Abstract
Dickkopf‐3 (DKK3) is frequently down‐regulated by promoter hypermethylation and is closely associated with a poor prognosis in many cancers. Our previous studies have shown that miR‐708 down‐regulates DKK3 at the post‐transcriptional level in B‐ALL. However, whether transcriptional mechanisms lead to DKK3 silencing remains unclear. Here, we analysed the promoter regions of DKK3 by bioinformatics and found binding sites for MYCN. A dual‐luciferase reporter gene assay and ChIP experiments revealed that MYCN negatively regulates DKK3 at the transcriptional level in B‐ALL cell lines, and using bisulphite sequencing PCR, we affirmed that MYCN has no effect on the methylation of the DKK3 promoter. MYCN silencing in B‐ALL cells resulted in reduced cell proliferation, increased apoptosis and G1 phase arrest. Treatment with MYCN siRNA or 5‐aza‐2′‐deoxycytidine (5‐AdC), a demethylating agent, significantly increased the levels of DKK3 mRNA and protein and decreased the protein levels of p‐GSK3β and nuclear β‐catenin, which indicates inhibition of the Wnt/β‐catenin pathway in vitro. MYCN knockdown significantly decreased the tumorigenic capacity of Nalm6 cells, which restored DKK3 levels and inhibited the Wnt/β‐catenin pathway in vivo. Our study provides an increased understanding of adult B‐ALL pathogenesis, which may be beneficial to the development of effective prognostic markers or therapeutic targets.
Keywords: 5‐AdC, adult B‐cell acute lymphoblastic leukaemia, DKK3, MYCN, Wnt/β‐catenin
1. INTRODUCTION
Despite ongoing improvements in the outcomes of patients with acute lymphoblastic leukaemia (ALL), only 30%‐40% of adult B‐cell ALL (B‐ALL) patients achieve long‐term remission due to its aggressive biological behaviour, even with allogeneic haematopoietic stem cell transplantation and chimeric antigen receptor T‐cell therapy.1, 2, 3, 4, 5, 6 Therefore, it is very important to understand the underlying mechanisms of adult B‐cell ALL carcinogenesis and progression to develop novel therapeutic targets and optimal treatment strategies for adult B‐ALL patients.
DKK3 is a member of the Dickkopf (DKK) gene family and is a putative Wnt antagonist. DKK3 may function as an anti‐oncogene, as it induces apoptosis and regulates the Wnt signalling pathway during tumorigenesis.7, 8, 9 DKK3 gene expression is frequently down‐regulated by promoter hypermethylation in many solid tumours and haematological malignancies.8, 10, 11 Moreover, some miRNAs can also down‐regulate the expression of DKK3 at the post‐transcriptional level in some cancers.9, 12, 13 Indeed, in support of this, our previous studies have shown that miR‐708 down‐regulates the expression and secretion of DKK3‐induced activation of Wnt/β‐catenin pathways in B‐ALL cell lines through direct targeting of the 3′‐UTR of DKK3.14 To determine whether transcription factors lead to DKK3 silencing, we analysed the promoter regions of DKK3 by bioinformatics and found multiple binding sites for MYCN, which indicates that the expression of DKK3 may be down‐regulated by MYCN at the transcriptional level.
MYCN, a member of the MYC proto‐oncogene family, encodes a nuclear transcriptional activator/repressor phosphoprotein that functions in the direct up‐ or down‐regulation of genes via promoter binding. MYCN also acts through indirect pathways to control cell proliferation, apoptosis and differentiation; MYCN is also extensively involved in oncogenesis.15, 16, 17, 18 MYCN is overexpressed in many malignancies, such as retinoblastoma, medulloblastoma and neuroblastoma, and MYCN overexpression is correlated with increased growth potential and poor prognosis.19, 20, 21 However, few studies to date have shown that MYCN can promote cell proliferation and inhibit the activity of tumour suppressor gene‐related signalling pathways that participate in adult B‐ALL, which cumulatively lead to a poor prognosis.
Here, we show that the MYCN mRNA level is negatively correlated with DKK3 mRNA in adult B‐ALL patient samples. Moreover, our data revealed that MYCN binds directly to the promoter region of DKK3 in B‐ALL cell lines. We also further determined that MYCN can directly down‐regulate DKK3 expression at the transcriptional level to activate Wnt/β‐catenin signalling, which in turn leads to proliferation of B‐ALL cell lines. Moreover, MYCN knockdown was shown to significantly inhibit cell proliferation and tumour growth in vitro and in vivo. Therefore, our results demonstrate that the targeting of MYCN upstream restores the high expression of DKK3 and may be a new treatment strategy for adult B‐ALL.
2. MATERIALS AND METHODS
2.1. Patient samples
We studied 12 matched samples of adult B‐ALL obtained at initial diagnosis, complete remission (CR) and after relapse from patients in the Department of Hematology of the First Affiliated Hospital of Harbin Medical University. The diagnosis was established according to the WHO diagnostic criteria.22 This study was approved by the Ethics Committee of Human Experimentation at Harbin Medical University. Informed consent was provided in accordance with the Declaration of Helsinki. Detailed patient information is described in Table S1. Bone marrow mononuclear cells from the patients and normal CD19+ B cells from the bone marrow of healthy volunteers (normal B cells) were obtained as previously reported.14
2.2. Reagents
5‐Aza‐2′‐deoxycytidine (5‐AdC) was purchased from Sigma (Sigma‐Aldrich Corporation, St. Louis, MO, USA), dissolved in 100% DMSO to generate a stock concentration of 10−2 M, stored at −20°C and diluted to the desired concentration in RPMI 1640 before use.
2.3. Cell culture
The human B‐ALL cell lines Nalm6 and BALL‐1 were used in this study. The characteristics and the culture conditions are described in the Supporting information.
2.4. Bisulphite sequencing PCR
We performed BSP as previously described.14 Five to ten clones from each sample were subjected to cycle sequencing (PE Applied Biosystems, Warrington, UK) and analysed using an ABI 310 sequencer (Applied Biosystems, Foster City, CA, USA). The primers used for BSP and the details of these experiments are given in the Supporting information.
