Abstract
Ganoderma lucidum is an edible medicinal mushroom known as “Lingzhi” in China and “Reishi or Manetake” in Japan. It is a highly prized vitality‐enhancing herb for more than 2000 years. G. lucidum polysaccharide (GLPS) has been identified as one of the major bioactive components and developed into a drug named “Ji 731 Injection” in China since 1973. The large‐scale production of the drug began in 1985 and approved by the Chinese FDA as “Polysaccharidum of G. lucidum Karst Injection” (Ling Bao Duo Tang Zhu She Ye) in 2000, which is applied intramuscularly. After more than forty years of clinical use, its efficacy, safety and long‐term tolerability have been recognized by neurologists. It is one of a few non‐hormonal drugs used for treating refractory myopathy. It is also used for combination therapy, which reduces the amount of glucocorticoid required for myopathy patient who is in remission. In addition, it reduces adverse reactions and improves the quality of life for cancer patients during chemotherapy. We found 81 qualified chemical, biochemical, preclinical and clinical studies of GLPS both in English and in Chinese spanning from 1973 to 2017 by searching CNKI (China National Knowledge Infrastructure), Wanfang database and PubMed. The molecular mechanisms underlying GLPS's antioxidant, anti‐tumour, immune‐modulatory, hypoglycaemic, hypolipidaemic and other activities are discussed. Both preclinical and clinical studies are either deliberated or indexed in the current article. We aimed at providing a molecular picture as well as a clinical basis to comprehend GLPS as one of few polysaccharide‐based modern medicines with complicated chemical and pharmacological properties that prevent it from entering the world's market.
Keywords: cancer, clinical studies, Ganoderma lucidum polysaccharide, immunotherapeutic, myopathy
1. INTRODUCTION
Ganoderma is a genus of polypore mushrooms that grow on wood that include about 80 species. Ganoderma lucidum, an edible medicinal mushroom known as “Lingzhi” in China and “Reishi or Manetake” in Japan 1 (Figure 1), has been used for promoting health and for extending life in China and in other East Asia countries for more than 2000 years.2 There are multiple species of Lingzhi, scientifically known to be within the Ganoderma lucidum species complex.3 “Lingzhi” includes both G. lucidum and G. sinense as recorded in Chinese Pharmacopoeia of 2010.4, 5, 6
Figure 1.

The picture of Ganoderma lucidum
The fungi, mycelia and spores of G. lucidum contain about 400 different bioactive substances, including polysaccharides, triterpenoids, nucleotides, sterols, steroids, fatty acids, proteins/peptides and trace elements. Among them, G. lucidum polysaccharide (GLPS) has been identified as one of the major bioactive components, showing most physiological and health‐promoting effects acclaimed for G. lucidum, such as anti‐tumour7, 8, immune‐modulatory,8, 9 antioxidant,10, 11, 12, 13 hypoglycaemic14, 15 and other activities.
Ganoderma lucidum polysaccharide is the major component by weight among all constituents in the spores of G. lucidum. Over 200 polysaccharides have been isolated and structurally articulated in the fruiting bodies, mycelia and spores of G. lucidum; however, modern analytical chemistry is still revealing new polysaccharides from G. lucidum.12 Glucose, mannose, galactose, xylose, fucose and arabinose have been identified in GLPS, and only β‐glucan, a pure glucose polymer, is believed to be one of the active ingredients in GLPS.10, 16 The β‐glucan structure in GLPS is shown in Figure 2.
Figure 2.
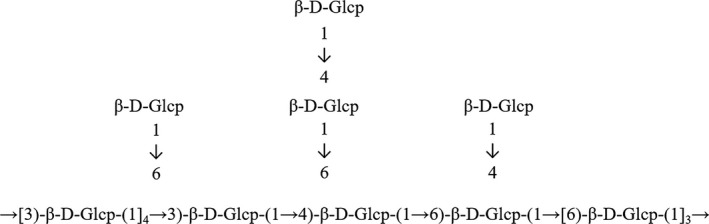
Structure of β‐glucan in Ganoderma lucidum polysaccharide, modified from Wang et al (2017)
Ganoderma lucidum is cultivated artificially on media of wood meal, rice bran and wood blocks in the last 10 years in China, Japan and the United States. However, these techniques do not guarantee a standardized yield as the compositions and structures of GLPS varied from batch to batch due to its non‐template biosynthesis. Baskar et al17, 18 reported that using statistical and evolutionary optimization methods and response surface methodology could enhance the production of G. lucidum MTCC 1039 in submerged culture. Thus far, how to standardize the biological activity and chemical structures of GLPS is still a pending issue.
So far, several G. lucidum‐based drugs are authorized by State Food and Drug Administration of China (SFDA) for clinical use. Table 1 shows the names, components, benefits, SFDA‐certified numbers and the number of manufacturers that produce the specific G. lucidum‐based drugs.
Table 1.
Ganoderma lucidum‐based drugs approved by SFDA in China
| Drug name | Components | Benefits | Drug numbers | No. of manufacturers |
|---|---|---|---|---|
| Ganoderma capsules |
Ganoderma
extracts |
Insomnia, forgetfulness, physical weakness, neurasthenia |
Z35020559 Z19993169 Z34020705 Z00812004 Z13021412 |
65 |
| Ganoderma tablet |
Ganoderma
extracts |
Insomnia, forgetfulness, physical weakness, neurasthenia |
Z45022158 Z45020362 Z21021884 Z13021413 Z36020672 |
23 |
| Compound Ganoderma lucidum granules |
Ganoderma
extracts |
Chronic hepatitis |
Z11021221 Z13020664 Z12020282 Z13020314 Z13020314 |
16 |
| Ganoderma lucidum syrup | – | Palpitations, insomnia, loss of appetite, neurasthenia |
Z53021201 Z32020893 Z45021449 Z36020812 Z32020921 |
12 |
| Ganoderma lucidum slices | Ganoderma lucidum extract | Chronic hepatitis, neurasthenia, dizziness, insomnia, gastrointestinal ulcers, chronic bronchitis Coronary heart disease |
Z20043150 Z20043199 Z20043459 Z34020483 Z20053282 |
7 |
| Ganoderma longan wine | Ganoderma lucidum extract | Frail, post‐partum weakness, anaemia |
Z45021420 Z45021095 Z36020578 Z45020259 Z36020662 Z45022143 |
6 |
| Ganoderma lucidum spore capsules | Ganoderma lucidum spores | Heart and spleen deficiency, adjuvant therapy for cancer patients |
B20040034 B20050008 B20040033 B20040035 |
4 |
| Ganoderma granules | Ganoderma lucidum extract |
Insomnia, forgetfulness, physical weakness, neurasthenia |
Z19993223 Z35020571 Z45021930 Z45020361 |
4 |
| Ganoderma oral liquid | – |
Insomnia, forgetful, fatigue |
Z20000010 Z20025750 |
2 |
| Ginseng Ganoderma capsules | – | Physical weakness | Z20028001 | 1 |
| Ganoderma dispersible tablets | Ganoderma lucidum paste | Insomnia, forgetfulness, physical weakness, neurasthenia, chronic bronchitis | Z20090141 | 1 |
| Ganoderma dripping pills | Ganoderma lucidum extract | Insomnia, forgetfulness, physical weakness, neurasthenia | Z20090778 | 1 |
| Ganoderma lucidum Gynostemma oral liquid | Ganoderma lucidum extract | Improve palpitations, insomnia | B20020517 | 1 |
| Polysaccharidum of Ganoderma lucidum Karst Injection | Ganoderma lucidum Karst spore powder extract |
Neurosis, polymyositis, dermatomyositis, atrophic myotonia Progressive muscular dystrophy |
H20003510 H20003123 |
2 |
| Polysaccharidum of Ganoderma lucidum Karst Injection | Ganoderma lucidum Karst spore powder extract |
Neurosis, polymyositis, dermatomyositis, atrophic myotonia Progressive muscular dystrophy |
H20051702 | 1 |
GLPS, Ganoderma lucidum polysaccharides.
A G. lucidum polysaccharide‐based product named “Ji 731 Injection” was used clinically for treating myopathy in China since 1973. The drug was independently developed by the Institute of Chinese Medical Sciences and its affiliated pharmaceutical factory (the Predecessor of Beijing Union Pharmaceutical Factory). Because of this, the institute won the 1978 National Award for Science and Technology. In 1985, it was renamed as “Ji Sheng Injection” before its large‐scale production. In 2000, State Food and Drug Administration of China (SFDA) approved and renamed the drug as “Polysaccharidum of G. lucidum Karst Injection” (Lin Bao Duo Tang Zhu She Ye) where the “Polysaccharidum of G. lucidum” is extracted from the spore of G. lucidum. The timeline for the drug development is shown in Figure 3.
Figure 3.
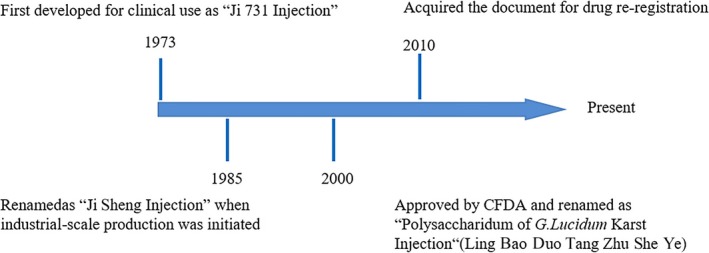
Timeline for developing Polysaccharidum of Ganoderma lucidum Karst Injection
Polysaccharidum of G. lucidum Karst Injection is applied intramuscularly. At present, this product is used for treating neurosis, polymyositis, dermatomyositis, atrophic myotonia and muscular dystrophy, and various diseases caused by a defective immune system. After more than forty years of clinical use, its efficacy, safety and long‐term tolerability have been recognized by neurologists. It is one of the few non‐hormonal drugs used for treating refractory myopathy. It is also used for combination therapy, which reduces the amount of glucocorticoid required for myopathy patient who is in remission. In addition, Polysaccharidum of G. lucidum Karst Injection reduces adverse reactions during chemotherapy and improves the quality of life of cancer patients significantly.
Based on CNKI (China National Knowledge Infrastructure), Wanfang database, and PubMed searches performed with keywords “Ganoderma lucidum polysaccharide,” “Ji 731,” “Ji Sheng,” “Polysaccharide of G. lucidum Karst Injection” and “Ling Bao Duo Tang Zhu She Ye” both in English and in Chinese, we pulled out all of the publications. After reviewing the description about the GLPS used, the methods, data, results and conclusions, we found 81 qualified studies of GLPS both in English and in Chinese spanning from 1979 to 2017. A summary of the major biological activities of GLPS described in the qualified reports is shown in Figure 4, which demonstrates its immune‐regulatory, antioxidant, anti‐tumour, hypoglycaemic, hypolipidaemic, anti‐myositis, anti‐radiation, cardiac‐protecting, sedative, hypnotic, lumbocrural pain relief, antidepressant, antibacterial, blood circulation–promoting, blood stasis–removing, hepatoprotective, anti‐facial paralysis, anti‐streptozotocin‐induced diabetic nephropathy, anti‐ageing, anti‐bleomycin‐induced pulmonary fibrosis and anti‐chronic pancreatitis effects of GLPS.
