Abstract
Sarcomatoid carcinoma (SC) of the lung is a rare and aggressive biphasic lung tumor with a 5-year survival of 20%. Early detection and treatment is the only way to improve outcomes in patients with SC of the lung. We present a case of primary SC identified early based on high suspicion. A 56-year-old female with a history of chronic obstructive pulmonary disease (COPD) presented with hemoptysis and exertional dyspnea. Chest X-ray revealed right upper lobe (RUL) opacity and patient was started on antibiotics for pneumonia. Due to the persistence of hemoptysis, a computed tomography scan was performed which showed RUL bronchiectasis with scattered nodular opacities suggestive of an infectious process. The patient underwent bronchoscopy which revealed a pedunculated mass in the RUL biopsy of which was consistent with poorly differentiated SC. Positron-emission tomography scan revealed Flourdeoxyglucose-avid right peri-hilar mass and another nodule in the RUL. The patient was not a surgical candidate because of severe COPD and was started on chemoradiation therapy. SC of the lung can have various presentations and is usually detected at a later stage and hence, difficult to treat. Our case highlights the importance of critical thinking and prompt diagnostic evaluation in high-risk patients with localized bronchiectasis even without an obvious lung mass on imaging.
Keywords: Sarcomatoid, Bronchiectasis, Biphasic, Carcinoma
Abbreviations
- CEA
Carcinoembryonic Antigen
- CK
Cytokeratin
- COPD
Chronic Obstructive Pulmonary Disease
- CT
Computed Tomography
- CXR
Chest X-Ray
- EMA
Epithelial Membrane Antigen
- FDG
Fluorodeoxyglucose
- GCDFP
Gross Cystic Disease Fluid Protein
- H & E
Hematoxylin and Eosin
- IHI
Immunohistochemical
- MRI
Magnetic Resonance Imaging
- NSCLC
Non-Small Cell Lung Carcinoma
- PET
Positron Emission Tomography
- PDL
Programmed Death Ligand
- SC
Sarcomatoid Carcinoma
- RUL
Right Upper Lobe
- SMA
Smooth Muscle Actin
- WHO
World Health Organization
- WT
Wilms Tumor
1. Introduction
Sarcomatoid carcinoma (SC) of the lung is a very uncommon biphasic lung tumor comprising of around 1% of all lung malignancies. It is known to be an aggressive tumor with a 5-year survival of only 20% and usually detected at later stages which make treatment options very limited especially when there are limited guidelines available for this rare malignancy. We present a case of SC of the lung confirmed by light microscopy and immunohistochemistry and diagnosed early in the course of disease based on a high index of suspicion. Smoking cessation, regular screening, early detection and treatment play a very crucial role in improving its morbidity and mortality. SC can have various presentations and pulmonologists need to be aware of this rare form of lung cancer and its presentations
