Abstract
Angiosarcomas are rare cancers accounting for less than 2% of all soft tissue sarcomas. We report the case of an unusual presentation of pleural epithelioid angiosarcoma in a patient with constrictive pericarditis and recurrent pleural effusion. A 62 year old smoker presented with acute chest pain. ECG showed diffuse elevation of ST segments in the precordial leads. After extensive evaluation, he was diagnosed with viral pericarditis and treated with colchicine. Two weeks later the patient presented to the emergency department with a large right pleural effusion. Evaluation of the pleural fluid obtained from a thoracentesis revealed an exudative effusion with negative microbial studies and no evidence of malignant cells. His pleural effusion re-accumulated rapidly, requiring repeated thoracenteses over several weeks. Medical thoracoscopy was performed and pleural biopsy revealed primary pleural epithelioid angiosarcoma. Staging PET scan revealed malignant enhancement of right pleura, pericardium, right iliac bone and right shoulder. He died suddenly within 6 weeks of diagnosis, prior to initiating palliative chemotherapy. Pleural angiosarcoma should be considered in the differential diagnosis of recurrent pleural effusions of unknown etiology. Negative cytology does not rule out the diagnosis; excisional biopsy is required. Reported risk factors include asbestos exposure, prior chest radiation, active smoking and history of complicated pleural tuberculosis. Pleural epithelioid angiosarcomas carry a very poor prognosis, with the majority of patients dying within months of diagnosis.
Keywords: Epithelioid angiosarcoma, Pleural effusion, Pericarditis
1. Introduction
Angiosarcomas are rare cancers accounting for less than 2% of all soft tissue sarcomas [1]. They originate from the endothelial cells of small blood vessels and may affect any organ – most commonly the liver, breasts, skin and heart [2]. Primary pleural epithelioid angiosarcomas are even less common; less than 30 cases have been reported in the literature. We report an unusual presentation of a primary pleural epithelioid angiosarcoma in a patient with pericarditis and recurrent cytology-negative exudative pleural effusion.
2. Case report
A 62-year-old African-American male smoker initially presented to the emergency department with acute sharp retrosternal pain that worsened with deep inspiration. He denied shortness of breath, fever, chills, chest trauma, sick contacts or recent travel. He worked as a custodian at a dairy factory and had no pets. His past medical history was notable for latent tuberculosis treated ten years prior. His physical examination revealed ectopic beats, but was otherwise normal. Laboratory studies demonstrated a normocytic anemia and elevated CRP (142 mg/L), normal troponin, complement levels, and negative ANA and ANCA. ECG showed diffuse elevation of ST segments in the precordial leads. Chest radiography was unremarkable. Computed tomography angiogram of the chest showed ground glass pulmonary nodules as well as a small pericardial effusion and pericardial enhancement. The initial concern was for pericarditis. Echocardiogram disclosed a reduced left ventricular ejection fraction of 38%, and a small circumferential pericardial effusion, without evidence of cardiac tamponade or constriction. Cardiac magnetic resonance imaging disclosed a thickened pericardium which enhanced on post contrast imaging suggesting active inflammation. He was presumptively diagnosed with viral myopericarditis and treated with colchicine.
Two weeks later, the patient again presented to the emergency department, this time with dyspnea. He was found to be hypoxemic, and chest radiography revealed a new large right-sided pleural effusion (Fig. 1).
Fig. 1.
Chest X-ray on admission showing large right pleural effusion.
A thoracentesis was performed with 2700 mL of dark amber fluid removed; evaluation of the pleural fluid revealed an exudative effusion. Gram stain and bacterial cultures were negative. Cytology did not reveal malignant cells. Cell differential demonstrated 2119 cells: 49% neutrophils, 18% lymphocytes, 24% monocytes/macrophages, and 7% mesothelial cells. Glucose was 105 mg/dL, and pH was 7.39. Echocardiogram was notable for newly found constrictive physiology. Left ventricular ejection fraction was 43%. After discharge, his pleural effusion re-accumulated rapidly, requiring multiple large volume thoracenteses over the next few weeks. Repeat pleural fluid cytology remained negative.
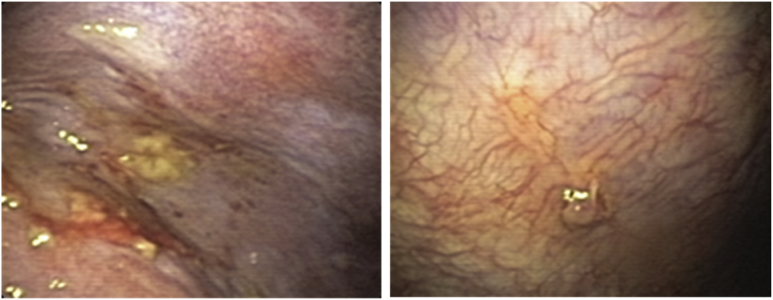
Given the recurrent unilateral pleural effusion and unrevealing fluid analysis, he underwent medical thorascopic pleural biopsy. Operative findings revealed scattered areas of fleshy nodularity involving the diaphragm and posterior sulcus, most consistent with fat (Fig. 2). Unexpectedly, pleural biopsies were consistent with high-grade pleural epithelioid angiosarcoma. Immunoperoxidase studies showed reactivity to CD31, FLI-1, AE1/3, OSCAR, WT-1, and D2-40 (Fig. 3).