Figure 4.
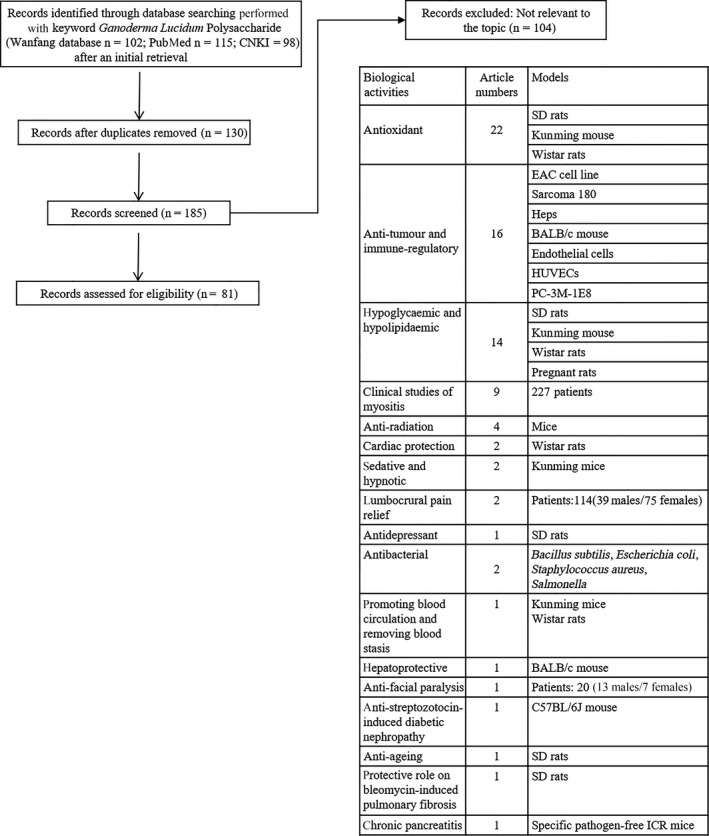
Flow chart of screened and analysed studies. Based on China National Knowledge Infrastructure (CNKI), Wanfang database and PubMed searches performed with keywords “Ganoderma lucidum polysaccharide,” “Ji 731,” “Ji Sheng,” “Polysaccharide of Ganoderma lucidum Karst Injection” and “Ling Bao Duo Tang Zhu She Ye,” we pulled out all of the publications. After reviewing the description about the Ganoderma lucidum polysaccharides (GLPS) used, the methods, data, results and conclusions, we found 81 qualified studies of GLPS both in English and in Chinese spanning from 1973 to 2017
2. IMMUNOMODULATORY AND ANTI‐TUMOUR ACTIVITIES OF GLPS
Multiple studies demonstrated that GLPS is a potent immunomodulator that exerts a significant and comprehensive impact on immune cells including B lymphocytes, T lymphocytes, NKs, macrophages and Dendritic cells (DCs). These immunomodulatory effects are likely to have been mediated by its complex multiple components and can be one of the underlying anti‐tumour mechanisms of GLPS to some extent. The potential effects of GLPS on immune cells are summarized in Figure 5.
Figure 5.
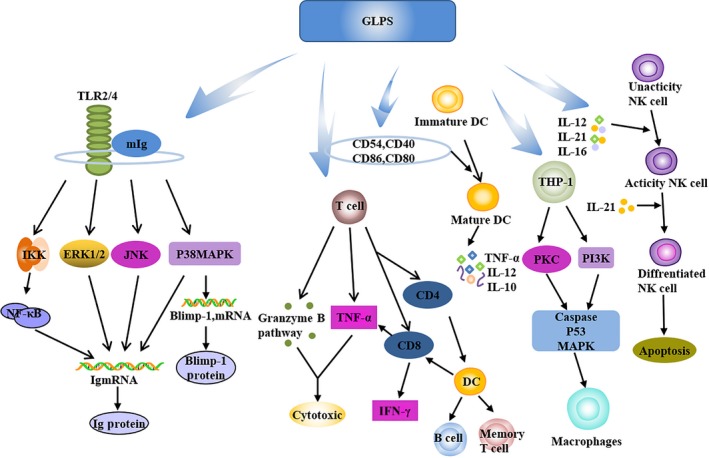
Immunomodulatory and anti‐tumour activities of Ganoderma lucidum polysaccharides (GLPS), modified from Xu et al (2011). Both innate and acquired immunities are enhanced by GLPS through activating T cells, B cells, NK cells, DC cells, antigen‐presenting cells and tissue macrophages accompanied by releasing a variety of chemokines, cytokines and growth factors
Bao et al19 found that GLPS enhances T‐cell and B lymphocyte proliferation to some extent. Shao et al found that GLPS‐mediated B‐cell activation requires cell membrane Ig (mIg) and toll‐like receptor 4 (TLR4), which is also involved in GLPS‐mediated macrophage activation. GLPS enhances antibody secretion associated with Blimp‐1 mRNA induction. The biological function of Reishi‐F3 (a preparation of GLPS) is TLR4/TLR2‐dependent, and the interaction of Reishi‐F3 with TLR4/TLR2 is followed by the induction of the signal transduction of p38 mitogen‐activated protein kinase (p38 MAPK) in Blimp‐1 mRNA. At the same time, Reishi‐F3‐mediated Ig secretion is involved in the signalling of Extracellular signal‐regulated kinase, p38 MAPK, JNK and IκB kinase complexes. Cao et al20 found that GLPS can increase the expression of IFN‐γ mRNA and granzyme B protein expression. It can also promote the cytotoxicity of T lymphocytes induced by DC‐specific, and the mechanism of cytotoxicity is presumed to be mediated by IFN‐γ and granzyme‐mediated B pathway. GLPS promotes the release of TNF‐γ and IFN‐γ in T lymphocytes in a dose‐dependent manner.21 Zhang et al22 showed that GLPS is a new B cell–stimulating factor, B‐cell percentage and mouse spleen B lymphocyte activation increased by three‐ to fourfolds.
Lin et al23 showed that the treatment of DC with GLPS results in enhanced cell surface expression of CD80, CD86, CD83, CD40, CD54 and human leucocyte antigen‐DR. GLPS‐induced activation and maturation of human monocyte–derived DCs are mediated by NF‐κB and p38 MAPK pathways. GLPS can also stimulate monocyte‐derived DC maturation. GLPS and Granulocyte macrophage colony‐stimulating factor (GMCSF)/IL‐4 simultaneously induced THP‐1 cells to transform into a typical DC form, whereas GLPS alone induced proliferation in THP‐1 and U937 cells alone. Furthermore, the transformation of THP‐1, DCs results in a significant increase in the expression of HLA‐DR, CD40, CD80, and CD86 and in similar antigen‐uptake capability. Thus, GLPS can effectively promote the activation and maturation of immature DCs, preferring the Th1 response.24
GLPS from G. lucidum spores can increase the volume of macrophages and their ability to devour milk beads.25 The pharmacological inhibitor assay showed that the ability of GLPS to enhance phagocytosis and chemotactic neutrophil function is mediated by PI3K, p38 MAPK, Src tyrosine kinase and Protein kinase C.26 Chien et al27 showed that the fucose‐containing glycoprotein fraction (F3) isolated from the water‐soluble extract of G. lucidum stimulated CD56+ NK activity in umbilical cord blood. After the F3 treatment, NK cell–mediated cytotoxicity is found to be significantly enhanced. The polysaccharide component of the branched (1→6)‐β‐D‐glucan moiety, named G. lucidum‐G (GS‐G) has been reported to exhibit anti‐tumour activity and to activate NK cells.28
3. BIOLOGICAL ACTIVITIES OF GLPS
3.1. Antioxidant activities of GLPS
3.1.1. Scavenging free radicals by GLPS
Free radicals refer to atoms, molecules or ions that have unpaired electrons. These unpaired electrons make free radicals highly chemically reactive towards other substances or even towards themselves. Reactive oxidative species (ROS) (such as ,˙OH, H2O2, R˙, RO˙, ROO˙) and Reactive nitrogen species (such as NO˙, ONOO−) are biologically relevant free radicals. Under normal circumstances, human body constantly produces and removes free radicals to maintain a dynamic balance. A moderate number of free radicals in the body play an active role in cell division, differentiation, growth and elimination of bacteria and parasites. If free radicals are excessively produced or insufficiently removed, they can cause host protein denaturation, enzyme inactivation, nucleic acid metabolic abnormalities and the formation of lipid peroxides. The free radicals also cause the formation of protein aggregates through cross‐linking, which damages the structure and function of a cell, promotes senility and creates various pathological states in human and animals.
In general, all known polysaccharides have antioxidant activity that can be measured by mixing with a free radical compound DPPH. Several studies showed that GLPS can directly scavenge oxygen free radicals in a GLPS concentration–dependent manner.29, 30 Malondialdehyde (MDA) is a lipid peroxide formed when oxygen free radicals attack polyunsaturated fatty acids. Pretreatment of GLPS significantly reduced levels of ROS and MDA produced by myocardial cells; thus, GLPS plays a direct role in reducing myocardial oxidative stress.31 Similarly, when PC12 cells are pre‐treated for 2 hours with GLPS, it antagonizes the oxidative stress induced by Amyloid β(25‐35) (Aβ25‐35) that elevates the level of intracellular ROS.32
3.1.2. Inhibiting oxidative stress in different animal models
In reality, by catching ROS, GLPS blocks or slows oxidative stress in different animal models (Table 2). In a rat model of Aβ‐induced Alzheimer's disease, GLPS inhibits the increased level of MDA both in serum and in hippocampus and protects rat neurons from oxidative stress.33, 34 GLPS also reduces lipid peroxidation in skeletal muscle of exhausted mice and ultimately protects the liver and skeletal muscle from oxidative stress.16 In a rabbit model of liver ischemia‐reperfusion injury, GLPS reduces the level of MDA significantly in liver and prevents liver from damage caused by oxygen free radical–mediated lipid peroxidation.31
Table 2.