2.5. Dual‐luciferase gene reporter assay
Luciferase assays were performed in Nalm6 cells. Luciferase activity was measured in the transfected cells using a Dual‐Luciferase Reporter Assay System (Promega, WI, USA).
2.6. Chromatin immunoprecipitation (ChIP) assay
ChIP analysis was performed according to the manufacturer's instructions (ChIP kit; Upstate Biotechnology, Waltham, USA) using an anti‐MYCN antibody (Becton Dickinson Pharmingen, San Diego, USA). DKK3 promoter‐specific primers and detailed methods are included in the Supporting information.
2.7. Cell proliferation analysis
Cell proliferation was assayed using the Cell Counting Kit‐8 method (CCK‐8; Sigma‐Aldrich).
2.8. Flow cytometric analysis of the cell cycle and apoptosis
For the cell cycle analysis, the cells were stained with propidium iodide (PI, Sigma‐Aldrich). The apoptosis analysis was performed using Annexin V‐FITC/PI according to the manufacturer's protocol (Sigma‐Aldrich).
2.9. In vivo experiments
In vivo experiments performed in NOD/SCID mice are described in the Supporting information.
2.10. Statistical analyses
The data are presented as the means ± SD. Comparisons of two or more data sets were analysed using one‐way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test. The size of the tumours from the in vivo experiments and the cell proliferation analysis from the in vitro experiments were analysed by two‐way ANOVA followed by Bonferroni's multiple comparisons test. The correlation analysis between MYCN and DKK3 mRNA expression was performed using Spearman's correlation analysis. Values were considered significant at P < .05. All analyses were performed using GraphPad Prism 5.0 (GraphPad Software, USA). Additional methods and details are described in the Supporting information.
3. RESULTS
3.1. MYCN and DKK3 mRNA expression in adult B‐ALL patients and cell lines
To analyse the relationship between MYCN and DKK3 expression, we performed quantitative real‐time PCR (qRT‐PCR) and assayed MYCN and DKK3 mRNA levels in adult B‐ALL patients and cell lines. We detected higher MYCN mRNA levels in Nalm6 and BALL‐1 cells than in normal B cells (Figure 1A). MYCN mRNA expression was remarkably increased in paired samples obtained at initial diagnosis and relapse compared with matched adult B‐ALL patient samples obtained after CR (Figure 1C). Subsequently, we examined DKK3 mRNA expression in paired adult B‐ALL patient samples and cell lines. Compared with normal B cells, DKK3 mRNA expression was lower in Nalm6 and BALL‐1 cells (Figure 1B). The levels of DKK3 mRNA in samples obtained from adult B‐ALL patients at initial diagnosis and after relapse were lower than those in samples from the same patients after a CR was achieved (Figure 1D). Next, Spearman's correlation analysis showed that MYCN mRNA expression was negatively correlated with DKK3 mRNA in adult B‐ALL patients (Figure 1E,F,G).
Figure 1.
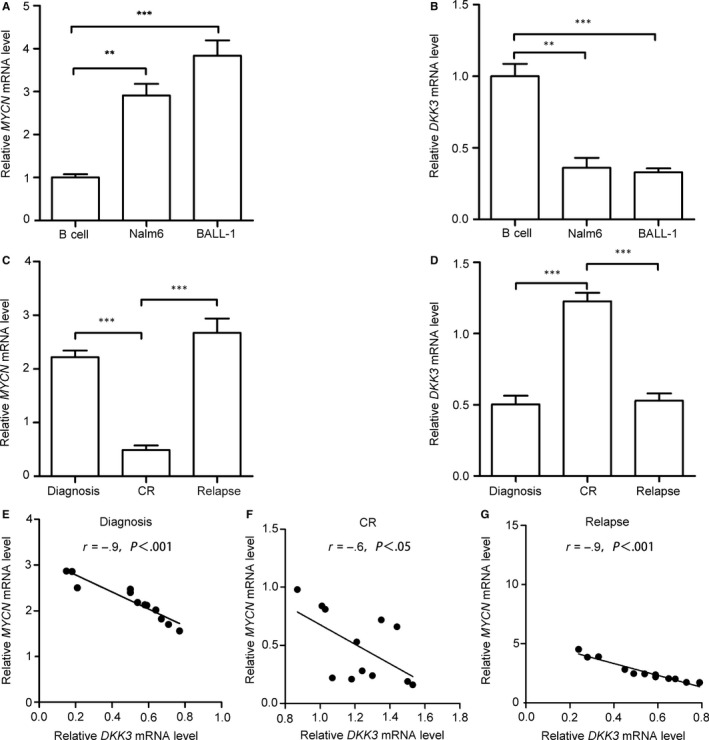
MYCN and DKK3 mRNA expression in B‐cell acute lymphoblastic leukaemia (B‐ALL) cell lines and adult B‐ALL patient samples. Compared with normal B cells, MYCN mRNA (A) was highly expressed, and DKK3 mRNA (B) was expressed at low levels in B‐ALL cell lines. (C) MYCN mRNA expression was high in adult B‐ALL samples obtained at initial diagnosis and at relapse (n = 12) compared with patient samples obtained after complete remission (CR). (D) DKK3 mRNA expression was low in adult B‐ALL samples obtained at initial diagnosis and at relapse (n = 12) compared with patient samples obtained after CR. The MYCN and DKK3 mRNA expression levels were negatively correlated in adult B‐ALL patient samples obtained at initial diagnosis (E), CR (F) and relapse (G). MYCN and DKK3 mRNA levels were measured by quantitative real‐time PCR (qRT‐PCR), and the data are presented as the means ± SD from three separate experiments. **P < .01; ***P < .001
3.2. MYCN directly binds to the DKK3 promoter but has no effect on DKK3 promoter methylation
To elucidate the relationship between MYCN and DKK3 in adult B‐ALL, we constructed firefly luciferase reporters containing the DKK3 gene promoter region and predicted MYCN binding sites (Figure 2A). Co‐transfection of Nalm6 cells with a MYCN overexpression plasmid greatly reduced the luciferase activity driven by the DKK3 promoter region (Figure 2B). Next, a ChIP analysis was performed to further investigate whether MYCN binds to the promoter region of DKK3 in Nalm6 cells. As shown in Figure 2D, DNA sequence fragments from the DKK3 promoter onto which MYCN was recruited were amplified by PCR using specific primers. In addition, MYCN overexpression significantly decreased the DKK3 mRNA and protein levels and MYCN siRNA increased DKK3 mRNA and protein expression in Nalm6 and BALL‐1 cells (Figure 2E,F). These data indicated that MYCN binds directly to the promoter region of DKK3 and significantly down‐regulates DKK3 mRNA and protein expression.
