Abstract
Septins are a conserved family of cytoskeletal GTPases present in different organisms, including yeast, drosophila, Caenorhabditis elegans and humans. In humans, septins are involved in various cellular processes, including exocytosis, apoptosis, leukemogenesis, carcinogenesis and neurodegeneration. Septin 7 is unique out of 13 human septins. Mammalian septin 6, septin 7, septin 2 and septin 9 coisolate together in complexes to form the core unit for the generation of the septin filaments. Physiological septin filaments are hetero‐oligomeric complexes consisting of core septin hexamers and octamers. Furthermore, septin 7 plays a crucial role in cytokinesis and mitosis. Septin 7 is localized to the filopodia and branches of developing hippocampal neurons, and is the most abundant septin in the adult rat forebrain as well as a structural component of the human and mouse sperm annuli. Septin 7 is crucial to the spine morphogenesis and dendrite growth in neurons, and is also a structural constituent of the annulus in human and mouse sperm. It can suppress growth of some tumours such as glioma and papillary thyroid carcinoma. However, the molecular mechanisms of involvement of septin 7 in human disease, especially in the development of cancer, remain unclear. This review focuses on the structure, function and mechanism of septin 7 in vivo, and summarizes the role of septin 7 in cell proliferation, cytokinesis, nervous and reproductive systems, as well as the underlying molecular events linking septin 7 to various diseases, such as Alzheimer's disease, schizophrenia, neuropsychiatric systemic lupus erythematosus, tumour and so on.
Keywords: cell proliferation and cytokinesis, filament formation, nervous and reproductive systems, septin7
1. INTRODUCTION
Septins were first found in budding yeast Saccharomyces cerevisiae as a protein family associated with cytokinesis and cell morphology.1 Because of the role of this protein family in the septum formation during yeast budding as well as in fungi, insects and vertebrates, they were named septins.2, 3, 4 Higher eukaryotic organisms have different numbers of septin isoforms ranging from 2 in Caenorhabditis elegans, 5 in drosophila and 13 in humans.5 Based on phylogenetic analysis, human septins can be divided into 4 groups (septin 2, septin 6, septin 7 and septin 9),6, 7 and 1 septin from each group can form a canonical complex8 to generate a number of redundant heteromeric complexes.7, 8 Septins have a unique ability to assemble into heteropolymers and form a variety of high‐order structures, including filaments, loops and cages.9 These unique structures can control cellular processes and localize at various cellular locations,10 including the plasma membrane,11 the annulus of spermatozoa,12 the bases of cilia13 and dendrites,14 as well as surrounding invasive bacteria.15, 16
Septins have been identified as the “cell‐division cycle” proteins,17 and they play a critical role during cytokinesis.18 It has been reported that septins are indispensable in co‐ordinating myosin motor proteins and bind with non‐muscle myosin II to activate myosin II in interphase and dividing cells19, 20 and reorganizingx membrane during cytokinesis,21 and anchoring the midbody ring structure in the membrane22 when a daughter cell separates from its mother cell. Septins can assemble into hetero‐oligomeric protein complexes which can further form filaments and microscopic bundles or ring structures in vitro and in vivo to control cellular processes. Septin filaments and intermediate filaments are non‐polar, distinguishing them from actin filaments and microtubules.17 Septins have been suggested to be cytoskeletal components owing to these structural features and their association with the membrane, F‐actin and microtubules.8, 23 Recent data indicate that they also serve as scaffolds which recruit factors to particular sites in a cell and/or act as blocks to separate different membrane areas into discrete domains to promote changes in cytoskeletal and membrane organization.24
The common septin structure consists of a highly variable N‐terminal domain, a central GTP‐binding domain and a C‐terminal domain, which normally includes sequences compatible with a coiled coil structure.25 Septin filaments are usually 7‐9 nm in width and vary in length, with unit length of 25‐32 nm observed under high salt concentration.26, 27, 28 G‐domains can form linear filaments using either the guanine nucleotide binding site (G interface) or N‐ and C‐terminal extensions (NC interface).17 Furthermore, exploring the physiological significance of GTP/GDP binding and/or GTP hydrolytic activity of septins may contribute to further understanding of their structural organization and functions.29
2. THE STRUCTURE OF THE SEPTIN 7
Septin 7, an important member of septin protein family, has an open reading frame containing 1254 nucleotides on chromosome 7P14.4‐14.1 and encodes 418 amino acids, including a GTP binding motif.30 cDNA sequence of septin 7 in humans is homologous to Cdc10 in yeast, and septin 7 in humans was even named hCdc10.
Three‐dimensional X‐ray structures of individual septins have shown that septin 7 shares with other septins a canonical Ras‐like G‐domain consisting of 6 ß‐strands and 5 α‐helixes.31 Septin 7 forms a dimer via a G interface in solution, as verified below by mutational analysis, and the monomer‐dimer equilibrium is influenced by the presence of nucleotides.32 Septin 7 is expected to form a septin 7‐septin 7 G interface in the polymeric form, however, the structure of the septin 7 G interface is dramatically different from the G interface of septin 2. This difference is almost entirely because of a well‐defined switch II region that was not detected in septin 2.32 But it is not obvious what the nature of such an interface would be and what makes septin 7 unique in the 4 human septin groups, allowing it alone among the 13 human septins to polymerize into non‐polarized filaments by occupying the ends of hexameric building blocks.
3. SEPTIN 7 AND CDC42 EFFECTOR PROTEINS (CDC42EPS)
Septin 7 can assemble into multimeric complexes and form filaments by combining with other septin proteins. Nevertheless, Cdc42eps can markedly alter the organization of septins within the cell, an effect that has been simultaneously discovered by the independent analysis of Cdc42‐GTP and TC10/RhoQ proteins.33, 34 Meanwhile, Cdc42‐GTP makes use of the association of Cdc42ep5 with septins to interfere with the reorganization of the septin filaments by binding to Cdc42ep5. It has been reported that the Cdc42eps are the first known negative regulators of septin reorganization providing a unique link between septins and Cdc42 GTPases. They can also be repressed by Cdc42‐GTP, a first example of the CRIB domain effect.33 Cdc42ep5 and Cdc42ep2 can bind septins via their BH3 domain and induce septin filament bundling.23, 33 Further characterization demonstrated that Cdc42ep5 binds specifically to septin 6/7 heterodimers or septin 2/6/7 trimers, but not to septin monomers.35 Using super‐resolution microscopy, it was shown that Cdc42ep3 forms an intricate filamentous network in cancer‐associated fibroblasts that colocalized with septin filaments. Budding yeast does not contain homologues of the Cdc42ep genes, indicating that the pathway that involves interaction of Cdc42eps with septin 7 has no apparent counterpart in the budding yeast.36 However, a similar functional linkage to septins may exist because Cdc42p deletion or mutation disrupts the septin ring structure at the yeast bud emergence site.37
4. SEPTIN 7‐ASSOCIATED COMPLEXES AND FILAMENT FORMATION
Septin family members in humans can polymerize into filamentous structures through forming homo‐ and hetero‐oligomeric complexes29, 38. Human septins are divided into the septin 2, septin 6, septin 7 and septin 9 groups. The septin 7 group seems to be unique compared to other groups, as it contains only one member in all organisms. The absence of septin 7 will lead to loss of other septin proteins in homo‐ and heterooligomeric complexes, and this protein appears essential to the generation of filaments.8 Abbey et al9 indicated that filaments are formed by alternating N‐C interfaces (formed by the interaction of N‐ and C‐termini of the septin subunits) and G‐G interfaces (formed by the interaction of the GTPase domains) between the subunits in a dual approach combining X‐ray crystallographic analysis with electron microscopy.