Antioxidant effect of GLPS in different mouse and rat models
| Models | MDA/(μmol/L) | SOD/(U/L) | GSH‐Px/(U/L) | References | ||||
|---|---|---|---|---|---|---|---|---|
| Groups | Concentration | P‐value | Concentration | P‐value | Concentration | P‐value | ||
| SD rats cardiomyocytes | Normal control | 0.27 ± 0.032 | – | 40.85 ± 2.98 | – | – | – | 88 |
| H2O2 | 1.01 ± 0.078 | <.01 | 16.77 ± 2.01 | <.01 | – | – | ||
| PSG‐1‐L (25 mg/mL) | 0.59 ± 0.068 | <.01 | 26.98 ± 2.21 | <.01 | – | – | ||
| PSG‐1‐M (50 mg/mL) | 0.43 ± 0.045 | <.01 | 32.69 ± 2.89 | <.01 | – | – | ||
| PSG‐1‐H (100 mg/mL) | 0.32 ± 0.043 | <.01 | 38.98 ± 3.09 | <.01 | – | – | ||
| SD rats (10/group) | Normal control | 3.61 ± 1.27 | – | 149.10 ± 5.93 | – | – | – | 33 |
| AD model | 20.53 ± 3.34 | <.01 | 84.79 ± 7.7 | <.01 | – | – | ||
| GLPS treatment | 8.67 ± 2.67 | <.01 | 128.75 ± 11.67 | <.01 | – | – | ||
| SD rats (12/group) | Normal control | 66.11 ± 7.64 | – | 113.14 ± 18.61 | – | – | – | 34 |
| AD model | 14.18 ± 1.99 | – | 257.79 ± 38. 55 | – | – | – | ||
| GLPS treatment | 21.95 ± 5.79 | <.05 | 135.76 ± 7. 97 | <.05 | – | – | ||
| Kunming mouse (10/group) | Normal control (myocardium) | – | – | 84.30 ± 11.3 | – | 5.71 ± 0.77 | – | 34 |
| GLPS (myocardium) | – | – | 87.30 ± 10.2 | – | 7.11 ± 1.32 | <.01 | ||
| Normal control (skeletal muscle) | – | – | 43.2 ± 7.3 | – | 30.7 ± 3.1 | – | ||
| GLPS (skeletal muscle) | – | – | 54.2 ± 7.7 | <.01 | 29.3 ± 1.98 | – | ||
| Rabbits (10/group) | Normal control | 3.12 ± 0.43 | – | 326.59 ± 28.63 | – | – | – | 31 |
| Normal saline | 6.03 ± 0.29 | <.05 | 223.32 ± 35.50 | <.05 | – | – | ||
| GLPS | 4.86 ± 0.33 | <.05 | 278.15 ± 24.41 | <.05 | – | – | ||
| SD rats (10/group) | Normal control | 2.71 ± 0.87 | – | 249.56 ± 16.38 | – | 23.76 ± 4.56 | – | 38 |
| DM | 7.95 ± 1.49 | <.01 | 131.06 ± 9.40 | <.01 | 13.61 ± 2.53 | <.01 | ||
| GLPS‐L (200 mg/kg) | 7.95 ± 1.21 | – | 131.94 ± 11.43 | – | 13.79 ± 4.47 | – | ||
| GLPS‐M (400 mg/kg) | 4.11 ± 0.69 | <.01 | 178.15 ± 5.53 | <.01 | 19.46 ± 3.65 | <.05 | ||
| GLPS‐H (800 mg/kg) | 2.79 ± 0.49 | <.01 | 187.66 ± 6.59 | <.01 | 23.32 ± 2.54 | <.05 | ||
| Mouse (10/group) | Ovarian cancer | – | – | 261.21 ± 13.01 | – | 36.20 ± 6.20 | – | 40 |
| GLPS‐L (50 mg/kg) | – | – | 304.11 ± 13.13 | – | 44.82 ± 5.62 | – | ||
| GLPS‐M (100 mg/kg) | – | – | 315.84 ± 11.09 | – | 45.60 ± 7.72 | – | ||
| GLPS‐H (200 mg/kg) | – | – | 330.83 ± 12.17 | <.05 | 50.88 ± 15.42 | <.05 | ||
| Wistar rats (10/group) | Normal control | 205.71 ± 18.94 | – | 28.75 ± 1.83 | – | 39.75 ± 2.17 | – | 41 |
| Gastric cancer | 137.89 ± 11.27 | <.01 | 58.05 ± 4.01 | <.01 | 61.52 ± 4.31 | <.01 | ||
| GLPS‐L (400 mL/kg) | 189.33 ± 16.08 | <.01 | 49.05 ± 3.28 | <.01 | 61.52 ± 4.31 | <.01 | ||
| GLPS‐H (800 mL/kg) | 216.48 ± 18.49 | <.01 | 32.17 ± 2.17 | <.01 | 45.06 ± 2.22 | <.01 | ||
| SD rats (10/group) | Normal control | 2.82 ± 0.99 | – | 246.59 ± 16.18 | – | 23.72 ± 4.76 | – | 42 |
| DM | 7.98 ± 1.52 | <.01 | 132.05 ± 9.10 | <.01 | 13.52 ± 2.55 | <.01 | ||
| GLPS‐L (200 mg/kg) | 7.98 ± 1.15 | <.01 | 132.91 ± 11.33 | <.01 | 13.70 ± 4.41 | <.01 | ||
| GLPS‐M (400 mg/kg) | 4.06 ± 0.73 | <.01 | 176.16 ± 5.41 | <.01 | 19.42 ± 3.62 | <.01 | ||
| GLPS‐H (800 mg/kg) | 2.82 ± 0.55 | <.01 | 189.68 ± 6.55 | <.01 | 23.16 ± 2.41 | <.01 | ||
| Wistar rats (6/group) | Normal control | – | – | 9.61 ± 0.49 | – | 11.15 ± 0.95 | – | 43 |
| DM | – | – | 4.53 ± 0.07 | <.01 | 5.76 ± 0.43 | <.01 | ||
| GLPS‐L (60 mL/kg) | – | – | 5.07 ± 0.12 | <.01 | 7.39 ± 0.29 | <.01 | ||
| GLPS‐M (80 mL/kg) | – | – | 6.82 ± 0.54 | <.01 | 7.39 ± 0.29 | <.01 | ||
| GLPS‐H (120 mL/kg) | – | – | 8.67 ± 0.42 | <.01 | 9.26 ± 0.28 | <.01 | ||
| Models | Catalase/(U/L) | ROS | References | |||
|---|---|---|---|---|---|---|
| Groups | Concentration | P‐value | Concentration | P‐value | ||
| SD rats | Normal control | – | – | 15.11 ± 0.95 | – | 88 |
| H2O2 | – | – | 62.54 ± 3.35 | <.01 | ||
| PSG‐1‐L (25 mg/mL) | – | – | 26.09 ± 2.01 | <.01 | ||
| PSG‐1‐M (50 mg/mL) | – | – | 19.38 ± 1.83 | <.01 | ||
| PSG‐1‐H (100 mg/mL) | – | – | 16.52 ± 1.21 | <.01 | ||
| SD rats (10/group) | Normal control | – | – | – | – | 33 |
| AD model | – | – | – | – | ||
| GLPS | – | – | – | – | ||
| SD rats (12/group) | Normal control | – | – | – | – | 34 |
| AD model | – | – | – | – | ||
| GLPS treatment | – | – | – | – | ||
| Kunming mouse (10/group) | Normal control (myocardium) | – | – | – | – | 16 |
| GLPS (myocardium) | – | – | – | – | ||
| Normal control (skeletal muscle) | – | – | – | – | ||
| GLPS (skeletal muscle) | – | – | – | – | ||
| Rabbits (10/group) | Normal control | – | – | – | – | 31 |
| Normal saline | – | – | – | – | ||
| GLPS | – | – | – | – | ||
| SD rats (10/group) | Normal control | 25.34 ± 6.61 | – | – | – | 38 |
| DM | 12.89 ± 1.69 | <.01 | – | – | ||
| GLPS‐L (200 mg/kg) | 14.45 ± 2.68 | – | – | – | ||
| GLPS‐M (400 mg/kg) | 19.68 ± 3.72 | <.01 | – | – | ||
| GLPS‐H (800 mg/kg) | 21.45 ± 3.21 | <.01 | – | – | ||
| Mouse (10/group) | Ovarian cancer | 7.57 ± 1.16 | – | – | – | 40 |
| GLPS‐L (50 mg/kg) | 8.32 ± 0.89 | – | – | – | ||
| GLPS‐M (100 mg/kg) | 8.57 ± 0.76 | – | – | – | ||
| GLPS‐H (200 mg/kg) | 8.57 ± 0.76 | <.01 | – | – | ||
| Wistar rats (10/group) | Normal control | – | – | – | – | 41 |
| Gastric cancer | – | – | – | – | ||
| GLPS‐L (400 mL/kg) | – | – | – | – | ||
| GLPS‐H (800 mL/kg) | – | – | – | – | ||
| SD rats (10/group) | Normal control | 25.88 ± 6.62 | – | – | – | 42 |
| DM | 12.98 ± 1.95 | <.01 | – | – | ||
| GLPS‐L (200 mg/kg) | 14.37 ± 2.79 | <.01 | – | – | ||
| GLPS‐M (400 mg/kg) | 19.79 ± 3.75 | <.05 | – | – | ||
| GLPS‐H (800 mg/kg) | 21.47 ± 3.33 | <.01 | – | – | ||
| Wistar rats (6/group) | Normal control | 96.38 ± 6.29 | – | – | – | 43 |
| DM | 53.86 ± 3.21 | <.01 | – | – | ||
| GLPS‐L (60 mL/kg) | 70.65 ± 9.06 | <.05 | – | – | ||
| GLPS‐M (80 mL/kg) | 78.86 ± 6.02 | <.01 | – | – | ||
| GLPS‐H (120 mL/kg) | 88.12 ± 6.73 | <.01 | – | – | ||
DM, Diabetes mellitus; GSH‐Px, Glutathione peroxidase; GLPS, Ganoderma lucidum polysaccharides; ROS, Reactive oxidative species; SOD, Superoxide dismutase; MDA, Malondialdehyde.
