Abstract
Background
The aim of the study was to examine the characteristics of alpha wave peak frequency, power, and coherence in patients with schizophrenia.
Methods
Thirty-one patients with schizophrenia and age- and sex-matched subjects with no psychopathology were enrolled. All study participants underwent quantitative electroencephalography (QEEG). Alpha-related values, including peak frequency, power, and coherence, were evaluated.
Results
Alpha peak frequency on the Oz area was slower in the schizophrenia group than that in the control group. However, no differences in absolute or relative power were observed between the two groups. Significant reductions in absolute and relative coherence were observed at the C3–C4 and T3–T4 nodes in the patients with schizophrenia. Relative coherence was reduced at the P3–P4 nodes.
Conclusion
This study focused on alpha variables detected in QEEG as intrinsic values to distinguish schizophrenia from a healthy control. The results suggest decreased alpha peak frequency of the occipital lobe and decreased coherence between the two hemispheres in patients with schizophrenia. A further study could elucidate the causal relationship and biological meaning of the variations in alpha waves in patients with schizophrenia.
Keywords: Schizophrenia, Quantitative Electroencephalography, Alpha Peak Frequency, Alpha Coherence
Graphical Abstract
INTRODUCTION
Electroencephalography (EEG) is a physiological test that monitors and records electrical activity of the brain. EEG abnormalities have been suggested to occur in patients with psychiatric disorders.1,2 The emergence of quantitative electroencephalography (QEEG) has enabled researchers to extract a multitude of variables that can be quantitatively measured1,3; thus, more objective studies on EEG profiles related to psychiatric disorders have become possible. The relevant variables include power, frequency, and coherence between two arbitrary electrodes.
Alpha waves originate from the occipital lobe when a person is awake with his eyes closed. In general, alpha waves have the largest absolute power among the brain waves and can be easily recognized by the naked eye. Furthermore, individual differences are readily apparent in alpha waves. However, few studies have investigated the meaning of differences in alpha waves among individuals. In recent studies, researchers reported that alpha waves may have an important role in the mechanisms of attention and consciousness.4 A close functional relationship has been reported between thalamic activity and alpha rhythm in humans mediated by corticothalamic loops, which are independent of sensory afferents, supporting the thalamus as the generator and modulator of EEG alpha rhythm.5 Several studies have reported thalamic abnormalities in patients with schizophrenia. For example, a meta-analysis performed by Konick and Friedman6 showed a significant reduction in thalamic size in patients with schizophrenia; postmortem and in vivo imaging studies indicated metabolic changes, including changes in the neurochemical substrate, in the thalamus of patients with schizophrenia.7 Andreasen et al.8 developed a model of schizophrenia that defined dysfunction in the cortical-thalamic-cerebellar-cortical circuit. Therefore, it can be assumed that there may be some changes in the alpha rhythm in patients with schizophrenia and related disorders, reflecting the thalamic pathology.
In this research, we investigated the association between alpha wave activity, including peak frequency, power, and coherence, and schizophrenia. The hypothesis of the study was that patients with schizophrenia would show a significantly different alpha wave profile on a QEEG compared with age- and sex-matched controls, indicating dysfunction in brain activity.
METHODS
Subjects
Among the patients who were diagnosed with schizophrenia in the psychiatry department of Seoul National University Hospital, 31 patients who underwent QEEG from January 2013 to August 2016 were recruited for this study. Those with comorbid medical or neurological disorders that could affect the electrophysiological results were excluded.
Subjects free of any psychiatric or neurological disorders that could affect the EEG results were recruited through advertising and referrals as the control group. The control group was age- and sex-matched to the patient group, and they underwent QEEG in January 2013.
To verify the longitudinal stability of the QEEG profile, data of additional 10 patients on medication with major depressive disorder, schizophrenia, or post-traumatic stress disorder, who underwent QEEG twice from 2013 to 2016, were used in a preliminary analysis.