Figure 2.
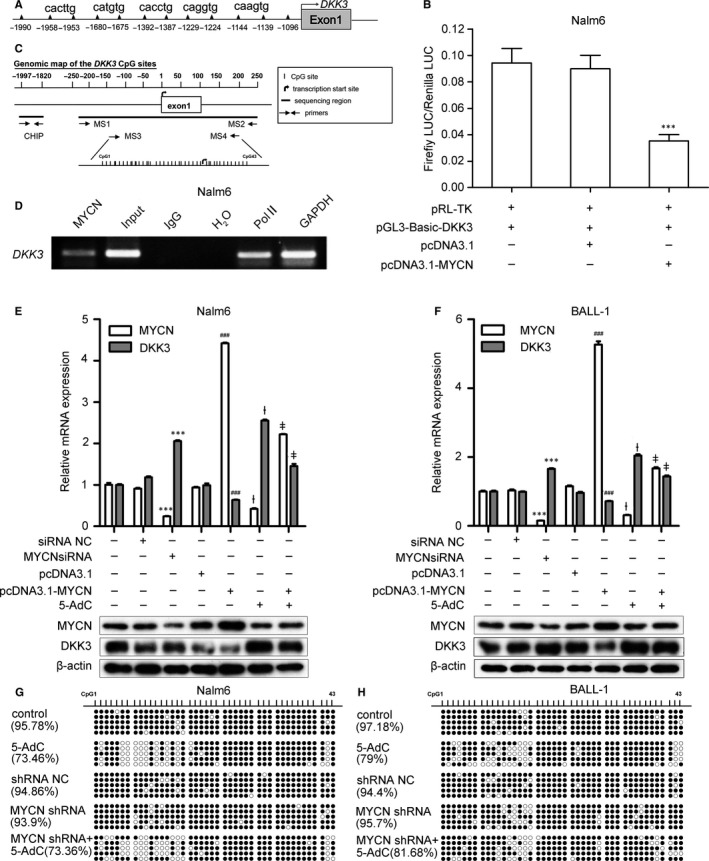
MYCN directly binds to the DKK3 promoter but has no effect on DKK3 promoter methylation. (A) Schematic of the putative DKK3 promoter region containing five potential binding sites for MYCN. (B) Luciferase assays show that the relative luciferase activity decreased in Nalm6 cells co‐transfected with pcDNA3.1‐MYCN and pGL3‐Basic‐DKK3. ***P < .001 vs. pRL‐TK+pGL‐Basic‐DKK3 + pcDNA3.1. (C) CpG site distribution in the promoter region of DKK3 and locations of the primers used in BSP and CHIP assays.(D) Chromatin immunoprecipitation (ChIP) analysis using an anti‐MYCN antibody shows that MYCN was bound to the DKK3 promoter region. (E, F) MYCN and DKK3 mRNA (top) and protein (bottom) expression levels changed after transfection with pcDNA3.1‐MYCN or MYCN siRNA and/or treatment with 5‐AdC in Nalm6 and BALL‐1 cells. The MYCN and DKK3 mRNA levels were measured by quantitative real‐time PCR (qRT‐PCR). MYCN and DKK3 protein expression was measured by Western blot, and β‐actin was used as a loading control. (G, H) After treatment with MYCN shRNA and/or 5‐AdC, the methylation status of the promoter region of DKK3 in CpG islands in B‐ALL cell lines was determined by bisulphite sequencing PCR (BSP). The percentage of methylation was determined via the ratios of methylated cytosine in 5 to 10 sequenced clones. The solid spots indicate methylated CpG dinucleotides; the hollow spots indicate unmethylated CpG dinucleotides. All regions are shown relative to the transcription start site (TSS). Each experiment was repeated three times. The data are presented as the means ± SD. ***P < .001 vs. siRNA NC; ### P < .001 vs. pcDNA3.1; ƚ P < .001 vs. control; ǂ P < .001 vs. 5‐AdC. Non‐transfected cells were used as a control. NC: negative control
DKK3 is reported to be silenced by promoter CpG methylation in ALL.11, 23 In this study, 5‐AdC treatment increased DKK3 mRNA and protein expression in both cell lines. However, MYCN mRNA and protein levels were significantly decreased in Nalm6 and BALL‐1 cells after 5‐AdC treatment. Interestingly, when MYCN‐overexpressing Nalm6 and BALL‐1 cells were treated with 5‐AdC, DKK3 mRNA and protein levels were decreased compared with 5‐AdC treatment alone (Figure 2E,F). To further determine whether MYCN expression was associated with DKK3 promoter methylation, we silenced MYCN in Nalm6 and BALL‐1 cells and examined DNA methylation at 43 CpG sites in the DKK3 promoter region via BSP (Figure 2C). No significant difference was observed between the MYCN shRNA and the negative control shRNA groups (Figure 2G,H). These results suggest no significant involvement of MYCN in the maintenance of DKK3 methylation and that MYCN knockdown and 5‐AdC increase DKK3 expression through two independent mechanisms.