All animals generate multiple antioxidant enzymes, such as Glutathione peroxidase (GSH‐Px), Superoxide dismutase (SOD) and Catalase (CAT). GLPS not only increases the expression of GSH‐Px, SOD and CAT that directly scavenges free radicals but also decreases the expression level of the enzymes that produce , including NOX, XO and NOS. In fact, NOX is the main producer of ROS in vascular endothelial cells.35, 36, 37
As shown in Table 2, GLPS increases the activities of skeletal muscle SOD and myocardial GSH‐Px in mice and protects liver and skeletal muscle from damage in exhausted mice.16 Moreover, GLPS enhances the activity of GSH‐Px, SOD and CAT in serum and pancreas.38, 39 In an Alzheimer's disease model, GLPS stimulates the activity of SOD in serum and hippocampus in addition to accelerate the process of eliminating free radicals in the body.35 GLPS also increases the serum level of SOD, GSH‐Px and CAT in a mouse model of ovarian orthotopic tumour, indicating that GLPS enhances its sensitivity to chemotherapeutic drugs.40 In the rabbit model of liver ischemia‐reperfusion injury, GLPS significantly enhances the activity of SOD in liver of rabbit.31 GLPS‐treated rats have increased levels of serum and gastric tissue SOD, CAT and GSH‐Px compared to that of control in a dose‐dependent manner.41 GLPS at >400 mg/kg significantly enhances the GSH‐Px, CAT and SOD activities in the heart of type II diabetic rat and decreases myocardial MDA level at the same time.42 GLPS increases SOD, GSH‐Px and CAT levels in STZ‐diabetic rats as well.43
3.2. Anti‐tumour and immune‐regulatory activities of GLPS
Since 1970s, the anti‐tumour effect has been demonstrated for GLPS. Joseph et al44 found that GLPS at 100 mg/kg showed 80.8% inhibitory ratio of Ehrlich's ascites carcinoma (EAC) tumour cells (Table 3). Pang et al45 found that GLPS at 100 and 300 mg/kg exhibits stronger growth inhibition against S180, Hepatoma solidity cell and EAC tumour cells. GLPS almost has no adverse reactions to human body, and this advantage is not possessed in many tumour chemotherapy drugs and other immune promoter. When combined with chemo‐ or radiation therapy, GLPS can improve the healthy state of cancer patients and strengthen anti‐cancer effect of chemo‐ or radiation therapy, which makes GLPS an excellent adjuvant therapeutic drug for cancer patients.
Table 3.
Anti‐tumour and immune‐regulatory effects of GLPS in both cell‐ and animal‐based models
| Models | Inhibitory ratio/% | Other test indicators | References | |||||
|---|---|---|---|---|---|---|---|---|
| Groups | ||||||||
| Swiss albino mouse EAC | Normal saline | – | – | 44 | ||||
| Cyclophosphamide | 71.8 | |||||||
| GLPS‐L (25 mg/mL) | 64.3 | |||||||
| GLPS‐M (50 mg/mL) | 73.4 | |||||||
| GLPS‐H (100 mg/mL) | 80.8 | |||||||
| ICR species mouse | Normal saline | – | – | 45 | ||||
| 1.S180 | Cyclophosphamide | 63.15 | ||||||
| GLPS‐L (33.3 mg/kg) | 23.73 | |||||||
| GLPS‐M (100 mg/kg) | 31.97 | |||||||
| GLPS‐H (300 mg/kg) | 38.56 | |||||||
| 2.EAC | Normal saline | – | ||||||
| Cyclophosphamide | 63.22 | |||||||
| GLPS‐L (33.3 mg/kg) | 19.66 | |||||||
| GLPS‐M (100 mg/kg) | 31.58 | |||||||
| GLPS‐H (300 mg/kg) | 35.89 | |||||||
| 3. Heps | Normal saline | – | ||||||
| Cyclophosphamide | 63.55 | |||||||
| GLPS‐L (33.3 mg/kg) | 24.08 | |||||||
| GLPS‐M (100 mg/kg) | 31.05 | |||||||
| GLPS‐H (300 mg/kg) | 35.73 | |||||||
| BALB/c mouse (10/group) | NK cytotoxic activity/% | Phagocytosis ratio/% | IL‐2 (pg/mL) | IFN‐γ (pg/mL) | TNF‐α (pg/mL) | 46 | ||
| Normal control | – | 35.6 ± 2.3 | 100.1 ± 11.0 | – | – | – | ||
| Normal saline | – | 8.3 ± 1.7 | 48.2 ± 5.0 | – | 11.73 ± 3.53 | – | ||
| Cyclophosphamide | 81 | 0.6 ± 0.5 | – | – | – | – | ||
| GLPS‐L (50 mg/kg) | 30.7 | 13.8 ± 1.0 | 75.0 ± 4.0 | – | 13.24 ± 3.48 | – | ||
| GLPS‐M (100 mg/kg) | 49.1 | 21.4 ± 2.9 | 134.1 ± 7.8 | – | 20.13 ± 2.93 | 20.62 ± 16.8 | ||
| GLPS‐H (200 mg/kg) | 59.9 | 30.3 ± 1.1 | 141.6 ± 27.8 | 3.4 ± 2.46 | 67.42 ± 5.47 | – | ||
|
BALB/c mouse HL‐60 S180 L929 cells |
Normal saline | – | Apoptosis ratio/% | P‐value | 48 | |||
| Cy (cyclophosphamide) | 78.02 | 7.44 ± 1.07 | – | |||||
| GLPS‐L (50 μg/mL) | 27.70 | 18.81 ± 0.93 | <.01 | |||||
| GLPS‐M (100 μg/mL) | 55.83 | 20.98 ± 1.57 | <.01 | |||||
| GLPS‐H (200 μg/mL) | 66.70 | 23.00 ± 0.56 | <.01 | |||||
|
Chick chorioallantoic membrane PC‐3M‐1E8 |
Angiogenesis inhibitory ratio/% | Adhesion of PC‐3M‐1E8 to laminin inhibitory ratio/% | 50 | |||||
| Normal saline | – | – | – | |||||
| GLPS‐L (0.2 μg) | – | 14.8 | 23.89 | |||||
| GLPS‐M (1 μg) | – | 28.1 | 35.4 | |||||
| GLPS‐H (5 μg) | – | 46 | 39.82 | |||||
|
BALB/c mouse S180 |
Normal saline | – | – | 51 | ||||
| GLPS‐L (1 μg/mL) | 35.2 | |||||||
| GLPS‐M (10 μg/mL) | 45.25 | |||||||
| GLPS‐H (100 μg/mL) | 61.88 | |||||||
| HUVECs PC‐23M | Normal saline | – | Adhesion activity of PC‐23M inhibitory ratio/% | 52 | ||||
| GLPS‐L (1 mg/mL) | – | 43.90 | ||||||
| GLPS‐M (10 mg/mL | – | 41.46 | ||||||
| GLPS‐H (100 mg/mL | – | 58.54 | ||||||
| Endothelial cells | Normal saline | – | Adhesion activity inhibitory ratio/% | Migration inhibitory ratio/% | 53 | |||
| GLPS‐L (50 μg/mL) | – | 43.94 | 42.37 | |||||
| GLPS‐M (100 μg/mL) | – | 42.83 | 44.86 | |||||
| GLPS‐H (200 μg/mL) | – | 59.12 | 59.43 | |||||
| Cy (cyclophosphamide) | 78.02 | – | ||||||
| GLPS‐L (50 μg/mL) | 27.7 | |||||||
| GLPS‐M (100 μg/mL) | 55.83 | |||||||
| GLPS‐H (200 μg/mL) | 66.7 | |||||||
EAC, Ehrlich's ascites carcinoma; GLPS, Ganoderma lucidum polysaccharides; Heps, Hepatoma solidity cell.
3.2.1. Inhibiting tumour cell proliferation
Studies showed that GLPS does not kill cancer cells directly in vitro,46, 47, 48 but it inhibits cancer cell proliferation, such as S180, PG and HL‐60 cells, in vitro, when GLPS‐treated animal serum is used in cell culture. It is proposed that one or more endogenous active substances are induced by GLPS, which are responsible for inhibiting cancer cell growth in vitro.
3.2.2. Inducing apoptosis of cancer cells
Shang et al49 demonstrated that SeGLPS‐2B‐1, a Se‐containing polysaccharide purified and characterized from the Se‐enriched G. lucidum, induces MCF‐7 cell (human breast adenocarcinoma cell line) apoptosis via a mitochondrial pathway.
3.2.3. Inhibiting tumour angiogenesis
Endothelial cell proliferation is one of the key steps in angiogenesis, which is essential for tumour growth. Thus, inhibition of vascular endothelial cell proliferation can inhibit tumour growth. As shown in Table 3, Zhang et al50 demonstrated that GLPS could significantly inhibit the angiogenesis in Chick embryo chorioallantoic membrane (CAM; at the administered dosages (0.2~5 g CAM); GLPS also remarkably inhibits with the adhesion of PC‐3M‐1E8 to laminin in a dose‐dependent manner at the concentrations of 0.33~3 g/L. Cao et al51 showed that the proliferation of HUVECs is inhibited by GLPS in a dose‐dependent fashion, and GLPS treatment of HUVECs could induce cell apoptosis directly. Human lung carcinoma cells when exposed to high dose of GLPS in hypoxia for 18 hours result in a decrease in VEGF secretion. They concluded that GLPS may directly inhibit vascular endothelial cell proliferation or indirectly decrease growth factor expression of the cancer cells.
3.2.4. Inhibiting tumour metastasis
Li et al and Liang et al52, 53 reported that GLPS has no cytotoxicity towards PC‐3M in vitro, but GLPS could inhibit tumour cell adhesion and migration through the endothelium.
3.2.5. Regulating the immune system
Studies showed that GLPS could enhance host immune function, activate the immune response and thereby inhibit tumour growth. As shown in Table 3, Wang et al found that the GLPS has no cytotoxicity towards S180 and PG cells in vitro. However, serum from GLPS‐treated S180‐bearing mice significantly inhibited S180 and PG cell proliferation in vitro. Moreover, GLPS promotes the splenic lymphocyte proliferation induced by ConA or LPS and enhances the cytotoxic activity of NK cells. The levels of serum IL‐2, IFN‐γ, TNF‐α and nitric oxide are also increased after GLPS treatment.46
3.3. Hypoglycaemic and hypolipidaemic activities of GLPS
As shown in Table 4, GLPS has both hypoglycaemic and hypolipidaemic effects, which are evidenced by the changed values of Fasting blood glucose (FBG), TC, TG, High‐density lipoprotein‐associated cholesterol (HDL‐C) and LDL‐C. Luo et al54 found that at gavage dose of 100 mg/kg or more, GLPS can reduce blood sugar levels in hyperglycaemia mice, and at gavage dose of 200 mg/kg, GLPS reduces blood sugar levels in normal mice. In addition, GLPS stimulates insulin secretion significantly in hyperglycaemic mice. In another study, Luo et al demonstrated that at gavage dose of 100 mg/kg, GLPS could significantly decrease the levels of serum TC, TG and LDL‐C. At gavage dose of 200 mg/kg, GLPS decreases the levels of serum TG and LDL‐C and increases the level of serum HDL‐C.55
Table 4.
