Abstract
Mosquitoes transmit a diverse group of human flaviviruses including West Nile, dengue, yellow fever, and Zika viruses. Mosquitoes are also naturally infected with insect‐specific flaviviruses (ISFs), a subgroup of the family not capable of infecting vertebrates. Although ISFs are not medically important, they are capable of altering the mosquito's susceptibility to flaviviruses and may alter host fitness. Wolbachia is an endosymbiotic bacterium of insects that when present in mosquitoes limits the replication of co‐infecting pathogens, including flaviviruses. Artificially created Wolbachia‐infected Aedes aegypti mosquitoes are being released into the wild in a series of trials around the globe with the hope of interrupting dengue and Zika virus transmission from mosquitoes to humans. Our work investigated the effect of Wolbachia on ISF infection in wild‐caught Ae. aegypti mosquitoes from field release zones. All field mosquitoes were screened for the presence of ISFs using general degenerate flavivirus primers and their PCR amplicons sequenced. ISFs were found to be common and widely distributed in Ae. aegypti populations. Field mosquitoes consistently had higher ISF infection rates and viral loads compared to laboratory colony material indicating that environmental conditions may modulate ISF infection in Ae. aegypti. Surprisingly, higher ISF infection rates and loads were found in Wolbachia‐infected mosquitoes compared to the Wolbachia‐free mosquitoes. Our findings demonstrate that the symbiont is capable of manipulating the mosquito virome and that Wolbachia‐mediated viral inhibition is not universal for flaviviruses. This may have implications for the Wolbachia‐based DENV control strategy if ISFs confer fitness effects or alter mosquito susceptibility to other flaviviruses.
Keywords: biocontrol, dengue, flavivirus, insect‐specific flavivirus, mosquitoes, virome, Wolbachia
1. INTRODUCTION
Mosquitoes transmit a wide range of pathogens including viruses deemed arboviruses that cause widespread morbidity and mortality in humans and animals (Mackenzie, Gubler, & Petersen, 2004; Mackenzie et al., 1994). These viruses belong to diverse families including the Flaviviridae (genus: Flavivirus) that are positive single‐stranded RNA viruses (Fauquet, Mayo, Maniloff, Desselberger, & Ball, 2005; Karabatsos, 1978). Flaviviruses include Japanese encephalitis virus and Murray Valley encephalitis virus transmitted by Culex species (Erlanger, Weiss, Keiser, Utzinger, & Wiedenmayer, 2009; Kay, Fanning, & Carley, 1984), West Nile virus (WNV) transmitted by a diverse group of mosquitoes including Aedes and Culex species (Mackenzie et al., 2004) and yellow fever virus (YFV), Zika virus (ZIKV), and dengue virus (DENV) that are all transmitted by Aedes aegypti and Aedes albopictus (Black et al., 2002; Hall‐Mendelin et al., 2016; Hayes, 2009). Dengue fever caused by DENV is a severely debilitating disease with 40% of the world's population at risk of infection and an estimated 300 new infections reported yearly (Bhatt et al., 2013). A newly emerging threat to global health is Zika fever caused by the ZIKV with outbreaks reported in both tropical and subtropical regions (Pujhari & Rasgon, 2016; Younger, 2016).
Mosquitoes are also known to naturally harbor flaviviruses that are incapable of infecting humans or other vertebrate animals and are therefore known as insect‐specific flaviviruses (ISFs). Cell fusing agent virus was the first ISF to be discovered in Ae. aegypti in 1975 (Stollar & Thomas, 1975). Since then, several others have been described including Culex flavivirus (CxFv) in Culex pipiens, Kamiti River virus in Ae. aegypti, and Palm Creek virus in Coquillettidia xanthogaster mosquitoes (Crabtree, Sang, Stollar, Dunster, & Miller, 2003; Hobson‐Peters et al., 2013; Hoshino et al., 2007). Even though ISFs are not directly associated with disease in vertebrates, there is growing interest in their effect on co‐infecting arboviruses. In mosquito cell culture, some studies have shown that ISFs suppress flaviviruses, including WNV by CxFV and Murray Valley virus by Palm creek virus (Bolling, Olea‐Popelka, Eisen, Moore, & Blair, 2012; Hobson‐Peters et al., 2013). However, this effect, known as superinfection exclusion (Billecocq, Vazeille‐Falcoz, Rodhain, & Bouloy, 2000; Geib et al., 2003; Karpf, Lenches, Strauss, Strauss, & Brown, 1997; McAlister & Barrett, 1977; Nethe, Berkhout, & van der Kuyl, 2005; Pesko & Mores, 2009), was not observed in field mosquito populations where a positive association was found between WNV and CxFV. This suggests that the presence of CxFV may make mosquitoes more susceptible to WNV (Newman et al., 2011). Conflicting results were also observed in other studies where CxFV did not have an effect on replication, transmission, or dissemination of WNV in Culex quinquefaciatus (Kent, Crabtree, & Miller, 2010).
Wolbachia pipientis is an endosymbiotic bacterium of insects that is currently being developed as a biocontrol agent (Iturbe‐Ormaetxe, Walker, & LO'Neill, 2011; Moreira et al., 2009; Walker et al., 2011). Wolbachia is naturally present in about 52% of arthropods and is maternally inherited (Weinart, Arauju‐Jnr, Ahamed, & Welch, 2015). The symbiont manipulates host reproduction, such that the eggs of Wolbachia‐free females do not hatch when they have been fertilized by a Wolbachia‐infected male (Serbus, Casper‐Lindley, Landmann, & Sullivan, 2008). This phenomenon, referred to as cytoplasmic incompatibility (CI), leads to the spread and invasion of Wolbachia into wild populations. Another desirable phenotype of Wolbachia is its ability to inhibit infection of the host with other pathogens (Bian, Xu, Lu, Xie, & Xi, 2010; Bian et al., 2013; Frentiu et al., 2014; Walker et al., 2011). This “pathogen blocking” by Wolbachia was first observed in Drosophila melanogaster (Hedges, Brownlie, O'Neill, & Johnson, 2008; Teixeira, Ferreira, & Ashburner, 2008) where flies infected with Drosophila C virus (DCV) and cricket paralysis virus and accumulated virus at a slower rate leading to higher survival rates compared to Wolbachia‐free controls (Hedges et al., 2008).
While present in 28% of mosquitoes such as Ae. albopictus, Cx. pipiens, and Cx. quinquefaciatus, Wolbachia was not thought to be present in the malaria vectors (Anopheles species) or the primary vector of DENV (Ae. aegypti) (Kittayapong, Baisley, Baimai, & O'Neill, 2000). There have been recent reports however of sporadic infections in Anopheles coluzzii (Shaw et al., 2016) and Anopheles gambiae (Gomes et al., 2017) and in a single population of Ae. aegypti (Coon, Brown, & Strand, 2016). Over the last decade three different Wolbachia strains have been artificially introduced into Ae. aegypti where they form stably inherited infections. These are wMelPop‐CLA and wMel from Drosophila (McMeniman et al., 2009; Walker et al., 2011), wAlbB from Ae. albopictus (Xi, Dean, Khoo, & Dobson, 2005), and wMelwAlbB (Joubert et al., 2016) that is a superinfection of wMel and wAlbB. Wolbachia‐mediated pathogen blocking has now been observed for arboviruses such as WNV (Glaser & Meola, 2010), YFV (van den Hurk et al., 2012), DENV (Bian et al., 2010; Frentiu, Robinson, Young, McGraw, & O'Neill, 2010; Moreira et al., 2009; Walker et al., 2011), ZIKV (Aliota, Peinado, Velez, & Osorio, 2016; Dutra et al., 2016), and Chikungunya virus (van den Hurk et al., 2012; Moreira et al., 2009). Wolbachia is currently being released into populations of Ae. aegypti globally to test whether it may be effective at limiting DENV and ZIKV transmission to humans (Ritchie, 2014) (see http://www.eliminatedengue.com). The first releases in Australia demonstrated that Wolbachia was able to invade wild Ae. aegypti populations and remain at near 100% frequency (Hoffmann et al., 2011). Subsequent releases in DENV endemic regions are being used to test for efficacy of human infection control (Ritchie, 2014).
Although largely consistent, there are some reports of Wolbachia enhancing rather than preventing pathogen co‐infection including Plasmodium and WNV within Anopheles gambiae and Culex tarsalis, respectively (Dodson et al., 2014; Hughes, Vega‐Rodriguez, Xue, & Rasgon, 2012). In both of these instances, however, the mosquitoes were only transiently infected with Wolbachia via artificial micro‐injection and so may not be representative of insects with germline tissue infections (Joubert & O'Neill, 2017). Several vectors naturally infected with Wolbachia have also exhibited increased susceptibility to pathogens. This has been shown in Cx. pipiens and Spodoptera exempta (African armyworm moth) with increased susceptibility to Plasmodium and nucleopolyhedrovirus (double‐stranded DNA virus), respectively (Graham, Grzywacz, Mushobozi, & Wilson, 2012; Zele et al., 2014). In contrast, the natively infected Ae. albopictus exhibits reduced susceptibility and transmission of DENV (Mousson et al., 2012). These studies suggest that Wolbachia‐mediated pathogen blocking may depend on several factors that are influenced by specific Wolbachia strain and pathogen–host interactions including history of association.