Septin 7 can bind to other members of the septin family and is a core component of most multimeric septin complexes,39 such as septin 2/6/7,8, 24 septin 7/9b/1129 and septin 5/7/11.14, 40, 41, 42 Septin 2/6/7 hetero‐polymer is the only one of septin 7‐associated complexes for which a crystal structure is currently available.31 Septin 2/6/7 is the most abundant septin complex out of those affinity purified from brain tissues or HeLa cells.8 Recent analysis revealed that this heterotrimeric complex can be reconstituted in vitro. Li et al24 indicated that the structure of the complex shows a universal bipolar polymer, composed of an extended G domain, which forms oligomers and filaments by conserved interactions between the adjacent nucleotide binding sites and/or the N‐ and C‐terminal extensions. Kinoshita et al8 identified that septin 2/6/7 is a nonpolar hexamer, ~25 nm in length and ~5 nm in diameter, with 2 copies of each septin symmetrically arranged (septin 7‐septin 6‐septin 2‐septin 2‐septin 6‐septin 7) (Figure 1A). Sirajuddin et al31 clarified that the basic repeat unit consists of a hexamer‐septin 7:6:2:2:6:7, where septin 2‐septin 2 and septin 6‐septin 7 interactions occur via N‐C interfaces and septin 2‐septin 6 and septin 7‐septin 7 interactions occur via the G‐G interface. Septin 2/6/7 may represent a physiological complex as septin 7 provides a predominate framework for human septin complexes, and reconstituted septin complexes composed of the 3 septins are indistinguishable from the endogenous ones.8 Furthermore, in drosophila, dseptin 7 can form a complex with dseptin 1 and dseptin 2 in a similar fashion to human septin 7, which forms linear hexamers with septin 2 and septin 6.43
Figure 1.
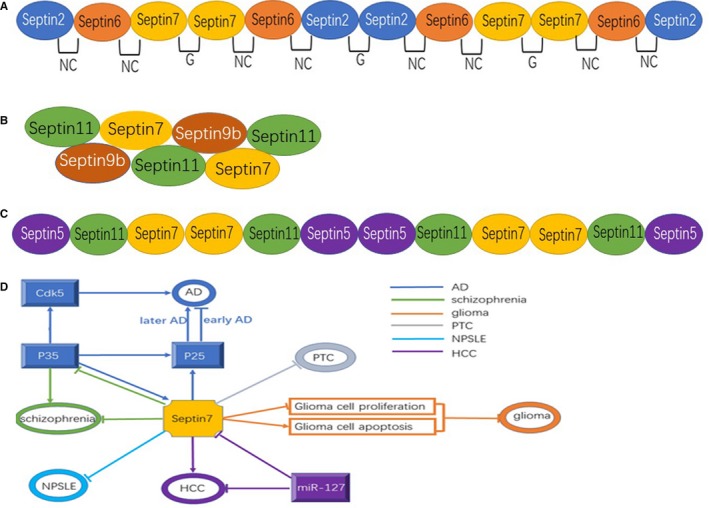
A, Organization chart of the septin2/6/7 complex. Septin 7 is a core component of septin 2/6/7. Septin 2/6/7 is the most abundant septin complex and the structure of the complex shows a universal bipolar polymer, composed of an extended G domain and/or the N‐ and C‐terminal extensions.24 Kinoshita et al identified that septin 2/6/7 is a non‐polar hexamer and 2 copies of each septin symmetrically arranged (septin 7/septin 6/septin 2/septin 2/septin 6/septin 7). B, Organization chart of the septin7/9b/11 complex. Septin 9b binds to C termini of both septin 7 and septin 11 through its long N‐terminal extension and septin 7/9b/11 forms a filamentous pattern along actin stress fibres in the actin filament‐dependent manner. Structure of the filaments containing septins 7/9b/11 depends on the integrity of actin filaments in REF52 cells. C, Organization chart of the septin5/7/11 complex. Septin 5 and septin 11 can colocalize and coimmunoprecipitate with septin 7. The existence of septin 5/7/11 complexes does not contradict the existence of previously reported septin 2/6/7 or septin 7/9b/11 complexes. D, Schematic Organization chart of the role of septin 7 in different diseases and the related regulation mechanism. The common structure of the septin 7 in human and other organisms and its molecular mechanism of action in physiology and disease pathology was summarized in various diseases including Alzheimer's disease (AD), schizophrenia, neuropsychiatric systemic lupus erythematosus (NPSLE), glioma, papillary thyroid carcinoma (PTC) and hepatocellular carcinoma (HCC)
The in vitro septin 7‐septin 6‐septin 2‐septin 2‐septin 6‐septin 7 hexamer is an incomplete mammalian septin complex. Mammalian septin complexes can form octamers that are arranged as setpin 9‐septin 7‐septin 6‐septin 2‐septin 2‐septin 6‐septin 7‐septin 9. Septin 9 occupies the ends of an octameric mammalian septin complex.44 Septin 9b binds to C termini of both septin 7 and septin 11 through its long N‐terminal extension, which lacks a predicted coiled‐coil region and does not contain any predicted domain structure.29 Nagata et al argued that septin 7/9b/11 forms a filamentous pattern along actin stress fibres which is distributed in REF52 cells (rat embryonic fibroblast cells) in the actin filament‐dependent manner (Figure 1B). The 3 septins interact in vitro and in vivo. Structure of the filaments containing septins 7/9b/11 depends on the integrity of actin filaments in REF52 cells.29
Moreover, septin 5 and septin 11 can colocalize and coimmunoprecipitate with septin 7, and expression levels of both septin 5 and septin 11 decreased in septin 7‐deficient neurons.14, 40 These data suggest the existence of a septin 5/7/11 complex in neuronal dendrites, a result consistent with an earlier finding that septin 7 level is significantly decreased in homozygotic septin 5 null mice.41 Interestingly, several human septins might be exchangeable in septin complexes.14, 40 It has been suggested that septin 2 can be replaced by septin 5 (or septin 1/septin 4) and septin 6 by septin 11 (or possibly by septin 8/septin 10) in a septin 2/6/7 complex.25 Therefore, the existence of septin 5/7/11 complexes does not contradict the existence of previously reported septin 2/6/7 or septin 7/9b/11 complexes, and is consistent with earlier findings that down‐regulation of septin 7 decreased the expression of other septin complex members (Figure 1C).8, 42
Septin 7 occupies terminal positions in above mentioned hexamers,31 which is further associated with forming non‐polar linear septin filaments.14, 17 Septin complexes have been purified from human tissues, and some components have been identified, but mutual influences of the septins in the complexes have not been studied.