3.3. MYCN depletion reduces proliferation and increases apoptosis of B‐ALL cells and restores the DKK3‐mediated inhibition of the Wnt/β‐catenin pathway
To examine the effects of MYCN expression on leukemogenesis, we evaluated the effects of MYCN on cell proliferation and the induction of apoptosis in B‐ALL cell lines. As shown in Figure 3A,D, MYCN siRNA inhibited cell proliferation, whereas MYCN overexpression promoted proliferation of Nalm6 and BALL‐1 cells. To evaluate the mechanism of MYCN inhibition in the suppression of cell proliferation, the cell cycle distribution and cell apoptosis were examined by flow cytometry. After cells were treated with MYCN siRNA, the proportions of Nalm6 cells (Figure 3B,G) and BALL‐1 cells (Figure 3E,H) in G1 phase increased from 41.8% to 53.7% and from 37.3% to 45.8%, respectively. In addition, apoptosis assays revealed that MYCN siRNA treatment increased apoptosis but that MYCN overexpression decreased apoptosis of Nalm6 (Figure 3C,I) and BALL‐1 (Figure 3F,J) cells. Next, we examined the cell cycle and apoptosis‐relevant proteins cyclin D1, Bcl‐2 and Bax via Western blotting (Figure 4A,B). MYCN siRNA decreased cyclin D1 and Bcl‐2 expression and increased Bax expression in these cell lines. These results demonstrated that the inhibition of cell proliferation by MYCN depletion is most likely mediated by G1 cell cycle arrest and apoptosis.
Figure 3.
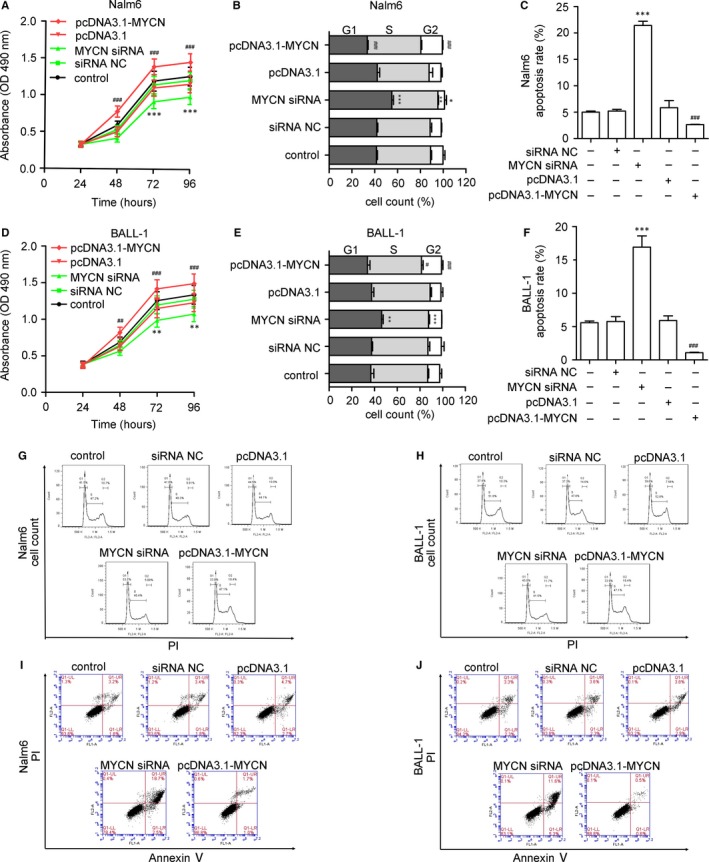
Influence of MYCN expression on cell proliferation, the cell cycle and apoptosis. (A, D) MYCN siRNA significantly decreased the proliferation rates of Nalm6 and BALL‐1 cells as measured by CCK‐8 assays. MYCN siRNA treatment significantly increased the proportions of Nalm6 and BALL‐1 cells in G1 phase, as measured by flow cytometry after PI staining. Histograms (B, E) and representative flow cytometry plots (G, H) of cell cycle alterations. MYCN siRNA treatment increased the number of apoptotic Nalm6 and BALL‐1 cells, as measured by flow cytometry after annexin V/PI staining. Histograms (C, F) and representative flow cytometry plots (I, J) of cell apoptosis alterations. LR: early apoptotic cells; UR: late apoptotic cells. The numbers represent the combined percentages of apoptotic cells in the LR and UR quadrants. Non‐transfected cells were used as a control. The data are presented as the means ± SD from three separate experiments. *P < 0.05 vs. siRNA; **P < 0.01 vs. siRNA; ***P < .001 vs. siRNA NC; # P < 0.05 vs. pcDNA3.1; ## P < 0.1 vs. pcDNA3.1; ### P < .001 vs. pcDNA3.1. NC: negative control
Figure 4.