The mechanistic basis of Wolbachia‐mediated pathogen blocking is still not well understood (Terradas & McGraw, 2017). Currently, pathogen blocking has been partly attributed to the ability of Wolbachia to increase the innate immune responses of the host, thereby making it resist subsequent pathogen infection (Bian et al., 2010; Pan et al., 2012; Rances, Ye, Woolfit, McGraw, & O'Neill, 2012). It has also been hypothesized that competition between Wolbachia and pathogens for key host resources such as lipids (Caragata et al., 2013) and intracellular space (Moreira et al., 2009) may underpin blocking. This may be particularly relevant for viruses that require lipids for attachment and entry into host cells and for replication (Lu, Cassese, & Kielian, 1999; Mackenzie, Khromykh, & Parton, 2007). Most recently, there is some evidence primarily from Drosophila that Wolbachia may be modifying host cellular structures or organelles rendering them less hospitable to viral replication (Rainey et al., 2016; White et al., 2017). A range of studies also point to a correlation between Wolbachia densities and the strength of blocking (Frentiu et al., 2010; Lu, Bian, Pan, & Xi, 2012; Osborne, Iturbe‐Ormaetxe, Brownlie, O'Neill, & Johnson, 2012), a trend that would be expected with any of the above explanations for blocking.
While Wolbachia appears to shift the composition of the microbiome in mosquitoes (Audsley, Seleznev, Joubert, O'Neill, & McGraw, 2018), little is known about its effects on ISFs. There is also little known about the effects of ISFs on mosquito health. If these infections affect survival or reproduction, Wolbachia‐infected insects in the field may receive an advantage in carrying the symbiont. For example, native viruses in Drosophila such as DCV and cricket paralysis virus reduce host fitness and Wolbachia infections are hence beneficial (Hedges et al., 2008). A survey in wild populations of D. melanogaster demonstrated that Wolbachia infection was not associated with changes in the diversity of native viruses in the insect (Webster et al., 2015). Wolbachia infections in Ae. aegypti differ significantly from those found in D. melanogaster however, exhibiting higher symbiont loads (McGraw, Merritt, Droller, & O'Neill, 2002; McMeniman et al., 2009; Moreira et al., 2009; Walker et al., 2011), broader tissue distributions (Moreira et al., 2009; Walker et al., 2011), greater activation of the immune response (McGraw et al., 2002; McMeniman et al., 2009; Moreira et al., 2009; Walker et al., 2011), and greater fitness costs (McMeniman, Hughes, & O'Neill, 2011; Min & Benzer, 1997). These discrepancies may result from different periods of association/evolutionary history, long (~5,000 years) in the case of D. melanogaster (Richardson et al., 2012) and short (<10 years) in the case of the newly infected Ae. aegypti (Walker et al., 2011). Understanding the fitness consequences of Wolbachia for Ae. aegypti is necessary to effectively model the long‐term stability and success of the symbiont as a biocontrol agent in wild populations.
Our work focused on determining if Wolbachia‐mediated viral blocking extends to naturally occurring flaviviruses in mosquitoes. We sampled Wolbachia‐infected mosquitoes from field release sites in Cairns, Australia, and symbiont‐free mosquitoes from nearby control areas outside of the Wolbachia release zone. Using flavivirus general degenerate primers, we amplified the NS5 region of the virus genome and sequenced the PCR amplicons of individual positive mosquitoes using Miseq Illumina sequencing. We further screened laboratory colonies and field mosquitoes using primers designed specifically for several of the ISF sequences. We found that ISFs are common and widely distributed in Ae. aegypti populations with infection rates and abundance consistently higher in field mosquitoes compared to the laboratory colonies. This possibly indicates that variations in environmental conditions could be playing a role in controlling ISF infection in Ae. aegypti. Unexpectedly, we found that Wolbachia enhanced ISF infection rates and loads in Ae. aegypti demonstrating that the antivirus effect associated with Wolbachia is not common to all flaviviruses. These findings may have implications for Wolbachia‐DENV control if ISFs affect host fitness or play a role in mosquito susceptibility to flaviviruses.
2. MATERIALS AND METHODS
2.1. Mosquito sampling
Wolbachia‐infected and uninfected (wild‐type) Ae. aegypti mosquitoes were sampled from three different communities in Cairns, Australia. The Wolbachia‐infected mosquitoes were sampled in 2013 from two sites where wMel mosquitoes were released in 2011 (Hoffmann et al., 2011) and 2013 (Ritchie, 2014). These are Gordonvale (GV) and Parramatta Park (PP), respectively. Wild‐type mosquitoes were sampled from Holloways Beach (HB) that is outside the original release zone. BG‐sentinel mosquito traps (Biogen, Germany) were set randomly in these areas, and adult mosquitoes were collected overnight. Ae. aegypti mosquitoes were morphologically identified and placed in vials containing 80% ethanol. A total of 95 individual mosquitoes were assessed across the three collection sites (39 from GV, 21 from PP, and 35 from HB) for the presence of ISFs.
2.2. Screening for insect‐specific flaviviruses
RNA and DNA were simultaneously extracted from each mosquito using the TRIzol® method from Invitrogen (Life technologies, Carlsbad, CA, USA). The DNA was used to screen for the presence of Wolbachia infection via qPCR as previously described (Frentiu et al., 2014). Two samples each from PP and GV were found to be Wolbachia negative and were excluded from further analysis. All HB samples were confirmed to be Wolbachia negative as expected. The RNA was DNase‐treated to remove genomic DNA contamination using DNase 1 recombinant RNase‐free (Roche, Germany). Reverse transcription of RNA to cDNA and the PCR amplification of the NS5 region using general degenerate flavivirus primers were carried out following the protocol of Sanchez‐Seco et al. (2005). Briefly, reverse transcription of RNA to cDNA and subsequent first‐round amplification were carried out using 1 μg of RNA in the Access RT‐PCR System (Promega, Madison, WI, USA). Reverse transcription controls that did not include enzyme were included in each run to rule out genomic DNA contamination. One microliter of the first‐round amplification was then used for the second round of nested PCR. All PCR products were run in a C1000™Thermal Cycler (Bio‐Rad, CA, USA). PCR products (143 bp) were analyzed using gel electrophoresis on a 2% Agarose gel (Sigma, Life Science, USA) stained with RedSafe™ (iNtRON Biotechnology). Products were then visualized on the Quantum gel documentation system (Fisher Biotec).
2.3. Sequencing of PCR products
A subset of six individual mosquitoes from each of the three sites were selected for further processing for sequencing. To ensure a good representation of an area, the samples were selected from different traps that were not in close proximity. One microliter of the second‐round PCR product of each of the selected individual samples was used as the template for a 5‐cycle amplification with primers barcoded with Illumina sequence adapters (Berry, Ben Mahfoudh, Wagner, & Loy, 2011). The PCR amplicons were analyzed using gel electrophoresis as described above. The amplicons were then excised and gel extracted using QIAquick gel extraction kit (Qiagen, Germany) following manufactures instructions. Extracted samples were then paired‐end sequenced using MiSeq at Ramaciotti sequencing center, NSW, Australia.
2.4. Processing and clustering of sequences
All sequences were processed with cutadapt (Martin, 2011) as the very first step, to remove degenerate primers used for the PCR amplicons. Cutadapt was run with the following settings; minimum overlap 10 nucleotides, minimum read length: 1 nucleotide (this is mainly to allow downstream R1 and R2 merging), and number of attempts to trim a primer was set to 2. To classify sequences into Operational Taxonomical Units (OTUs), vsearch (Rognes, Flouri, Nichols, Quince, & Mahe, 2016) was used to merge reads with number of mismatches set to two nucleotides, the number of allowed N's set to 0, and the minimum overlap set to 32 bases. This was followed by filtering reads based on expected error of 1. Identical sequences were then collapsed into a single sequence (dereplication) and then clustered using 97% identity. Contingency table of cluster counts was subsequently generated using usearch (Edgar, 2010). The OTUs were finally imported into the flavivirus Database under The Virus Pathogen Resource (http://www.viprbrc.org) and the Basic Local Alignment Search Tool (BLAST) used to find the closest match or hit of each OTU.
2.5. Phylogenetic reconstruction
Individual OTUs were aligned with their best hits using the multiple sequence comparison by log‐expectation (MUSCLE) tool (Edgar, 2004) provided by The European Bioinformatics Institute (EMBL‐EBI). The aligned sequences were manually trimmed and then imported into Phylogeny.fr together with other common flaviviruses (Dereeper, Audic, Claverie, & Blanc, 2010; Dereeper et al., 2008). The one click mode of Phylogeny.fr that uses MUSCLE for sequence alignment and maximum likelihood (PhyML) for tree building with aLRT (approximate likelihood‐ratio test) statistical test for branch support values, and TreeDyn for tree drawing was used for the phylogeny tree (Anisimova & Gascuel, 2006; Chevenet, Brun, Banuls, Jacq, & Christen, 2006; Guindon & Gascuel, 2003).
2.6. Screening of field mosquitoes using OTU‐specific primers
Primers (Table 1) were designed for 7 ISF OTUs that were selected based on their abundance and diversity in the sequenced samples as well as their phylogenetic positions. All primers were designed using the Primer3 tool in The Virus Pathogen Resource database (viprbrc.org). Quantitative PCR using SYBR Green (Roche, Applied Science, Switzerland) in a LightCycler480 (Roche, Applied Science, Switzerland) was then used to validate the presence and abundance of OTUs in all mosquitoes sampled from the field. This was performed using 1 μl of the first‐round amplification, 5 μl of 5X SYBR Green master mix, and 0.5 μl of 10 mmol/L each of forward and reverse primers in a total volume of 10 μl. The cycling conditions were pre‐incubation at 95°C for 5 min, 45 amplification cycles of 95°C for 10 s, 60°C for 10 s, and 72°C for 10 s followed by a melting curve at 95°C for 5 s, 65°C for 1 min, and a continuous acquisition mode at 97°C. The housekeeping gene RPS17 (Cook et al., 2006; Thellin et al., 1999) was used to normalize virus abundance.
