbers [4,6]. A recently published study has also shown that the prevalence of PI*SZ genotype was very low in Tunisia and in Morocco [19]. In some Mediterranean regions, PI*Mmalton allele prevails over the commonest deficient variants PI*S and PI*Z. Effectively, screening for AATD in patients with obstructive lung diseases from Central Tunisia and COPD subjects from Eastern Tunisia [6,18] noted a frequency of the rare AATD PI*Mmalton mutation as large as that reported in the targeted screening in Central and Southern Italy, peaking in Sardinia [20]. So, the necessity of combining a biochemical with a genetic approach appears crucial, otherwise rare AAT variants will be missed [21]. In the same way, a recent study highlights in Saudi adults with COPD, the importance of using genotyping in order to detect rare variants in subjects with AATD [22].
The present study reports for the first time the Q0cairo mutation in homozygosis in three siblings, featuring early onset and severity of pulmonary manifestations: in particular bilateral bronchiectasis, that is classically rare or at least occurs later in life, and the presence of mild chronic obstructive pulmonary disease (COPD) in the index case and one sibling with rapid deterioration of lung function. These features contrast sharply with the cases of AATD reported in pediatric series. In general, these severe aspects are observed in the third or fourth decade of life and are linked with tobacco use. Pulmonary manifestations are proportionally related to low circulating levels of AAT, which would favor the occurrence of inflammation and destruction of the bronchial epithelium [8]. In the three siblings, the AAT protein was undetectable in serum (<0.1 g/L), which probably explains the early onset and the severity of respiratory symptoms in two of them (patients 1 and 2). However, patient 3 is intriguing by being both homozygous for the Q0cairo mutation and lacking pulmonary manifestations. She presented with neutrophilic panniculitis, which is a very rare disease with an estimated prevalence of 1/1000 subjects with severe AATD [23]. Clinically, patient 3 presented with painful, recurrent ulcerating subcutaneous nodules, histologically characterized by dense infiltrates of neutrophils in the deep dermis and connective tissue septae. Panniculitis may be the only clinical expression of AATD, although it can also occur together with the classical pulmonary or hepatic manifestations. It is puzzling that dermatological manifestations occurred in patient 3 at the age of 16.
An association between AATD and other inflammatory diseases observed in the mother and patient 3, both affected by ASA, has already been reported in the literature [24,25]. It could be explained by the destruction of cartilage by chronic inflammation in the absence of protease inhibitors. Indeed, AAT is a key serine protease inhibitor with anti-inflammatory, anti-thrombotic, and anti-apoptotic effects [24,26]. As AAT secretion sharply rises in response to inflammation, AAT may modulate both duration and magnitude of inflammation [27]. Effectively, AAT contributes to the suppression of pro-inflammatory cytokine synthesis, as described [28].
In the index case, the occurrence of recurrent infectious episodes concomitant with severe neutropenia led us to suppose that neutropenia was probably due to consumption. This could be explained by a significant inflammatory reaction with excessive recruitment of neutrophils in tissues and therefore a peripheral neutropenia.
No liver disease was clinically or biologically observed in this family, as expected for the Null genotypes [29]. Liver disorders are induced by the deposition in the endoplasmic reticulum of the hepatocytes of polymerized PI*Z or PI*Mmalton variants accumulated in the liver [9]. In our three patients, the AAT variant corresponds to a Null Q0cairo homozygous genotype which explains the absence of circulating AAT. This Null genotype can lead to unstable mRNA or to the synthesis of a truncated polypeptide chain, ending prematurely in position 259 and therefore devoid of its mobile reactive loop. It is highly likely that these structural abnormalities lead to a misfolded protein quickly degraded by the ERAD system and consequently not secreted into blood circulation [29]. This could also explain why it does not polymerize into hepatocytes and therefore does not lead to liver damage.