5. SEPTIN 7 AND INTRACELLULAR CALCIUM
Septin 7 can be regarded as a novel regulator of neuronal Ca2+ homoeostasis based on physiological and behavioural phenotypes.43 Drosophila has been identified to have 5 septin‐encoding genes45, 46, 47 including dseptin 7, a homologue of human septin 7. The septin 7 group is unique because it consists of a single protein both in drosophila and in humans.17, 47 Overexpression of dseptin 7 in neurons of wild‐type drosophila causes significant flight defects.43 Furthermore, knockdown or partial genetic depletion of dseptin 7 rescues the flight defects of animals when the reduction of inositol‐1,4,5‐trisphosphate receptor (IP3R), a protein which contributes to the release of intracellular Ca2+,48, 49 and the septin7 in drosophlia (dseptin7)‐deficient can compensate for the lessened function of IP3R.43 dseptin 7 down‐regulates the dOrai‐mediated spontaneous Ca2+ entry into drosophila neurons in that the dseptin7‐deficient contributes to activate the dOrai, a calcium release‐activated calcium channel protein interacting with the stromal interaction molecule (STIM) protein and other non‐canonical patterns to keep the store‐operated Ca2+ entry (SOCE) function.14, 43 Together, the septin7 affects the cytosolic Ca2+ by down‐regulating the expression of the Orai and IP3R which can cause the deficient flight ability in drosophlia. Disruption of Ca2+ homoeostasis has been shown to play a negative role in several neurodegenerative diseases,43, 50, 51 which may provide a new therapeutic target to some nervous diseases in human.
6. THE ROLE OF SEPTIN 7 IN THE NERVOUS SYSTEM
Septin 7 is widely distributed in the brain39 and has been suggested to be the most common septin in human and rat forebrain postsynaptic density (PSD) fractions by semi‐quantitative mass‐spectrometric analysis.52, 53 Moreover, the phosphorylation of septin 7 mediated by TAOK2, a gene which contributes to the spine mature,5 stabilizes the PSD95 in dendritic spine via its C‐terminal tail to inhibit the formation of mislocalized synapses. Furthermore the non‐phosphorylated Septin7 cannot function in the PSD95.54 Endogenous septin 7 is expressed in axons and clusters in dendrites of cultured hippocampal neurons localizing beneath the presynaptic membrane.14, 39 In dendritic protrusions septin 7 can form complex structures, such as an arc8 or gauze,55 similar to ring and hourglass structures formed by the yeast septin during cytokinesis.56, 57, 58 Interestingly, depletion of septin 7 does not decrease total protrusion density, but causes the appearance of many thin filopodia‐like protrusions. Moreover, the phenotype of septin 7 loss of function could result from the damage to the protein forming developing protrusions or may be a secondary response to the loss of mature spines.14
Septin 7 is crucial for regulation of dendrite branching and dendritic spine morphology. Phosphorylated septin 7 mediated by TAOK2 in the spine head facilitates to the formation and the maturation of the dendritic spine54. It has been identified that septin 7 is expressed at all stages of neuronal differentiation by Western blot analysis,8, 59 and redistributes and accumulates in the formation of protrusions.60 Septin 7 has been found at the bases of filopodia and at the branch points in developing hippocampal neurons.54, 60, 61 Dendrite branching can be impaired by down‐regulation of septin 7. In mature neurons, septin 7 bound to the plasma membrane and was localized at the bases of dendritic spines. Xie et al40 indicated that septin 7 was associated with the plasma membrane in hippocampal neurons based on significant reduction in septin 7 immunoreactivity in permeabilized hippocampal neurons after treatment with 0.2% Triton X‐100, and the down‐regulation of septin 7 disturbed the dendritic outgrowth during cell culture. Furthermore, mature septin‐deficient neurons exhibited elongated spines.40 Meanwhile, formation of normal branches in developing hippocampal neurons required the GTP‐binding activity of the septin 7 which may be because of GTP binding being required for septin filament polymerization, a process potentially associated with the formation of cytoskeleton.40 Septin 7 interacting with HDAC6 decreases the microtubule stability during the formation of the collateral in cortical neurons.61 In addition, septin 7 can maintain the shape of the dorsal root ganglia (DRG) neuron and their bipolar processes, meanwhile, septin 7 is required for regulating the polarity of cortical neuron rather than the DRG neuron.60
Overall, down‐regulation of septin 7 alters the morphology of dendritic protrusions in mature neurons.40 Interestingly, dendrites can be altered to form elongated protuberances, similar to the elongated buds of the septin‐deficient yeast cells.62 These similarities indicate that septin 7 plays a conserved function in neurons. Similar to that in yeast bud neck, it may be a key component of the dendrite diffusion barrier. This function will be a long sought after molecular association to explain the fact that dendrites are unique compartments, and that their motility is crucial for synaptic plasticity.
Alzheimer's disease (AD) is a chronic neurodegenerative disease that usually starts slowly and worsens over time.63 The pathological change in the AD is cerebral cortex atrophy, loss of cortical neurons, accumulation and progressive deposition of β‐amyloid in brain parenchyma and vessel wall, glial proliferation and formation of neurofibrillary tangles accompanied by the appearance of hyperphosphorylated tau.64 The cyclin‐dependent kinase 5 (Cdk5) is a tau kinase up‐regulated in AD,65 and the Cdk5 activator p35 can be cleaved to p25 to increase the Cdk5 activity.66 Interestingly, expression of p25 varies depending on AD stage. Reduction of p25 levels contributes to memory formation in early AD,67, 68 while the ongoing overexpression of p25 leads to neurodegeneration in later AD.69, 70 Septin 7 has been suggested to be a p25‐regulated protein localized in spine necks, where it can control the formation and shape of spines.12, 38, 71 Low expression of septin 7 induces lower spine density and increased size of spines in vivo.14, 40 Interestingly, septin 7 expression was specifically up‐regulated in female, but not male mice. Septin 7 can increase the spine density and reduce spine size in female p25 transgenic mice.72 Meanwhile, in water maze experiments septin 7 level was increased in trained mice but not in control mice, which indicates that septin 7 expression is up‐regulated during spatial memory formation.66 As the synapse has been identified to be affected in early AD73 and the association between the septin 7 and p25, investigating the role of septin 7 can have a profound effect on AD treatment.
Cdk5 signalling can alter expression of various candidate genes related to schizophrenia.74, 75, 76 Reduction of the level of p35, as Cdk5 activator,66 is sufficient to reduce septin 7 expression.72 Cdk5 activity can be regulated by the glutamatergic and dopaminergic cell signalling.77, 78, 79, 80 In addition,the dysfunction of the TAOK2 and septins are found in the neurofibrillary tangles in AD,81 which may offer a novel pathway to treat AD. Schizophrenia is a common and chronic psychiatric disorder with imprecise etiopathogenesis82 and has various symptoms, including disorders in sensory perception, cognition, emotions and behaviour, that could be induced by the glutamatergic dysfunction and dopaminergic disbalance.83 Septin 7 expression is changed in schizophrenia post‐mortem tissue and down‐regulated in the prefrontal cortex, but not the hippocampus, and expression of p35 and septin 7 are not altered by clozapine treatment. Clozapine is a kind of antipsychotics by regulating dopamine receptor D2 and other neurotransmitter receptors.72 Low expression of septin 7 leads to the abnormal spine density in the prefrontal cortex of schizophrenia patients.84, 85 Interestingly, septin 7 levels are reduced in prefrontal cortex of male mice, but not in female mice86 and the sex differences in the septin 7 levels in schizophrenia and AD are the complete opposites. However, the cause of the gender differences during the schizophrenia and AD is not still explored. Epigenetic dysregulation of septin 7 expression may result in the reduction of the p35 level which induces cognitive impairments in schizophrenia that would in turn influence septin 7 expression.72 Thus, enhancing the expression of septin 7 may offer us a novel way to cure the schizophrenia.