Table 1.
OTU‐specific primer pairs used for PCR amplification
| OTUs | Forward primer | Reverse primer |
|---|---|---|
| OTU1 | AGAAGCAACCGACCATAGCT | CCAGATATCGACTTCCCAGCC |
| OTU2 | AGAAGGAGAAAAAGCCCAGCC | GCTAGAGCCTCAAATTCAAGGA |
| OTU3 | TAGCTGGGGAGCCGAAAG | GGCCTCATATTCCAGATATCGACT |
| OTU16 | GTGTGCACAACATGATGGGG | TTGAGGAAGCCCAATGGTCC |
| OTU20 | TCAACACGGACCACTGGAAG | TGTTGAGAAAGCCCATGGTGT |
| OTU21 | TTCCTCAACACGGACCAGTG | GTGGTCTTGTAGAGAAGCCCC |
| OTU25 | GCCACTGGGAGCATTAACCT | GTCCGTGTTAGAAAGCCCCA |
2.7. Screening of laboratory mosquitoes
Wolbachia‐infected and wild‐type lines maintained in the laboratory were screened for ISFs to ascertain whether they showed similar patterns as seen in field‐caught mosquitoes. The Wolbachia‐infected mosquitoes were previously sampled from field release sites in Cairns, Australia (Hoffmann et al., 2011), and the wild‐type mosquitoes was from Babinda, Australia (outside the release zone). To avoid genetic drift between the two lines, 20% of wild‐type males were outcrossed with the Wolbachia‐infected females at every generation. Mosquitoes were maintained only on 10% sucrose, and 4‐ to 7‐day‐old females were used for this study. RNA was extracted from 59 and 56 individual mosquitoes each from the Wolbachia‐infected and the wild‐type populations, respectively. The RNA extraction and DNase treatment were carried out as above. Using random primers (125 ng/μl), the first‐strand cDNA synthesis was carried out with SuperScript III reverse transcriptase (Invitrogen, California USA) following the manufacturer's instructions. The cDNA synthesis was run in a C1000™ Thermal Cycler (Bio‐Rad, California USA). Quantitative PCR using SYBR Green (Roche, Applied Science, Switzerland) was then carried out in a LightCycler480 (Roche, Applied Science, Switzerland) using 1.5 μl of cDNA, 5 μl of 5X SYBR Green master mix, and 0.5 μl of 10 mmol/L each of forward and reverse OTU‐specific primers in a total volume of 10 μl. The cycling conditions were as above.
2.8. Statistical analysis
To determine whether there was an association between Wolbachia infection and the presence/absence of ISFs, a binary logistic regression was carried out with presence/absence of ISFs as a dependent variable and Wolbachia infection status as a predictor in a generalized linear model to analyze the following: (1) infection rates in all the field mosquitoes, (2) infection rates in the sequenced mosquitoes, (3) infection rates in the field mosquitoes after RT‐qPCR, and (4) infection rates in the laboratory samples. These analyses were performed in SPSS® (IBM Statistics for Windows, Version 20.0). Where multiple models were run for individual OTUs, we utilized a Bonferroni multiple test correction. A Mann–Whitney test in GraphPad Prism (version 6) was used to analyze differences in ISF abundance between the wild‐type and wMel mosquitoes.
3. RESULTS
3.1. Wolbachia infection is associated with higher rates of ISF infection as measured by ISF generalist primers in PCR
We observed high ISF infection rates in the all field‐collected samples as measured by PCR; 100% and 95% for GV (Gordonvale) and PP (Parramatta Park), respectively, and 74% for HB (Holloways Beach). If we test for the effect of Wolbachia in dictating infection frequency, we see that it is significant (Wald = 7.80; df = 1; p = .005). Our findings suggest that ISFs are a common feature of the mosquito virome and that Wolbachia may be enhancing the frequency of infection in the field.
3.2. The ISFs include well‐characterized viruses as well as what appear to be novel viruses
To identify specific ISFs in the Ae. aegypti field populations, we sequenced a subset of samples that were ISF positive for both the wild‐type (n = 6) and wMel (n = 12) mosquitoes. Following clustering analysis, a total of 26 “unique” ISF OTUs were identified in the sequenced samples (Figure 1). A list of all the OTUs and their sequences can be found in Table S1. Four OTUs (1, 2, 3, and 13) were very similar (>80%, Table 2) to the previously described ISFs Kamiti river virus, Cell fusing agent, and CbaAr4001. This group also forms a strongly supported (95%) phylogenetic cluster (Figure 1). OTU2's closest relative (75% bootstrap support) was Cell fusing agent. The majority of the OTUs, however, had low similarity (<50%) to known ISFs and OTUs 25 and 26, which had no match (Table 2). This lack of close relatives is recapitulated in the poor resolution within the phylogeny (Figure 1). There is some evidence of relatedness for OTU 9 that clusters with a group containing other ISFs including Mosquito_flavivirus and Marisma virus (90% support). The similarity of OTUs 4, 11, 14, 21, 22, 24 with one another suggest they may all be variants of a single virus. In summary, our results show evidence of several well‐characterized ISFs but also a large number of novel viruses in our Ae. aegypti population.
Figure 1.
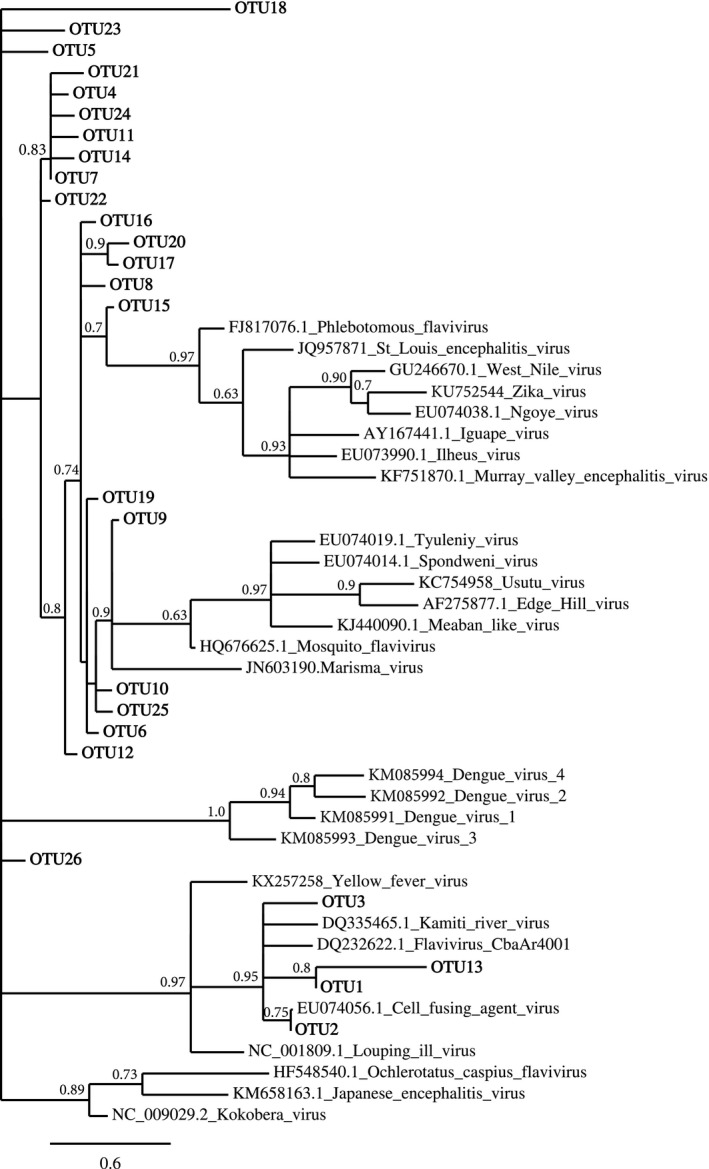
Maximum likelihood tree for the 26 ISF OTUs, their best reference hits, and key flaviviruses. Numbers at the nodes depict branch support values
Table 2.