Hypoglycaemic and hypolipidaemic effect of GLPS
| Models | FBG/(mmol/L) | TC/(mmol/L) | TG/(mmol/L) | References | ||||
|---|---|---|---|---|---|---|---|---|
| Groups | Concentration | P‐value | Concentration | P‐value | Concentration | P‐value | ||
| Wistar rats (8/group) | Normal control | 4.90 ± 0.70 | – | 0.78 ± 0.06 | – | 0.35 ± 0.08 | – | 62 |
| DM | 16.93 ± 2.42 | <.01 | 0.81 ± 0.07 | – | 0.51 ± 0.11 | <.05 | ||
| DM+GLPS | 11.80 ± 1.71 | <.01 | 0.74 ± 0.08 | <.05 | 0.46 ± 0.05 | <.05 | ||
| DS (DM + sporting) | 12.30 ± 2.10 | <.01 | 0.75 ± 0.06 | <.05 | 0.44 ± 0.06 | <.05 | ||
| DS+GLPS | 9.18 ± 1.28 | <.01 | 0.72 ± 0.04 | <.01 | 0.40 ± 0.10 | <.05 | ||
| Wistar rats (8/group) | Normal control | 4.7 ± 0.2 | – | 2.46 ± 0.23 | – | 0.61 ± 0.04 | – | 61 |
| High‐fat/fructose diet | 4.8 ± 0.2 | – | 2.55 ± 0.15 | – | 0.65 ± 0.07 | – | ||
| DM | 16.8 ± 0.7 | – | 3.78 ± 0.91 | – | 1.10 ± 0.05 | – | ||
| PSG‐1 | 12.4 ± 1.0 | <.01 | 2.59 ± 0.12 | <.05 | 0.89 ± 0.08 | <.05 | ||
| Cyclosporin A | 17.1 ± 1.4 | – | 2.80 ± 0.22 | – | 1.14 ± 0.04 | – | ||
| N‐acetyl‐L‐cysteine | 15.6 ± 1.2 | – | 2.78 ± 0.10 | – | 1.09 ± 0.13 | – | ||
| SD rats (10/group) | Normal control | 2.99 ± 0.47 | – | 1.90 ± 0.12 | <.01 | 0.84 ± 0.11 | <.05 | 59 |
| DM | 12.93 ± 1.91 | – | 2.87 ± 0.18 | – | 2.56 ± 0.73 | – | ||
| GLPS‐L (100 mg/mL) | 7.64 ± 0.94 | <.01 | 2.38 ± 0.24 | <.01 | 2.04 ± 0.18 | <.01 | ||
| GLPS‐M (200 mg/mL) | 7.47 ± 0.87 | <.01 | 2.29 ± 0.21 | <.01 | 1.77 ± 0.27 | <.01 | ||
| GLPS‐H (400 mg/mL) | 5.51 ± 0.77 | <.01 | 2.15 ± 0.13 | <.01 | 1.01 ± 0.19 | <.01 | ||
| Metformin | 4.63 ± 0.80 | <.01 | 2.08 ± 0.12 | <.01 | 1.62 ± 0.12 | <.01 | ||
| SD rats (10/group) | Normal control | – | – | 1.35 ± 0.21 | – | 0.42 ± 0.23 | – | 64 |
| Hyperlipidaemia | – | – | 9.13 ± 2.17 | <.01 | 1.19 ± 0.21 | <.01 | ||
| GLPS‐L (200 mg/kg) | – | – | 5.52 ± 1.29 | <.01 | 0.83 ± 0.22 | <.01 | ||
| GLPS‐M (400 mg/kg) | – | – | 6.23 ± 1.75 | <.01 | 0.82 ± 0.22 | <.01 | ||
| GLPS‐H (800 mg/kg) | – | – | 5.85 ± 1.62 | <.01 | 0.80 ± 0.26 | <.01 | ||
| SD rats (10/group) | Normal control | 4.46 ± 0.44 | – | 1.49 ± 0.28 | – | 1.32 ± 0.24 | – | 63 |
| DM | 22.60 ± 2.46 | – | 1.68 ± 0.20 | – | 1.60 ± 0.46 | – | ||
| GLPS‐L (100 mg/kg) | 12.04 ± 1.21 | <.05 | 1.32 ± 0.24 | <.05 | 1.04 ± 0.21 | <.05 | ||
| GLPS‐M (200 mg/kg) | 9.82 ± 1.31 | <.05 | 1.02 ± 0.20 | <.05 | 0.92 ± 0.18 | <.05 | ||
| GLPS‐H (400 mg/kg) | 8.69 ± 1.25 | <.01 | 0.89 ± 0.19 | <.01 | 0.85 ± 0.15 | <.01 | ||
| SD rats (10/group) | Hyperlipidaemia | – | – | 9.13 ± 2.17 | – | 1.19 ± 0.21 | – | 61 |
| GLPS‐L (100 mg/kg) | – | – | 5.52 ± 1.29 | <.01 | 0.83 ± 0.17 | <.01 | ||
| GLPS‐M (200 mg/kg) | – | – | 6.23 ± 1.75 | <.01 | 0.82 ± 0.22 | <.01 | ||
| GLPS‐H (400 mg/kg) | – | – | 5.85 ± 1.62 | <.01 | 0.80 ± 0.26 | <.01 | ||
| SD rats (10/group) | Normal control | 5.00 ± 0.00 | – | 2.01 ± 0.29 | – | 1.13 ± 0.16 | – | 60 |
| DM | 25.00 ± 3.92 | <.05 | 4.38 ± 0.34 | <.05 | 1.74 ± 0.18 | <.05 | ||
| Metformin | 13.53 ± 1.82 | <.05 | 2.68 ± 0.36 | <.05 | 1.38 ± 0.09 | <.05 | ||
| GLPS | 16.61 ± 2.26 | <.05 | 3.57 ± 0.64 | <.05 | 1.54 ± 0.12 | <.05 | ||
| Pregnant rats (8/group) | Normal control | 5.6 ± 1.5 | <.01 | – | – | – | – | 57 |
| DM | 21.3 ± 1.9 | – | – | – | – | – | ||
| GLPS | 8.7 ± 1.6 | <.01 | – | – | – | – | ||
| Kunming species mouse (8/group) | Normal control | 5.98 ± 0.27 | <.05 | – | – | – | – | 89 |
| DM | 32.53 ± 0.37 | – | – | – | – | – | ||
| GLPS‐L (100 mg/kg) | 31.97 ± 0.22 | – | – | – | – | – | ||
| GLPS‐M (200 mg/kg) | 20.01 ± 0.14 | <.05 | – | – | – | – | ||
| GLPS‐H (400 mg/kg) | 17.96 ± 0.49 | <.05 | – | – | – | – | ||
| SD rats (16/group) | Normal control | 4.38 ± 0.40 | – | – | – | – | – | 56 |
| DM | 22.81 ± 1.41 | – | – | – | – | – | ||
| GLPS‐L (0.1 g/kg) | 11.33 ± 0.40 | <.05 | – | – | – | – | ||
| GLPS‐M (0.2 g/kg) | 9.56 ± 0.23 | <.05 | – | – | – | – | ||
| GLPS‐H (0.3 g/kg) | 7.23 ± 0.36 | <.05 | – | – | – | – | ||
| Kunming species mouse (10/group) | DM | 37.01 ± 7.80 | – | – | – | – | – | 54 |
| GLPS‐L (100 mg/kg) | 22.40 ± 6.01 | <.01 | – | – | – | – | ||
| GLPS‐M (200 mg/kg) | 23.87 ± 7.77 | <.01 | – | – | – | – | ||
| GLPS‐H (400 mg/kg) | 23.82 ± 6.43 | <.01 | – | – | – | – | ||
| Wistar rats (6/group) | Normal control | 5.71 ± 0.7 | – | – | – | – | – | 43 |
| DM | 22.14 ± 1.91 | <.01 | – | – | – | – | ||
| GLPS‐L (60 mg/kg) | 17.32 ± 0.98 | <.01 | – | – | – | – | ||
| GLPS‐M (120 mg/kg) | 14.38 ± 1.23 | <.01 | – | – | – | – | ||
| GLPS‐H (180 mg/kg) | 8.43 ± 0.72 | <.01 | – | – | – | – | ||
GLPS, Ganoderma lucidum polysaccharides; TC, triglycerides; TG, total cholesterol; FBG, fasting blood glucose.
Zhang et al56 established an alloxan‐induced diabetic mouse model. Their data showed that GLPS decreases blood glucose level, increases insulin secretion, recovers the B cells and improves the liver glucokinase activity in the mouse model. Chen et al47, 48, 49 published three studies related to hypoglycaemic and hypolipidaemic effects of GLPS. They found that when STZ‐induced diabetic mice were treated with GLPS, levels of serum FBG, TC, TG and LDL‐C are decreased (P < .05) and levels of serum insulin and HDL‐C are significantly increased (P < .01). Zhao et al50 divided alloxan‐induced diabetic mice into normal saline, GLPS low‐dosage (GLPS‐L), GLPS middle‐dosage (GLPS‐M) and GLPS high‐dosage (GLPS‐H) groups and measured blood glucose levels after 0, 1, 2 and 4 hours. They found that all doses reduce blood glucose levels at all conditions tested with statistical significance (P < .05). Moreover, Shan et al57 found that GLPS could decrease blood glucose level and increase serum insulin level in gestational diabetic rats (P < .01). Decreased level of serum insulin and increased level of blood glucose are observed in the plasmas of untreated diabetic control rats. GLPS treatment increases serum insulin level and reduced blood glucose levels in STZ diabetic rats significantly and dose‐dependently.43 Huang et al58, 59 found that the high dose of GLPS is able to lower the levels of blood glucose, TC and TG and increase the level of HDL‐C in diabetic rats. GLPS exerts noticeable hypoglycaemic and hypolipidaemic effects (P < .05) in diabetic rats, and all the beneficial effects are better than those of Cyclosporine A and N‐acetyl‐L‐cysteamine (NAC).60, 61 Gong et al62 also found that combined sporting with GLPS could lower the levels of blood glucose and lipids in diabetic mice.
4. CLINICAL STUDIES OF GLPS ON MYOPATHY
As shown in Table 5, after treating with “Ji 731 Injection” for patients with mild, moderate and severe atrophic rhinitis, the effective rates are 80%, 67% and 33%, respectively.63 Subsequently, Fu et al64 reported that the G. Lucidium Karst extract has the ability to treat atrophic myotonia with 50% efficacy. Wang et al65, 66, 67 demonstrated that “Ji Sheng Injection” could be used to treat various myopathy diseases and the diseases of the nervous system including demyelinate diseases. Acupoint injection of G. lucidum Karst Injection had 75% significant efficiency of neurodermatitis.68 When acupoint injection of the Polysaccharidum of G. lucidum Karst Injection is combined with spleen‐arousing decoction, the efficacy ratio of treating low muscle strength in children was up to 90%.69
Table 5.