1.1. Case
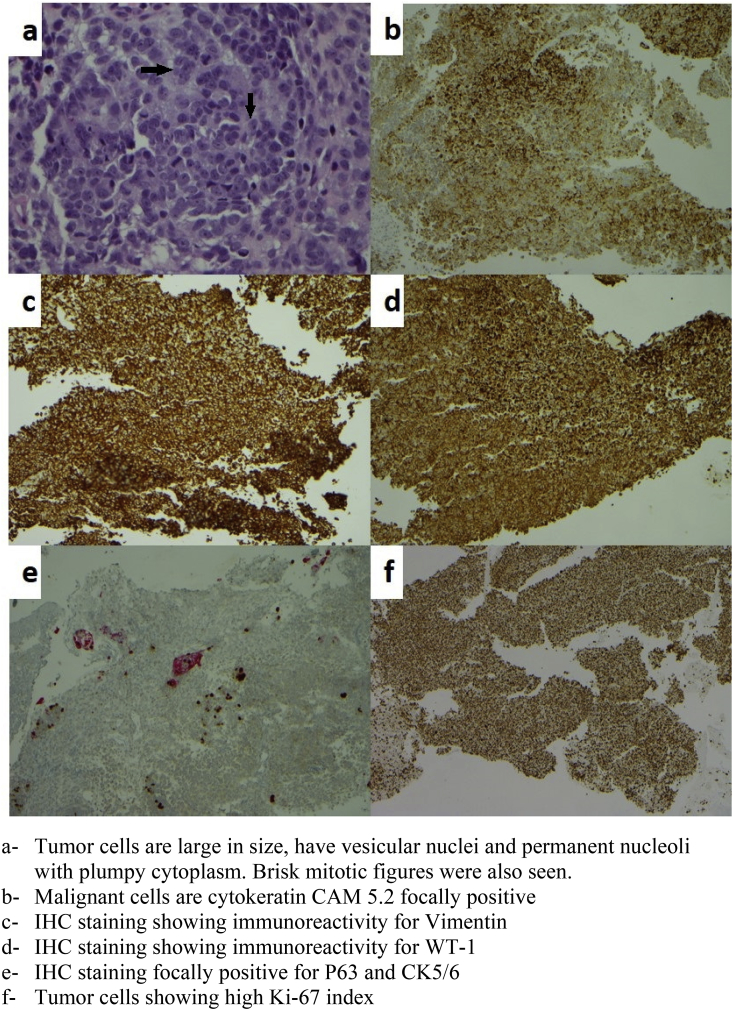
A 56-year-old female with a history of heavy smoking presented to the emergency room with complaints of cough, fatigue, and hemoptysis for few days prior to presentation. She reported productive cough with blood mixed in sputum, along with shortness of breath and pleuritic chest pain. Her other notable medical history included severe chronic obstructive pulmonary disease (COPD), depression and family history of lung cancer in her mother. Upon physical examination, she appeared cachectic and had bilateral wheezing. Chest X-ray (CXR) revealed a right upper lobe consolidation. The patient was admitted to the hospital and started on antibiotics in the setting of COPD exacerbation and community-acquired pneumonia. After a few days of treatment, she had improvement in her dyspnea and chest pain. However, due to the persistence of hemoptysis, she underwent a computed tomography (CT) scan of the chest which showed right upper lobe (RUL) bronchiectasis with scattered nodular opacities suggestive of an infectious process. There was a high suspicion of underlying malignancy in context of her medical history and imaging findings. Therefore, a bronchoscopy was subsequently performed that showed a right upper lobe endobronchial lesion. The rest of the airways were patent. Bronchial washing and endobronchial biopsy specimen analysis revealed a poorly differentiated malignant tumor with sarcomatoid carcinoma features. Bronchoalveolar lavage was negative for an underlying bacterial, fungal or tubercular infection. Immunohistochemical (IHC) staining showed immunoreactivity for vimentin and Wilms Tumor-1 (WT-1), and focally positive for pan-Cytokeratin (CK) AE1/AE3, P63, CK5/6, CK 8/18, CAM 5.2, Epithelial Membrane Antigen (EMA) and Gross Cystic Disease Fluid Protein-15 (GCDFP-15). Focal weak positivity was noted in CD34 and Smooth Muscle Actin (SMA), consistent with nonspecific staining pattern. MIB1/Ki-67 labeling index was high (up to 30–50%). ( Fig. 1)
Fig. 1.
a- Tumor cells are large in size, have vesicular nuclei and permanent nucleoli with plumpy cytoplasm. Brisk mitotic figures were also seen. b- Malignant cells are cytokeratin CAM 5.2 focally positive. c- IHC staining showing immunoreactivity for Vimentin. d- IHC staining showing immunoreactivity for WT-1. e- IHC staining focally positive for P63 and CK5/6. f- Tumor cells showing high Ki-67 index.
Positron-emission tomography (PET) scan revealed a 1.1 cm right peri-hilar mass which was Fluorodeoxyglucose (FDG)-avid (SUV 8.9) and another nodule in the RUL. Magnetic Resonance Imaging (MRI) of her brain was negative for any metastasis. Peripheral blood cytometry did not show any evidence of a lymphoproliferative disorder.