OTUs with best hits/match reference
| OTU | Best match name | Best match accession | Length of match | Bits score | e‐score | Identity | % Similarity |
|---|---|---|---|---|---|---|---|
| 1 | Kamiti River virus | DQ335465.1 | 124 | 178 (90) | 5.00E‐44 | 106/110 (96%) | 96.00 |
| 2 | Cell fusing agent virus | EU074056.1 | 229 | 190 (96) | 9.00E‐48 | 99/100 (99%) | 99.00 |
| 3 | Flavivirus CbaAr4001 | DQ232622.1 | 87 | 155 (78) | 5.00E‐37 | 84/86 (97%) | 97.67 |
| 4 | West Nile virus | GU246670.1 | 186 | 46.1 (23) | 2.00E‐04 | 26/27 (96%) | 57.81 |
| 5 | Meaban‐like virus | KJ440090.1 | 124 | 48.1 (24) | 6.00E‐05 | 24/24 (100%) | 52.00 |
| 6 | Dengue 2 virus | FJ392598.1 | 144 | 48.1 (24) | 2.00E‐05 | 24/24 (100%) | 43.04 |
| 7 | Iguape virus | EU074054.1 | 229 | 48.1 (24) | 6.00E‐05 | 24/24 (100%) | 52.17 |
| 8 | Tyuleniy virus | EU074019.1 | 232 | 48.1 (24) | 1.00E‐04 | 24/24 (100) | 52.92 |
| 9 | Usutu virus | KC754958.1 | 10,745 | 44.1 (22) | 1.00E‐03 | 22/22 (100%) | 43.93 |
| 10 | Spondweni virus | EU074014.1 | 232 | 44.1 (22) | 1.00E‐03 | 25/26 (96%) | 55.10 |
| West Nile virus | GU246670.1 | 186 | 44.1 (22) | 1.00E‐03 | 25/26 (96%) | 50.00 | |
| 11 | Spondweni virus | EU074014.1 | 232 | 44.1 (22) | 1.00E‐03 | 25/26 (96%) | 53.12 |
| West Nile virus | GU246670.1 | 186 | 44.1 (22) | 1.00E‐03 | 25/26 (96%) | 53.12 | |
| St Louis encephalitis virus | JQ957871.1 | 2,718 | 44.1 (22) | 1.00E‐03 | 25/26 (96%) | 45.56 | |
| Ilheus virus | EU073990.1 | 232 | 44.1 (22) | 1.00E‐03 | 25/26 (96%) | 46.94 | |
| 12 | Mosquito flavivirus | HQ676625.1 | 165 | 44.1 (22) | 4.00E‐03 | 24/25 (96%) | 44.87 |
| Phlebotomus flavivirus | FJ817076.1 | 157 | 42.1 (21) | 4.00E‐03 | 24/25 (96%) | 48.15 | |
| 13 | Mosquito flavivirus | HQ676625.1 | 165 | 42.1 (21) | 4.00E‐03 | 24/25 (96%) | 96.97 |
| Kamiti River virus | DQ335465.1 | 124 | 63.9 (32) | 1.00E+09 | 32/32 (100%) | 80.95 | |
| Flavivirus CbaAr4001 | DQ232622.1 | 87 | 63.9 (32) | 1.00E‐09 | 32/32 (100%) | 59.49 | |
| 14 | Mosquito flavivirus | HQ676625.1 | 165 | 42.(21) | 4.00E‐03 | 24/25 (96%) | 47.95 |
| Phlebotomus virus | FJ817076.1 | 157 | 42.1 (21) | 4.00E‐03 | 24/25 (96%) | 49.37 | |
| 15 | Louping ill‐like virus | NC_001809.1 | 10,871 | 40.1 (20) | 2.80E‐02 | 20/20 (100%) | 45.70 |
| 16 | Japanese encephalitis virus | HQ223287.1 | 10,296 | 38.2 (19) | 5.10E‐02 | 25/27 (92%) | 50.72 |
| Edge Hill virus | AF275877.1 | 986 | 38.2 (19) | 5.10E‐02 | 25/27 (92%) | 50.72 | |
| 17 | Marisma virus | JN603190.1 | 1,008 | 44.1 (22) | 1.00E‐03 | 22/24 (100%) | 50.00 |
| 18 | Kokobera virus | NC_009029.2 | 10,874 | 42.1 (21) | 5.00E‐03 | 21/21 (100%) | 42.39 |
| Tick‐borne encephalitis virus | KT224352.1 | 10,619 | 42.1 (21) | 5.00E‐03 | 21/21 (100%) | 45.75 | |
| Dengue 2 virus | FJ392595.1 | 144 | 42.1 (21) | 5.00E‐03 | 21/21 (100%) | 37.11 | |
| 19 | Murray Valley encephalitis virus | KF751870.1 | 11,012 | 32.2 (16) | 3.60E+00 | 19/20 (95%) | 57.79 |
| St Louis encephalitis virus | JQ957871 | 2,718 | 32.2 (16) | 3.60E+00 | 19/20 (95%) | 49.35 | |
| Dengue 2 virus | FJ392598.1 | 144 | 32.2 (16) | 3.60E+00 | 19/20 (95%) | 42.86 | |
| 20 | Ochlerotatus caspius flavivirus‐like virus | HF548540 | 9,839 | 34.2 (17) | 8.80E‐01 | 17/17 (100%) | 43.42 |
| 21 | Hepatitis C virus | JQ060123.1 | 336 | 32.2 (16) | 3.30E+00 | 16/16 (100%) | 41.67 |
| Dengue 1 virus | M87512.1 | 10,717 | 32.2 (16) | 3.30E+00 | 16/16 (100%) | 52.11 | |
| Usutu virus | NC_006551.1 | 11,066 | 32.2 (16) | 3.30E+00 | 16/16 (100%) | 45.33 | |
| West Nile virus | KX547594.1 | 10,787 | 32.2 (16) | 3.30E+00 | 16/16 (100%) | 49.33 | |
| Japanese encephalitis virus | KM658163.1 | 10,965 | 32.2 (16) | 3.30E+00 | 16/16 (100%) | 46.67 | |
| 22 | West Nile virus | GU246670.1 | 186 | 44.1 (22) | 1.00E‐03 | 25/26 (96%) | 61.8 |
| Ngoye virus | EU074038.1 | 232 | 44.1 (22) | 1.00E‐03 | 25/26 (96%) | 50.00 | |
| 23 | West Nile virus | JX041630.1 | 10,810 | 40.1 (20) | 1.40E‐02 | 23/24 (95%) | 50.00 |
| Meaban virus | KJ440090.1 | 124 | 40.1 (20) | 1.40E‐02 | 23/24 (95%) | 51.39 | |
| 24 | Meaban virus | KJ440090.1 | 124 | 40.1 (20) | 1.50E‐02 | 20/20 (100) | 53.33 |
| Iguape virus | AY167441.1 | 2,669 | 40.1 (20) | 1.50E‐02 | 20/20 (100) | 51.35 | |
| 25 | Unidentified flavivirus 1 | – | – | – | – | – | – |
| 26 | Unidentified flavivirus 2 | – | – | – | – | – | – |
3.3. Wolbachia is often associated with higher ISF frequencies
All but five OTUs (16, 20, 21, 23, and 25) were fixed in wild‐type and wMel‐infected field populations based on sequence analysis. In the group of viruses not fixed, we found a significant effect of Wolbachia on infection frequency (Wald = 5.49, df = 1, p = .019), suggesting that Wolbachia was associated with higher rates of infection in the sequenced samples (Figure 2). We then tested whether these same trends were also present in the total set of samples (sequenced and not, n = 93) from the field using RT‐qPCR primers designed specifically for seven of the OTUs (Table 1). OTUs 1–3 were selected as they are closely related to well‐characterized ISFs (Figure 1, Table 2) and because they were fixed in both wMel and wild‐type populations. Four additional OTUs (16, 20, 21, and 25) (Figure 2, Table 1) were selected given their differential distributions by sequencing. OTUs 1–3 were shown to be at 100% frequency (Figure 3a) in the larger set of field samples, recapitulating what was seen by sequence analysis. We then tested whether the frequencies of the remaining OTUs varied with respect to Wolbachia infection status and found there was a significant interaction between OTU and Wolbachia infection status (Wald = 12.3, df = 3, p = .006) and so proceeded with the four individual comparisons and a multiple test correction (revised α = 0.0125). OTUs 16 (Wald chi‐square = 6.9, df = 1, p = .009), 20 (18.3, df = 1, p < .001), and 21 (41.0, df = 1, p < .001) were significantly different, whereas OTU25 (5.26, df = 1, p = .022) was not. In each case of significance, OTU frequencies were higher in wMel mosquitoes (Figure 3a). OTUs 16 and 21 that were previously not found in the sequenced wild‐type samples were detected through RT‐qPCR. This may be due to the sensitivity cutoff employed with the sequence data whereby we excluded OTUs with <10 sequence reads. Lastly, we then determined if these differences were also seen in laboratory lines of wMel‐infected and wild‐type mosquitoes. Unlike in the field, the rates of infection appear similar (Wald = 1.177; p = .27) between the two lines (Figure 3b).
Figure 2.
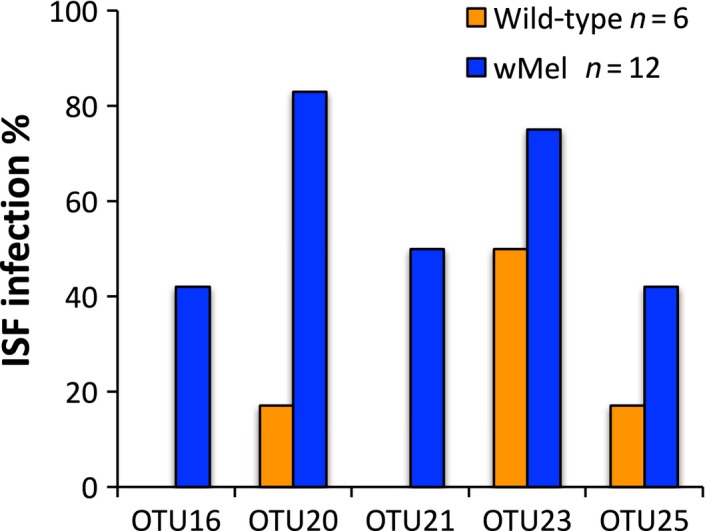
ISF infection rates for OTUs not fixed in both WT and Wolbachia‐infected mosquitoes from the field as determined by sequencing. Across these 5 OTUs, wMel mosquitoes exhibited higher infection rates (p = .019)
Figure 3.