Effect of Polysaccharidum of G. lucidum Karst Injection
| Indications | Total number of cases (male/female) | Treatment group | Control | Effective rate (treatment/control) | References |
|---|---|---|---|---|---|
| Low muscle strength | 51(35/16) | 30, Polysaccharidum of G. lucidum Karst Injection | 21, Current therapy | 90%/47.6% | 69 |
| Neurodermatitis | 64(24/40) | Acupoint injection of Ji Sheng | – | 75% | 68 |
| Duchenne muscular dystrophy | 17(14/3) | Ji Sheng Injection | – | 64.71% | 65 |
| Atrophic myotonia | 10 | Ji Sheng Injection | – | 50% | |
| Polymyositis and dermatomyositis | 30(10/20) | Ji Sheng Injection | – | 47.1% | |
| Hypothyroidism myopathy | 1 | Ji Sheng Injection +oral thyroid hormone tablets | – | Completely cured | |
| Myasthenia gravis | 2 | Ineffective when used alone and when shared with neostigmine enhance the efficacy | |||
| Multiple sclerosis | 15(6/9) | 40% complete remission, 13.33% significant and 26.67% invalid | |||
| JE vaccine injections after encephalomyelitis | 1 | Progress was not ideal, but therapeutic effect was better than any previously accepted | |||
| Chronic multiple polyradiculoneuritis | 4(3/1) | Ji Sheng Injection | – | 50% completely cured | |
| Visceral ADHD | – | – | – | Completely cured | |
| Concomitant in medical illness | – | – | – | Completely cured | |
| Paralysis after cerebral arteriosclerosis and stroke | Enhance memory, improve their mood, and a variety of recovery after stroke has a certain effect | ||||
| Visceral ADHD (attention deficit/hyperactivity disorder) | 2 | Complete remission in a relatively short period of time, without recurrence | 66 | ||
| Atrophic myotonia | 10(8/2) | 50% effective, 20% increased and 30% improved | 64 | ||
| Atrophic rhinitis | 20(3/17) | Significant efficiency: mild (80%), moderate (67%) and severe (33%) | 63 | ||
5. OTHER PRECLINICAL AND CLINICAL STUDIES ON GLPS
As shown in Table 6, Polysaccharidum of G. lucidum Karst Injection has a curative effect on depression in a combination therapy.70 When combined with glucocorticoids, Polysaccharidum of G. lucidum Karst Injection is effective in treating facial paralysis in children.71 Zhang et al72, 73 conducted experimental studies on isolated heart preservation with Ji Sheng Injection and found that it can improve long‐term preservation of the isolated arrested rat hearts. Acupoint injection of Ji Sheng Injection could treat lumbar hyperplasia and lumbocrural pain with improved efficacy.74, 75 When intravenously injected at 80 mg/kg to rats, GLPS could prolong sleeping time and improve the sleeping quality.76, 77
Table 6.
Other biological activities of GLPS
| Function | Models | Groups | Test indicator | References | |
|---|---|---|---|---|---|
| Facial paralysis | Patients: 20 (13 males/7 females) | 10, Ling Bao Duo Tang Injection | After combination, glucocorticoid dosage reduced, reduced fast, course of treatment shorted and completely healed | 71 | |
| + prednisone | Forehead symptom disappeared, mouth askew and other conditions improved significantly compared with before treatment | ||||
| 10, prednisone + VB1, VB12 | In muscle function after four weeks of combination, there are very significant differences in the muscle function (P < .01) | ||||
| Lumbar hyperplasia | 180 patients | 90, acupoint injection of Ji Sheng | Efficacy: 98.9% | 74 | |
| 30, Ji Sheng Injection | 73.4% | ||||
| 60, electrotherapy | 96.7% | ||||
| Lumbocrural pain | 114 (39 males/75 females) | 58(18/40), Ji Sheng + VB12 | 79.3% | 75 | |
| 56(21/35), Stauntonia + VB12 | 64.3%, (P > .05) | ||||
| Depression | SD rats (8/group) | Normal control, depression | Level of 5‐HT, NE and DA increased than depression group (P < .05) | 70 | |
| Normal saline, GLPS | After treatment of GLPS 28 days, level of 5‐HT, NE and DA were equal to normal (P > .05) | ||||
| Cardiac protection | Wistar rats (10/group) | Modified Euro‐Collins solution (mEC) | Cardiac function and coronary flow were significantly better than mEC (P < .01) | 72 | |
| mEC + Ji Sheng Injection | The content of water and Malondialdehyde, in myocardium, was lower than mEC (P < .05) | ||||
| Activity of LDH and CK was lower than mEC (P < .01), and SOD was higher (P < .05) | |||||
| Heart protection | Kunming mice (10/group) | Ji Sheng Injection‐L (400 mg/L) | Cardiac function and coronary flow were significantly better in three groups of Ji Sheng than mEC, especially in Ji Sheng‐H | 73 | |
| Ji Sheng Injection‐M (800 mg/L) | The content of water in myocardium in Ji Sheng‐M/H was lower than mEC (P < .01) | ||||
| Ji Sheng Injection‐H (1600 mg/L) | Resurrection of heart rate was higher than mEC (P < .01) | ||||
| Sedative and hypnotic | Kunming mice (10/group) | Normal control, sodium pentobarbital + Ji Sheng Injection, Valium, Ji Sheng Injection | Extend sleeping time when administered along with sodium pentobarbital and sodium pentobarbital in a dose‐dependent manner | 77 | |
| Drug independence for Ji Sheng, but for Valium | |||||
| Abnormal locomotor activity is significantly decreased | |||||
| Prolonged sleeping time | SD rats | Prolong sleeping time and improve sleeping quality | 76 | ||
| Anti‐radiation | Mice | Leucocyte/×109/L | SOD/(U/mL) | 79 | |
| Normal control | 12.28 ± 2.51 | 411.07 ± 9.18 | |||
| Radiation | 1.41 ± 0.26 | 357.39 ± 13.52 | |||
| GLPS | 1.92 ± 0.55 | 397.16 ± 5.92 | |||
| Anti‐radiation | Mouse | Leucocyte/×109/L | 78 | ||
| Normal control | 12.08 ± 1.15 | ||||
| Radiation | 2.14 ± 1.83 | ||||
| GLPS | 2.37 ± 1.48 | ||||
| Antibacterial | Inhibited Bacillus subtilis, Escherichia coli, Staphylococcus aureus and Salmonella | 80 | |||
| Antibacterial | Inhibited Bacillus subtilis, Escherichia coli, Staphylococcus aureus and Salmonella | 81 | |||
| Antibacterial | A strong inhibiting effect on Erwinia carotovora and a weak inhibiting effect on Penicillium digitatum | 82 | |||
| Blood stasis | Kunming mice | Prolonged clotting time and reduced serum TG levels in hyperlipidaemia mice inhibited thrombus formation in vitro | 86 | ||
| Wistar rats | |||||
| Hepatoprotective | BALB/c mouse | Significantly mitigated hepatic tumefaction and decreased both ALT release and NO production | 84 | ||
| STZ‐induced diabetic nephropathy | C57BL/6J mouse | Reduced the serum Cr and BUN levels and oxidative stress | 85 | ||
| Anti‐skin ageing | SD rats (10/group) | Enhanced both hydroxyproline and SOD contents in a GLPS dose‐dependent manner | 83 | ||
| Protective roles on bleomycin‐induced pulmonary fibrosis | SD rats | Increased levels of glutathione, glutathione peroxidase, catalase and superoxide dismutase and decreased contents of malondialdehyde and hydroxyproline in the lung | 90 | ||
| Chronic pancreatitis | ICR mice | Alleviated the pancreatitis in mice through decreasing lipase, AMS, IFN‐γ and TNF‐α levels as well as increasing SOD and total antioxidant activity | 91 | ||
GLPS, Ganoderma lucidum polysaccharides; SOD, Superoxide dismutase.
The level and activity of SOD is one of the important indicators to measure the body recover after irradiation. Studies demonstrated that GLPS could increase the level of SOD and the numbers of leucocytes.78, 79 Three independent studies have investigated the antibacterial activity of GLPS.80, 81, 82 Lin observed the anti‐skin ageing function of GLPS. In this model, GLPS increases both hydroxyproline and SOD contents in a concentration‐dependent manner, indicating that GLPS slows the skin ageing process.83 Furthermore, studies showed that GLPS is hepatoprotective84 in that it corrects the metabolic abnormalities of diabetic mice and prevents or delays the progression of diabetic renal complications.85 Finally, GLPS promotes blood circulation and removes blood stasis.86
6. FUTURE PERSPECTIVES
The data collected from published studies and presented in Figures 1, 2, 3, 4, 5 and Tables 1, 2, 3, 4, 5, 6 in the current manuscript provide solid evidence that GLPS has multiple biological functions and should be considered as an effective modern medicine. Both advantages and disadvantages of GLPS as a drug rely on their complicated molecular structures and multiple biological functions. When compared with small molecule‐ and/or protein‐based drugs, GLPS has broader spectrum of therapeutic properties but lacks specific molecular targets. Interestingly, it was reported that among 656 US FDA‐approved drugs tested, each drug hits more than 7 targets in the 73 total targets tested.87 In addition, many drugs are less effective to the previously known targets compared to off‐targets. Therefore, multiple or unknown biological targets in vivo might be a common but not a particular problem for GLPS.
In reality, multiple ingredients with multiple beneficial effects are essence of traditional Chinese medicines, which explains why GLPS is approved only by SFDA so far. However, there are multiple issues needed to be addressed before GLPS is accepted by governments and clinicians worldwide, such as how to comprehend the pharmacodynamics of GLPS, how to standardize the quality of GLPS and how to perform reliable pharmacokinetic studies of GLPS. Perhaps, the efficacy but not the homogeneity of polysaccharide‐based drugs, such as GLPS, should be emphasized by the drug regulators worldwide in the near future.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
This research was supported by the Natural Science Foundation of China (Grant No. 81672585); Key Technology Fund of Shandong Province (Grant No. 2016ZDJS07A07); the “Double First‐Class” Fund of Shandong Province; and National Institutes of Health (Grant No. R01GM069968) to LZ.