QEEG data
All QEEG tests were conducted and recorded by one skilled electroencephalographic technician using SynAmps2 (Compumedics, Abbotsford, VIC, Australia) and the Neuroscan system (Scan 4.3; Compumedics) in the QEEG room at Seoul National University Hospital. The participants were seated on a comfortable single sofa located in an isolated sound-shielded room, in a stable state with their eyes closed. The EEG recording lasted about 20 minutes. A total of 21 electrodes, including 2 electrooculography electrodes to trace eye movements, were placed on the scalp, based on the international 10–20 system (FP1, FP2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, Oz, and O2).
Electrode impedance was < 5 kΩ, and reference electrodes were attached to the mastoids. Data were acquired at a frequency of 500 Hz and band-pass filtered at 0.1–60 Hz. The recorded QEEG data were analyzed using Neuroguide software (NG 2.7.8; Applied Neuroscience, St. Petersburg, FL, USA). The recorded signals were visually inspected to eliminate signals disturbed by eye movements or other artifacts, and epochs of 90 seconds were selected for spectral analysis.
Statistical analysis
The Wilcoxon signed-rank test was used to verify the effect of use of psychotropic medication on QEEG results in the preliminary analysis. For the main analysis, Student's t-test was used to detect differences in QEEG alpha peak frequency, power, and coherence between patients with schizophrenia and the matched control group participants. All analyses were performed using SPSS ver. 21 for Windows (SPSS Inc., Chicago, IL, USA). A P value < 0.05 was considered significant.
Ethics statement
This study protocol was approved by the Institutional Review Board, Seoul National University Hospital (H-1611-029-805). Written informed consent was obtained from all study subjects.
RESULTS
Effect of medication on the QEEG profile
A preliminary analysis was performed using the data of patients who took the QEEG twice to verify longitudinal stability of the QEEG profile, and the results are presented in Table 1. Ten participants were composed of four males and six females (mean age, 42.5 years; standard deviation [SD], 10.63 years). The mean time interval between the QEEG analysis of the patients was 289 days (SD, 287.68 days). Between the first and second QEEG tests, participants could change the dose of antipsychotics (amisulpride, aripiprazole, clozapine, paliperidone, and quetiapine) and antidepressants (escitalopram, fluoxetine, paroxetine, and venlafaxine). A Wilcoxon signed-rank test showed no significant differences between the two recordings for all alpha-related variables used in the current analysis. Therefore, in this study, the alpha-related parameters were regarded as independent of time and medication use, and they were not considered confounders.
Table 1. Change of the alpha-related parameters according to time and medication use.
| Parameters | Test 1 (n = 10) | Test 2 (n = 10) | P value | |
|---|---|---|---|---|
| Peak frequency, Oz | 9.75 ± 0.36 | 9.87 ± 0.30 | 0.514 | |
| Absolute power, Oz | 161.28 ± 171.41 | 136.87 ± 121.16 | 0.260 | |
| Relative power, Oz | 64.92 ± 13.40 | 66.28 ± 12.07 | 0.767 | |
| Coherence | O–F | 21.18 ± 20.50 | 22.33 ± 19.84 | 0.575 |
| O–C | 37.78 ± 24.51 | 39.28 ± 20.83 | 0.678 | |
| C3–C4 | 71.20 ± 9.01 | 73.36 ± 10.07 | 0.515 | |
| T3–T4 | 18.72 ± 16.56 | 20.01 ± 19.03 | 0.569 | |
| T5–T6 | 24.33 ± 14.68 | 22.87 ± 15.34 | 0.412 | |
| P3–P4 | 68.12 ± 15.16 | 64.21 ± 15.97 | 0.305 | |
| Coherence (z) | C3–C4 | 0.90 ± 0.46 | 1.07 ± 0.48 | 0.374 |
| T3–T4 | 0.95 ± 1.23 | 1.49 ± 1.98 | 0.313 | |
| T5–T6 | 0.54 ± 1.01 | 0.45 ± 1.33 | 0.485 | |
| P3–P4 | 0.95 ± 1.16 | 1.17 ± 0.64 | 0.405 | |
Values are presented as mean ± standard deviation.