Fig. 2.
Thorascopic view of right pleura showing the fleshy nodularity that was biopsied.
Fig. 3.
a. Highly atypical, infiltrating epithelioid cells, with focal areas of discohesiveness. H&E stain, 100x magnification; b. Higher power (200x magnification); c. Solid areas of the tumor, showing highly atypical epithelioid cells with numerous mitotic figures (arrows); d. Fli-1 (vascular marker) immunohistochemistry showing expression in tumor cells. CD-31 (another vascular marker) was also positive. The tumor was negative for epithelial, neuroendocrine, and mesothelial markers.
Staging PET scan (Fig. 4) revealed malignant FDG-PET avidity of the pericardium, with a loculated pericardial effusion (SUV max 16.1). It also showed FDG activity in the right pleural effusion, right iliac bone, left shoulder soft tissue, and bilateral pulmonary nodules.
Fig. 4.
PET image of FDG-avid pericardium (a) and pleural effusion (b).
A few hours after the PET scan, he presented to the Emergency Department with a near-syncopal episode, and was found to be in shock. Electrocardiogram revealed ST-segment abnormalities concerning for acute myocardial infarction. The patient went into pulseless electrical activity arrest. Despite chest compressions, intravenous epinephrine, and emergent pericardiocentesis, the patient did not regain a pulse. Family requested cessation of resuscitation efforts, and the patient passed. Autopsy was declined.
3. Discussion
Epithelioid angiosarcomas are rare, typically fatal neoplasms that may arise from any organ, though the skin, liver, spleen, and heart are frequently involved. Primary pleural epithelioid angiosarcoma is a rarer form of the disease. Published reports describe typical patients as men with similar age as our case [1]. Here we report a unique presentation of epithelioid angiosarcoma involving both the pericardium and pleura, masquerading as a constrictive pericarditis with recurrent pleural effusions of presumed viral origin until the thorascopic biopsy. Prior case reports have presented with atraumatic hemothorax, pleural effusion, or chest pain [1]. Only one previous case report described a case of epithelioid angiosarcoma presenting as recurrent pleural and pericardial effusions [3].
Our case demonstrates the difficulty in diagnosing this rare disease, especially since no mass was seen on chest CT or cardiac MRI and cytology did not reveal malignant cells. This highlights the utility of pleural biopsy in recurrent exudative pleural effusions to rule out malignancy. In our case, thorascopic pleural biopsy resulted in diagnosis, and has a higher diagnostic yield compared to closed pleural biopsy [5,6]. Angiosarcoma can be difficult to differentiate from other malignancies like mesothelioma and immunohistochemistry stains play an important part in diagnosis [7]. Thus, in the case of recurrent effusions of unknown etiology, lack of a mass or positive cytology does not rule out malignant disease, and excisional biopsy should be considered. In this case, staging PET scan was consistent with a metastatic cancer, and may be beneficial in both diagnosis and staging of the disease.
This patient's only potential risk factor for epithelioid angiosarcoma was active smoking status. While active pleural tuberculosis has been associated with epithelioid angiosarcoma, this patient had latent tuberculosis that was treated and did not have any mycobacterial growth on his biopsy specimens. Other possible risk factors include asbestos exposure and prior chest radiation, none of which were evident in this case [1].
Although this patient died unexpectedly prior to any therapy, previous studies have shown that epithelioid angiosarcomas are typically very aggressive and incurable at diagnosis, with most patients succumbing within months of diagnosis [8,9]. Surgery is the best option for patients with localized disease [9]. In patients with metastatic disease, chemotherapy is palliative. Treatments with reported activity include doxorubicin-containing regimens as well as single-agent paclitaxel, vinorelbine, sorafenib and bevacizumab [[10], [11], [12], [13], [14]]. In the open label phase 1b randomized phase 2 study that led to the recent approval of the anti-platelet derived growth factor receptor α antibody, olaratumab, in combination with doxorubicin, 7 patients with angiosarcoma were included [15]. On the basis of dramatic response rates exhibited by patients with angiosarcoma on the phase 1b/2 study of the anti-endoglin antibody, TRC105, in combination with pazopanib in patients with advanced soft tissue sarcoma, there is an ongoing randomized phase 3 study evaluating the combination vs pazopanib alone in patients with angiosarcoma (TAPPAS, clinical trials.gov identifier: NCT02979899). Had this patient survived, the plan was to proceed with palliative chemotherapy with weekly paclitaxel on the basis of poor performance status [16].
Though rare, pleural epithelioid angiosarcoma should be considered in patients with recurrent unexplained exudative pleural effusions. If suspected, thorascopic or open pleural biopsy should be considered for diagnosis.