Zeng P, Guo Z, Zeng X, et al. Chemical, biochemical, preclinical and clinical studies of Ganoderma lucidum polysaccharide as an approved drug for treating myopathy and other diseases in China. J Cell Mol Med. 2018;22:3278–3297. https://doi.org/10.1111/jcmm.13613
REFERENCES
- 1. Zhang H, He J, Yuan L, et al. In vitro and in vivo protective effect of Ganoderma lucidum polysaccharides on alloxan‐induced pancreatic islets damage. Life Sci. 2003;73:2307‐2319. [DOI] [PubMed] [Google Scholar]
- 2. Pang X, Chen Z, Gao X, et al. Potential of a novel polysaccharide preparation (GLPP) from Anhui‐Grown Ganoderma lucidum in tumor treatment and immunostimulation. J Food Sci. 2007;72:S435‐S442. [DOI] [PubMed] [Google Scholar]
- 3. Zhou L, Cao Y, Wu SH, et al. Global diversity of the Ganoderma lucidum complex (Ganodermataceae, Polyporales) inferred from morphology and multilocus phylogeny. Phytochemistry. 2015;115:7‐15. [DOI] [PubMed] [Google Scholar]
- 4. Meng LZ, Xie J, Lv GP, et al. A comparative study on immunomodulatory activity of polysaccharides from two official species of Ganoderma (Lingzhi). Nutr Cancer. 2014;66:1124‐1131. [DOI] [PubMed] [Google Scholar]
- 5. Zhou ZJ, Han ZR, Zeng YY, et al. Chinese FDA approved fungal glycan‐based drugs: an overview of structures, Mechanisms and Clinical Related Studies. Transl Med. 2014;4:1‐11. [Google Scholar]
- 6. Jiang Y, Chang Y, Liu Y, et al. Overview of Ganoderma Sinense polysaccharide–an adjunctive drug used during concurrent chemo/radiation therapy for cancer treatment in China. Biomed Pharmacother. 2017;96:865‐870. [DOI] [PubMed] [Google Scholar]
- 7. Zjawiony JK. Biologically active compounds from Aphyllophorales (Polypore) fungi. J Nat Prod. 2004;67:300‐310. [DOI] [PubMed] [Google Scholar]
- 8. Zhu X, Chen A, Lin Z. Ganoderma lucidum polysaccharides enhance the function of immunological effector cells in immunosuppressed mice. J Ethnopharmaco. 2007;111:219‐226. [DOI] [PubMed] [Google Scholar]
- 9. Lin ZB, Zhang HN. Anti‐tumor and immunoregulatory activities of Ganoderma lucidum and its possible mechanisms. Acta Pharmacol Sin. 2004;25:1387‐1395. [PubMed] [Google Scholar]
- 10. Zhong W, Liu N, Xie Y, et al. Antioxidant and anti‐aging activities of mycelial polysaccharides from Lepista sordida . Int J Biol Macromol. 2013;60:355‐359. [DOI] [PubMed] [Google Scholar]
- 11. Yang Q, Wang S, Xie Y, et al. HPLC analysis of Ganoderma lucidum polysaccharides and its effect on antioxidant enzymes activity and Bax, Bcl‐2 expression. Int J Biol Macromol. 2010;46:167‐172. [DOI] [PubMed] [Google Scholar]
- 12. Zhang TX, Liu HB, Zhang WZ, et al. Study on enzymatic extraction process of polysaccharide from Ganoderma lucidum and its oxidation resistance. J Anhui Agri. 2011;39:4496‐4498. [Google Scholar]
- 13. Yan Y, Zhang H, Nie SP, et al. Physico‐chemical characteristics and antioxidant activity of polysaccharide from Ganoderma atrum fruitbody. Food Sci. 2009;30:55‐60. [Google Scholar]
- 14. Xiao C, Wu Q, Cai W, et al. Hypoglycemic effects of Ganoderma lucidum polysaccharides in type 2 diabetic mice. Arch Pharm Res. 2012;35:1793‐1801. [DOI] [PubMed] [Google Scholar]
- 15. Zhu K, Nie S, Li C, et al. A newly identified polysaccharide from Ganoderma atrum attenuates hyperglycemia and hyperlipidemia. Int J Biol Macromol. 2013;57:142‐150. [DOI] [PubMed] [Google Scholar]
- 16. Shi YL, Liu YX, Han LM, et al. Research on the effect of Glossy Ganoderma polysaccharide on anti‐oxidation in exhausted mice. Mod Prev Med. 2006;33:889‐890. [Google Scholar]
- 17. Baskar G, Shree Rajesh LK. Enhanced production of medicinal polysaccharide by Submerged Fermentation of Lingzhi or Reishi medicinal mushroom Ganoderma lucidum (W.Curt.:Fr.) P. Karst using statistical and evolutionary optimization methods. Int J Med Mushrooms. 2011;13:455‐464. [DOI] [PubMed] [Google Scholar]
- 18. Baskar G, Shree Rajesh LK, Riswana B, et al. Statistical optimization of polysaccharide production by submerged cultivation of Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (W.Curt.: Fr.) P. Karst. MTCC 1039 (Aphyllophoromycetideae). Int J Med Mushrooms. 2011, 13:41‐49. [DOI] [PubMed] [Google Scholar]
- 19. Bao XF, Wang XS, Dong Q, et al. Structural features of immunologically active polysaccharides from Ganoderma lucidum . Phytochemistry. 2002;59:175‐181. [DOI] [PubMed] [Google Scholar]
- 20. Cao LZ, Lin ZB. Regulation on maturation and function of dendritic cells by Ganoderma lucidum polysaccharides. Immunol Lett. 2002;83:163‐169. [DOI] [PubMed] [Google Scholar]
- 21. Cao LZ, Lin ZB. Regulatory effect of Ganoderma lucidum polysaccharides on cytotoxic T‐lymphocytes induced by dendritic cells in vitro. Acta Pharmacol Sin. 2003;24:321‐326. [PubMed] [Google Scholar]
- 22. Wang SY, Hsu ML, Hsu HC, et al. The anti‐tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int J Cancer. 1997;70:699‐705. [DOI] [PubMed] [Google Scholar]
- 23. Lin YL, Liang YC, Lee SS, et al. Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte‐derived dendritic cells by the NFκB and p38 mitogen‐activated protein kinase pathways. J Leukoc Biol. 2005;78:533‐543. [DOI] [PubMed] [Google Scholar]
- 24. Lin YL, Lee SS, Hou SM, et al. Polysaccharide purified from Ganoderma lucidum induces gene expression changes in human dendritic cells and promotes T helper 1 immune response in BALB/c mice. Mol Pharmacol. 2006;70:637‐644. [DOI] [PubMed] [Google Scholar]
- 25. Tang QJ, Zhang JS, Pan YJ, et al. Activation of mouse macrophages by the alkali‐extracted polysaccharide from spore of Ganoderma lucidum . Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2004;20:142‐144. [PubMed] [Google Scholar]
- 26. Hsu JW, Huang HC, Chen ST, et al. Ganoderma lucidum polysaccharides induce macrophage‐like differentiation in human leukemia THP‐1 cells via caspase and p53 activation. Evid Based Complement Alternat Med. 2009;107:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chien CM, Cheng JL, Chang WT, et al. Polysaccharides of Ganoderma lucidum alter cell immunophenotypic expression and enhance CD56þ NK‐cell cytotoxicity in cord blood. Bioorg Med Chem. 2004;12:5603‐5609. [DOI] [PubMed] [Google Scholar]
- 28. Lin YL, Liang YC, Lee SS, et al. Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte‐derived dendritic cells by the NFκB and p38 mitogen‐activated protein kinase pathways. J Leukoc Biol. 2005;78:533‐543. [DOI] [PubMed] [Google Scholar]
- 29. Zhang ZJ, Li SF, Wei XS, et al. Ganoderma lucidum polysaccharides antioxidant activity. Chem Bioeng. 2011;28:63‐65. [Google Scholar]
- 30. Shi M, Zhang Z, Yang Y. Antioxidant and immunoregulatory activity of Ganoderma lucidum polysaccharide(GLPS). Carbohyde Polym. 2013;95:200‐206. [DOI] [PubMed] [Google Scholar]
- 31. Chen J, Yang HM, Pei R, et al. Ganoderma lucidum polysaccharides on hepatic ischemia‐reperfusion injury. J Pract Med. 2010;28:700‐701. [Google Scholar]
- 32. Yu FL, Liu YS, Yu HQ. Protective effect of Ganoderma lucidum polysaccharide against‐amyloid‐induced neurotoxicity in PC12 cells. J Apopl Nerv Dis. 2012;29:633‐635. [Google Scholar]
- 33. Yan T, Chen XB, Xu L, et al. Effect of Ganoderma lucidum polysaccharide on learning and memory ability and oxygen stress of model rats with Alzheimer's disease. Shaanxi Med J. 2011;40:387‐389+425. [Google Scholar]
- 34. Guo YJ, Yuan H, Zhang LN, et al. Effect of Ganoderma lucidum polysaccharides on antioxidant activity and modality in the Hippocampus of AD rats. Acta Anatomica Sinica. 2006;37:509‐513. [Google Scholar]
- 35. Zalba G, José GS, Moreno MU, et al. Oxidative stress in arterial hypertension: role of NAD (P) H oxidase. Hypertension. 2001;38:1395‐1399. [DOI] [PubMed] [Google Scholar]
- 36. Cifuentes ME, Rey FE, Carretero OA, et al. Upregulation of p67phox and gp91phox in aortas from angiotensin II‐infused mice. Am J Physiol‐Heart Circ Physiol. 2000;279:H2234‐H2240. [DOI] [PubMed] [Google Scholar]
- 37. Hu TP, Cai YB. Effect of Ganoderma lucidum polysaccharides on activity of NADPH oxidase in aortas of DOCA salt hypertensive rats. J Community Med. 2007;5:1‐3. [Google Scholar]
- 38. Tang ZG, Xue H, Qiao J, et al. Protect effect of Ganoderma lucidum polysaccharide on Pancreatic Islet in Type 2 Diabetes Mellitus Rats. J SuZhou Univ Med Sci. 2010;30:922‐925+935+1128. [Google Scholar]
- 39. You YH, Lin ZB. Antioxidant effect of Ganoderma lucidum polysaccharide peptide. Acta Pharm Sin. 2003;2:85‐88. [PubMed] [Google Scholar]
- 40. Qu HG, Qu HY, Gao L, et al. Ganoderma lucidum polysaccharides on ovarian cancer in mice and its mechanisms. Chin J Gerontol. 2011;31:3760‐3762. [Google Scholar]
- 41. Pan K, Jiang QG, Liu GQ, et al. Optimization extraction of Ganoderma lucidum polysaccharides and its immunity and antioxidant activities. Int J Bio Macromol. 2013;55:301‐306. [DOI] [PubMed] [Google Scholar]
- 42. Xue H, Qiao J, Meng GL, et al. Influence of Ganoderma lucidum polysaccharides on blood type 2 diabetic rats dynamics and oxidative stress. China J Chin Materia Med. 2010;35:339‐343. [DOI] [PubMed] [Google Scholar]