Demographic data
Table 2 shows the demographic data of the schizophrenia and age- and sex-matched control groups. Thirty-one patients (15 males and 16 females; age, 23–44 years) diagnosed with schizophrenia were involved in the study. The average time from the diagnosis of schizophrenia to performance of the QEEG was 5.39 months, and three of the participants were using clozapine at the time of the test. The mean age of the age- and sex-matched control participants was 32.96 years.
Table 2. Demographic characteristics of the study participants.
| Parameters | Schizophrenia group (n = 31) | Control group (n = 31) | P value |
|---|---|---|---|
| Age | 33.07 ± 5.88 | 32.96 ± 5.88 | 0.940 |
| Gender (male) | 15 (48.39) | 15 (48.39) | - |
| Test time from onset, mon | 5.39 ± 4.70 | - | - |
| Clozapine use | 3 (9.68) | - | - |
Values are presented as mean ± standard deviation or number (%).
Analysis of the QEEG alpha variables
Peak frequency, absolute and relative power of alpha wave on Oz area, and absolute and relative coherence of the alpha waves between each two nodes were compared and analyzed (Table 3). The patient group had significantly lower peak alpha wave frequency at the Oz scalp site compared with the control group (mean ± SD, 9.87 ± 0.53 vs. 10.14 ± 0.42, P = 0.026). However, absolute and relative powers of the alpha wave at Oz were not different between the two groups.
Table 3. QEEG findings related to alpha wave of the study participants.
| Parameters | Schizophrenia group (n = 31) | Control group (n = 31) | P valuea | |
|---|---|---|---|---|
| Peak frequency, Oz | 9.87 ± 0.53 | 10.14 ± 0.42 | 0.026 | |
| Absolute power, Oz | 91.30 ± 71.41 | 82.91 ± 57.50 | 0.612 | |
| Relative power, Oz | 54.15 ± 17.54 | 59.64 ± 14.91 | 0.190 | |
| Coherence | O–F | 19.40 ± 13.13 | 18.71 ± 16.06 | 0.853 |
| O–C | 30.14 ± 18.27 | 22.72 ± 19.46 | 0.127 | |
| C3–C4 | 68.19 ± 16.81 | 79.26 ± 15.45 | 0.009 | |
| T3–T4 | 16.75 ± 17.86 | 35.68 ± 21.07 | < 0.001 | |
| T5–T6 | 25.77 ± 15.68 | 15.78 ± 14.64 | 0.012 | |
| P3–P4 | 69.45 ± 12.05 | 69.02 ± 15.97 | 0.905 | |
| Coherence (z) | C3–C4 | 0.69 ± 0.82 | 3.32 ± 0.73 | < 0.001 |
| T3–T4 | 0.89 ± 1.65 | 2.99 ± 1.89 | < 0.001 | |
| T5–T6 | 0.53 ± 1.19 | 0.55 ± 1.01 | 0.958 | |
| P3–P4 | 0.50 ± 0.96 | 1.67 ± 0.64 | < 0.001 | |
Values are presented as mean ± standard deviation.
QEEG = quantitative electroencephalography.
aSignificant findings at a level of P < 0.05 are in bold.