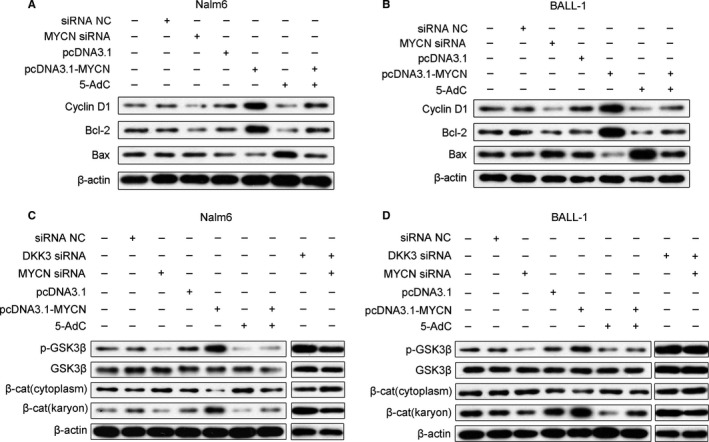
Effects of MYCN and 5‐AdC on the expression of proteins related to the cell cycle, apoptosis and the Wnt/β‐catenin signalling pathway. Nalm6 and BALL‐1 cells were transfected with MYCN siRNA or pcDNA3.1‐MYCN and/or treated with 5‐AdC. (A, B) Representative Western blots show the expression of cyclin D1, Bcl‐2 and Bax in Nalm6 and BALL‐1 cells. (C, D) Representative Western blots show the expression of p‐GSK3β, GSK3β and β‐catenin (cytoplasmic and nuclear) in Nalm6 and BALL‐1 cells. Untreated cells were used as a control, and the protein levels were measured by Western blots from three separate experiments; β‐actin was used as a loading control. NC: negative control, β‐cat: β‐catenin
MYCN can directly down‐regulate DKK3 expression (Figure 2). DKK3 is reported to be a putative antagonist of the Wnt/β‐catenin signalling pathway,7 and activation of this pathway has been implicated in the pathogenesis of leukaemia.24, 25, 26 To explore the effects of MYCN on the Wnt/β‐catenin signalling pathway, we measured the protein levels of p‐GSK3β, GSK3β and both cytoplasmic and nuclear β‐catenin in Nalm6 and BALL‐1 cells by Western blotting. In contrast to DKK3 protein levels (Figure 2E,F), the p‐GSK3β and nuclear β‐catenin protein levels were increased in MYCN‐overexpressing cells. However, p‐GSK3β and nuclear β‐catenin expression were decreased after MYCN siRNA and 5‐AdC treatment (Figure 4C,D). These results suggested that MYCN activates the Wnt/β‐catenin signalling pathway but that MYCN siRNA or 5‐AdC suppresses this pathway. Interestingly, knockdown of both MYCN and DKK3 increased p‐GSK3β and nuclear β‐catenin protein levels, suggesting activation of the Wnt/β‐catenin pathway. When MYCN‐overexpressing Nalm6 and BALL‐1 cells were treated with 5‐AdC, nuclear β‐catenin and p‐GSK3β protein levels were increased compared with 5‐AdC treatment alone (Figure 4C,D).
3.4. MYCN shRNA exerts antitumour effects in mice with Nalm6 cell xenografts
To assess the potential of MYCN shRNA therapy in vivo, we tested the antitumour effects of MYCN shRNA in a mouse model. During the 22‐day observation period, tumour growth was inhibited in mice treated with MYCN shRNA (Figure 5A). At the end of the 22 days, the tumour weights (Figure 5B) and the tumour volumes (Figure 5C) were significantly lower in the MYCN shRNA‐treated mice. The morphologies of Nalm6 tumour xenograft cells were examined after H&E staining. A greater percentage of the tumour cells derived from MYCN shRNA‐treated mice exhibited characteristics of apoptosis, such as cell volume shrinkage, nuclear pyknosis and prominent apoptotic bodies (Figure 5D). The number of TUNEL‐positive cells significantly increased (Figure 5D,E) upon MYCN shRNA treatment, which suggested that MYCN shRNA induces apoptosis in vivo.
Figure 5.
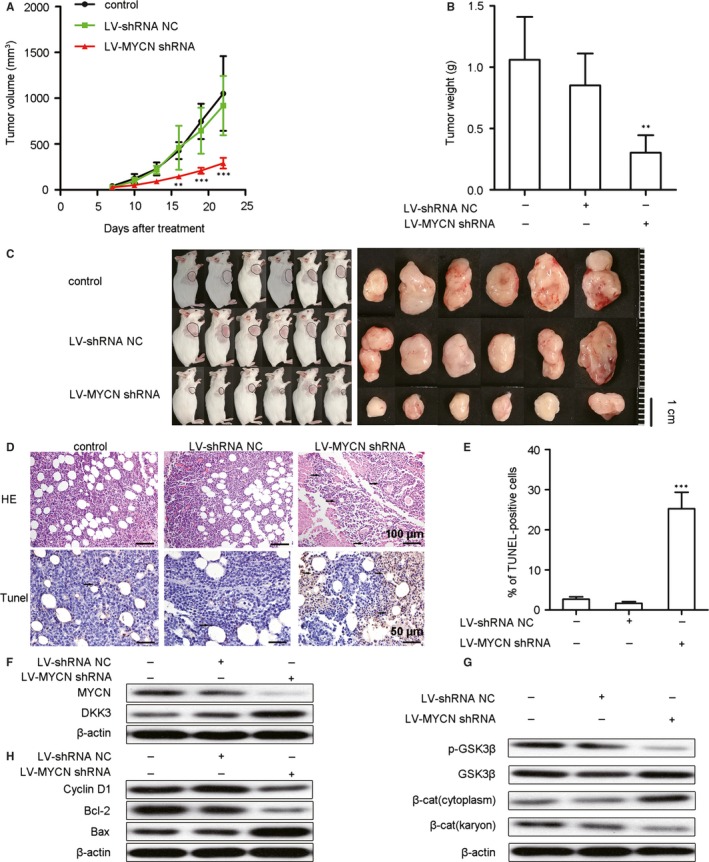
MYCN shRNA suppresses tumour growth via the restoration of DKK3‐mediated inhibition of the Wnt/β‐catenin pathway in a murine Nalm6 xenograft model. Mice burdened with growing Nalm6 tumours were infected with Lv‐shRNA NC or Lv‐MYCN shRNA or left untreated (control), and the tumour sizes (A) were measured for 22 days (n = 6). The tumour weights (B) were also measured after 22 days (n = 6). Images of tumour‐bearing mice (C) show that the tumours were smaller in MYCN shRNA‐treated animals. (D) Haematoxylin and eosin (H&E) (upper) and TUNEL (lower) staining of the xenograft tumour tissues from 3 mice of each group. In the H&E‐stained sections, the original magnification was 200 × . In the TUNEL‐stained sections, positive cells are indicated by brown staining, with an original magnification of 400 × . Representative fields are shown. Arrows indicate tumour cells. (E) The TUNEL‐positive cells were measured from 3 randomly chosen fields (3 × 104 μm2/field; n = 9). (F) Representative Western blots show the protein expression of MYCN and DKK3. (G) Representative Western blots show the expression of p‐GSK3β, GSK3β and β‐catenin (cytoplasmic and nuclear). (H) Representative Western blots show the expression of Bax, Bcl‐2 and cyclin D1. Expression of proteins was measured by Western blotting, and β‐actin was used as a loading control; we used 3 mice from each group. All data are presented as the means ± SD. **P < .01 vs. Lv‐shRNA NC; ***P < .001 vs. Lv‐shRNA NC. NC: negative control, β‐cat: β‐catenin
To further address the mechanisms that underlie the effects of MYCN shRNA, we analysed proteins downstream of Wnt/β‐catenin signalling and apoptosis‐relevant proteins by Western blotting. In contrast to the increased DKK3 protein levels in murine tumour tissues (Figure 5F), both p‐GSK3β and nuclear β‐catenin expression decreased after MYCN shRNA treatment (Figure 5G). The suppression of MYCN expression increased the levels of Bax and decreased the levels of Bcl‐2 and cyclin D1 proteins in cell‐derived tumour xenografts (Figure 5H). These findings suggested that MYCN shRNA inhibits the Wnt/β‐catenin signalling pathway, enhances apoptosis and restores DKK3 levels in vivo.
