Abstract
Myasthenia gravis (MG) and experimental autoimmune MG (EAMG) are T-cell regulated, antibody-mediated diseases. Peptides p195–212 and p259–271 of the human acetylcholine receptor (AChR) α-subunit, were previously shown to be immunodominant T cell epitopes in MG patients as well as in SJL and BALB/c mice, respectively. A dual altered peptide ligand (APL) composed of the two single amino acid analogs of the myasthenogenic peptides was shown to inhibit, in vitro and in vivo, MG-associated autoimmune responses. Furthermore, the dual APL was shown to down-regulate the clinical manifestations of an established EAMG in C57BL/6 mice injected with Torpedo AChR (TAChR). In the present study we attempted the elucidation of the mechanism(s) by which the dual APL down-regulates EAMG-associated responses. It is shown here that the dual APL acts by actively suppressing, in a specific manner, myasthenogenic T cell responses. The active suppression is mediated, at least partially, by the up-regulation of the secretion of TGF-β following administration of the dual APL. The up-regulated secretion of TGF-β is accompanied by down-regulation of IFN-γ and IL-2 [T helper (Th) 1-type cytokine] secretion and by an up-regulation of IL-10 secretion (Th2-type cytokine). Furthermore, the inhibitory effect of the dual APL could be adoptively transferred to p195–212 or TAChR-immunized mice. The down-regulation of IL-2 secretion and the ability of recombinant IL-2 to rescue lymph node cells of mice treated with the dual APL from a state of unresponsiveness suggests that the dual APL acts also, at least partially, by causing the cells to undergo anergy.
Myasthenia gravis (MG) and its experimental animal model, experimental autoimmune myasthenia gravis (EAMG), are immune disorders characterized by circulating antibodies and lymphocyte autoreactivity to nicotinic acetylcholine receptor (AChR). Although the production of antibodies specific to the acetylcholine receptor is directly attributed to B cells, there is extensive evidence that T cells have a key role in the etiopathology of the disease in humans and animals (1–5). Because the α-subunit of the AChR was shown to be predominant for T cell epitopes (2), we used peptides representing different sequences of the human AChR α-subunit to study the role of T cells in the initiation, development, and immunomodulation of myasthenia gravis.
It was previously demonstrated by our laboratory that peptides p195–212 and p259–271 of the human AChR α-subunit stimulated peripheral blood lymphocytes (PBL) from human MG patients to proliferate (as opposed to PBL of healthy controls that hardly proliferated; ref. 6). Peptides p195–212 and p259–271 were found to be immunodominant T cell epitopes in SJL mice and BALB/c mice, respectively (7). T cell lines specific to p195–212 and to p259–271 of SJL and BALB/c origin, respectively, were established and found to induce autoimmune responses that were manifested by the presence of anti-self AChR antibodies and compound muscle action–potential decrements (4).
To inhibit T cell responses to both myasthenogenic peptides p195–212 and p259–271, we synthesized a dual altered peptide ligand (APL) composed of the tandemly arranged two single amino acid substituted analogs, designated Lys-262–Ala-207. The dual APL Lys-262–Ala-207 was shown to inhibit the proliferation of p195–212- and p259–271-specific T cell lines (8). It was also capable of inhibiting efficiently (90–100%) the in vivo priming of lymph node cells (LNC) of mice to either myasthenogenic peptides (8). Furthermore, the dual APL, when administered orally, could reverse EAMG manifestations induced (in BALB/c mice) by the p259–271 pathogenic T cell line (8) or by immunization (C57BL/6 mice) with the multideterminant native Torpedo AChR (TAChR) (9). Treatment with the dual APL down-regulated the clinical manifestations of the ongoing disease as assessed by the clinical score, grip strength, and electromyography. The amelioration of the clinical disease correlated with a reduction in the titers of AChR-specific antibodies and with a decrease in the levels of IL-2 and especially IFN-γ (9).
The present study deals with the possible mechanisms by which the dual APL exerts its down regulating effects. It is shown here that the dual APL, when administered to mice by different routes (i.p., s.c., per os) either concomitantly or before immunization with p195–212, down-regulates the secretion of the T helper (Th) 1-type cytokines (IL-2 and IFN-γ), whereas the secretion of the Th2-type cytokines (IL-4 and IL-10) and of the Th3-type cytokine (TGF-β) is elevated. Furthermore, splenocytes of treated mice could adoptively transfer their inhibitory effect and inhibit in vivo priming of LNC of p195–212-immunized SJL mice, suggesting that the dual APL may exert its inhibitory effect by actively suppressing T cell responses specific to the myasthenogenic peptide. The dual APL may also cause the LNC to undergo anergy, because LNC of treated mice could be rescued from their state of unresponsiveness following their coculturing with peptide p195–212 in the presence of recombinant IL-2 (rIL-2).
Materials and Methods
Mice.
Female mice of the inbred strains SJL (The Jackson Laboratory) and C57BL/6 (Harlan Breeders, Indianapolis) were used at the age of 8–12 weeks.
Synthetic Peptides and Peptide Analogs.