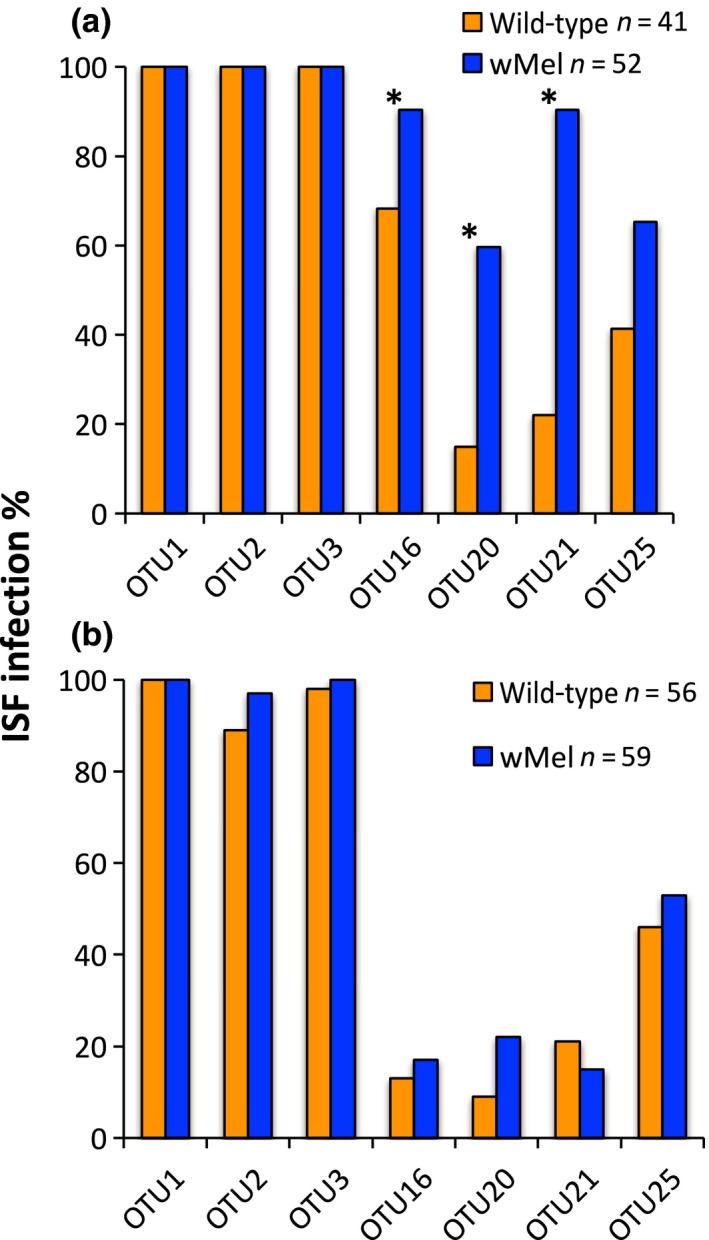
ISF infection rates in mosquitoes for a subset of OTUs as determined by RT‐qPCR. (a) In the field, three of the OTUs were more common in wMel‐infected mosquitoes than WT (*p < .0125). (b) In the laboratory, there were no differences in ISF infection rates between wMel and wild‐type mosquitoes in the laboratory (p = .27)
3.4. Wolbachia infection is associated with differences in abundance of ISFs
To determine whether the presence of Wolbachia had an effect on the abundance of ISFs, we compared the viral load of the seven selected OTUs between the wild‐type and wMel mosquitoes under field and laboratory conditions by RT‐qPCR. Even though Wolbachia reduced the load of OTU2 in the field (Figure 4a), with the wild‐type having a higher load (p = .017) compared to wMel mosquitoes, the opposite effect was observed in the laboratory where the wMel mosquitoes had a significantly higher load (p < .0001) than that in the wild type. In the field, OTU20 (p = .0009) and OTU21 (p < .0001) were significantly more abundant in wMel mosquitoes compared to the wild type (Figure 4b,c). This effect was not observed in the laboratory lines with no significant differences observed in loads of OTU20 (p = .17) and OTU21 (p = .91) between wMel and wild‐type mosquitoes (Figure 4e,f). There were no significant differences between loads of OTU1 (p = .94) and OTU3 (p = .63) in wMel and wild‐type mosquitoes in the field. In the laboratory lines however, wMel mosquitoes consistently had a higher abundance of OTU1 (p < .0001) and OTU3 (p < .0001) compared to the wild type. In both the field and laboratory mosquito lines, there were no differences in the loads of OTU16 (p > .05) and the OTU25 (p > .05) between Wolbachia‐infected and wild‐type mosquitoes (Figure S1). In summary, regardless of the mosquito line, ISF loads varied considerably between field and laboratory environments with the former consistently harboring higher ISF density than the latter. This suggests that environmental conditions and differences in host genetic background may influence abundance of ISFs. Our findings demonstrate that in general, Wolbachia does not inhibit ISF loads in Ae. aegypti mosquitoes and in some cases may enhance them. It also suggests that the effect of Wolbachia on ISFs is virus‐specific and environmental conditions may influence this effect, such that the laboratory environment may not be predictive of the field.
Figure 4.
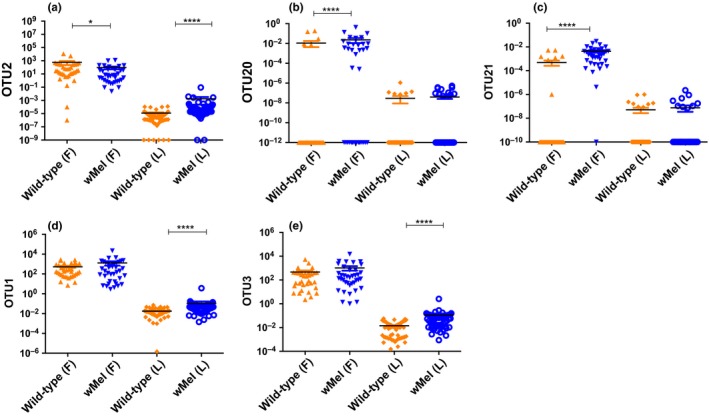
Relative abundance of identified ISF OTUs. (a) OTU2 decreased in abundance in wMel mosquitoes in the field but increased in wMel in the laboratory lines (b) OTU20 and (c) OTU21 were more abundant in wMel mosquitoes in the field but were not different in the laboratory. (d) OTU1 and (e) OTU3 increased in abundance in wMel laboratory mosquitoes only. *p < 05; ***p < .001; ****p < .0001
4. DISCUSSION
Despite large‐scale field releases of Wolbachia‐infected Ae. aegypti mosquitoes (Hoffmann et al., 2011; McGraw & O'Neill, 2013; Ritchie, 2014), the antivirus or blocking effect of the symbiont on the naturally occurring ISFs in Ae. aegypti mosquitoes is currently not known. This work therefore examined whether wMel Wolbachia‐infected mosquitoes sampled from field release sites in Australia and in laboratory populations exhibit symbiont‐associated changes in their ISFs. Generally, we found that in the field wMel mosquitoes had both higher ISF infection rates and abundances compared to wild‐type mosquitoes. We should point out that the number of field populations tested is small and so the findings could be the result of environmental or stochastic factors. Mosquitoes from other field release sites around the globe should be profiled to determine whether these relationships are robust and generalizable. In the laboratory where Ae. aegypti are reared under optimal conditions, there was no difference in ISF infection rates between wild‐type and wMel mosquitoes. However, wMel mosquitoes did exhibit higher loads of ISFs compared to the wild type. These findings were unexpected given that Wolbachia has been extensively shown to inhibit flaviviruses of medical importance in Ae. aegypti (Bian et al., 2010; van den Hurk et al., 2012; Moreira et al., 2009; Walker et al., 2011).
A total of 26 OTUs were observed in the sequenced field samples with all mosquitoes harboring multiple, concurrent infections. Some of these OTUs were closely related to each other indicating they are likely variants of the same virus. Very few of the OTUs clustered with characterized ISFs present in the databases. This confirms observations in invertebrates (Shi et al., 2016), and more specifically in Drosophila (Webster et al., 2015), that there are novel insect‐specific viruses that are yet to be classified. Our study is limited by sequencing a short fragment (143 bp) that probably affects the ability to have unambiguous matches and could explain why some OTUs matched to more than one virus in the database.
ISFs were found to be ubiquitous in both the laboratory and field mosquito populations suggesting that these flaviviruses may be transmitted vertically. Studies by Bolling et al. (2012) and Lutomiah, Mwandawiro, Magambo, and Sang (2007) demonstrated that Culex flavivirus and Kamiti river virus are maintained in Cx. pipiens and Ae. aegypti mainly through vertical transmission with venereal transmission playing a minor role. Other studies carried out both under laboratory and field conditions further demonstrated that flaviviruses of medical importance including WNV (Baqar, Hayes, Murphy, & Watts, 1993), DENV (Bosio, Thomas, Grimstad, & Rai, 1992), and YFV (Beaty, Tesh, & Aitken, 1980) can be maintained in nature through vertical transmission.