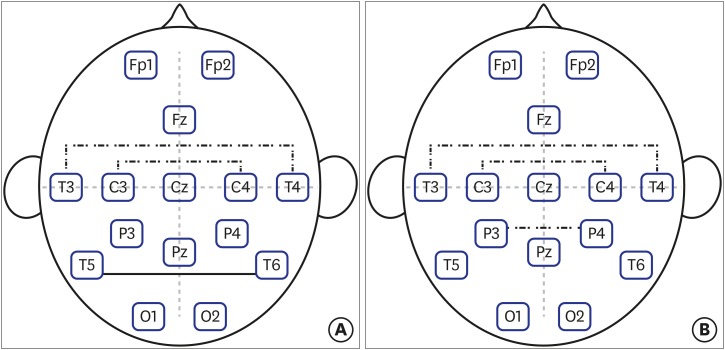
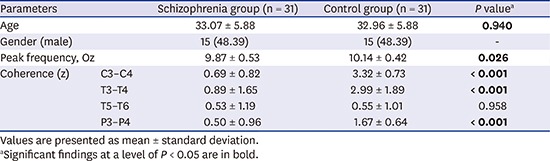
Fig. 1 shows the differences in alpha coherence between the schizophrenia group and the control group in absolute and relative values. The coherence values of alpha activity between the occipital pole and frontal pole, and the occipital pole and central pole were not different between the schizophrenia and control groups. The schizophrenia group showed lower alpha coherence between the C3 and C4 poles in both the absolute (68.19 ± 16.81 vs. 79.26 ± 15.45, P = 0.009) and relative (0.69 ± 0.82 vs. 3.32 ± 0.73, P < 0.001) coherence values compared with the control group. This trend was also true for coherence between the T3 and T4 poles (absolute coherence, 16.75 ± 17.86 vs. 35.68 ± 21.07, P < 0.001; relative coherence, 0.89 ± 1.65 vs. 2.99 ± 1.89, P < 0.001). The absolute coherence value between the T5 and T6 poles was significantly higher in the schizophrenia group than that in the control group (25.77 ± 15.68 vs. 15.78 ± 14.64, P = 0.012), but relative coherence was not different. Only the relative coherence value was significantly lower between the P3 and P4 poles in schizophrenia group than that in the control group (0.50 ± 0.96 vs. 1.67 ± 0.64, P < 0.001); however, no difference in the absolute value was observed between the two groups.
Fig. 1. Differences in alpha coherence between the schizophrenia group and the control group. Dashed lines indicate a decrease in alpha coherence in the schizophrenia group, and straight lines indicate an increase. (A) The absolute values showed significant decrease between the C3–C4 poles, T3–T4 poles, and increase between the T5–T6 poles. (B) The relative values showed significant decrease between the C3–C4 poles, T3–T4 poles, and P3–P4 poles.
DISCUSSION
This study focused on the EEG alpha rhythm as an intrinsic value of each individual, and validated the difference in alpha-related values, including power, coherence, and frequency between a schizophrenia group and an age- and sex-matched control group. The analysis showed that peak frequency of the alpha wave at the Oz area was significantly lower in the schizophrenia group, and the absolute and relative interhemispheric coherence values were also lower in patients with schizophrenia than those in the control group. Considering that the relative coherence value is more valid because it evaluates normalized data, relative coherence was lower in the patient group generally in the central area, including the C3, T3, and P3 nodes.
Superior memory performance is related to higher alpha frequency.9 Moreover, significantly lower peak alpha frequency is observed in individuals with traumatic brain injury,10 as well as in older subjects.11 Our results are consistent with previous analogue EEG findings12; we assumed that patients with conditions involving cognitive decline show lower peak alpha frequency compared to a control group. Peak alpha frequency in EEG is associated with memory performance and cognitive function13; as such, a decrease in peak alpha frequency in schizophrenia patients indicates cognitive impairment. The causal relationship could be validated with further longitudinal studies.
Previous studies that analyzed coherence of EEG in patients with schizophrenia focused on changes associated with cognitive activities or psychiatric symptoms of patients. For example, Ford et al.14 identified reduced coherence between the frontal and temporal lobes while talking in patients with schizophrenia; changes in alpha coherence according to negative symptoms15 or auditory hallucinations16 were also reported. In the present study, alpha variables were intrinsic to each individual and were significantly different between the two study groups. Alpha EEG coherence implicates brain functional states and cognitive arousal level17,18; however, the exact meaning of alpha coherence in brain electrophysical activity remains unknown. One important assumption is that thalamic dysfunction and volume reduction in schizophrenia could be associated with both a change in alpha waves and the symptoms of perception, thinking, and feeling.19,20 A connection deficiency between the left and right hemisphere could be another explanation considering the study results, as schizophrenia has often been conceived as a disorder of connectivity of brain networks.21,22 Consistent reports have found connection abnormalities in the anterior temporal region, including the planum temporale in patients with schizophrenia, suggesting that schizophrenia is a misconnection syndrome.23,24,25 Alpha waves can be easily distinguished with the naked eye and digital analysis is accessible. Therefore, if the relationship with clinical features and the mechanism of the alpha wave are investigated further, it could be a useful clinical marker related to the schizophrenia.