Peptide p195–212 (DTPYLDITYHFVMQRLPL) and the dual APL Lys-262–Ala-207 (VIVKLIPSTSSAVDTPYLDITYHFVAQRLPL), which is composed of the single analogs of p195–212 (Ala-207 in which methionine was substituted by alanine) and of another myasthenogenic peptide p259–271 (VIVELIPSTSSAV; Lys-262 in which glutamic acid was substituted by arginine), were synthesized and characterized as described (8). A batch of the dual APL Lys-262–Ala-207 synthesized (97% purity) by UCB-Bioproducts was also used in the present study.
Torpedo AChR.
AChR was purified from Torpedo marmorata as described (10) and was kindly provided by Y. Paas (Pasteur Institute, Paris).
In Vivo Inhibition of Priming of LNC by the Dual APL.
The dual APL was administered to mice for a week at 2-day intervals before [i.p. (200 μg/mouse) or orally (500 μg/mouse)] or concomitantly with [i.p., s.c. (200 μg/mouse) or orally (500 μg/mouse)] the immunization with p195–212, TAChR, or ovalbumin (OVA). Ten days after immunization with p195–212, TAChR, or OVA (10 μg) in complete Freund's adjuvant (CFA; Difco), popliteal LNC (1 × 106) obtained from immunized mice were cultured in enriched RPMI medium 1640 (11) supplemented with 1% normal mouse serum, in the presence of various concentrations of p195–212, TAChR, or OVA, for 96 h [3H]thymidine [0.5 μCi of 5 Ci/mmol (1 Ci = 37 GBq), Nuclear Research Center] was then added, and 16 h later plates were harvested onto filter paper and radioactivity was counted.
The Effect of Anti-TGF-β and Anti-IL-4 Neutralizing Antibodies on the Proliferative Responses of LNC of Dual APL-Treated Mice.
LNC (1 × 106) of mice administered with the dual APL (s.c.) and immunized with p195–212 were cocultured with p195–212 (5 μM) and anti-TGF-β (5 μg/ml, R & D Systems) or anti-IL-4 (10 μg/ml, PharMingen) neutralizing antibodies or with their matched isotype controls, for 96 h [3H]thymidine (0.5 μCi of 5 Ci/mmol, Nuclear Research Center) was then added, and 16 h later plates were harvested onto filter paper and radioactivity was counted.
Effect of Recombinant IL-2 on the Proliferative Responses of LNC of Dual APL-Treated Mice.
LNC (1 × 106) of SJL mice pretreated with the dual APL Lys-262–Ala-207 and immunized with p195–212, were incubated in the presence of p195–212 (2.5 μM) and various concentrations of rIL-2 for 96 h. Thereafter, [3H]thymidine (0.5 μCi of 5 Ci/mmol, Nuclear Research Center) was added and 16 h later cells were harvested.
Inhibition of in Vivo Priming of LNC of SJL Mice Immunized with p195–212 by Adoptive Transfer of Splenocytes of Dual APL-Treated Mice.
SJL and C57BL/6 mice were injected either i.p. or s.c., respectively, with the dual APL (200 μg/mouse in PBS) or with PBS seven times at 2-day intervals. Splenocytes obtained from these mice were injected to SJL and C57BL/6 mice concomitantly with immunization with p195–212 or TAChR (10 μg/mouse), respectively. Ten days after immunization with p195–212 or TAChR, proliferative responses of the LNC were determined as described above.
Secretion and Detection of Cytokines.
Splenocytes and LNC (5 × 106/ml) of the tested mice were stimulated with p195–212 (5 μM), TAChR, or OVA (1 μg/ml) for 24–72 h. Supernatants were collected and analyzed for cytokine content by ELISA, using the relevant standard capture and detecting antibodies (from PharMingen and R & D Systems).
Results
The Effect of the Dual APL on the Cytokine Pattern of p195–212-Immunized SJL Mice When Administered Concomitantly with the Myasthenogenic Peptide.
It was of interest to determine the effect of the dual APL on the cytokine network in mice following its administration by different routes concomitantly with the immunization with p195–212.
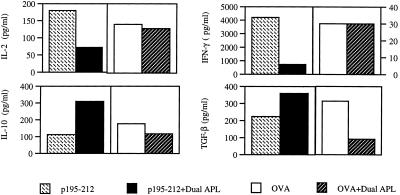
The representative results of Fig. 1 demonstrate that a s.c. administration of the dual APL down-regulated the secretion of Th1-type cytokines (IL-2 and IFN-γ) by the splenocytes (Fig. 1) of the treated mice, whereas the secretion of the Th2-type cytokine (IL-10) and of the Th3-type cytokine (TGF-β) was up-regulated. The specificity of the effect of the dual APL on the cytokine network was determined when OVA was used as the priming antigen instead of the myasthenogenic peptide p195–212. It can be seen in Fig. 1 that the immunomodulation by the dual APL is specific to the myasthenogenic peptide p195–212, because it could not be observed with splenocytes of mice immunized with OVA and treated with the dual APL. A similar effect of the dual APL on the cytokine profile could be observed when the secretion of cytokines by LNC of mice administered either s.c., i.p., or orally with the dual APL (Table 1) was tested. In the case of the i.p. administration, the dual APL also caused an elevation in IL-4 secretion, but the levels secreted by the cells were very low (3–32 pg/ml). As can be seen, the effect of the dual APL is specific to the myasthenogenic peptide p195–212 and was not observed in mice immunized with OVA. In some of the cases the dual APL, when administered to OVA-immunized mice, had the opposite effect on the cytokine secretion of the splenocytes and LNC than on p195–212-immunized mice; however, the reason for that is unclear. The data in Table 1 represent ten experiments performed with similar results. Because the concentration of cytokines varied between experiments, the results represented in the table are not the mean of all experiments but are of a representative experiment.