Neuropsychiatric systemic lupus erythematosus (NPSLE) is a type of systemic lupus erythematosus, a common autoimmune disease, and is characterized by multi‐systemic manifestations with both neurological and psychiatric symptoms.87 Septin 7 was detected in the sera of non‐NPSLE patients, but not in the sera of the NPSLE patients.88 This difference may reflect the deletion of pathogenic antibodies associated with alteration of brain tissue or the lack of regulatory antibodies required for maintaining neuroprotection,88 and it has been previously identified in normal brain tissue by control sera.71 Septin 7 is involved in neuronal microtubule stability, suggesting a role of microtubules in the pathophysiology of NPSLE.88 Taken together, septin 7 can be used to investigate the mechanism of the NPSLE.
7. THE ROLE OF SEPTIN 7 IN REPRODUCTIVE SYSTEM
Septin 7 is a structural constituent of the annulus of mouse and human sperm.89 Spermatocytes, round spermatids and elongated spermatids located in the lumen of seminiferous tubules, all can express septin 7. Also, septin 7 deficiency can cause different types of damage to the sperm.12, 38, 90, 91, 92 Chao et al12 have found the association between septin 7 and spermiogenesis: Septin 7 is widely distributed in the cytoplasm of round spermatozoa at the early stages of mouse spermatogenesis and can polymerize into a circular structure at the perinuclear area. It is also located in the caudal region of the cytoplasm which can colocalize with the mitochondria; then, the mitochondrial and septin 7 signals are shifted to the caudal part of the sperm. During the sperm tail development, the septin 7 signal becomes denser within the mitochondria in the elongated tail of the cell. At the more advanced stages of spermatogenesis, septin 7 is identified as 2 dots in the neck and annulus of the sperm with disappearance of the perinuclear ring. Finally, septin 7 is well distributed in the cytoplasm. At this stage, the proteins colocalize with mitochondria and nucleus. In mature mouse sperm, septin 7 is mainly expressed in the head, ring and, weakly, in the midpiece. Septin 7 entirely moves from the cytoplasm and extends sperm cells into the annulus in the elongated sperm cells and mature sperm.12
In human mature sperm, septin 7 is mainly expressed in the annulus, where it is colocalized with septin 4, and in the sperm head,89, 91 with the highest expression in the tail. The absence of the septin 7 signal often appears in the sperm with abnormal morphology and immature sperm. In the patients with asthenospermia the percentage of septin 7 deficient signals was significantly higher compared to controls, and the degree of asthenospermia appeared to be related to the percentage of defective septin 7 signals.12
Septin 7 may interact with δ‐tubulin during polymerization or localization of the perinuclear ring during spermatogenesis.38, 90, 92 Dysfunction of septin 7 may interfere with the formation of the manchette/perinuclear ring and play a negative role in resultant sperm head because of its role in formation of the perinuclear ring of the manchette.12 Furthermore, septin 7 expression is similar to the septin 12 expression which has been observed (as a component of the sperm annulus)89, 91 in the post‐meiotic germ cells.38 Septin 7/septin 12 may co‐regulate formation of all 4 subcellular compartments (acrosome, head, midpiece and tail) during spermiogenesis. In conclusion, septin 7 filaments may play a role in different intracellular diffusion events in sperm as an intracellular diffusion barrier.12
8. THE ROLE OF SEPTIN 7 IN CELL PROLIFERATION AND CYTOKINESIS
Successful cytokinesis relies on septin‐dependent and septin‐independent pathways. During septin‐dependent human cytokinesis, the presence of septin 7 is indispensable to cytokinesis for fibroblasts, but non‐essential in the hematopoietic system.93 Septin‐deficient T cells fail to complete cytokinesis when prompted by pharmacological activation or cytokines. Reversely, cell division is dispensable in septins when cell‐cell contacts, such as those with APCs (antigen‐presenting cells), provide a niche.94 Septin 7 deficiency causes embryonic lethality in early mouse embryos. Meanwhile, Menon et al93 indicated that septin 7‐deficient fibroblasts display incomplete cytokinesis and constitutive multinucleation by affecting mitotic spindle and midbody rather than the contractile ring. Septin 7 deficiency causes depletion of other septins but leads to near‐normal cell division in response to cues given by D10 cell lines.95 It is interesting to note that T cell cytokinesis in the absence of septins has also been identified in septin 7 knockout mice.93 Furthermore, the absence of the central subunit septin 7 did not affect the mitosis in T lymphocytes.95 Meanwhile, septin 7 is dispensable during the cytokinesis of myeloid cells.
Menon et al93 also elucidated that sufficient supplementation of stathmin could override the depletion of septin 7 and complete cytokinesis in fibroblasts, leading to a passive rescue as a result of general microtubule destabilization, and thus cytokinesis could proceed in a septin‐independent manner in the haematopoietic system. Abundant expression of stathmin in early embryos96 may explain the dispensability of septin up to mid‐gestation. Menon et al93 found that synergistic action of septins and stathmin is crucial in the completion of cytokinesis and midbody abscission. This gives us a new way to explore the mechanism of cytokinesis in vivo. Accordingly, septin 7 can be a promising target in that a solid tumour‐selective anti‐proliferative therapy against septin 7 would not damage haematopoietic cells.
Cdc10 dominates the G1/S transition in yeast,10 but its role in the cell cycle is unclear. Meanwhile, septin 7‐CENP‐E (septin 7‐centromere associated protein‐E) interaction can affect the distribution of CENP‐E for the kinetochore and chromosome alignment.97 Septin 7 localizes in the spindles from the pro‐MI stage to the MII stage in mouse by immunofluorescence analysis. Li et al. have found that knockdown of septin with siRNA microinjection caused high rate of formation of abnormal spindles and affected the extrusion of the first polar body. Overexpression of septin 7 hindered the alignment of chromosomes and recruitment of α‐tubulin to the spindles to affect the extrusion of the second polar body,24 which suggests that septin 7 plays a specific role in meiosis. In human mitosis, septin isoform may form new scaffolds in the midplane of mitotic spindles which occupy several key steps.24 Meanwhile, the dseptin 7 and other septins are suggested to be dispensable for the orthogonal cell division in the single‐layer neuroepithelium of the dorsal thorax except for planar cell cytokinesis.98 These data indicate that septin 7 plays a unique role in cytokinesis of diverse organisms.
9. DIFFERENT VIEWS ON SEPTIN 7 IN THE DEVELOPMENT OF CANCER
There are few reports about the role of septin 7 in cancer. To date, studies of the role of septin 7 in glioma,99, 100, 101, 102, 103, 104, 105 papillary thyroid carcinoma (PTC)106 and hepatocellular carcinoma (HCC)107 have been reported. In glioma and PTC, septin 7 negatively regulated the growth and progression of tumour. However, in HCC, septin 7 inhibited the growth of HCC. The opposite views about septin 7 in different kinds of cancer may be associated with the subcellular localization and post‐translational modifications of this protein.