A diagnosis of SC of the lung, stage IIb (T3N0M0) was made. The patient was not a candidate for surgical resection secondary to her advanced COPD and poor pulmonary function (FEV1 of 29%). Hence, she was started on chemoradiation therapy with weekly carboplatin and paclitaxel and received a total of 6000 cGy of radiotherapy over 30 fractions followed by three additional cycles of consolidation chemotherapy. A PET scan performed 6 months after the initial PET scan showed a significant decrease in FDG uptake in the right peri-hilar mass and RUL nodule, suggestive of a response to the treatment. However, a new FDG avid 0.7 cm left upper lobe nodule and 0.5 cm left lower lobe nodule was noted concerning for the spread of malignancy. A biopsy of the new left-sided nodules was not done as she was deemed high risk for the procedure. Her programmed death ligand-1 (PDL-1) expression status was not known; therefore she was started on Nivolumab. Follow-up CT scan showed shrinkage of the nodules and our patient is currently continuing immunotherapy. She still continues to smoke despite smoking cessation counseling.
2. Discussion
Sarcomatoid carcinoma (SC) of the lung is an uncommon biphasic lung tumor and comprises around 1% of all primary lung malignancies [[1], [2], [3]]. It is characterized as a poorly differentiated non-small cell lung carcinoma (NSCLC) with a component of either sarcoma-like differentiation (spindle and/or giant cell) or a component of true sarcoma (malignant bone, cartilage, or skeletal muscle). The malignant carcinomatous component in SC is usually composed of squamous carcinoma, adenocarcinoma, undifferentiated carcinoma, or a mixture of these elements. The sarcomatous component is usually poorly differentiated spindle cell sarcoma [4]. In 2004, World Health Organization (WHO) classified SC into five subtypes based on specific histological criteria: spindle cell carcinoma, giant cell carcinoma, pleomorphic carcinoma, pulmonary blastoma, and carcinosarcoma. Carcinosarcoma refers to a group of tumors that contains a mixture of carcinoma and true sarcoma with differentiated sarcomatous elements [3].
The likely pathogenesis of SC includes malignant transformation of cancer derived stroma, malignant transformation of hamartoma, simultaneous malignant transformation of epithelial elements and stroma, sarcomatous change in carcinoma, and carcinomatous change in sarcoma [[5], [6], [7]]. Some studies indicate a stem cell origin of SC from a single totipotent stem cell that has the capacity to pursue both mesenchymal and epithelial differentiation [[8], [9], [10], [11]].
SC of the lung primarily occurs in male smokers with a higher male to female ratio. Smoking is the main risk factor for SC, although there have been few cases linked to asbestos exposure. The mean age for diagnosis is 65–75 years. The average size at the time of its diagnosis is 7 cm in diameter and often invades the chest wall [3,8,12,13]. It can be divided into two types depending on location: peripheral parenchymal and central endobronchial. It most commonly arises as a large solitary, peripheral mass in the upper lobes like the other smoking-related NSCLC, but can also arise centrally [1,2,8]. There is no specific clinical presentation for this cancer. The usual symptoms are cough, hemoptysis, chest pain, shortness of breath, fever and weight loss. Hemoptysis is seen in around half of the cases of proximal or central tumors, peripheral tumors may be asymptomatic or may present with chest pain [[14], [15], [16]]. Paraneoplastic syndromes due to SC have not been reported so far, although they are seen in around 5–8% of NSCLC [11]. Metastasis to skin, stomach, pancreas, esophagus, jejunum, rectum, kidneys, bones, and adrenal glands and brain have been reported with SC of the lung [7]. Park et al. reported an unusual case where a patient initially presented with gingival exophytic mass which was rapidly growing. Biopsy revealed metastatic carcinoma and the patient was eventually found to have SC of the lung as the primary tumor [17]. Hence, it is also important to pay attention to the appearance of metastatic symptoms of SC which could be prominent and can mislead the diagnosis.
On gross examination, the tumor highly varies in its morphology and is soft and fleshy to firm, hard or rubbery in consistency. The cut surface varies from white gray to tan-yellow in color with frequent areas of hemorrhage and necrosis and occasionally shows cavitation [2]. Imaging modalities like CT scan, MRI, and PET scan seem to be less efficient in diagnosing SC of the lung as it resembles and mimics malignant pleural mesothelioma and pulmonary aspergillosis on imaging [18]. The IHC phenotype and histological characteristics of SC are very different from NSCLC, which result in its aggressive nature. Hematoxylin and eosin (H & E) staining may not be adequate in all cases for its diagnosis. It is difficult to distinguish SC from true sarcomas, such as fibrosarcoma and malignant fibrous histiocytoma if no carcinomatous areas are recognized on the H & E stained sections [11]. IHC stains positive for carcinoembryonic antigen (CEA), pancreatin, EMA, CK, CD 56, chromogranin A, and synaptophysin are very specific for carcinomatous elements, while vimentin, desmin, and smooth muscle/sarcomeric actin confirm the presence of sarcomatous components. The tumor cells often co-express vimentin, CK, smooth muscle markers and CEA. Therefore, the final diagnosis is attained through IHC which has become the gold standard for diagnosis of this tumor [13].