We observed differences between field and laboratory ISF infection rates and loads with the field mosquitoes consistently having higher infection rate and abundance regardless of mosquito line. These differences between the two populations may be partly attributed to selection and founder effects (Lorenz, Beaty, Aitken, Wallis, & Tabachnick, 1980; Munstermann, 1994) as is common with laboratory colonies (Lorenz et al., 1980; Munstermann, 1980, 1994). There is also a possibility that environmental conditions in the field (Huber et al., 2002) predispose Ae. aegypti to increased ISF infection as factors such as temperature influence mosquito immunity and therefore mosquito–pathogen interactions (Huber et al., 2002; Murdock, Moller‐Jacobs, & Thomas, 2013; Murdock, Paaijmans, Cox‐Foster, Read, & Thomas, 2012; Murdock, Paaijmans, Bell, et al., 2012). High larval crowding (Alto, Lounibos, Mores, & Reiskind, 2008; Baqar, Hayes, & Ahmed, 1980), nutritional restrictions (Alto et al., 2008; Baqar et al., 1980; Grimstad & Haramis, 1984; Kho, Hugo, Lu, Smith, & Kay, 2016), and low temperature (Chambers & Klowden, 1990) have independently been shown to cause small body size with an accompanying increased susceptibility to arboviruses such as WNV (Baqar et al., 1980), DENV (Alto et al., 2008; Kho et al., 2016), and La Crosse virus (Grimstad & Haramis, 1984). Environmental variables therefore need to be tested empirically to establish their effects on ISF infection rates and load in Ae. aegypti mosquitoes. It is also possible that age could contribute to variation in ISF infection (Bolling et al., 2012), but the sampling of adults from wild populations did not allow for age control.
It was unexpected to observe a higher ISF infection rate in wMel mosquitoes compared to the wild type in field Ae. aegypti populations. This sharply contrasts previous studies where Wolbachia infection significantly reduced the proportion of individuals infected with other flaviviruses such as DENV (Amuzu & McGraw, 2016; Amuzu, Simmons, & McGraw, 2015; Bian et al., 2013; Frentiu et al., 2014; Moreira et al., 2009; Walker et al., 2011), Zika (Aliota et al., 2016; Dutra et al., 2016), and YFV (van den Hurk et al., 2012). Our findings are, however, supported by studies performed in Cx. tarsalis and An. gambiae where the presence of Wolbachia increased the infection rate of WNV and P. berghei, respectively (Dodson et al., 2014; Hughes et al., 2012). In addition, Wolbachia has been observed to increase susceptibility of the DNA virus nucleopolyhedrovirus in the African armyworm, S. exempta (Graham et al., 2012). In laboratory lines, Wolbachia does not influence ISF infection rates suggesting that population genetic variation and differences in environmental conditions between the laboratory and field could be influencing Wolbachia interaction with ISFs. This hypothesis is not supported by Kho et al. (2016) and Caragata et al. (2013) who demonstrated that larval nutrition and adult carbohydrate intake did not affect DENV infection rates in wMel mosquitoes. Temperature, in contrast, has been shown to determine whether the wAlbB Wolbachia strain inhibits, enhances, or has a neutral effect on oocyte infection rate and intensity of Plasmodium yoelii in An. stephensi (Murdock, Blanford, Hughes, Rasgon, & Thomas, 2014). Based on these studies and our findings, there is a need to further investigate the effect of environmental conditions on Wolbachia–ISF interactions in order to establish the role the environment may be playing in modulating ISF infection.
Wolbachia suppressed the abundance of OTU2, that is most similar to cell fusing agent virus, in the field populations and this is supported by other studies that found inhibition of this ISF by the wMelPop Wolbachia strain in Ae. aegypti cell lines (Schnettler, Sreenu, Mottram, & McFarlane, 2016; Zhang, Etebari, & Asgari, 2016). This suppressive effect, however, was lost in the laboratory where Wolbachia significantly enhanced loads of OTU2. Generally, we observed wMel either enhanced loads of ISFs or had no significant effect, signifying that Wolbachia does not inhibit the success of these flaviviruses. The load of the insect‐specific virus Phasi Charoen‐like bunyavirus present in Ae. aegypti cells infected with the wMelPop Wolbachia was not shown to differ from those without the symbiont (Schnettler et al., 2016) thus supporting our observation that Wolbachia does not have an effect on ISF loads. Still, other studies have demonstrated pathogen enhancement by Wolbachia where the number of Plasmodium relictum oocytes that develop in the midgut of Cx. pipiens increased in the presence of the symbiont (Zele et al., 2014). This effect was also observed in An. gambiae where wAlbB Wolbachia strain significantly increased oocytes levels of P. berghei (Hughes et al., 2012). The fact that Wolbachia did not have a significant effect on loads of OTU16 and OTU25 suggests that the effect of Wolbachia on ISF are virus‐specific. This supports other studies in which contrasting results were observed for closely related species where the wAlbB Wolbachia strain enhanced P. berghei (Hughes et al., 2012) but inhibits P. falciparum (Hughes, Koga, Xue, Fukatsu, & Rasgon, 2011) in An. gambiae.
The effect of the Wolbachia–ISF relationship on viruses of medical importance has not been examined in mosquitoes. It is possible that ISF enhancement by Wolbachia may not have an effect on arboviruses (Crockett et al., 2012; Kent et al., 2010). Alternatively, it could lead to inhibition of arboviruses as was observed in the case of WNV and Murray Valley encephalitis (Bolling et al., 2012; Hobson‐Peters et al., 2013), further strengthening the pathogen blocking effect of Wolbachia. Given that ISF is common and widely distributed in Ae. aegypti mosquitoes, this could be advantageous to the current Wolbachia‐dengue control strategy. More concerning is the possibility of ISF enhancement resulting in increased susceptibility of mosquitoes for arboviruses as was reported in a different study for WNV (Newman et al., 2011). This would have serious consequences for the current Wolbachia–DENV control strategy as Wolbachia‐infected mosquitoes will facilitate arbovirus proliferation instead of limiting them. Finally, our findings point to the need to carefully examine environmental conditions before embarking on Wolbachia–Ae. aegypti field releases as the Wolbachia‐pathogen effects observed in the laboratory may not be representative of the field.
CONFLICT OF INTEREST
None declared.
DATA ACCESSIBILITY
All data are available at Figshare https://doi.org/10.4225/03/5aa1a8b0af9f3.
AUTHOR CONTRIBUTIONS
EAM conceptualized and supervised the project. CK provided field samples. HEA did the experimental work. KT, HEA, EAM, RH, and DP analyzed data. The manuscript was written by EAM and HEA.
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank the Cairns Eliminate Dengue team at James Cook University for assistance with sample collection. The research was supported by an ARC Discovery Grant to EAM (DP160100588).
Amuzu HE, Tsyganov K, Koh C, Herbert RI, Powell DR, McGraw EA. Wolbachia enhances insect‐specific flavivirus infection in Aedes aegypti mosquitoes. Ecol Evol. 2018;8:5441–5454. https://doi.org/10.1002/ece3.4066
REFERENCES
- Aliota, M. T. , Peinado, S. A. , Velez, I. D. , & Osorio, J. E. (2016). The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti . Scientific Reports, 6, 28792 https://doi.org/10.1038/srep28792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto, B. W. , Lounibos, L. P. , Mores, C. N. , & Reiskind, M. H. (2008). Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proceedings. Biological sciences/The Royal Society, 275, 463–471. https://doi.org/10.1098/rspb.2007.1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amuzu, H. E. , & McGraw, E. A. (2016). Wolbachia‐based dengue virus inhibition is not tissue‐specific in Aedes aegypti . PLoS Neglected Tropical Diseases, 10, e0005145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amuzu, H. E. , Simmons, C. P. , & McGraw, E. A. (2015). Effect of repeat human blood feeding on Wolbachia density and dengue virus infection in Aedes aegypti . Parasit Vectors, 8, 246 https://doi.org/10.1186/s13071-015-0853-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimova, M. , & Gascuel, O. (2006). Approximate likelihood‐ratio test for branches: A fast, accurate, and powerful alternative. Systematic Biology, 55, 539–552. https://doi.org/10.1080/10635150600755453 [DOI] [PubMed] [Google Scholar]
- Audsley, M. D. , Seleznev, A. , Joubert, D. A. , O'Neill, S. L. , & McGraw, E. A. (2018). Wolbachia infection alters the relative abundance of resident bacteria in adult Aedes aegypti mosquitoes, but not larvae. Molecular Ecology, 27, 297–309. https://doi.org/10.1111/mec.14436 [DOI] [PubMed] [Google Scholar]
- Baqar, S. , Hayes, C. , & Ahmed, T. (1980). The effect of larval rearing conditions and adult age on the susceptibility of Culex tritaeniorhynchus to infection with West Nile virus. Mosq News, 40, 165–171. [Google Scholar]
- Baqar, S. , Hayes, C. G. , Murphy, J. R. , & Watts, D. M. (1993). Vertical transmission of West Nile virus by Culex and Aedes species mosquitoes. American Journal of Tropical Medicine and Hygiene, 48, 757–762. https://doi.org/10.4269/ajtmh.1993.48.757 [DOI] [PubMed] [Google Scholar]