- 43. Jia J, Zhang X, Hu YS, et al. Evaluation of in vivo antioxidant activities of Ganoderma lucidum polysaccharides in STZ‐diabetic rats. Food Chem. 2009;115:32‐36. [Google Scholar]
- 44. Joseph S, Sabulal B, George V, et al. Antitumor and anti‐inflammatory activities of polysaccharides isolated from Ganoderma lucidum . Acta Pharmaceut. 2011;61:335‐342. [DOI] [PubMed] [Google Scholar]
- 45. Yang G, Yang L, Zhuang Y, et al. Ganoderma lucidum polysaccharide exerts anti‐tumor activity via MAPK pathways in HL‐60 acute leukemia cells. J Recept Signal Transduct Res. 2016;36:6‐13. [DOI] [PubMed] [Google Scholar]
- 46. Wang P, Zhu X, Lin Z. Antitumor and immunomodulatory effects of polysaccharides from broken‐spore of Ganoderma lucidum . Front Pharmacol. 2012;3:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ning AH, Sun XD, Cao J, et al. Elementarily the inhibition of Ganoderma lucidum polysaccharides for mice tumor. Chinese J Micro Ecol. 2003;15:24‐25. [Google Scholar]
- 48. Zhang QH, Lin ZB. Studies on antitumor activity and mechanism of Ganoderma lucidum polysaccharides B. Chin J Int Tradit West Med. 1999;19:544‐547. [PubMed] [Google Scholar]
- 49. Shang D, Li Y, Wang C, et al. A novel polysaccharide from Se‐enriched Ganoderma lucidum induces apoptosis of human breast cancer cells. Oncol Rep. 2011;25:267‐272. [PubMed] [Google Scholar]
- 50. Zhang XC, Chen GL, Ma B, et al. Ganoderma lucidum polysaccharides inhibit angiogenesis and cell adhesion in chicken chorioallantoic membrane model. Basic Clin Med. 2005;25:63‐66. [Google Scholar]
- 51. Cao Q, Lin Z. Ganoderma lucidum polysaccharides peptide inhibits the growth of vascular endothelial cell and the induction of VEGF in human lung cancer cell. Life Sci. 2006;78:1457‐1463. [DOI] [PubMed] [Google Scholar]
- 52. Li YB, Li YH, Wang R, et al. Influence of Ganoderma lucidum polysaccharides on tumor cells and endothelial cell interactions. Chin Pharmacol Bull. 2008;24:250‐253. [Google Scholar]
- 53. Liang L. Influence of Ganoderma lucidum polysaccharides on tumor cells and endothelial cell interactions. China Pharm. 2012;43:4055‐4056. [Google Scholar]
- 54. Luo SH, Yang H. Experimental study on the role of Ganoderma lucidum polysaccharides regulate blood sugar. J Guangdong Pharm Univ. 2000;16:119‐120. [Google Scholar]
- 55. Luo SH, Chen WQ, Huang T, et al. Influence of Ganoderma lucidum polysaccharides on blood lipid levels in rats with high cholesterol. J Guangdong College Pharm. 2005;30:77‐78. [Google Scholar]
- 56. Zhang LZ, Feng L. Researching the mechanism of GLPS's hypoglycem. Fujian Med J. 2004;26:137‐140. [Google Scholar]
- 57. Shan F. The effect of Ganoderma lucidum polysaccharides on plasma glucose and insulin levels in gestational diabetic rats. J Nantong Univ. 2010;30:441‐442. [Google Scholar]
- 58. Huang ZX, Shi X, OuYang PY. Study on Hypoglycemic and Hypolipidemic effect of Ganoderma lucidum polysaccharides. J Heilongjiang Med. 2008;21:39‐41. [Google Scholar]
- 59. Huang ZX, OuYang PY. Research on Ganoderma lucidum polysaccharides for lowering blood sugar. Edible Fungi. 2009;1:60‐61. [Google Scholar]
- 60. Yang B. Hypoglycemic effect of Ganoderma lucidum polysaccharides and its possible mechanism. Zhejiang Univ. 2011;1:1‐80. [Google Scholar]
- 61. Zhu KX, Nie SP, Song D, et al. Protective effect of polysaccharide from Ganoderma atrum on fasting blood glucose, serum lipids and arteriosclerotic narrowing of superior mesenteric arteries in type II diabetic rats. Food Sci. 2013;34:300‐304. [Google Scholar]
- 62. Gong Y, Wang C, Kang JG. Effects of exercise and Ganoderan intervention on blood fat of rats with diabetes mellitus. West J Tradit Chin Med. 2013;26:8‐10. [Google Scholar]
- 63. Xu HR, Xue JW. Applications “Ji 731 Solution” treatment of atrophic rhinitis preliminary summary. J Peking Univ. 1979;03:180. [Google Scholar]
- 64. Fu HD, Wang ZY. The clinical effects of Ganoderma lucidum spore preparations in 10 cases of atrophic myotonia. J Tradit Chin Med. 1981;6:22‐23. [PubMed] [Google Scholar]
- 65. Wang ZY, Fu HD, Gao LH. Jisheng Injection advent and application of the nervous system diseases. J Youyi Med. 1998;21:17‐19. [Google Scholar]
- 66. Wang ZY, Zhang ZY. Ganoderma lucidum spore powder preparations for treatment of ADHD report two cases of visceral. J Tradit Chin Med. 1983;06:52. [Google Scholar]
- 67. Gu X, Wang ZY, Liu GT. Pharmacological studies on fungus spores –1. The protective effect of chemical and animal allergic myositis. Pharmacol Clin. 1993;2:23‐26. [Google Scholar]
- 68. Cai SL. Point injection therapy observed 64 cases of neurodermatitis. Materia Medica. 2000;11:724. [Google Scholar]
- 69. Qiang F, Yin KJ. Clinical observation of qi spleen decoction and acupuncture point injection therapy for the treatment of low strength of 30 cases. Shaanxi TCM. 2014;35:741‐742. [Google Scholar]
- 70. Liu M, Meng CY, Zhu YM, et al. Polysaccharides in treatment of depression. Prog Mod Biomed. 2014;14:656‐662. [Google Scholar]
- 71. Song LX. Efficacy GLPSS injection combined with glucocorticoid treatment facial paralysis. Capital Med. 2010;22:27. [Google Scholar]
- 72. Zhang SQ, Shi XY, Xi SF, et al. Experimental studies on isolated heart preservation with Jisheng injection. Tradit Chin Drug Res Clin Pharmacol. 2013;14:155‐158. [Google Scholar]
- 73. Zhang SQ, Shi XY. Experimental studies on cardiac protection and anti‐oxidation effect of Jisheng injection. Chin J Int Tradit West Med. 2003;23:455‐457. [PubMed] [Google Scholar]
- 74. Liang Z, Li SZ, Zhang ZR. Acupuncture point injection raw treatment on lumbar hyperplasia clinical observation on 90 cases. Chin Acupun Moxibus. 1998;5:306. [Google Scholar]
- 75. Wang HY, Liu B. Ji Sheng injection point injection treatment of 58 cases of lumbocrural pain. Chin J Int Tradit West Med. 1997;17:644. [Google Scholar]
- 76. Cui XY, Cui SY, Zhang J, et al. Extract of Ganoderma lucidum prolongs sleep time in rats. J Ethnopharmacol. 2012;139:796‐800. [DOI] [PubMed] [Google Scholar]
- 77. Wei HL, Yu LH, Liu GT. Ganoderma lucidum spore water‐soluble extract (Jisheng Injection) hypnotic sedative effect in mice. Pharmacol Clin Chin Materia Medica. 2000;16:12‐14. [Google Scholar]
- 78. Fang LQ, Wei H, Su Y, et al. Experimental study of Placenta Powder and Ganoderma lucidum Polysaccharide radiation function synergies. Food Sci. 2003;24:118‐120. [Google Scholar]
- 79. Wu JY, Chen GY, Jiang HT, et al. Research on Ganoderma lucidum polysaccharides mixture of anti‐radiation health functions. J Nanjing Norm Univ. 2003;26:79‐81. [Google Scholar]
- 80. Zhao CP, Feng CP, Chang XM. Research on inhibitory effect of Ganoderma lucidum polysaccharides. Mushroom. 2012;2:60‐61+64. [Google Scholar]
- 81. Pan M, Xu YT, Xu YL, et al. Bacteriostatic activity of polysaccharides produced by Ganoderma lucidum . China Brewing. 2010;2:56‐58. [Google Scholar]
- 82. Bai D, Chang NT, Li DH, et al. Antiblastic activity of Ganoderma lucidum polysaccharides. Acta Agriculture Boreali‐Sinica. 2008;S1:282‐285. [Google Scholar]
- 83. Lin X, Pan WJ. Ganoderma lucidum polysaccharide anti‐skin senile function research. J Liaoning Univ TCM. 2009;11:174‐175. [Google Scholar]
- 84. Zhang GL, Wang YH, Ni W, et al. Hepatoprotective role of Ganoderma lucidum polysaccharide against BCG‐induced immune liver injury in mice. World J Gastroenterol. 2002;8:728‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. He CY, Li WD, Guo SX, et al. Effect of polysaccharides from Ganoderma lucidum on streptozotozin‐induced diabetic nephropathy in mice. J Asian Nat Prod Res. 2006;8:705‐711. [DOI] [PubMed] [Google Scholar]
- 86. Jin CH, Jiang XL, Wang YJ, et al. Experimental study on the role of Ganoderma lucidum polysaccharides blood circulation. Chin Herbal Med. 1998;7:470‐472. [Google Scholar]
- 87. Lounkine E, Keiser MJ, Whitebread S, et al. Large‐scale prediction and testing of drug activity on side‐effect targets. Nature. 2012;7403:361‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li WJ, Nie SP, Yu Q, et al. Protective effect of polysaccharides from Ganoderma atrum against oxidative stress‐induced cardiomyocyte injury in neonatal rats. Food Sci. 2010;31:221‐225. [Google Scholar]
- 89. Zhao J, Li P, Hu Y. Effect of Ganoderma lucidum polysaccharides on reducing blood sugar in mice with diabetes. J North China Coal Medical College. 2006;8:287‐288. [Google Scholar]
- 90. Chen J, Shi Y, He L, et al. Protective roles of polysaccharides from Ganoderma lucidum on bleomycin‐induced pulmonary fibrosis in rats. Int J Biol Macromol. 2016;92:278‐281. [DOI] [PubMed] [Google Scholar]
- 91. Li K, Zhuo C, Teng C, et al. Effects of Ganoderma lucidum polysaccharides on chronic pancreatitis and intestinal microbiota in mice. Int J Biol Macromol. 2016, 93(Pt A): 904‐912. [DOI] [PubMed] [Google Scholar]