The treatment of SC of the lung is as challenging as the diagnosis. There are no clearly defined guidelines for the treatment of SC of the lung as it is a rare malignancy. SC of the lung is considered to have grave prognosis because it is an aggressive tumor with advanced local stage and metastasis at the time of diagnosis; and refractory to chemoradiation therapy [19]. Radical surgical resection remains the mainstay of treatment, especially for localized tumors. Radiotherapy and chemotherapy are being used as adjuvant therapy or in cases where the patient is a poor surgical candidate because there seems to be a little benefit [7]. Chemotherapy used for the treatment of SC is the same as used for NSCLCs. For metastatic cancer, no data is currently available and SC is usually treated as NSCLC [16]. It has been seen that survival rate at 6 months is only around 27% and the median survival is around 9 months to 1 year after a potentially curative surgical resection of the tumor [19]. SC of the lung has a worse outcome than conventional NSCLC. The 5-year survival rate is approximately 20% for sarcomatoid carcinoma as compared with non-small cell lung cancer which has a 5-year survival of 50% [7]. The early identification of the tumor followed by surgical and adjuvant therapy might lead to prolonged survival in patients with SC of the lung.
3. Conclusion
Our case highlights the importance of having a high index of suspicions for usual and unusual lung cancers in patients presenting with non-specific symptoms. Pursuing further investigation can lead to a prompt diagnosis at an early stage such as in our case allowing for definitive treatment.
Consent
Informed consent was obtained for publication of this manuscript.
Disclosures
None.
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Footnotes
The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans; Uniform Requirements for manuscripts submitted to biomedical journals.
Contributor Information
Kartikeya Rajdev, Email: kartikeyarajdev@gmail.com.
Abdul Hasan Siddiqui, Email: drsiddiqui07@gmail.com.
Uroosa Ibrahim, Email: uroosaibrahim@gmail.com.
Shivika Agarwal, Email: shivika.agarwal1990@gmail.com.
Juan Ding, Email: jding1@northwell.edu.
Michel Chalhoub, Email: mchalhoub1@northwell.edu.
References
- 1.Rossi G., Cavazza A., Sturm N., Migaldi M., Facciolongo N., Longo L., Brambilla E. Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements: a clinicopathologic and immunohistochemical study of 75 cases. Am. J. Surg. Pathol. 2003;27(3):311–324. doi: 10.1097/00000478-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Franks T.J., Galvin J.R. Sarcomatoid carcinoma of the lung: histologic criteria and common lesions in the differential diagnosis. Arch. Pathol. Lab Med. 2010;134(1):49–54. doi: 10.5858/2008-0547-RAR.1. [DOI] [PubMed] [Google Scholar]
- 3.Travis W.D., Brambilla E., Müller-Hermelink K., Harris C., Kleihues C., Sobin P. IARC Press;; Lyon: 2004. World Health Organization Classification of Tumours; pp. 53–58. Pathology and genetics of tumors of the lung, pleura, thymus, and heart. [Google Scholar]
- 4.Rainosek D.E., Ro J.Y., Ordonez N.G., Kulaga A.D., Ayala A.G. Sarcomatoid carcinoma of the lung. A case with atypical carcinoid and rhabdomyosarcomatous components. Am. J. Clin. Pathol. 1994;102(3):360–364. doi: 10.1093/ajcp/102.3.360. [DOI] [PubMed] [Google Scholar]
- 5.Goto T., Maeshima A., Tajima A., Kato R. A resected case of pulmonary carcinosarcoma. Ann. Thorac. Cardiovasc. Surg. 2010;16(3):190–193. [PubMed] [Google Scholar]