4. DISCUSSION
In this study, we identified MYCN as a strong marker for the onset and progression of adult B‐ALL. We provide evidence that MYCN expression is markedly up‐regulated in newly diagnosed and relapsed adult B‐ALL but is down‐regulated in patients who achieve CR. MYCN overexpression has been reported in haematologic malignancies, such as lymphoma,27 chronic lymphocytic leukaemia (CLL) 28 and paediatric T‐ALL,29 and it is considered a well‐established marker of a poor prognosis in these diseases. In addition, a previous study showed that MYCN overexpression rapidly led to acute myeloid leukaemia (AML) in mice.30 MYCN was able to induce pre‐B‐ALL/LBL directly from the progenitor B cells of mice in the absence of Ink4a and Arf.31 In our experiments, MYCN promoted cell proliferation and inhibited apoptosis. However, MYCN knockdown significantly inhibited tumour growth and promoted apoptosis in vivo and in vitro. This finding suggests that MYCN plays a proto‐oncogenic role and indicates that MYCN is a potential component of adult B‐ALL pathogenesis and may therefore be a viable candidate for targeted therapy.
The reduced expression of DKK3 has become a hallmark of several haematologic malignancies, such as ALL, CLL, AML and myelodysplastic syndrome, and its down‐regulation is associated with a poor prognosis in patients.11, 32, 33, 34 DKK3 promoter hypermethylation is associated with DKK3 silencing in ALL cells, and DKK3 expression was restored after exposure to 5‐AdC, which indicates that hypermethylation is one of the mechanisms by which DKK3 is silenced in ALL cells.11, 23 These previous results are consistent with our current work. However, when MYCN‐overexpressing Nalm6 and BALL‐1 cells were treated with 5‐AdC, DKK3 mRNA and protein levels decreased compared with 5‐AdC treatment alone, which indicates that methylation is not the only mechanism of DKK3 silencing. MYCN might induce another mechanism of DKK3 silencing such that 5‐AdC alone is unable to restore DKK3 expression. In previous studies, MYC has been shown to associate with DNA methyltransferases, which induce the transcriptional silencing of target genes in neuroblastoma18, 35, 36; this suggests that MYCN might play a similar role in the hypermethylation of DKK3. However, we found that the modulation of MYCN silencing had no effects on DKK3 promoter methylation in B‐ALL cell lines. This finding indicates that MYCN might silence DKK3 gene expression but not via methylation.
In this study, we also found a low DKK3 expression level in B‐ALL cell lines and in patient samples from adult B‐ALL obtained at initial diagnosis and at relapse. Our results show that DKK3 may be a tumour suppressor in adult B‐ALL. DKK3 may contribute to the suppression of tumours by virtue of its ability to antagonize Wnt signalling. The Wnt/β‐catenin pathway plays a crucial role in haematopoietic differentiation,37 and aberrant activation Wnt/β‐catenin signalling has been linked to haematologic malignancies, including AML and ALL.26, 38, 39 Therefore, it can be speculated that the silencing of DKK3 gene expression leads to the activation of the Wnt/β‐catenin signalling pathway, which is involved in cancer development. In this study, the knockdown of MYCN suppressed the Wnt/β‐catenin signalling pathway in vitro and in vivo. Depletion of DKK3 by siRNA eliminated the inhibition effect of MYCN siRNA on the Wnt/β‐catenin pathway in vitro. These results demonstrated that MYCN siRNA restored the DKK3‐mediated inhibition of the Wnt/β‐catenin signalling pathway. When MYCN‐overexpressing Nalm6 and BALL‐1 cells were treated with 5‐AdC, the nuclear β‐catenin and p‐GSK3β protein levels remained increased, which suggests that 5‐AdC cannot by itself suppress the Wnt/β‐catenin signalling pathway in vitro. The suppression of MYCN expression may be another key factor for the inhibition of the Wnt/β‐catenin signalling pathway, and this effect may result from the restoration of DKK3 levels.
In this manuscript, we also found that MYCN directly binds to the DKK3 promoter; furthermore, MYCN overexpression significantly down‐regulated DKK3 mRNA and protein levels in two B‐ALL cell lines. This finding suggests that MYCN may regulate DKK3 at both the transcriptional and translational levels. DKK3 expression has previously been related to MYCN and c‐MYC gene expression levels.40, 41 We confirmed the previously reported inverse relationship between DKK3 and MYCN gene expression in neuroblastic cell lines.40 MYCN has been shown to directly bind to the promoter of some genes to drive transcription; these genes include SKP2, NDRG1, TG2 and HMGA1.17, 42 However, previous studies did not find a direct relationship between MYCN and DKK3. Here, we confirmed direct targeted down‐regulation of DKK3 by MYCN in B‐ALL cell lines, which is a strong indication that this newly discovered molecular interaction between MYCN and the upstream Wnt/β‐catenin pathway is relevant to the biology of adult B‐ALL.