Serum protein electrophoresis is a simple and inexpensive way to detect at least severe forms of AATD [11] but the individual screening is based on the determination of the serum AAT by immunological methods. However, the possible existence of non-functional AAT variants for which serum concentrations remain normal could cause false negatives. If the serum AAT level is below the lower limit of normal, confirmatory testing with EIA measurement and AAT phenotyping [14] and/or genotyping is required. Genetic screening by RFLP-PCR or allele specific-PCR can detect the most common deficient alleles (PI*Z, PI*S, PI*Mmalton) but will miss the other rare deficient alleles [1,14]. Genotyping by complete sequencing of the coding exons of the SERPINA1 gene is the optimal technique to characterize the common alleles but also the Null genotypes and the rare alleles such as PI*Mprocida, PI*Mheerlen, PI*Mwürzburg, PI*Plowell [7].
4. Conclusion
We have identified the rare PI*Q0cairo mutation in homozygosis for the first time in a non-consanguineous Moroccan family. It is noticeable that this homozygous mutation leads to early onset and severity of respiratory symptoms for the index case and one sibling, whereas it was clinically expressed in the third sibling by ankylosing spondyloarthritis and subsequent necrotizing panniculitis without any pulmonary disorders. This specific phenotypic heterogeneity encountered in this family could be attributed to genetic rearrangements, modifier genes, epigenetics or environmental factors. This study underlines the importance of genotyping by whole SERPINA1 gene sequencing, in addition to serum AAT determination, in order to enable detection of AATD in populations well known for their very low incidence of PI*S and PI*Z alleles.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
The authors are grateful to Pr Fatima Ailal and Pr Bouchra Slaoui for patient management and Mrs Evelyne Crème, Désirée Duhem, Catherine Lelorne and Nadine Houriez for skillful technical assistance. They are also grateful to Dr Laura Ravasi for help in writing and editing the English version of the manuscript on behalf of the University of Lille, and Mike Howsam for proofreading.
References
- 1.Stoller J.K., Aboussouan L.S. A review of alpha1-antitrypsin deficiency. Am. J. Respir. Crit. Care Med. 2012;185(3):246–259. doi: 10.1164/rccm.201108-1428CI. [DOI] [PubMed] [Google Scholar]
- 2.Cuvelier A., Mornex J.F. The alpha-1 antitrypsin deficiency: advances in knowledge and unsolved questions. Rev. Mal. Respir. 2014;31(4):295–299. doi: 10.1016/j.rmr.2014.04.087. [DOI] [PubMed] [Google Scholar]
- 3.de Serres F., Blanco I. Role of alpha-1 antitrypsin in human health and disease. J. Intern. Med. 2014;276(4):311–335. doi: 10.1111/joim.12239. [DOI] [PubMed] [Google Scholar]
- 4.Chaabani H., Martin J.P., Frants R.R., Lefranc G. Genetic study of Tunisian Berbers. II. Alpha 1-antitrypsin (Pi) polymorphism: report of a new allele (Pi S Berber) Exp. Clin. Immunogenet. 1984;1(1):19–24. [PubMed] [Google Scholar]