Figure 1.
The effect of the dual APL on the secretion of cytokines by splenocytes of SJL mice treated s.c. with the dual APL. SJL mice were injected i.d. in the hind footpads with 10 μg of p195–212 or 10 μg of OVA in CFA and treated s.c. with the dual APL (200 μg in 100 μl of PBS). Ten days after immunization with p195–212 or OVA, splenocytes (5 × 106 per well) harvested from the mice were incubated with 5 μM of p195–212 or 1 μg/ml of OVA, respectively, and the supernatants were collected for cytokine detection. The results represent one experiment of two performed. The concentrations of cytokines varied by 10–30% between experiments.
Table 1.
Secretion of cytokines by LNC of mice administered with the dual APL by different routes
| IL-2, pg/ml | IFN-γ, pg/ml | IL-10, pg/ml | TGF-β, pg/ml | |
|---|---|---|---|---|
| s.c. administration of the dual APL | ||||
| p195–212 | 208 | 3680 | 290 | 100 |
| +Dual APL | 190 | 1462 | 372 | 140 |
| OVA | 78 | 464 | 240 | 100 |
| +Dual APL | 84 | 1292 | 164 | 116 |
| i.p. administration of the dual APL | ||||
| p195–212 | 92 | 242 | 202 | 368 |
| +Dual APL | 48 | 126 | 354 | 512 |
| OVA | 56 | 80 | 153 | 132 |
| +Dual APL | 80 | 84 | 140 | 106 |
| Oral administration of the dual APL | ||||
| p195–212 | 74 | 72 | 50 | 56 |
| +Dual APL | 26 | 38 | 82 | 385 |
| OVA | 56 | 183 | 142 | 132 |
| +Dual APL | 72 | 176 | 88 | 114 |
SJL mice were injected i.d. in the hind footpads with 10 μg of p195–212 or 10 μg of OVA in CFA and administered s.c. (200 μg in 100 μl of PBS), i.p. (200 of μg in 200 μl of PBS) or orally (500 μg in 300 μl of PBS) with the dual APL. Ten days after immunization with p195–212 or OVA, LNC (5 × 106 per well) obtained from the mice were incubated with 5 μM of p195–212 or 1 μg/ml OVA, respectively, and the supernatants were collected for cytokine detection.
The Effect of the Dual APL on the Cytokine Pattern of TAChR-Immunized C57BL/6 Mice.
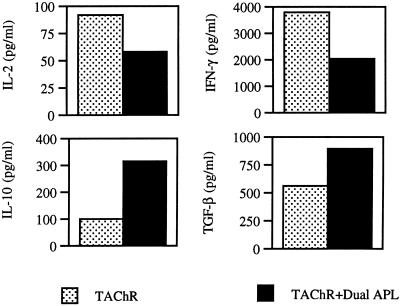
The dual APL was previously shown to have beneficial effects on the clinical manifestations of an established EAMG in C57BL/6 mice (9). It was of interest to determine whether the immunomodulation of the cytokine network observed in p195–212-immunized SJL mice could also be observed in C57BL/6 mice immunized with the whole TAChR and treated with the dual APL. As can be seen from representative results in Fig. 2, the s.c. administration of the dual APL caused a significant decrease in the secretion of IFN-γ, whereas secretion of TGF-β was up-regulated. It is noteworthy that, as shown in Fig. 1 for mice immunized with p195–212, the administration of the dual APL to TAChR-injected mice down-regulated IL-2 secretion and up-regulated IL-10 secretion.
Figure 2.
The effect of the dual APL on the secretion of cytokines by splenocytes of C57BL/6 mice administered s.c. with the dual APL. C57BL/6 mice were injected i.d. in the hind footpads with 10 μg of TAChR in CFA and treated s.c. with the dual APL (100 μg in 100 μl of PBS). Ten days after immunization with TAChR, splenocytes (5 × 106 per well) obtained from the mice were incubated with 1 μg/ml of TAChR, and the supernatants were collected for cytokine detection. The results represent one of two experiments. The concentrations of cytokines varied by 10–30% between experiments.
The Effect of Anti-TGF-β and Anti-IL-4 Neutralizing Antibodies on the Inhibition of LNC Proliferation by the Dual APL.