- Beaty, B. J. , Tesh, R. B. , & Aitken, T. H. (1980). Transovarial transmission of yellow fever virus in Stegomyia mosquitoes. American Journal of Tropical Medicine and Hygiene, 29, 125–132. https://doi.org/10.4269/ajtmh.1980.29.125 [DOI] [PubMed] [Google Scholar]
- Berry, D. , Ben Mahfoudh, K. , Wagner, M. , & Loy, A. (2011). Barcoded primers used in multiplex amplicon pyrosequencing bias amplification. Applied and Environment Microbiology, 77, 7846–7849. https://doi.org/10.1128/AEM.05220-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt, S. , Gething, P. W. , Brady, O. J. , Messina, J. P. , Farlow, A. W. , Moyes, C. L. , … Hay, S. I. (2013). The global distribution and burden of dengue. Nature, 496, 504–507. https://doi.org/10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian, G. , Joshi, D. , Dong, Y. , Lu, P. , Zhou, G. , Pan, X. , … Xi, Z. (2013). Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science, 340, 748–751. https://doi.org/10.1126/science.1236192 [DOI] [PubMed] [Google Scholar]
- Bian, G. , Xu, Y. , Lu, P. , Xie, Y. , & Xi, Z. (2010). The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti . PLoS Pathogens, 6, e1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billecocq, A. , Vazeille‐Falcoz, M. , Rodhain, F. , & Bouloy, M. (2000). Pathogen‐specific resistance to Rift Valley fever virus infection is induced in mosquito cells by expression of the recombinant nucleoprotein but not NSs non‐structural protein sequences. Journal of General Virology, 81, 2161–2166. https://doi.org/10.1099/0022-1317-81-9-2161 [DOI] [PubMed] [Google Scholar]
- Black, W. Ct. , Bennett, K. E. , Gorrochotegui‐Escalante, N. , Barillas‐Mury, C. V. , Fernández‐Salas, I. , de Lourdes Muñoz, M. , … Beaty, B. J. (2002). Flavivirus susceptibility in Aedes aegypti . Archives of Medical Research, 33, 379–388. https://doi.org/10.1016/S0188-4409(02)00373-9 [DOI] [PubMed] [Google Scholar]
- Bolling, B. G. , Olea‐Popelka, F. J. , Eisen, L. , Moore, C. G. , & Blair, C. D. (2012). Transmission dynamics of an insect‐specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co‐infection on vector competence for West Nile virus. Virology, 427, 90–97. https://doi.org/10.1016/j.virol.2012.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosio, C. F. , Thomas, R. E. , Grimstad, P. R. , & Rai, K. S. (1992). Variation in the efficiency of vertical transmission of dengue‐1 virus by strains of Aedes albopictus (Diptera: Culicidae). Journal of Medical Entomology, 29, 985–989. https://doi.org/10.1093/jmedent/29.6.985 [DOI] [PubMed] [Google Scholar]
- Caragata, E. P. , Rances, E. , Hedges, L. M. , Gofton, A. W. , Johnson, K. N. , O’ Neill, S. L. , & McGraw, E. A. (2013). Dietary cholesterol modulates pathogen blocking by Wolbachia . PLoS Pathogens, 9, e1003459 https://doi.org/10.1371/journal.ppat.1003459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers, G. M. , & Klowden, M. J. (1990). Correlation of nutritional reserves with a critical weight for pupation in larval Aedes aegypti mosquitoes. Journal of the American Mosquito Control Association, 6, 394–399. [PubMed] [Google Scholar]
- Chevenet, F. , Brun, C. , Banuls, A. L. , Jacq, B. , & Christen, R. (2006). TreeDyn: Towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics, 7, 439 https://doi.org/10.1186/1471-2105-7-439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, P. E. , Hugo, L. E. , Iturbe‐Ormaetxe, I. , Williams, C. R. , Chenoweth, S. F. , Ritchie, S. A. , … O'Neill, S. L. (2006). The use of transcriptional profiles to predict adult mosquito age under field conditions. Proceedings of the National Academy of Sciences of the United States of America, 103, 18060–18065. https://doi.org/10.1073/pnas.0604875103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon, K. L. , Brown, M. R. , & Strand, M. R. (2016). Mosquitoes host communities of bacteria that are essential for development but vary greatly between local habitats. Molecular Ecology, 25, 5806–5826. https://doi.org/10.1111/mec.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree, M. B. , Sang, R. C. , Stollar, V. , Dunster, L. M. , & Miller, B. R. (2003). Genetic and phenotypic characterization of the newly described insect flavivirus, Kamiti River virus. Archives of Virology, 148, 1095–1118. https://doi.org/10.1007/s00705-003-0019-7 [DOI] [PubMed] [Google Scholar]
- Crockett, R. K. , Burkhalter, K. , Mead, D. , Kelly, R. , Brown, J. , Varnado, W. , … Nasci, R. (2012). Culex flavivirus and West Nile virus in Culex quinquefasciatus populations in the southeastern United States. Journal of Medical Entomology, 49, 165–174. https://doi.org/10.1603/ME11080 [DOI] [PubMed] [Google Scholar]
- Dereeper, A. , Audic, S. , Claverie, J. M. , & Blanc, G. (2010). BLAST‐EXPLORER helps you building datasets for phylogenetic analysis. BMC Evolutionary Biology, 10, 8 https://doi.org/10.1186/1471-2148-10-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper, A. , Guignon, V. , Blanc, G. , Audic, S. , Buffet, S. , Chevenet, F. , … Claverie, J. M. (2008). Phylogeny.fr: Robust phylogenetic analysis for the non‐specialist. Nucleic Acids Research, 36, W465–W469. https://doi.org/10.1093/nar/gkn180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson, B. L. , Hughes, G. L. , Paul, O. , Matacchiero, A. C. , Kramer, L. D. , & Rasgon, J. L. (2014). Wolbachia enhances West Nile virus (WNV) infection in the mosquito Culex tarsalis . PLoS Neglected Tropical Diseases, 8, e2965 https://doi.org/10.1371/journal.pntd.0002965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra, H. L. , Rocha, M. N. , Dias, F. B. , Mansur, S. B. , Caragata, E. P. , & Moreira, L. A. (2016). Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host & Microbe, 19, 771–774. https://doi.org/10.1016/j.chom.2016.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, R. C. (2004). MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–1797. https://doi.org/10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics, 26, 2460–2461. https://doi.org/10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- Erlanger, T. E. , Weiss, S. , Keiser, J. , Utzinger, J. , & Wiedenmayer, K. (2009). Past, present, and future of Japanese encephalitis. Emerging Infectious Diseases, 15, 1–7. https://doi.org/10.3201/eid1501.080311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauquet, C. M. , Mayo, M. , Maniloff, J. , Desselberger, U. , & Ball, L. (2005). Virus taxonomy: VIIIth report of the International Committee on Taxonomy of Viruses. Elsevier/Academic Press, London. [Google Scholar]
- Frentiu, F. D. , Robinson, J. , Young, P. R. , McGraw, E. A. , & O'Neill, S. L. (2010). Wolbachia‐mediated resistance to dengue virus infection and death at the cellular level. PLoS ONE, 5, e13398 https://doi.org/10.1371/journal.pone.0013398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentiu, F. D. , Zakir, T. , Walker, T. , Popovici, J. , Pyke, A. T. , van den Hurk, A. , … O’ Neill, S. L. (2014). Limited dengue virus replication in field‐collected Aedes aegypti mosquitoes infected with Wolbachia . PLoS Neglected Tropical Diseases, 8, e2688 https://doi.org/10.1371/journal.pntd.0002688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geib, T. , Sauder, C. , Venturelli, S. , Hässler, C. , Staeheli, P. , & Schwemmle, M. (2003). Selective virus resistance conferred by expression of Borna disease virus nucleocapsid components. Journal of Virology, 77, 4283–4290. https://doi.org/10.1128/JVI.77.7.4283-4290.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser, R. L. , & Meola, M. A. (2010). The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS ONE, 5, e11977 https://doi.org/10.1371/journal.pone.0011977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, F. M. , Hixson, B. L. , Tyner, M. D. W. , Ramirez, J. L. , Canepa, G. E. , e Silva, T. L. , … Sogoba, N. (2017). Effect of naturally occurring Wolbachia in Anopheles gambiae s.I. mosquitoes from Mali on Plasmodium falciparum malaria transmission. Proceedings of the National Academy of Sciences of the United States of America, 114, 12566–12571. https://doi.org/10.1073/pnas.1716181114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, R. I. , Grzywacz, D. , Mushobozi, W. L. , & Wilson, K. (2012). Wolbachia in a major African crop pest increases susceptibility to viral disease rather than protects. Ecology Letters, 15, 993–1000. https://doi.org/10.1111/j.1461-0248.2012.01820.x [DOI] [PubMed] [Google Scholar]
- Grimstad, P. R. , & Haramis, L. D. (1984). Aedes triseriatus (Diptera: Culicidae) and La Crosse virus. III. Enhanced oral transmission by nutrition‐deprived mosquitoes. Journal of Medical Entomology, 21, 249–256. https://doi.org/10.1093/jmedent/21.3.249 [DOI] [PubMed] [Google Scholar]
- Guindon, S. , & Gascuel, O. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology, 52, 696–704. https://doi.org/10.1080/10635150390235520 [DOI] [PubMed] [Google Scholar]