Some limitations of this study should be discussed. First, medication was not controlled. According to the previous studies, psychotropic medications, such as antipsychotics can affect alpha activity on QEEG.26,27 However, the results of the medication effect on QEEG are rather inconsistent.28 Also, only a few participants were taking clozapine in this study, which is known to affect the QEEG profile.29 Furthermore, when additional analyses were performed after excluding the patients who were taking clozapine, the significance of the results did not change. The second limitation is that the patient group was categorized only by the schizophrenia diagnosis, and their symptoms or duration of illness were not considered. Therefore, it is possible that the patient group was rather heterogeneous. Finally, this was a cross-sectional study, so causal relationships between QEEG abnormalities and the disease could not be assessed.
In conclusion, alpha activity of the peak frequency in the occipital area and the coherence between the two hemispheres are intrinsic values in subjects, and a low peak frequency value and reduced absolute and relative coherence values are characteristics of the QEEG profile in patients with schizophrenia. The results indicate that the thalamic abnormality, represented by the change of alpha coherence and frequency, could be one of the main pathophysiology of schizophrenia. Further large sample studies and an evaluation of biophysiological mechanisms would help elucidate the pathophysiology of schizophrenia and related disorders with regard to brain electric activity.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Yeum TS, Kang UG. Data curation: Kang UG. Formal analysis: Yeum TS. Investigation: Yeum TS, Kang UG. Writing - original draft: Yeum TS. Writing - review & editing: Kang UG.
References
- 1.Hughes JR, John ER. Conventional and quantitative electroencephalography in psychiatry. J Neuropsychiatry Clin Neurosci. 1999;11(2):190–208. doi: 10.1176/jnp.11.2.190. [DOI] [PubMed] [Google Scholar]
- 2.Abrams R, Taylor MA. Differential EEG patterns in affective disorder and schizophrenia. Arch Gen Psychiatry. 1979;36(12):1355–1358. doi: 10.1001/archpsyc.1979.01780120085010. [DOI] [PubMed] [Google Scholar]
- 3.Prichep LS, John ER. QEEG profiles of psychiatric disorders. Brain Topogr. 1992;4(4):249–257. doi: 10.1007/BF01135562. [DOI] [PubMed] [Google Scholar]
- 4.Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007;30(4):150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Schreckenberger M, Lange-Asschenfeldt C, Lochmann M, Mann K, Siessmeier T, Buchholz HG, et al. The thalamus as the generator and modulator of EEG alpha rhythm: a combined PET/EEG study with lorazepam challenge in humans. Neuroimage. 2004;22(2):637–644. doi: 10.1016/j.neuroimage.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 6.Konick LC, Friedman L. Meta-analysis of thalamic size in schizophrenia. Biol Psychiatry. 2001;49(1):28–38. doi: 10.1016/s0006-3223(00)00974-4. [DOI] [PubMed] [Google Scholar]
- 7.Clinton SM, Meador-Woodruff JH. Thalamic dysfunction in schizophrenia: neurochemical, neuropathological, and in vivo imaging abnormalities. Schizophr Res. 2004;69(2-3):237–253. doi: 10.1016/j.schres.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Andreasen NC, Paradiso S, O'Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24(2):203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 9.Klimesch W, Schimke H, Pfurtscheller G. Alpha frequency, cognitive load and memory performance. Brain Topogr. 1993;5(3):241–251. doi: 10.1007/BF01128991. [DOI] [PubMed] [Google Scholar]