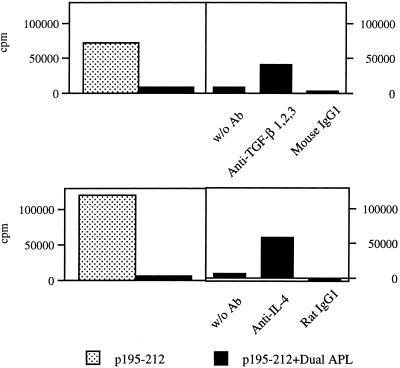
To confirm the role of TGF-β in the inhibitory effect of the dual APL, the in vitro effect of anti-TGF-β neutralizing antibodies on the proliferative responses of LNC of mice administered with the dual APL s.c. was tested. Further, because the levels of secreted IL-4 were very low and thus the effect of the dual APL on this cytokine was not clear cut, the in vitro effect of anti-IL-4 neutralizing antibodies was studied as well. As can be seen from Fig. 3, the s.c. administration of the dual APL inhibited (as expected) the in vivo priming of the LNC. When the inhibited LNC were cocultured with the myasthenogenic peptide p195–212 and with anti-TGF-β neutralizing antibodies, the cells regained more than 50% of their ability to respond to p195–212. This effect is specific to the anti-TGF-β antibodies because LNC that were cocultured with an Ig isotype control did not proliferate in response to the priming peptide. Similarly, when LNC of mice administered s.c. with the dual APL were cocultured with anti-IL-4 neutralizing antibodies and the myasthenogenic peptide, the cells regained about 50% of their proliferative capacity. As can be seen in this case as well, the Ig isotype control did not have a significant effect on the LNC proliferation. The combination of anti-TGF-β and anti-IL-4 had an additive effect. Nevertheless, the addition of both types of neutralizing antibodies did not abolish completely the inhibition of the LNC proliferation (data not shown).
Figure 3.
The effect of anti-TGF-β and anti-IL-4 neutralizing antibodies on the inhibitory effect of the dual APL on the proliferative responses of LNC. SJL mice were injected i.d. in the hind footpads with 10 μg p195–212 in CFA and administered s.c. with the dual APL (200 μg in 100 μl of PBS). Ten days after immunization with p195–212, a proliferation assay was performed as described in Materials and Methods, in the presence of anti-TGF-β/IL-4 neutralizing antibodies and their matched isotype controls. Results are expressed as mean delta cpm of triplicates and they represent one experiment of four performed. SD values did not exceed 10%.
The Effect of the Dual APL on the Cytokine Network of Pretreated SJL Mice.
It was of interest to determine whether the dual analog has the same immunomodulating effect on the cytokine network when it is administered before the immunization with p195–212 as when it is administered concomitantly with immunization with the myasthenogenic peptide. To this end SJL mice were pretreated (i.p. or orally) with the dual APL (or with PBS) before their immunization with p195–212. Ten days postimmunization the splenocytes of the pretreated and the nontreated mice were stimulated with p195–212. The supernatants were collected and the levels of IL-2, IL-10, IFN-γ, and TGF-β were determined. The results in Table 2 represent six experiments performed with similar results. Because the concentration of cytokines varied between experiments, the results represented in the table are not the mean of all experiments but are of a representative experiment. As can be seen, on stimulation with p195–212 the splenocytes of mice pretreated i.p. or orally with the dual analog secreted lower amounts of Th1-type cytokines (IL-2 and IFN-γ) in comparison to splenocytes of mice pretreated with PBS. In contrast, the pretreatment with the dual APL led, on stimulation with p195–212, to an elevated secretion of IL-10 (Th2-type cytokine) and TGF-β (Th3-type cytokine) in comparison to cells of nontreated mice. No IL-4 secretion was detected in the supernatants of the splenocytes. Similar results were obtained when the levels of cytokines were measured in supernatants of LNC of pretreated mice (data not shown). Thus, the dual APL affects cytokine secretion in a similar manner whether administrated before or concomitantly with the immunization.
Table 2.
The effect of pretreatment with the dual APL on the secretion of cytokines by splenocytes of SJL mice
| Cytokine | Amount secreted, pg/ml
|
|||
|---|---|---|---|---|
| i.p.
|
Oral
|
|||
| PBS | Dual APL | PBS | Dual APL | |
| IL-2 | 70 | 24 | 192 | 156 |
| IFN-γ | 752 | 148 | 676 | 132 |
| IL-10 | 546 | 1184 | 314 | 1072 |
| TGF-β | 95 | 140 | 195 | 343 |
SJL mice were pretreated i.p. or orally with the dual APL (200 μg in 200 μl of PBS and 500 μg in 300 μl of PBS, respectively) or with PBS and then immunized i.d. in the hind footpads with 10 μg of p195–212 in CFA. Ten days after immunization with p195–212, splenocytes (5 × 106 per well) obtained from the mice were incubated with p195–212 (5 μM) and the supernatants were collected for cytokine detection.
Reconstitution of Proliferative Responses to p195–212 by rIL-2.