Declaration of interest
The authors have no conflicts of interest to declare.
Acknowledgment
This work was funded in part by the National Institutes of Health (NIH) grant K12-CA090628.
Contributor Information
Urshila Durani, Email: durani.urshila@mayo.edu.
Alice Gallo de Moraes, Email: gallo.alice@mayo.edu.
Joel Beachey, Email: beachey.joel@mayo.edu.
Darlene Nelson, Email: nelson.darlene@mayo.edu.
Steven Robinson, Email: robinson.steven@mayo.edu.
Nandan S. Anavekar, Email: anavekar.nandan@mayo.edu.
References
- 1.Dainese E., Pozzi B., Milani M., Rossi G., Pezzotta M.G., Vertemati G. Primary pleural epithelioid angiosarcoma. A case report and review of the literature. Pathol. Res. Pract. 2010;206(6):415–419. doi: 10.1016/j.prp.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Carillo G.A., Carretero M.A., Vazquez J.E., Fontan E.G., Ramos M.B., Ventura J.A. Epithelioid angiosarcoma of the lung with pleural metastases: a rare cause of haemoptysis clinicopathological conference. Heart Lung Circ. 2010;19(10):624–628. doi: 10.1016/j.hlc.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Patwardhan M., Ben-Or S. vol. 185. 2012. Unusual and fatal presentation of epithelioid angiosarcoma. (American Journal of Respiratory and Critical Care Medicine Conference: American Thoracic Society International Conference, ATS). (1 MeetingAbstracts):A5956. [Google Scholar]
- 5.Mishra A.K., Verma S.K., Kant S., Kushwaha R.A., Garg R., Kumar S. A study to compare the diagnostic efficacy of closed pleural biopsy with that of the thoracoscopic guided pleural biopsy in patients of pleural effusion. South. Asian j. cancer. 2016;5(1):27–28. doi: 10.4103/2278-330X.179700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haridas N., K PS, T PR, P TJ, Chetambath R. Medical thoracoscopy vs closed pleural biopsy in pleural effusions: a randomized controlled study. J. Clin. Diagn. Res. J. Clin. Diagn. Res.CDR. 2014;(5):8. doi: 10.7860/JCDR/2014/7476.4310. Mc01-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kao Y.C., Chow J.M., Wang K.M., Fang C.L., Chu J.S., Chen C.L. Primary pleural angiosarcoma as a mimicker of mesothelioma: a case report VS. Diagn. Pathol. 2011;6:130. doi: 10.1186/1746-1596-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel A.M., Ryu J.H. Angiosarcoma in the lung. Chest. 1993;103(5):1531–1535. doi: 10.1378/chest.103.5.1531. [DOI] [PubMed] [Google Scholar]
- 9.Zhang S., Zheng Y., Liu W., Yu X. Primary epithelioid angiosarcoma of the pleura: a case report and review of literature. Int. J. Clin. Exp. Pathol. 2015;8(2):2153–2158. [PMC free article] [PubMed] [Google Scholar]
- 10.Fury M.G., Antonescu C.R., Van Zee K.J., Brennan M.F., Maki R.G. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer j. (Sudbury, Mass) 2005;11(3):241–247. doi: 10.1097/00130404-200505000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Ray-Coquard I., Italiano A., Bompas E., Le Cesne A., Robin Y.M., Chevreau C. Sorafenib for patients with advanced angiosarcoma: a phase II Trial from the French Sarcoma Group (GSF/GETO) Oncol. 2012;17(2):260–266. doi: 10.1634/theoncologist.2011-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maki R.G., D'Adamo D.R., Keohan M.L., Saulle M., Schuetze S.M., Undevia S.D. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J. Clin. Oncol. Offic. J. Am. Soc. Clin. Oncol. 2009;27(19):3133–3140. doi: 10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George S., Merriam P., Maki R.G., Van den Abbeele A.D., Yap J.T., Akhurst T. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J. Clin. Oncol. Offic. J. Am. Soc. Clin. Oncol. 2009;27(19):3154–3160. doi: 10.1200/JCO.2008.20.9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agulnik M., Yarber J.L., Okuno S.H., von Mehren M., Jovanovic B.D., Brockstein B.E. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann. Oncol. : Official Journal of the European Society for Medical Oncology. 2013;24(1):257–263. doi: 10.1093/annonc/mds237. [DOI] [PubMed] [Google Scholar]
- 15.Tap W.D., Jones R.L., Van Tine B.A., Chmielowski B., Elias A.D., Adkins D. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet (London, England) 2016;388(10043):488–497. doi: 10.1016/S0140-6736(16)30587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penel N., Bui B.N., Bay J.O., Cupissol D., Ray-Coquard I., Piperno-Neumann S. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J. Clin. Oncol. Official J. Am. Soc. Clin. Oncol. 2008;26(32):5269–5274. doi: 10.1200/JCO.2008.17.3146. [DOI] [PubMed] [Google Scholar]