- 10.Angelakis E, Lubar JF, Stathopoulou S, Kounios J. Peak alpha frequency: an electroencephalographic measure of cognitive preparedness. Clin Neurophysiol. 2004;115(4):887–897. doi: 10.1016/j.clinph.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 11.Richard Clark C, Veltmeyer MD, Hamilton RJ, Simms E, Paul R, Hermens D, et al. Spontaneous alpha peak frequency predicts working memory performance across the age span. Int J Psychophysiol. 2004;53(1):1–9. doi: 10.1016/j.ijpsycho.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Omori M, Koshino Y, Murata T, Murata I, Nishio M, Sakamoto K, et al. Quantitative EEG in never-treated schizophrenic patients. Biol Psychiatry. 1995;38(5):305–309. doi: 10.1016/0006-3223(95)00300-6. [DOI] [PubMed] [Google Scholar]
- 13.Angelakis E, Lubar JF, Stathopoulou S. Electroencephalographic peak alpha frequency correlates of cognitive traits. Neurosci Lett. 2004;371(1):60–63. doi: 10.1016/j.neulet.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 14.Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51(6):485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- 15.Merrin EL, Floyd TC. Negative symptoms and EEG alpha activity in schizophrenic patients. Schizophr Res. 1992;8(1):11–20. doi: 10.1016/0920-9964(92)90056-b. [DOI] [PubMed] [Google Scholar]
- 16.Sritharan A, Line P, Sergejew A, Silberstein R, Egan G, Copolov D. EEG coherence measures during auditory hallucinations in schizophrenia. Psychiatry Res. 2005;136(2-3):189–200. doi: 10.1016/j.psychres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Cantero JL, Atienza M, Salas RM, Gómez CM. Alpha EEG coherence in different brain states: an electrophysiological index of the arousal level in human subjects. Neurosci Lett. 1999;271(3):167–170. doi: 10.1016/s0304-3940(99)00565-0. [DOI] [PubMed] [Google Scholar]
- 18.Merrin EL, Floyd TC, Fein G. EEG coherence in unmedicated schizophrenic patients. Biol Psychiatry. 1989;25(1):60–66. doi: 10.1016/0006-3223(89)90147-9. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS. Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry. 2001;158(4):618–624. doi: 10.1176/appi.ajp.158.4.618. [DOI] [PubMed] [Google Scholar]
- 20.Cronenwett WJ, Csernansky J. Thalamic pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:509–528. doi: 10.1007/7854_2010_55. [DOI] [PubMed] [Google Scholar]
- 21.Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164(3):450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 22.Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30(28):9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearlson GD. Superior temporal gyrus and planum temporale in schizophrenia: a selective review. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21(8):1203–1229. doi: 10.1016/s0278-5846(97)00159-0. [DOI] [PubMed] [Google Scholar]
- 24.DeLisi LE, Hoff AL, Neale C, Kushner M. Asymmetries in the superior temporal lobe in male and female first-episode schizophrenic patients: measures of the planum temporale and superior temporal gyrus by MRI. Schizophr Res. 1994;12(1):19–28. doi: 10.1016/0920-9964(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 25.Falkai P, Bogerts B, Schneider T, Greve B, Pfeiffer U, Pilz K, et al. Disturbed planum temporale asymmetry in schizophrenia. A quantitative post-mortem study. Schizophr Res. 1995;14(2):161–176. doi: 10.1016/0920-9964(94)00035-7. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura M, Koenig T, Irisawa S, Isotani T, Yamada K, Kikuchi M, et al. A pharmaco-EEG study on antipsychotic drugs in healthy volunteers. Psychopharmacology (Berl) 2007;191(4):995–1004. doi: 10.1007/s00213-007-0737-8. [DOI] [PubMed] [Google Scholar]
- 27.Centorrino F, Price BH, Tuttle M, Bahk WM, Hennen J, Albert MJ, et al. EEG abnormalities during treatment with typical and atypical antipsychotics. Am J Psychiatry. 2002;159(1):109–115. doi: 10.1176/appi.ajp.159.1.109. [DOI] [PubMed] [Google Scholar]
- 28.Mucci A, Volpe U, Merlotti E, Bucci P, Galderisi S. Pharmaco-EEG in psychiatry. Clin EEG Neurosci. 2006;37(2):81–98. doi: 10.1177/155005940603700206. [DOI] [PubMed] [Google Scholar]
- 29.Hyun J, Baik MJ, Kang UG. Effects of psychotropic drugs on quantitative EEG among patients with schizophrenia-spectrum disorders. Clin Psychopharmacol Neurosci. 2011;9(2):78–85. doi: 10.9758/cpn.2011.9.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]