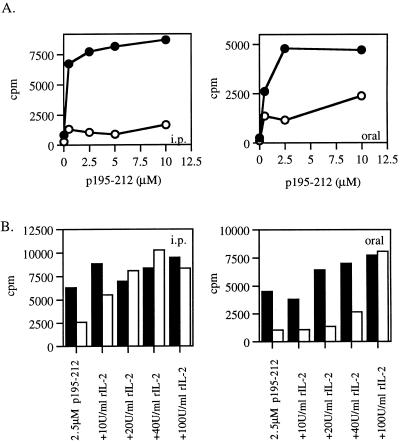
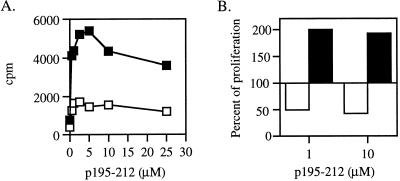
The reproducible decrease in IL-2 secretion observed on administration of the dual APL may suggest that at least part of its inhibitory effect is due to its ability to cause the LNC to undergo anergy. One approach to examining this possibility is attempting to restore the proliferative capacity of the cells by addition of rIL-2. To this end, a series of experiments were performed in which SJL mice were pretreated (for a period of 7 days) with the dual APL either i.p. or orally, followed by an immunization with p195–212 in CFA. Ten days postimmunization the capacity of the LNC of the pretreated mice to proliferate in response to their priming peptide was tested. The results shown in Fig. 4A represent numerous experiments indicating that the proliferation of the LNC of the pretreated mice (i.p. and orally) was inhibited efficiently under these conditions. The LNC were cocultured with the priming peptide p195–212 in the presence of different concentrations of rIL-2. As can be seen in Fig. 4B, the addition of rIL-2 diminished in a dose-dependent manner the inhibitory effect of the dual APL. In the case of i.p. pretreatment, the rIL-2 concentration that enabled the LNC of the pretreated mice to reach the proliferation levels of the nontreated cells was 20 units/ml, whereas in the case of the oral pretreatment a concentration of 100 units/ml of rIL-2 was required to restore the proliferative capacity of the LNC of the pretreated mice. These results were reproducible and may indicate that one of the mechanisms by which the dual analog exerts its inhibitory effect is by induction of anergy.
Figure 4.
The effect of recombinant IL-2 on the proliferative responses of the LNC of mice pretreated with the dual APL. (A) SJL mice were pretreated (i.p. and orally) with the dual APL (○) or with PBS (●) three times in 2-day intervals for a week (200 μg and 500 μg in 200 μl and 300 μl of PBS for i.p. and oral administration, respectively). Mice were then immunized i.d. in the hind footpads with 10 μg p195–212 in CFA. Ten days after immunization with p195–212, a proliferation assay was performed as described in Materials and Methods. Results are expressed as mean cpm of triplicates and they represent 20 experiments. SD values did not exceed 10%. (B) Mice were pretreated as described in A. Ten days after immunization with p195–212, lymph node cells (106 per well) obtained from mice pretreated with PBS (■) or with the dual APL (□) were incubated in the presence of p195–212 (2.5 M) with or without recombinant IL-2 for 96 h. Thereafter, [3H]thymidine was added and 16 h later cells were harvested. Results are expressed as mean cpm of triplicates and they represent four experiments. SD values did not exceed 10%.
Transfer of Suppression of Autoimmune-Associated Responses by Splenocytes of Mice Treated with Dual APL.
Because administration of the dual APL up-regulated the levels of secreted TGF-β, which has been shown to be an immunosuppressive cytokine, it was of interest to determine whether the inhibitory effect can be actively transferred by cells of dual APL-treated mice. Fig. 5 represents one of two experiments performed. Fig. 5A shows that injection of splenocytes (10 × 106 cells per mouse) obtained from SJL mice that were treated (i.p. four times, 200 μg per injection) with the dual APL could inhibit the in vivo priming of LNC of mice immunized with the myasthenogenic peptide p195–212 at the time of cell transfer. It is noteworthy that a dose of 5 × 106 splenocytes was capable of inhibiting (up to 50%) the proliferative responses of LNC of p195–212-immunized mice (data not shown). The inhibitory effect of the injected splenocytes from the treated mice was shown to be specific because, as can be seen in Fig. 5B, splenocytes of PBS-treated mice (20 × 106 per mouse) did not inhibit the p195–212-specific proliferative response, whereas injection of the same number of cells obtained from dual APL-treated mice did inhibit the priming of LNC to the myasthenogenic peptide. Similar results were obtained when the dual APL-treated (s.c. or orally) splenocytes were injected to C57BL/6 mice concomitantly with the immunization with the TAChR. Thus, 10 × 106 splenocytes of mice administered orally with the dual APL inhibited significantly the proliferation to the TAChR. Similarly, 20 × 106 splenocytes of mice administered s.c. with the dual APL inhibited in a specific manner the proliferative responses to the TAChR. Thus, 27,690 ± 8,780 counts per minute (cpm) were determined for LNC proliferation of mice injected with splenocytes of dual APL-treated mice as compared with 48,620 ± 1,320 cpm and 79,800 ± 7,750 cpm measured for LNC proliferation of TAChR-immunized mice that were not treated and LNC proliferation of mice injected with splenocytes of PBS-treated mice, respectively.
Figure 5.