- 5.Makni S., Zitouni M., Ayed K., Mhirii S., Azabi S., Cherif F., Maalej M., Martin J.P., Sesboue R. Absence of the alpha-1-antitrypsin PI*Z allele in Tunisia substantiates the particular genetic structure of African populations. Am. J. Hum. Biol. 1997;9(2):223–224. doi: 10.1002/(SICI)1520-6300(1997)9:2<223::AID-AJHB8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Denden S., Zorzetto M., Amri F., Knani J., Ottaviani S., Scabini R., Gorrini M., Ferrarotti I., Campo I., Chibani J.B., Khelil A.H., Luisetti M. Screening for Alpha 1 antitrypsin deficiency in Tunisian subjects with obstructive lung disease: a feasibility report. Orphanet J. Rare Dis. 2009;4:12. doi: 10.1186/1750-1172-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balduyck M., Odou M.F., Zerimech F., Porchet N., Lafitte J.J., Maitre B. Diagnosis of alpha-1 antitrypsin deficiency: modalities, indications and diagnosis strategy. Rev. Mal. Respir. 2014;31(8):729–745. doi: 10.1016/j.rmr.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Brode S.K., Ling S.C., Chapman K.R. Alpha-1 antitrypsin deficiency: a commonly overlooked cause of lung disease. CMAJ (Can. Med. Assoc. J.) 2012;184(12):1365–1371. doi: 10.1503/cmaj.111749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Townsend S.A., Edgar R.G., Ellis P.R., Kantas D., Newsome P.N., Turner A.M. Systematic review: the natural history of alpha-1 antitrypsin deficiency, and associated liver disease. Aliment. Pharmacol. Ther. 2018;47(7):877–885. doi: 10.1111/apt.14537. [DOI] [PubMed] [Google Scholar]
- 10.Greene C.M., Marciniak S.J., Teckman J., Ferrarotti I., Brantly M.L., Lomas D.A., Stoller J.K., McElvaney N.G. alpha1-Antitrypsin deficiency. Nat Rev Dis Primers. 2016;2:16051. doi: 10.1038/nrdp.2016.51. [DOI] [PubMed] [Google Scholar]
- 11.Laurell C.B., Eriksson S. The electrophoretic alpha1-globulin pattern of serum in alpha1-antitrypsin deficiency. Scand. J. Clin. Lab. Invest. 1963;15:132–140. [Google Scholar]
- 12.Costa X., Jardi R., Rodriguez F., Miravitlles M., Cotrina M., Gonzalez C., Pascual C., Vidal R. Simple method for alpha1-antitrypsin deficiency screening by use of dried blood spot specimens. Eur. Respir. J. 2000;15(6):1111–1115. doi: 10.1034/j.1399-3003.2000.01521.x. [DOI] [PubMed] [Google Scholar]
- 13.Klumpp T., Bieth J.G. Automated measurement of the elastase-inhibitory capacity of plasma with a centrifugal analyzer. Clin. Chem. 1979;25(6):969–972. [PubMed] [Google Scholar]
- 14.Zerimech F., Hennache G., Bellon F., Barouh G., Jacques Lafitte J., Porchet N., Balduyck M. Evaluation of a new Sebia isoelectrofocusing kit for alpha 1-antitrypsin phenotyping with the Hydrasys System. Clin. Chem. Lab. Med. 2008;46(2):260–263. doi: 10.1515/CCLM.2008.036. [DOI] [PubMed] [Google Scholar]
- 15.Zorzetto M., Ferrarotti I., Campo I., Balestrino A., Nava S., Gorrini M., Scabini R., Mazzola P., Luisetti M. Identification of a novel alpha1-antitrypsin null variant (Q0Cairo) Diagn. Mol. Pathol. 2005;14(2):121–124. doi: 10.1097/01.pas.0000155023.74859.d6. [DOI] [PubMed] [Google Scholar]
- 16.Haj-Khelil A., Denden S., Hlioui L., Hattab N., Daimi H., Leban N., Perrin P., Lefranc G., Ben Chibani J. Alpha 1 antitrypsin polymorphism associated to asthma and emphysema in a central Tunisian population. Ann. Biol. Clin. 2008;66(4):379–384. doi: 10.1684/abc.2008.0243. [DOI] [PubMed] [Google Scholar]
- 17.de Serres F.J., Blanco I. Prevalence of alpha1-antitrypsin deficiency alleles PI*S and PI*Z worldwide and effective screening for each of the five phenotypic classes PI*MS, PI*MZ, PI*SS, PI*SZ, and PI*ZZ: a comprehensive review. Ther. Adv. Respir. Dis. 2012;6(5):277–295. doi: 10.1177/1753465812457113. [DOI] [PubMed] [Google Scholar]