Our analyses therefore show that MYCN directly down‐regulates DKK3, which results in Wnt/β‐catenin signalling pathway activation. This novel regulatory cascade might function in the regulation of the Wnt/β‐catenin signalling pathway, which is involved in the genesis and development of adult B‐ALL. The inhibition of this pathway by DKK3 and the release of this MYCN‐mediated suppression might therefore have important biological consequences in the prevention and treatment of adult B‐ALL. However, due to the small number of patients in our study, we could not definitively address whether MYCN has any predictive value for the prognosis of adult B‐ALL. Therefore, we analysed published gene expression data from The Cancer Genome Atlas and Gene Expression Omnibus, but we did not find abnormal MYCN expression or prognosis‐related data for adult B‐ALL. Thus, further research with a larger cohort will be needed to assess whether MYCN is predictive for adult B‐ALL relapse. In addition, the HDAC inhibitor vorinostat has been shown to down‐regulate MYCN mRNA and protein levels in neuroblastoma.43, 44, 45 In the present study, we confirmed that 5‐AdC has a similar effect. We therefore believe that siRNA either alone or in combination with epigenetic drugs may be of potential therapeutic significance for MYCN‐amplified adult B‐ALL.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
ACKNOWLEDGEMENTS
This study was supported by the Foundation for the Returned Overseas Chinese Scholars of Heilongjiang Province (LCO7C22) and the Overseas Scholars Education Department of Heilongjiang Province (1153h12).
Kong D, Zhao L, Sun L, et al. MYCN is a novel oncogenic target in adult B‐ALL that activates the Wnt/β‐catenin pathway by suppressing DKK3 . J Cell Mol Med. 2018;22:3627–3637. https://doi.org/10.1111/jcmm.13644
Contributor Information
Jin Zhou, Email: jin_zhouxy@163.com.
Yinghua Li, Email: liyinghua1965@aliyun.com.
REFERENCES
- 1. Paul S, Kantarjian H, Jabbour EJ. Adult acute lymphoblastic leukemia. Mayo Clin Proc. 2016;91:1645‐1666. [DOI] [PubMed] [Google Scholar]
- 2. Al Ustwani O, Gupta N, Bakhribah H, et al. Clinical updates in adult acute lymphoblastic leukemia. Crit Rev Oncol Hematol. 2016;99:189‐199. [DOI] [PubMed] [Google Scholar]
- 3. Frey NV, Luger SM. How I treat adults with relapsed or refractory Philadelphia chromosome‐negative acute lymphoblastic leukemia. Blood. 2015;126:589‐596. [DOI] [PubMed] [Google Scholar]
- 4. Zhao Y, Huang H, Wei G. Novel agents and biomarkers for acute lymphoid leukemia. J Hematol Oncol. 2013;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hay KA, Turtle CJ. Chimeric antigen receptor (CAR) T cells: lessons learned from targeting of CD19 in B‐cell malignancies. Drugs. 2017;77:237‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor‐modified T cells for acute lymphoid leukemia. N Eng J Med. 2013;368:1509‐1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hara K, Kageji T, Mizobuchi Y, et al. Blocking of the interaction between Wnt proteins and their co‐receptors contributes to the anti‐tumor effects of adenovirus‐mediated DKK3 in glioblastoma. Cancer Lett. 2015;356:496‐505. [DOI] [PubMed] [Google Scholar]
- 8. Xiang T, Li L, Yin X, et al. Epigenetic silencing of the WNT antagonist Dickkopf 3 disrupts normal Wnt/beta‐catenin signalling and apoptosis regulation in breast cancer cells. J Cell Mol Med. 2013;17:1236‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fang L, Cai J, Chen B, et al. Aberrantly expressed miR‐582‐3p maintains lung cancer stem cell‐like traits by activating Wnt/beta‐catenin signalling. Nat Commun. 2015;6:8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Veeck J, Dahl E. Targeting the Wnt pathway in cancer: the emerging role of Dickkopf‐3. Biochem Biophys Acta. 2012;1825:18‐28. [DOI] [PubMed] [Google Scholar]
- 11. Roman‐Gomez J, Cordeu L, Agirre X, et al. Epigenetic regulation of Wnt‐signaling pathway in acute lymphoblastic leukemia. Blood. 2007;109:3462‐3469. [DOI] [PubMed] [Google Scholar]
- 12. Ueno K, Hirata H, Shahryari V, et al. microRNA‐183 is an oncogene targeting Dkk‐3 and SMAD4 in prostate cancer. Br J Cancer. 2013;108:1659‐1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Q, Shen K, Zhao Y, et al. MiR‐92b inhibitor promoted glioma cell apoptosis via targeting DKK3 and blocking the Wnt/beta‐catenin signaling pathway. J Transl Med. 2013;11:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Y, Li H, Cao R, et al. Suppression of miR‐708 inhibits the Wnt/beta‐catenin signaling pathway by activating DKK3 in adult B‐all. Oncotarget. 2017;8:64114‐64128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gustafson WC, Weiss WA. Myc proteins as therapeutic targets. Oncogene. 2010;29:1249‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xue C, Yu DM, Gherardi S, et al. MYCN promotes neuroblastoma malignancy by establishing a regulatory circuit with transcription factor AP4. Oncotarget. 2016;7:54937‐54951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Evans L, Chen L, Milazzo G, et al. SKP2 is a direct transcriptional target of MYCN and a potential therapeutic target in neuroblastoma. Cancer Lett. 2015;363:37‐45. [DOI] [PubMed] [Google Scholar]
- 18. Charlet J, Szemes M, Malik KT, et al. MYCN is recruited to the RASSF1A promoter but is not critical for DNA hypermethylation in neuroblastoma. Mol Carcinog. 2014;53:413‐420. [DOI] [PubMed] [Google Scholar]