Transfer of inhibition of the in vivo priming to p195–212 by splenocytes of dual APL-treated mice. (A) SJL mice were injected intraperitoneally with the dual APL (200 μg per mouse in PBS) seven times in 2-day intervals. Splenocytes obtained from these mice were injected intravenously (10 × 106 cells per mouse in PBS) to SJL mice concomitantly with immunization with 10 μg of p195–212 in CFA (□). Alternatively, SJL mice were immunized with 10 μg of p195–212 in CFA only (■). A proliferation assay was performed as described in Materials and Methods. (B) SJL mice were injected intraperitoneally either with the dual APL (200 μg per mouse in PBS; □) or with PBS (■) seven times in 2-day intervals. Splenocytes were obtained from these mice and injected intravenously (20 × 106 cells per mouse in PBS) to SJL mice concomitantly with immunization with 10 μg of p195–212 in CFA. A proliferation assay was performed as described in Materials and Methods.
Discussion
The results presented in this study show that the dual APL is capable of inhibiting proliferative responses specific to peptide p195–212 and to TAChR. The inhibitory effect of the dual APL is mediated by a specific shift in the cytokine pattern. Furthermore, the results indicate that the dual APL acts, at least partially, by induction of anergy and that its inhibitory effect can be transferred by splenocytes of treated mice.
Administration of the dual APL to mice that were immunized either with the myasthenogenic peptide or with the native TAChR down-regulated significantly the secretion of IFN-γ. Indeed, it has been shown that EAMG-resistant BALB/c mice developed the disease after IFN-γ expression within neuromuscular junctions (12). Balasa et al. (13) showed that IFN-γ knockout mice remained resistant to clinical EAMG. The role of IFN-γ in the pathogenesis of EAMG was further supported recently by Wang et al. (14), who showed that injection of recombinant IFN-γ at the time of immunization with AChR in CFA enhanced the disease in EAMG-susceptible Lewis rats and induced mild and transient EAMG in EAMG-resistant Wistar Furth rats. IFN-γ was also found to be strongly up-regulated in blood mononuclear cells of MG patients (15). Thus, the decrease in IFN-γ secretion seen following administration of the dual APL may play an important role in the mechanism by which the dual APL immunomodulates EAMG-associated T cell responses. In agreement, we have recently shown that oral administration of the dual APL to C57BL/6, which developed EAMG after immunization with TAChR, led to a dramatic decrease in IFN-γ level. The latter correlated with amelioration in disease manifestations (9).
One of the most significant effects of the dual APL on the cytokine balance was the up-regulation of TGF-β. The important role of the immunosuppressive cytokine TGF-β in the inhibitory effect of the dual APL was supported by the results of in vitro experiments in which the effect of anti-TGF-β neutralizing antibodies on p195–212-specific T cell proliferative responses was tested. These antibodies were shown to partially reverse the inhibitory effect of the dual APL. In accordance, it was demonstrated that secretion of TGF-β was up-regulated (in parallel to down-regulation in IFN-γ secretion) in mice and rats on oral or nasal administration of AChR, and that correlated with an improvement in their clinical status (16–19). Furthermore, a correlation was found between clinical remission and increase in TGF-β expression in mononuclear cells of MG patients (20).
The dual APL also decreased the secretion of IL-2 when it was administered either before or concomitantly with immunization with the myasthenogenic peptide. The role of IL-2 in MG and EAMG remains unclear (17, 21). A down-regulation in IL-2 secretion is characteristic of a state of anergy, which is known to be one of the possible mechanisms implicated in tolerance to protein antigens (22, 23). Because we have shown here that rIL-2 could rescue LNC of mice administered with the dual APL before immunization with the myasthenogenic peptide p195–212, it is likely that one of the routes by which the dual APL acts is the induction of anergy.
The role of IL-10 in MG is still not clear; nevertheless, the up-regulation in IL-10 secretion observed in this report is supported by the beneficial effect reported for this cytokine in MG patients (24). Similarly, the role of IL-4 in MG and EAMG is controversial. In some of the experiments of this study the administration of the dual APL resulted in an increase in IL-4 secretion, but the levels detected were very low. Nevertheless, the role of IL-4 was further shown in in vitro experiments using anti-IL-4 neutralizing antibodies (Fig. 3). Like the effect observed for the anti-TGF-β neutralizing antibodies, the anti-IL-4 neutralizing antibodies reversed only partially the inhibition of proliferation of the LNC exerted by the dual APL. These results may indicate that the inhibitory effect of the dual APL is not mediated by the immunomodulation of one cytokine, but that a number of cytokines and other factors are involved. Indeed, a combination of anti-TGF-β and anti-IL-4 neutralizing antibodies had a synergistic effect; however in this case as well, the proliferative responses were not completely restored.
All routes of administration of the dual APL, namely i.p., oral, and s.c., resulted basically in a similar cytokine pattern—namely, a significant reduction in the levels of Th1-type cytokines and a marked increase in TGF-β secretion. Thus, these results support previous data showing that the i.p. route (25–27), the oral route (28–32), and the s.c. route (33) can be effective for tolerance induction.