9.1. Septin 7 inhibits the growth and invasion of glioma
Glioma is the most common primary malignant brain tumour, characterized by high mortality and poor prognosis.108 Septin 7 can suppress the growth of glioma cells by inhibiting cell proliferation and arresting the cell cycle progression at G0/G1 phase99 and can induce apoptosis of tumour cells.103 Meanwhile, depletion of septin 7 can improve glioblastoma cells migration and invasion,103 which lead to a proposal that septin 7 contributes to the reorganization of the actin cytoskeleton in glioblastoma cells.100 Expression of septin 7 in brain tumours is much lower than in normal brain tissue.101, 102 Low expression of septin 7 induces poor clinical outcomes and poor prognosis in neuroblastoma patients.109 Knocking down the septin 7 with siRNA in U251 xenograft tumours enhanced tumour growth compared to control tumours, and proliferation of the septin 7‐transfected U251 cells was significantly lower than that of control cells.104 These studies suggest that septin 7 can inhibit the growth and proliferation and induce apoptosis in glioma cells acting as a tumour‐suppressor protein. In xenograft tumours in mice treated with septin 7, proliferating cell nuclear antigen (PCNA) is down‐regulated while glial fibrillary acidic protein (GFAP) is up‐regulated.104 In addition, down‐regulation of Bcl‐2 and up‐regulation of caspase‐3 may indicate that septin 7 functions as a tumour suppressor in glioma.104 Hence, inhibition of glioma cell proliferation or promotion of apoptosis by septin 7 may be regulated by the positive or negative cell‐cycle regulators.104 Furthermore, up‐regulation of GFAP in TJ905 and U251 xenograft tumours treated with septin 7 indicates that septin 7 can reverse the glioma phenotypes in differentiation.110 Down‐regulation of MMP2/9, MTI‐MMP,99 integrin αvβ3 and the up‐regulation of TIMP1/2 and the redistribution of α‐tubulin after transfection with septin 7 illustrate that septin 7 inhibits migration and invasion of glioma cells99, 103. Moreover, upon overexpression septin 7 can bind to actin filaments and promote F‐actin ring formation to inhibit the migration of glioma cells.99 Increased levels of septin 7 promoted depolymerization of actin filaments via cofilin phospho‐regulation, and the septin 7 knockdown by cofilin phospho‐regulation improved glioma cell motility and accelerated actin polymerization. Thus, interaction of septin 7 with cofilin phosphate modulates the homoeostasis of actin and cytoskeletal motility, providing a promising candidate for new therapeutic approaches to the treatment of gliomas.99
MiR‐30a‐5p is a small non‐coding RNA (microRNA) that may facilitate the formation of glioma since its expression is up‐regulated in glioma cell lines and specimens.105 Septin 7 gene contains the highly conserved putative binding sites to miR‐30a‐5p which regulate the post‐transcriptional expression of septin 7.105 Septin 7 expression in control glioma cells is much lower than in glioma cells treated with miR‐30a‐5p antisense oligonucleotide. Septin 7 can be negatively regulated by miR‐30a‐5p during its translation.105 Furthermore, adenovirus‐mediated overexpression of septin 7 can partly reverse the increased glioma cells growth because of the down‐regulation of miR‐30a‐5p.105 Hence, there is an inverse correlation between septin 7 and miR‐30a‐5p, and miR‐30a‐5p decreases septin 7 expression at the translational level in glioma cells.
9.2. The subcellular location of septin 7 related to the development and subtype of PTC
Septin 7 is also a tumour suppressor in PTC. Papillary thyroid carcinoma is the most common form of thyroid cancer based on the histopathological differentiation of subtypes of molecular patterns into different subtypes, such as the follicular variant of PTC (FVPTC) and the classic variant of PTC (CVPTC).111 Expression of septin 7 and its subcellular location have been shown to be associated with specific subtypes of PTC.111 Nuclear, cytoplasmic and overall septin 7 expression were much lower in FVPTC tissues in contrast with benign hyperfunctioning thyroid nodules. In CVPTC group, the septin 7 expression was only decreased in the nucleus while its overall and cytoplasmic expressions were stable.106 The difference in septin 7 expression patterns between FVPTC and CVPTC may be associated with different molecular regulatory mechanisms and signalling pathways.99, 112
9.3. Septin 7 inhibits proliferation of HCC
Hepatocellular carcinoma is the primary tumour of the liver. It may result from chronic alcoholism and viral hepatitis infection. MiR‐127 level is decreased in HCC, and it reduces Huh7 cell (a hepatocellular carcinoma cell line) growth and arrests the G2/M cell cycle via suppression of septin 7 in this cell line.107 MiR‐127 may act as an antitumour regulator in HCC.113 Overexpression of MiR‐127 reduces the expression of septin 7 at its post‐transcription state in HCC tissues, and suppresses the Huh7 cell growth by down regulation of septin 7.107
10. CONCLUSION
As a highly evolutionarily conserved GTPase, septin 7 is a member of septin family which includes 13 human septins involving in exocytosis, apoptosis, leukemogenesis, carcinogenesis and neurodegeneration.105 Septin 7 can combine with other septins to form heteropolymers and is a core component of these multimeric septin complexes.39 These heteropolymers can form a diverse array of higher order structures which include filaments, gauzes and rings.9 However, the function and molecular mechanism of action of these heteropolymers have not received enough attention. In this review, we described the common structure of the septin 7 in human and other organisms and its molecular mechanism of action in physiology and disease pathology, summarized recent studies of the function of septin 7 in nervous and reproductive systems and showed its diverse functions in various diseases including AD, schizophrenia, NPSLE, glioma, PTC and HCC (Figure 1D). The role of septin 7 in physiology and disease pathology may provide us novel ideas for exploration of the therapeutic targets in human disease.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ACKNOWLEDGEMENTS
This work was supported in part by grants from the National Science Foundation of China (#81472729 and #81672426), the Foundation of Tianjin Health Bureau (15KG112) and the Foundation of Committee on Science and Technology of Tianjin (17YFZCSY00700 and 17ZXMFSY00120).