- 6.Ito K., Oizumi S., Fukumoto S., Harada M., Ishida T., Fujita Y., Group H.L.C.C.S. Clinical characteristics of pleomorphic carcinoma of the lung. Lung Canc. 2010;68(2):204–210. doi: 10.1016/j.lungcan.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira M.F., Watanabe S.C., Andrade M.P., Rotta J.M., Pinto F.C. Sarcomatoid carcinoma of the lung with brain metastases. J. Bras. Pneumol. 2013;39(6):753–756. doi: 10.1590/S1806-37132013000600016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fishback N.F., Travis W.D., Moran C.A., Guinee D.G., McCarthy W.F., Koss M.N. Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer. 1994;73(12):2936–2945. doi: 10.1002/1097-0142(19940615)73:12<2936::aid-cncr2820731210>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Nappi O., Glasner S.D., Swanson P.E., Wick M.R. Biphasic and monophasic sarcomatoid carcinomas of the lung. A reappraisal of 'carcinosarcomas' and 'spindle-cell carcinomas'. Am. J. Clin. Pathol. 1994;102(3):331–340. doi: 10.1093/ajcp/102.3.331. [DOI] [PubMed] [Google Scholar]
- 10.Terzi A., Gorla A., Piubello Q., Tomezzoli A., Furlan G. Biphasic sarcomatoid carcinoma of the lung: report of 5 cases and review of the literature. Eur. J. Surg. Oncol. 1997;23(5):457. doi: 10.1016/s0748-7983(97)93733-1. [DOI] [PubMed] [Google Scholar]
- 11.Jia J., Ren J., Gu J., Di L., Song G. Predominant sarcomatoid carcinoma of the lung concurrent with jejunal metastasis and leukocytosis. Rare Tumors. 2010;2(3):e44. doi: 10.4081/rt.2010.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishida T., Tateishi M., Kaneko S., Yano T., Mitsudomi T., Sugimachi K., Ohta M. Carcinosarcoma and spindle cell carcinoma of the lung. Clinicopathologic and immunohistochemical studies. J. Thorac. Cardiovasc. Surg. 1990;100(6):844–852. [PubMed] [Google Scholar]
- 13.Koss M.N., Hochholzer L., Frommelt R.A. Carcinosarcomas of the lung: a clinicopathologic study of 66 patients. Am. J. Surg. Pathol. 1999;23(12):1514–1526. doi: 10.1097/00000478-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Yoshino N., Kubokura H., Yamauchi S., Ohaki Y., Koizumi K., Shimizu K. A true pulmonary carcinosarcoma that required diagnostic differentiation from a pleomorphic adenoma: a case report. Ann. Thorac. Cardiovasc. Surg. 2009;15(1):42–45. [PubMed] [Google Scholar]
- 15.Kim H.M., Shin B.S., Song Y.W., Lee S.M., Jung S.H., Kim C.W., Na D.J. A case of pulmonary carcinosarcoma with persistent mild fever. Korean J. Intern. Med. 2002;17(1):78–82. doi: 10.3904/kjim.2002.17.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouziane I., Boutayeb S., Mrabti H., Lalya I., Rimani M., Errihani H. Sarcomatoid carcinoma of the lung: a model of resistance of chemotherapy. N. Am. J. Med. Sci. 2014;6(7):342–345. doi: 10.4103/1947-2714.136920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park J.Y., Kim H.S., Zo J.I., Lee S., Choi S.W. Initial presentation of lung sarcomatoid carcinoma as a metastatic lesion in the mandibular gingiva. J. Periodontol. 2006;77(4):734–737. doi: 10.1902/jop.2006.050137. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y., Li B., Shi H., Cai L., Gu Y. Sarcomatoid carcinoma of the lung mimics aspergilloma on 1⁸F-FDG PET/CT. Hellenic J. Nucl. Med. 2015;18(3):268–270. doi: 10.1967/s002449910311. [DOI] [PubMed] [Google Scholar]
- 19.Romano A., Grassia M., Rossetti A.R., Esposito G., Braccio B., Pezzella M., Martino N.D. Sarcomatoid Carcinoma of the lung: a rare case of three small intestinal intussusceptions and literature review. Int J Surg Case Rep. 2015;13:48–50. doi: 10.1016/j.ijscr.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]