- 19. Wu N, Jia D, Bates B, et al. A mouse model of MYCN‐driven retinoblastoma reveals MYCN‐independent tumor reemergence. J Clin Investig. 2017;127:888‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wong M, Tee AE, Milazzo G, et al. The histone methyltransferase DOT1L promotes neuroblastoma by regulating gene transcription. Can Res. 2017;77:2522‐2533. [DOI] [PubMed] [Google Scholar]
- 21. von Bueren AO, Kortmann RD, von Hoff K, et al. Treatment of children and adolescents with metastatic medulloblastoma and prognostic relevance of clinical and biologic parameters. J Clin Oncol. 2016;34:4151‐4160. [DOI] [PubMed] [Google Scholar]
- 22. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937‐951. [DOI] [PubMed] [Google Scholar]
- 23. Roman‐Gomez J, Jimenez‐Velasco A, Agirre X, et al. Transcriptional silencing of the Dickkopfs‐3 (Dkk‐3) gene by CpG hypermethylation in acute lymphoblastic leukaemia. Br J Cancer. 2004;91:707‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luis TC, Ichii M, Brugman MH, et al. Wnt signaling strength regulates normal hematopoiesis and its deregulation is involved in leukemia development. Leukemia. 2012;26:414‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hu K, Gu Y, Lou L, et al. Galectin‐3 mediates bone marrow microenvironment‐induced drug resistance in acute leukemia cells via Wnt/beta‐catenin signaling pathway. J Hematol Oncol. 2015;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gang EJ, Hsieh YT, Pham J, et al. Small‐molecule inhibition of CBP/catenin interactions eliminates drug‐resistant clones in acute lymphoblastic leukemia. Oncogene. 2014;33:2169‐2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin YW, Beharry ZM, Hill EG, et al. A small molecule inhibitor of Pim protein kinases blocks the growth of precursor T‐cell lymphoblastic leukemia/lymphoma. Blood. 2010;115:824‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rinaldi A, Mian M, Kwee I, et al. Genome‐wide DNA profiling better defines the prognosis of chronic lymphocytic leukaemia. Br J Haematol. 2011;154:590‐599. [DOI] [PubMed] [Google Scholar]
- 29. Astolfi A, Vendemini F, Urbini M, et al. MYCN is a novel oncogenic target in pediatric T‐cell acute lymphoblastic leukemia. Oncotarget. 2014;5:120‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kawagoe H, Kandilci A, Kranenburg TA, et al. Overexpression of N‐Myc rapidly causes acute myeloid leukemia in mice. Can Res. 2007;67:10677‐10685. [DOI] [PubMed] [Google Scholar]
- 31. Sugihara E, Shimizu T, Kojima K, et al. Ink4a and Arf are crucial factors in the determination of the cell of origin and the therapeutic sensitivity of Myc‐induced mouse lymphoid tumor. Oncogene. 2012;31:2849‐2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moskalev EA, Luckert K, Vorobjev IA, et al. Concurrent epigenetic silencing of wnt/beta‐catenin pathway inhibitor genes in B cell chronic lymphocytic leukaemia. BMC Cancer. 2012;12:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Valencia A, Roman‐Gomez J, Cervera J, et al. Wnt signaling pathway is epigenetically regulated by methylation of Wnt antagonists in acute myeloid leukemia. Leukemia. 2009;23:1658‐1666. [DOI] [PubMed] [Google Scholar]
- 34. Wang H, Fan R, Wang XQ, et al. Methylation of Wnt antagonist genes: a useful prognostic marker for myelodysplastic syndrome. Ann Hematol. 2013;92:199‐209. [DOI] [PubMed] [Google Scholar]
- 35. Vasanthakumar A, Lepore JB, Zegarek MH, et al. Dnmt3b is a haploinsufficient tumor suppressor gene in Myc‐induced lymphomagenesis. Blood. 2013;121:2059‐2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Falco G, Ambrosio MR, Fuligni F, et al. Burkitt lymphoma beyond MYC translocation: N‐MYC and DNA methyltransferases dysregulation. BMC Cancer. 2015;15:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brandon C, Eisenberg LM, Eisenberg CA. WNT signaling modulates the diversification of hematopoietic cells. Blood. 2000;96:4132‐4141. [PubMed] [Google Scholar]
- 38. Man CH, Fung TK, Wan H, et al. Suppression of SOX7 by DNA methylation and its tumor suppressor function in acute myeloid leukemia. Blood. 2015;125:3928‐3936. [DOI] [PubMed] [Google Scholar]
- 39. Yang Y, Mallampati S, Sun B, et al. Wnt pathway contributes to the protection by bone marrow stromal cells of acute lymphoblastic leukemia cells and is a potential therapeutic target. Cancer Lett. 2013;333:9‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koppen A, Ait‐Aissa R, Koster J, et al. Dickkopf‐3 expression is a marker for neuroblastic tumor maturation and is down‐regulated by MYCN. Int J Cancer. 2008;122:1455‐1464. [DOI] [PubMed] [Google Scholar]
- 41. Bell E, Lunec J, Tweddle DA. Cell cycle regulation targets of MYCN identified by gene expression microarrays. Cell Cycle. 2007;6:1249‐1256. [DOI] [PubMed] [Google Scholar]
- 42. Bell E, Chen L, Liu T, et al. MYCN oncoprotein targets and their therapeutic potential. Cancer Lett. 2010;293:144‐157. [DOI] [PubMed] [Google Scholar]
- 43. Marshall GM, Gherardi S, Xu N, et al. Transcriptional upregulation of histone deacetylase 2 promotes Myc‐induced oncogenic effects. Oncogene. 2010;29:5957‐5968. [DOI] [PubMed] [Google Scholar]
- 44. Sun Y, Liu PY, Scarlett CJ, et al. Histone deacetylase 5 blocks neuroblastoma cell differentiation by interacting with N‐Myc. Oncogene. 2014;33:2987‐2994. [DOI] [PubMed] [Google Scholar]
- 45. Cortes C, Kozma SC, Tauler A, et al. MYCN concurrence with SAHA‐induced cell death in human neuroblastoma cells. Cell Oncol. 2015;38:341‐352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