- 18.Denden S., Lakhdar R., Keskes N.B., Hamdaoui M.H., Chibani J.B., Khelil A.H. PCR-based screening for the most prevalent alpha 1 antitrypsin deficiency mutations (PI S, Z, and Mmalton) in COPD patients from Eastern Tunisia. Biochem. Genet. 2013;51(9–10):677–685. doi: 10.1007/s10528-013-9597-6. [DOI] [PubMed] [Google Scholar]
- 19.Blanco I., Bueno P., Diego I., Perez-Holanda S., Lara B., Casas-Maldonado F., Esquinas C., Miravitlles M. Alpha-1 antitrypsin Pi*SZ genotype: estimated prevalence and number of SZ subjects worldwide. Int. J. Chronic Obstr. Pulm. Dis. 2017;12:1683–1694. doi: 10.2147/COPD.S137852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrarotti I., Baccheschi J., Zorzetto M., Tinelli C., Corda L., Balbi B., Campo I., Pozzi E., Faa G., Coni P., Massi G., Stella G., Luisetti M. Prevalence and phenotype of subjects carrying rare variants in the Italian registry for alpha1-antitrypsin deficiency. J. Med. Genet. 2005;42(3):282–287. doi: 10.1136/jmg.2004.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosella M., Accardo M., Molino A., Maniscalco M., Zamparelli A.S. Description of a new rare alpha-1 antitrypsin mutation in Naples (Italy): PI*M S-Napoli. Ann. Thorac. Med. 2018;13(1):59–61. doi: 10.4103/atm.ATM_234_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Jameil N., Hassan A.A., Buhairan A., Hassanato R., Isac S.R., Al-Otaiby M., Al-Maarik B., Al-Ajeyan I. Genotyping diagnosis of alpha-1 antitrypsin deficiency in Saudi adults with liver cirrhosis. Medicine. 2017;96(6):e6071. doi: 10.1097/MD.0000000000006071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanco I., Lipsker D., Lara B., Janciauskiene S. Neutrophilic panniculitis associated with alpha-1-antitrypsin deficiency: an update. Br. J. Dermatol. 2016;174(4):753–762. doi: 10.1111/bjd.14309. [DOI] [PubMed] [Google Scholar]
- 24.Stone H., Pye A., Stockley R.A. Disease associations in alpha-1-antitrypsin deficiency. Respir. Med. 2014;108(2):338–343. doi: 10.1016/j.rmed.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Janciauskiene S.M., Bals R., Koczulla R., Vogelmeier C., Kohnlein T., Welte T. The discovery of alpha1-antitrypsin and its role in health and disease. Respir. Med. 2011;105(8):1129–1139. doi: 10.1016/j.rmed.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Petrache I., Fijalkowska I., Zhen L., Medler T.R., Brown E., Cruz P., Choe K.H., Taraseviciene-Stewart L., Scerbavicius R., Shapiro L., Zhang B., Song S., Hicklin D., Voelkel N.F., Flotte T., Tuder R.M. A novel antiapoptotic role for alpha1-antitrypsin in the prevention of pulmonary emphysema. Am. J. Respir. Crit. Care Med. 2006;173(11):1222–1228. doi: 10.1164/rccm.200512-1842OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brantly M. Alpha1-antitrypsin: not just an antiprotease: extending the half-life of a natural anti-inflammatory molecule by conjugation with polyethylene glycol. Am. J. Respir. Cell Mol. Biol. 2002;27(6):652–654. doi: 10.1165/rcmb.F250. [DOI] [PubMed] [Google Scholar]
- 28.Pott G.B., Chan E.D., Dinarello C.A., Shapiro L. Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J. Leukoc. Biol. 2009;85(5):886–895. doi: 10.1189/jlb.0208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrarotti I., Carroll T.P., Ottaviani S., Fra A.M., O'Brien G., Molloy K., Corda L., Medicina D., Curran D.R., McElvaney N.G., Luisetti M. Identification and characterisation of eight novel SERPINA1 Null mutations. Orphanet J. Rare Dis. 2014;9(1):172. doi: 10.1186/s13023-014-0172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]