Wang X, Fei F, Qu J, Li C, Li Y, Zhang S. The role of septin 7 in physiology and pathological disease: A systematic review of current status. J Cell Mol Med. 2018;22:3298–3307. https://doi.org/10.1111/jcmm.13623
REFERENCES
- 1. Hartwell LH, Culotti J, Reid B. Genetic control of the cell‐division cycle in yeast. I. Detection of mutants. Proc Natl Acad Sci USA. 1970;66:352‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Longtine MS, DeMarini DJ, Valencik ML, et al. The septins: roles in cytokinesis and other processes. Curr Opin Cell Biol. 1996;8:106‐119. [DOI] [PubMed] [Google Scholar]
- 3. Spiliotis ET, Nelson WJ. Here come the septins: novel polymers that coordinate intracellular functions and organization. J Cell Sci. 2006;119:4‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pan F, Malmberg RL, Momany M. Analysis of septins across kingdoms reveals orthology and new motifs. BMC Evol Biol. 2007;7:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weirich CS, Erzberger JP, Barral Y. The septin family of GTPases: architecture and dynamics. Nat Rev Mol Cell Biol. 2008;9:478‐489. [DOI] [PubMed] [Google Scholar]
- 6. Dolat L, Hu Q, Spiliotis ET. Septin functions in organ system physiology and pathology. Biol Chem. 2014;395:123‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Macara IG, Baldarelli R, Field CM, et al. Mammalian septins nomenclature. Mol Biol Cell. 2002;13:4111‐4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ. Self‐ and actin‐templated assembly of Mammalian septins. Dev Cell. 2002;3:791‐802. [DOI] [PubMed] [Google Scholar]
- 9. Abbey M, Hakim C, Anand R, et al. GTPase domain driven dimerization of SEPT7 is dispensable for the critical role of septins in fibroblast cytokinesis. Sci Rep. 2016;6:20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kinoshita M, Noda M. Roles of septins in the mammalian cytokinesis machinery. Cell Struct Funct. 2001;26:667‐670. [DOI] [PubMed] [Google Scholar]
- 11. Mostowy S, Janel S, Forestier C, et al. A role for septins in the interaction between the listeria monocytogenes invasion protein InlB and the met receptor. Biophys J. 2011;100:1949‐1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chao HC, Lin YH, Kuo YC, Shen CJ, Pan HA, Kuo PL. The expression pattern of SEPT7 correlates with sperm morphology. J Assist Reprod Genet. 2010;27:299‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim SK, Shindo A, Park TJ, et al. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science. 2010;329:1337‐1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tada T, Simonetta A, Batterton M, Kinoshita M, Edbauer D, Sheng M. Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr Biol. 2007;17:1752‐1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mostowy S, Nam Tham T, Danckaert A, et al. Septins regulate bacterial entry into host cells. PLoS ONE. 2009;4:e4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mostowy S, Bonazzi M, Hamon MA, et al. Entrapment of intracytosolic bacteria by septin cage‐like structures. Cell Host Microbe. 2010;8:433‐444. [DOI] [PubMed] [Google Scholar]
- 17. Mostowy S, Cossart P. Septins: the fourth component of the cytoskeleton. Nat Rev Mol Cell Biol. 2012;13:183‐194. [DOI] [PubMed] [Google Scholar]
- 18. Mujal AM, Gilden JK, Gerard A, Kinoshita M, Krummel MF. A septin requirement differentiates autonomous and contact‐facilitated T cell proliferation. Nat Immunol. 2016;17:315‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oh Y, Bi E. Septin structure and function in yeast and beyond. Trends Cell Biol. 2011;21:141‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joo E, Surka MC, Trimble WS. Mammalian SEPT2 is required for scaffolding nonmuscle myosin II and its kinases. Dev Cell. 2007;13:677‐690. [DOI] [PubMed] [Google Scholar]
- 21. El Amine N, Kechad A, Jananji S, Hickson GR. Opposing actions of septins and Sticky on Anillin promote the transition from contractile to midbody ring. J Cell Biol. 2013;203:487‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kechad A, Jananji S, Ruella Y, Hickson GR. Anillin acts as a bifunctional linker coordinating midbody ring biogenesis during cytokinesis. Curr Biol. 2012;22:197‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kinoshita M. Diversity of septin scaffolds. Curr Opin Cell Biol. 2006;18:54‐60. [DOI] [PubMed] [Google Scholar]
- 24. Li S, Ou XH, Wei L, et al. Septin 7 is required for orderly meiosis in mouse oocytes. Cell Cycle. 2012;11:3211‐3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kinoshita M. Assembly of mammalian septins. J Biochem. 2003;134:491‐496. [DOI] [PubMed] [Google Scholar]
- 26. Byers B, Goetsch L. A highly ordered ring of membrane‐associated filaments in budding yeast. J Cell Biol. 1976;69:717‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Field CM, al‐Awar O, Rosenblatt J, Wong ML, Alberts B, Mitchison TJ. A purified Drosophila septin complex forms filaments and exhibits GTPase activity. J Cell Biol. 1996;133:605‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mendoza M, Hyman AA, Glotzer M. GTP binding induces filament assembly of a recombinant septin. Curr Biol. 2002;12:1858‐1863. [DOI] [PubMed] [Google Scholar]
- 29. Nagata K, Asano T, Nozawa Y, Inagaki M. Biochemical and cell biological analyses of a mammalian septin complex, Sept7/9b/11. J Biol Chem. 2004;279:55895‐55904. [DOI] [PubMed] [Google Scholar]
- 30. Nakatsuru S, Sudo K, Nakamura Y. Molecular cloning of a novel human cDNA homologous to CDC10 in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1994;202:82‐87. [DOI] [PubMed] [Google Scholar]
- 31. Sirajuddin M, Farkasovsky M, Hauer F, et al. Structural insight into filament formation by mammalian septins. Nature. 2007;449:311‐315. [DOI] [PubMed] [Google Scholar]
- 32. Zent E, Vetter I, Wittinghofer A. Structural and biochemical properties of Sept7, a unique septin required for filament formation. Biol Chem. 2011;392:791‐797. [DOI] [PubMed] [Google Scholar]
- 33. Joberty G, Perlungher RR, Macara IG. The Borgs, a new family of Cdc42 and TC10 GTPase‐interacting proteins. Mol Cell Biol. 1999;19:6585‐6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hirsch DS, Pirone DM, Burbelo PD. A new family of Cdc42 effector proteins, CEPs, function in fibroblast and epithelial cell shape changes. J Biol Chem. 2001;276:875‐883. [DOI] [PubMed] [Google Scholar]
- 35. Sheffield PJ, Oliver CJ, Kremer BE, Sheng S, Shao Z, Macara IG. Borg/septin interactions and the assembly of mammalian septin heterodimers, trimers, and filaments. J Biol Chem. 2003;278:3483‐3488. [DOI] [PubMed] [Google Scholar]
- 36. Joberty G, Perlungher RR, Sheffield PJ, et al. Borg proteins control septin organization and are negatively regulated by Cdc42. Nat Cell Biol. 2001;3:861‐866. [DOI] [PubMed] [Google Scholar]
- 37. Richman TJ, Sawyer MM, Johnson DI. The Cdc42p GTPase is involved in a G2/M morphogenetic checkpoint regulating the apical‐isotropic switch and nuclear division in yeast. J Biol Chem. 1999;274:16861‐16870. [DOI] [PubMed] [Google Scholar]
- 38. Hall PA, Russell SE. The pathobiology of the septin gene family. J Pathol. 2004;204:489‐505. [DOI] [PubMed] [Google Scholar]