The ability of splenocytes of mice that were treated with the dual APL to adoptively transfer their capacity to inhibit proliferative responses to p195–212 and TAChR, suggests that one of the mechanisms by which the dual APL exerts its inhibitory effect is by inducing an immunosuppressive response mediated by regulatory cells (34) and/or immunosuppressive cytokines like TGF-β. As a result of that, the secretion of cytokines supporting myasthenogenic immune responses is actively suppressed. The regulatory T cells—induced by the administration of the dual APL, which secrete the suppressive cytokines (especially TGF-β)—when encountering the autoantigen may secrete antigen-nonspecific antiinflammatory cytokines, which suppress the secretion of the pathogenic cytokine IFN-γ. Adoptive transfer of regulatory cells was proven to be an efficient means of protecting against different autoimmune diseases. It was recently shown by Quinn et al. (35) that CD4+ T cells specific to peptide p524 [a peptide originated from the glutamic acid decarboxylase 65 (GAD65) autoantigen] could inhibit insulin-dependent mellitus (IDDM) on adoptive transfer into young NOD mice (35). In addition, adoptive transfer of LNC from SJL mice treated with palmitoylated proteolipid protein peptide 139–151 (PAL139–151) to naive recipients resulted in the reduction of the acute phase of experimental autoimmune encephalomyelitis (EAE) and delayed relapses following challenge. The results suggest that treatment with PAL139–151 leads to both anergy and the generation of regulatory cells (36). These data are in agreement with the results of the present study, which indicate that the dual APL acts by actively suppressing myasthenogenic responses and at least partially by inducing anergy.
In conclusion, this study demonstrates that administration of the dual APL may affect EAMG-associated responses by inducing anergy and by immunomodulating the pattern of secreted cytokines that is manifested mainly in the down-regulation of the secretion of the pathogenic cytokine IFN-γ and the up-regulation of the secretion of the immunosuppressive cytokine TGF-β. Moreover, the inhibitory effect of the dual APL could be adoptively transferred by cells of treated mice into naive mice that were further immunized with the myasthenogenic peptide p195–212 or with the TAChR. Whether the inhibitory effect of the dual APL is mediated by regulatory cells or by secreted immunosuppressive cytokines is currently under investigation. Although these results were from short-term experiments in which EAMG-associated responses were elicited, the dual APL was already shown to immunomodulate an established EAMG (8, 9), and therefore it is likely that the mechanism of action of the dual APL shown above is the one effective in the amelioration of an established EAMG.
Acknowledgments
This research was supported by Peptor, Israel (M.S. and E.M.).
Abbreviations
- AChR
acetylcholine receptor
- APL
altered peptide ligand
- CFA
complete Freund's adjuvant
- MG
myasthenia gravis
- EAMG
experimental autoimmune MG
- LNC
lymph node cells
- Th
T helper
- TAChR
Torpedo AChR
- TGF-β
transforming growth factor β
- rIL-2
recombinant IL-2
- OVA
ovalbumin
- CPM
counts per minute
References
- 1.Ahlberg R, Yi Q, Pirskanen R, Matell G, Swerup C, Rieber E P, Riethmuller G, Holm G, Lefvert A K. Neurology. 1994;44:1732–1737. doi: 10.1212/wnl.44.9.1732. [DOI] [PubMed] [Google Scholar]
- 2.Lindstrom J, Shelton D, Fuji Y. Adv Immunol. 1988;42:233–284. doi: 10.1016/s0065-2776(08)60847-0. [DOI] [PubMed] [Google Scholar]
- 3.Drachman D B. N Engl J Med. 1994;25:1797–1810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- 4.Kirshner S L, Katz-Levy Y, Wirguin I, Argov Z, Mozes E. Cell Immunol. 1994;157:11–28. doi: 10.1006/cimm.1994.1201. [DOI] [PubMed] [Google Scholar]
- 5.Dedhia V, Goluszko E, Wu B, Deng C, Christadoss P. Clin Immunol Immunopathol. 1998;87:266–275. doi: 10.1006/clin.1998.4535. [DOI] [PubMed] [Google Scholar]
- 6.Brocke S, Brautbar C, Steinman L, Abramsky O, Rothbard J, Newmann D, Fuchs S, Mozes E. J Clin Invest. 1988;82:1894–1900. doi: 10.1172/JCI113807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brocke S, Dayan M, Rothbard J, Fuchs S, Mozes E. Immunology. 1990;69:495–500. [PMC free article] [PubMed] [Google Scholar]