- 39. Kinoshita A, Noda M, Kinoshita M. Differential localization of septins in the mouse brain. J Comp Neurol. 2000;428:223‐239. [DOI] [PubMed] [Google Scholar]
- 40. Xie Y, Vessey JP, Konecna A, Dahm R, Macchi P, Kiebler MA. The GTP‐binding protein Septin 7 is critical for dendrite branching and dendritic‐spine morphology. Curr Biol. 2007;17:1746‐1751. [DOI] [PubMed] [Google Scholar]
- 41. Peng XR, Jia Z, Zhang Y, Ware J, Trimble WS. The septin CDCrel‐1 is dispensable for normal development and neurotransmitter release. Mol Cell Biol. 2002;22:378‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kremer BE, Haystead T, Macara IG. Mammalian septins regulate microtubule stability through interaction with the microtubule‐binding protein MAP4. Mol Biol Cell. 2005;16:4648‐4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Deb BK, Pathak T, Hasan G. Store‐independent modulation of Ca(2 + ) entry through Orai by Septin 7. Nat Commun. 2016;7. doi: 10.1038/ncomms11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim MS, Froese CD, Estey MP, Trimble WS. SEPT9 occupies the terminal positions in septin octamers and mediates polymerization‐dependent functions in abscission. J Cell Biol. 2011;195:815‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Neufeld TP, Rubin GM. The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell. 1994;77:371‐379. [DOI] [PubMed] [Google Scholar]
- 46. Adam JC, Pringle JR, Peifer M. Evidence for functional differentiation among Drosophila septins in cytokinesis and cellularization. Mol Biol Cell. 2000;11:3123‐3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cao L, Ding X, Yu W, Yang X, Shen S, Yu L. Phylogenetic and evolutionary analysis of the septin protein family in metazoan. FEBS Lett. 2007;581:5526‐5532. [DOI] [PubMed] [Google Scholar]
- 48. Agrawal N, Venkiteswaran G, Sadaf S, Padmanabhan N, Banerjee S, Hasan G. Inositol 1,4,5‐trisphosphate receptor and dSTIM function in Drosophila insulin‐producing neurons regulates systemic intracellular calcium homeostasis and flight. J Neurosci. 2010;30:1301‐1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Agrawal N, Padmanabhan N, Hasan G. Inositol 1,4,5‐ trisphosphate receptor function in Drosophila insulin producing cells. PLoS ONE. 2009;4:e6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Egorova P, Popugaeva E, Bezprozvanny I. Disturbed calcium signaling in spinocerebellar ataxias and Alzheimer's disease. Semin Cell Dev Biol. 2015;40:127‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van de Leemput J, Chandran J, Knight MA, et al. Deletion at ITPR1 underlies ataxia in mice and spinocerebellar ataxia 15 in humans. PLoS Genet. 2007;3:e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peng J, Kim MJ, Cheng D, Duong DM, Gygi SP, Sheng M. Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry. J Biol Chem. 2004;279:21003‐21011. [DOI] [PubMed] [Google Scholar]
- 53. Collins MO, Husi H, Yu L, et al. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J Neurochem. 2006;97(Suppl 1):16‐23. [DOI] [PubMed] [Google Scholar]
- 54. Yadav S, Oses‐Prieto JA, Peters CJ, et al. TAOK2 kinase mediates PSD95 stability and dendritic spine maturation through Septin7 phosphorylation. Neuron. 2017;93:379‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rodal AA, Kozubowski L, Goode BL, Drubin DG, Hartwig JH. Actin and septin ultrastructures at the budding yeast cell cortex. Mol Biol Cell. 2005;16:372‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Longtine MS, Bi E. Regulation of septin organization and function in yeast. Trends Cell Biol. 2003;13:403‐409. [DOI] [PubMed] [Google Scholar]
- 57. Vrabioiu AM, Mitchison TJ. Structural insights into yeast septin organization from polarized fluorescence microscopy. Nature. 2006;443:466‐469. [DOI] [PubMed] [Google Scholar]
- 58. Lippincott J, Shannon KB, Shou W, Deshaies RJ, Li R. The Tem1 small GTPase controls actomyosin and septin dynamics during cytokinesis. J Cell Sci. 2001;114:1379‐1386. [DOI] [PubMed] [Google Scholar]
- 59. Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454‐1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Boubakar L, Falk J, Ducuing H, et al. Molecular memory of morphologies by septins during neuron generation allows early polarity inheritance. Neuron. 2017;95:834‐851, e5. [DOI] [PubMed] [Google Scholar]
- 61. Ageta‐Ishihara N, Miyata T, Ohshima C, et al. Septins promote dendrite and axon development by negatively regulating microtubule stability via HDAC6‐mediated deacetylation. Nat Commun. 2013;4:2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Barral Y, Mermall V, Mooseker MS, Snyder M. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol Cell. 2000;5:841‐851. [DOI] [PubMed] [Google Scholar]
- 63. Burns A, Iliffe S. Alzheimer's disease. BMJ. 2009;338:b158. [DOI] [PubMed] [Google Scholar]
- 64. Lovestone S, Reynolds CH. The phosphorylation of tau: a critical stage in neurodevelopment and neurodegenerative processes. Neuroscience. 1997;78:309‐324. [DOI] [PubMed] [Google Scholar]
- 65. Cruz JC, Tsai LH. A Jekyll and Hyde kinase: roles for Cdk5 in brain development and disease. Curr Opin Neurobiol. 2004;14:390‐394. [DOI] [PubMed] [Google Scholar]
- 66. Engmann O, Hortobagyi T, Thompson AJ, et al. Cyclin‐dependent kinase 5 activator p25 is generated during memory formation and is reduced at an early stage in Alzheimer's disease. Biol Psychiatry. 2011;70:159‐168. [DOI] [PubMed] [Google Scholar]
- 67. Angelo M, Plattner F, Irvine EE, Giese KP. Improved reversal learning and altered fear conditioning in transgenic mice with regionally restricted p25 expression. Eur J Neurosci. 2003;18:423‐431. [DOI] [PubMed] [Google Scholar]
- 68. Ris L, Angelo M, Plattner F, et al. Sexual dimorphisms in the effect of low‐level p25 expression on synaptic plasticity and memory. Eur J Neurosci. 2005;21:3023‐3033. [DOI] [PubMed] [Google Scholar]
- 69. Bian F, Nath R, Sobocinski G, et al. Axonopathy, tau abnormalities, and dyskinesia, but no neurofibrillary tangles in p25‐transgenic mice. J Comp Neurol. 2002;446:257‐266. [DOI] [PubMed] [Google Scholar]
- 70. Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai LH. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus‐dependent memory. Neuron. 2005;48:825‐838. [DOI] [PubMed] [Google Scholar]
- 71. Almeras L, Lefranc D, Drobecq H, et al. New antigenic candidates in multiple sclerosis: identification by serological proteome analysis. Proteomics. 2004;4:2184‐2194. [DOI] [PubMed] [Google Scholar]
- 72. Engmann O, Hortobágyi T, Pidsley R, et al. Schizophrenia is associated with dysregulation of a Cdk5 activator that regulates synaptic protein expression and cognition. Brain. 2011;134:2408‐2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Perl DP. Neuropathology of Alzheimer's Disease. Mt Sinai J Med. 2010;77:32‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Meyer DA, Richer E, Benkovic SA, et al. Striatal dysregulation of Cdk5 alters locomotor responses to cocaine, motor learning, and dendritic morphology. Proc Natl Acad Sci USA. 2008;105:18561‐18566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wen Y, Planel E, Herman M, et al. Interplay between cyclin‐dependent kinase 5 and glycogen synthase kinase 3 beta mediated by neuregulin signaling leads to differential effects on tau phosphorylation and amyloid precursor protein processing. J Neurosci. 2008;28:2624‐2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Singh KK, Ge X, Mao Y, et al. Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron. 2010;67:33‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bibb JA, Snyder GL, Nishi A, et al. Phosphorylation of DARPP‐32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669‐671. [DOI] [PubMed] [Google Scholar]