- 8.Katz-Levy Y, Paas Rozner M, Kirshner S, Dayan M, Zisman E, Fridkin M, Wirguin I, Sela M, Mozes E. Proc Natl Acad Sci USA. 1997;94:3200–3205. doi: 10.1073/pnas.94.7.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paas-Rozner M, Dayan M, Paas Y, Changeux J P, Wirguin Y, Sela M, Mozes E. Proc Natl Acad Sci USA. 2000;97:2168–2173. doi: 10.1073/pnas.040554597. . (First Published February 18, 2000; 10.1073/pnas.040554597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saoudi A, Bernard I, Hoedemaekers A, Cautain B, Martinez K, Druet P, De Baets M, Guery J C. J Immunol. 1999;162:7189–7197. [PubMed] [Google Scholar]
- 11.Axelrod O, Mozes E. Immunobiology. 1986;172:99–109. doi: 10.1016/S0171-2985(86)80056-0. [DOI] [PubMed] [Google Scholar]
- 12.Gu D, Wogensen L, Calcutt N A, Xia G, Zhu S, Merlie J P, Fox H S, Lindstrom J, Powell H C, Sarvetnick N. J Exp Med. 1995;181:547–557. doi: 10.1084/jem.181.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balasa B, Deng C, Lee J, Bradley L M, Dalton D K, Christadoss P, Sarvetnick N. J Exp Med. 1997;186:385–391. doi: 10.1084/jem.186.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H B, Shi F D, Li H, van der Meide P H, Ljunggren H G, Link H. Clin Immunol. 2000;95:156–162. doi: 10.1006/clim.2000.4850. [DOI] [PubMed] [Google Scholar]
- 15.Link J, Navikas V, Fredrikson S, Osterman P O, Link H. J Neuroimmunol. 1994;51:185–192. doi: 10.1016/0165-5728(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 16.Ma C G, Zhang G X, Xiao B G, Link J, Olsson T, Link H. J Neuroimmunol. 1995;58:51–60. doi: 10.1016/0165-5728(94)00187-s. [DOI] [PubMed] [Google Scholar]
- 17.Okumura S, McIntosh K, Drachman D B. Ann Neurol. 1994;36:704–713. doi: 10.1002/ana.410360504. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z Y, Qiao J, Link H. J Neuroimmunol. 1993;44:209–214. doi: 10.1016/0165-5728(93)90045-z. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z Y, Link H, Ljunnngdahl A, Hojeberg B, Link J, He B, Qiao J, Melms A, Olsson T. Cell Immunol. 1994;157:353–368. doi: 10.1006/cimm.1994.1233. [DOI] [PubMed] [Google Scholar]
- 20.Weissert R, Melms A, Link H. J Neurol Sci. 1997;151:49–55. doi: 10.1016/s0022-510x(97)00104-4. [DOI] [PubMed] [Google Scholar]
- 21.Bellone M, Ostlie N, Lei S, Manfredi A A, Conti-Tronconi B M. J Autoimmun. 1992;5:27–46. doi: 10.1016/s0896-8411(05)80049-6. [DOI] [PubMed] [Google Scholar]
- 22.Whitacre C C, Gienapp I E, Orosz C G, Bitar D. J Immunol. 1991;147:2155–2163. [PubMed] [Google Scholar]
- 23.Melamed D, Friedman A. Eur J Immunol. 1993;23:937–942. doi: 10.1002/eji.1830230426. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y M, Kivisakk P, Ozenci V, Pirskanen R, Link H. Clin Exp Immunol. 1999;118:304–308. doi: 10.1046/j.1365-2249.1999.01062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian J, Kaufman D L. J Immunol. 1998;161:5399–5403. [PubMed] [Google Scholar]
- 26.Tisch R, Wang B, Serreze D V. J Immunol. 1999;163:1178–1187. [PubMed] [Google Scholar]
- 27.Brocke S, Gijbels K, Allegretta M, Ferber I, Piercy C, Blankenstein T, Martin R, Utz U, Karin N, Mitchell D, et al. Nature (London) 1996;379:343–346. doi: 10.1038/379343a0. [DOI] [PubMed] [Google Scholar]
- 28.Miller A, Lider O, Roberts A B, Sporn M B, Weiner H L. Proc Natl Acad Sci USA. 1992;89:421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos L M, Al-Sabbagh A, Londono A, Weiner H L. Cell Immunol. 1994;157:439–447. doi: 10.1006/cimm.1994.1240. [DOI] [PubMed] [Google Scholar]
- 30.Hancock W W, Polanski M, Zhang J, Blogg N, Weiner H L. Am J Pathol. 1995;147:1193–1199. [PMC free article] [PubMed] [Google Scholar]
- 31.Polanski M, Melican N S, Zhang J, Weiner H L. J Autoimmun. 1997;10:339–346. doi: 10.1006/jaut.1997.0148. [DOI] [PubMed] [Google Scholar]
- 32.Baggi F, Andreetta F, Caspani E, Milani M, Longhi R, Mantegazza R, Cornelio F, Antozzi C. J Clin Immunol. 1999;104:1287–1295. doi: 10.1172/JCI7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karachunski P I, Ostlie N S, Okita D K, Garman R, Conti-Fine B M. J Neuroimmunol. 1999;93:108–121. doi: 10.1016/s0165-5728(98)00208-2. [DOI] [PubMed] [Google Scholar]
- 34.Shevach E M. J Exp Med. 2001;193:41–46. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quinn A, McInerney B, Reich E P, Kim O, Jensen K P, Sercarz E E. J Immunol. 2001;166:2982–2991. doi: 10.4049/jimmunol.166.5.2982. [DOI] [PubMed] [Google Scholar]
- 36.St Louis J, Uniyal S, Xu L, Chan E, Singh B, Chan B M, Strejan G H. J Neuroimmunol. 2001;115:79–90. doi: 10.1016/s0165-5728(01)00265-x. [DOI] [PubMed] [Google Scholar]