- 78. Chergui K, Svenningsson P, Greengard P. Cyclin‐dependent kinase 5 regulates dopaminergic and glutamatergic transmission in the striatum. Proc Natl Acad Sci USA. 2004;101:2191‐2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wei FY, Tomizawa K, Ohshima T, et al. Control of cyclin‐dependent kinase 5 (Cdk5) activity by glutamatergic regulation of p35 stability. J Neurochem. 2005;93:502‐512. [DOI] [PubMed] [Google Scholar]
- 80. Hawasli AH, Benavides DR, Nguyen C, et al. Cyclin‐dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tavares IA, Touma D, Lynham S, et al. Prostate‐derived sterile 20‐like kinases (PSKs/TAOKs) phosphorylate tau protein and are activated in tangle‐bearing neurons in Alzheimer disease. J Biol Chem. 2013;288:15418‐15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gaspar PA, Bustamante ML, Silva H, Aboitiz F. Molecular mechanisms underlying glutamatergic dysfunction in schizophrenia: therapeutic implications. J Neurochem. 2009;111:891‐900. [DOI] [PubMed] [Google Scholar]
- 84. Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65‐73. [DOI] [PubMed] [Google Scholar]
- 85. Kolomeets NS, Orlovskaya DD, Rachmanova VI, Uranova NA. Ultrastructural alterations in hippocampal mossy fiber synapses in schizophrenia: a postmortem morphometric study. Synapse. 2005;57:47‐55. [DOI] [PubMed] [Google Scholar]
- 86. Mizuno K, Giese KP. Towards a molecular understanding of sex differences in memory formation. Trends Neurosci. 2010;33:285‐291. [DOI] [PubMed] [Google Scholar]
- 87. Liang MH, Corzillius M, Bae SC, et al. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42:599‐608. [DOI] [PubMed] [Google Scholar]
- 88. Lefranc D, Launay D, Dubucquoi S, et al. Characterization of discriminant human brain antigenic targets in neuropsychiatric systemic lupus erythematosus using an immunoproteomic approach. Arthritis Rheum. 2007;56:3420‐3432. [DOI] [PubMed] [Google Scholar]
- 89. Ihara M, Kinoshita A, Yamada S, et al. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev Cell. 2005;8:343‐352. [DOI] [PubMed] [Google Scholar]
- 90. Surka MC, Tsang CW, Trimble WS. The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol Biol Cell. 2002;13:3532‐3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kissel H, Georgescu MM, Larisch S, Manova K, Hunnicutt GR, Steller H. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev Cell. 2005;8:353‐364. [DOI] [PubMed] [Google Scholar]
- 92. Nagata K, Kawajiri A, Matsui S, et al. Filament formation of MSF‐A, a mammalian septin, in human mammary epithelial cells depends on interactions with microtubules. J Biol Chem. 2003;278:18538‐18543. [DOI] [PubMed] [Google Scholar]
- 93. Menon MB, Sawada A, Chaturvedi A, et al. Genetic deletion of SEPT7 reveals a cell type‐specific role of septins in microtubule destabilization for the completion of cytokinesis. PLoS Genet. 2014;10:e1004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mujal AM, Krummel M. The subtle hands of self reactivity in peripheral T cells. Nat Immunol. 2015;16:10‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tooley AJ, Gilden J, Jacobelli J, et al. Amoeboid T lymphocytes require the septin cytoskeleton for cortical integrity and persistent motility. Nat Cell Biol. 2009;11:17‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yoshie M, Tamura K, Hara T, Kogo H. Expression of stathmin family genes in the murine uterus during early pregnancy. Mol Reprod Dev. 2006;73:164‐172. [DOI] [PubMed] [Google Scholar]
- 97. Zhu M, Wang F, Yan F, et al. Septin 7 interacts with centromere‐associated protein E and is required for its kinetochore localization. J Biol Chem. 2008;283:18916‐18925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Founounou N, Loyer N, Le Borgne R. Septins regulate the contractility of the actomyosin ring to enable adherens junction remodeling during cytokinesis of epithelial cells. Dev Cell. 2013;24:242‐255. [DOI] [PubMed] [Google Scholar]
- 99. Hou M, Liu X, Cao J, Chen B. SEPT7 overexpression inhibits glioma cell migration by targeting the actin cytoskeleton pathway. Oncol Rep. 2016;35:2003‐2010. [DOI] [PubMed] [Google Scholar]
- 100. Jiang H, Hua D, Zhang J, et al. MicroRNA‐127‐3p promotes glioblastoma cell migration and invasion by targeting the tumor‐suppressor gene SEPT7. Oncol Rep. 2014;31:2261‐2269. [DOI] [PubMed] [Google Scholar]
- 101. Hall PA, Jung K, Hillan KJ, Russell SE. Expression profiling the human septin gene family. J Pathol. 2005;206:269‐278. [DOI] [PubMed] [Google Scholar]
- 102. Huang H, Colella S, Kurrer M, Yonekawa Y, Kleihues P, Ohgaki H. Gene expression profiling of low‐grade diffuse astrocytomas by cDNA arrays. Cancer Res. 2000;60:6868‐6874. [PubMed] [Google Scholar]
- 103. Xu S, Jia ZF, Kang C, et al. Upregulation of SEPT7 gene inhibits invasion of human glioma cells. Cancer Invest. 2010;28:248‐258. [DOI] [PubMed] [Google Scholar]
- 104. Jia ZF, Huang Q, Kang CS, et al. Overexpression of septin 7 suppresses glioma cell growth. J Neurooncol. 2010;98:329‐340. [DOI] [PubMed] [Google Scholar]
- 105. Jia Z, Wang K, Wang G, Zhang A, Pu P. MiR‐30a‐5p antisense oligonucleotide suppresses glioma cell growth by targeting SEPT7. PLoS ONE. 2013;8:e55008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106. Igci YZ, Erkilic S, Arslan A. Septin 7 immunoexpression in papillary thyroid carcinoma: a preliminary study. Pathol Res Pract. 2014;210:426‐431. [DOI] [PubMed] [Google Scholar]
- 107. Zhou J, Lu S, Yang S, et al. MicroRNA‐127 post‐transcriptionally downregulates Sept7 and suppresses cell growth in hepatocellular carcinoma cells. Cell Physiol Biochem. 2014;33:1537‐1546. [DOI] [PubMed] [Google Scholar]
- 108. Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro‐oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Nagata T, Takahashi Y, Asai S, et al. The high level of hCDC10 gene expression in neuroblastoma may be associated with favorable characteristics of the tumor. J Surg Res. 2000;92:267‐275. [DOI] [PubMed] [Google Scholar]
- 110. Kang CS, Zhang ZY, Jia ZF, et al. Suppression of EGFR expression by antisense or small interference RNA inhibits U251 glioma cell growth in vitro and in vivo. Cancer Gene Ther. 2006;13:530‐538. [DOI] [PubMed] [Google Scholar]
- 111. Igci YZ, Arslan A, Akarsu E, et al. Differential expression of a set of genes in follicular and classic variants of papillary thyroid carcinoma. Endocr Pathol. 2011;22:86‐96. [DOI] [PubMed] [Google Scholar]
- 112. Igci YZ, Erkilic S, Igci M, Arslan A. MCM3 protein expression in follicular and classical variants of papillary thyroid carcinoma. Pathol Oncol Res. 2014;20:87‐91. [DOI] [PubMed] [Google Scholar]
- 113. Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857‐866. [DOI] [PubMed] [Google Scholar]