- Hall‐Mendelin, S. , Pyke, A. T. , Moore, P. R. , Mackay, I. M. , McMahon, J. L. , Ritchie, S. A. , … van den Hurk, A. F. (2016). Assessment of local mosquito species incriminates Aedes aegypti as the potential vector of Zika virus in Australia. PLoS Neglected Tropical Diseases, 10, e0004959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, E. B. (2009). Zika virus outside Africa. Emerging Infectious Diseases, 15, 1347–1350. https://doi.org/10.3201/eid1509.090442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges, L. M. , Brownlie, J. C. , O'Neill, S. L. , & Johnson, K. N. (2008). Wolbachia and virus protection in insects. Science, 322, 702 https://doi.org/10.1126/science.1162418 [DOI] [PubMed] [Google Scholar]
- Hobson‐Peters, J. , Yam, A. W. , Lu, J. W. , Setoh, Y. X. , May, F. J. , Kurucz, N. , … Melville, L. (2013). A new insect‐specific flavivirus from northern Australia suppresses replication of West Nile virus and Murray Valley encephalitis virus in co‐infected mosquito cells. PLoS ONE, 8, e56534 https://doi.org/10.1371/journal.pone.0056534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, A. A. , Montgomery, B. L. , Popovici, J. , Iturbe‐Ormaetxe, I. , Johnson, P. H. , Muzzi, F. , … Cook, H. (2011). Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature, 476, 454–457. https://doi.org/10.1038/nature10356 [DOI] [PubMed] [Google Scholar]
- Hoshino, K. , Isawa, H. , Tsuda, Y. , Yano, K. , Sasaki, T. , Yuda, M. , … Sawabe, K. (2007). Genetic characterization of a new insect flavivirus isolated from Culex pipiens mosquito in Japan. Virology, 359, 405–414. https://doi.org/10.1016/j.virol.2006.09.039 [DOI] [PubMed] [Google Scholar]
- Huber, K. , Loan, L. L. , Hoang, T. H. , Tien, T. K. , Rodhain, F. , & Failloux, A. B. (2002). Temporal genetic variation in Aedes aegypti populations in Ho Chi Minh City (Vietnam). Heredity (Edinb), 89, 7–14. https://doi.org/10.1038/sj.hdy.6800086 [DOI] [PubMed] [Google Scholar]
- Hughes, G. L. , Koga, R. , Xue, P. , Fukatsu, T. , & Rasgon, J. L. (2011). Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae . PLoS Pathogens, 7, e1002043 https://doi.org/10.1371/journal.ppat.1002043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, G. L. , Vega‐Rodriguez, J. , Xue, P. , & Rasgon, J. L. (2012). Wolbachia strain wAlbB enhances infection by the rodent malaria parasite Plasmodium berghei in Anopheles gambiae mosquitoes. Applied and Environment Microbiology, 78, 1491–1495. https://doi.org/10.1128/AEM.06751-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hurk, A. F. , Hall‐Mendelin, S. , Pyke, A. T. , Frentiu, F. D. , McElroy, K. , Day, A. , … O’ Neill, S. L. (2012). Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti . PLoS Neglected Tropical Diseases, 6, e1892 https://doi.org/10.1371/journal.pntd.0001892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturbe‐Ormaetxe, I. , Walker, T. , & LO'Neill, S. (2011). Wolbachia and the biological control of mosquito‐borne disease. EMBO Reports, 12, 508–518. https://doi.org/10.1038/embor.2011.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert, D. A. , & O'Neill, S. L. (2017). Comparison of stable and transient Wolbachia infection models in Aedes aegypti to block dengue and West Nile Viruses. PLoS Neglected Tropical Diseases, 11, e0005275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert, D. A. , Walker, T. , Carrington, L. B. , De Bruyne, J. T. , Kien, D. H. , Hoang, N. L. , … O’ Neill, S. L. (2016). Establishment of a Wolbachia Superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management. PLoS Pathogens, 12, e1005434 https://doi.org/10.1371/journal.ppat.1005434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabatsos, N. (1978). Supplement to international catalogue of arboviruses including certain other viruses of vertebrates. American Journal of Tropical Medicine and Hygiene, 27, 372. [DOI] [PubMed] [Google Scholar]
- Karpf, A. R. , Lenches, E. , Strauss, E. G. , Strauss, J. H. , & Brown, D. T. (1997). Superinfection exclusion of alphaviruses in three mosquito cell lines persistently infected with Sindbis virus. Journal of Virology, 71, 7119–7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay, B. H. , Fanning, I. D. , & Carley, J. G. (1984). The vector competence of Australian Culex annulirostris with Murray Valley encephalitis and Kunjin viruses. Australian Journal of Experimental Biology and Medical Science, 62(Pt 5), 641–650. https://doi.org/10.1038/icb.1984.61 [DOI] [PubMed] [Google Scholar]
- Kent, R. J. , Crabtree, M. B. , & Miller, B. R. (2010). Transmission of West Nile virus by Culex quinquefasciatus say infected with Culex flavivirus Izabal. PLoS Neglected Tropical Diseases, 4, e671 https://doi.org/10.1371/journal.pntd.0000671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho, E. A. , Hugo, L. E. , Lu, G. , Smith, D. D. , & Kay, B. H. (2016). Effects of larval nutrition on Wolbachia‐based dengue virus interference in Aedes aegypti (Diptera: Culicidae). Journal of Medical Entomology, 53, 894–901. https://doi.org/10.1093/jme/tjw029 [DOI] [PubMed] [Google Scholar]
- Kittayapong, P. , Baisley, K. J. , Baimai, V. , & O'Neill, S. L. (2000). Distribution and diversity of Wolbachia infections in Southeast Asian mosquitoes (Diptera: Culicidae). Journal of Medical Entomology, 37, 340–345. https://doi.org/10.1093/jmedent/37.3.340 [DOI] [PubMed] [Google Scholar]
- Lorenz, L. , Beaty, B. J. , Aitken, T. H. , Wallis, G. P. , & Tabachnick, W. J. (1980). The effect of colonization upon Aedes aegypti susceptibility to oral infection with yellow fever virus. American Journal of Tropical Medicine and Hygiene, 29, 125–132. [DOI] [PubMed] [Google Scholar]
- Lu, P. , Bian, G. , Pan, X. , & Xi, Z. (2012). Wolbachia induces density‐dependent inhibition to dengue virus in mosquito cells. PLoS Neglected Tropical Diseases, 6, e1754 https://doi.org/10.1371/journal.pntd.0001754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y. E. , Cassese, T. , & Kielian, M. (1999). The cholesterol requirement for sindbis virus entry and exit and characterization of a spike protein region involved in cholesterol dependence. Journal of Virology, 73, 4272–4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutomiah, J. J. , Mwandawiro, C. , Magambo, J. , & Sang, R. C. (2007). Infection and vertical transmission of Kamiti river virus in laboratory bred Aedes aegypti mosquitoes. Journal of Insect Science, 7, 1–7. https://doi.org/10.1673/031.007.5501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie, J. S. , Gubler, D. J. , & Petersen, L. R. (2004). Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nature Medicine, 10, S98–S109. https://doi.org/10.1038/nm1144 [DOI] [PubMed] [Google Scholar]
- Mackenzie, J. M. , Khromykh, A. A. , & Parton, R. G. (2007). Cholesterol manipulation by West Nile virus perturbs the cellular immune response. Cell Host & Microbe, 2, 229–239. https://doi.org/10.1016/j.chom.2007.09.003 [DOI] [PubMed] [Google Scholar]
- Mackenzie, J. S. , Lindsay, M. D. , Coelen, R. J. , Broom, A. K. , Hall, R. A. , & Smith, D. W. (1994). Arboviruses causing human disease in the Australasian zoogeographic region. Archives of Virology, 136, 447–467. https://doi.org/10.1007/BF01321074 [DOI] [PubMed] [Google Scholar]
- Martin, M. (2011). Cutadapt removes adapter sequences from high‐throughput sequencing reads. EMBnet Journal, 17, 10 https://doi.org/10.14806/ej.17.1.200 [Google Scholar]
- McAlister, W. T. , & Barrett, C. (1977). Superinfection exclusion by bacterophage T7. Journal of Virology, 24, 709–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw, E. A. , Merritt, D. J. , Droller, J. N. , & O'Neill, S. L. (2002). Wolbachia density and virulence attenuation after transfer into a novel host. Proceedings of the National Academy of Sciences of the United States of America, 99, 2918–2923. https://doi.org/10.1073/pnas.052466499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw, E. A. , & O'Neill, S. L. (2013). Beyond insecticides: New thinking on an ancient problem. Nature Reviews Microbiology, 11, 181–193. https://doi.org/10.1038/nrmicro2968 [DOI] [PubMed] [Google Scholar]
- McMeniman, C. J. , Hughes, G. L. , & O'Neill, S. L. (2011). A Wolbachia symbiont in Aedes aegypti disrupts mosquito egg development to a greater extent when mosquitoes feed on nonhuman versus human blood. Journal of Medical Entomology, 48, 76–84. https://doi.org/10.1603/ME09188 [DOI] [PubMed] [Google Scholar]
- McMeniman, C. J. , Lane, R. V. , Cass, B. N. , Fong, A. W. , Sidhu, M. , Wang, Y. F. , & O'neill, S. L. (2009). Stable introduction of a life‐shortening Wolbachia infection into the mosquito Aedes aegypti . Science, 323, 141–144. https://doi.org/10.1126/science.1165326 [DOI] [PubMed] [Google Scholar]
- Min, K. T. , & Benzer, S. (1997). Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proceedings of the National Academy of Sciences of the United States of America, 94, 10792–10796. https://doi.org/10.1073/pnas.94.20.10792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira, L. A. , Iturbe‐Ormaetxe, I. , Jeffery, J. A. , Lu, G. , Pyke, A. T. , Hedges, L. M. , & Hugo, L. E. (2009). A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell, 139, 1268–1278. https://doi.org/10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- Mousson, L. , Zouache, K. , Arias‐Goeta, C. , Raquin, V. , Mavingui, P. , & Failloux, A. B. (2012). The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus . PLoS Neglected Tropical Diseases, 6, e1989 https://doi.org/10.1371/journal.pntd.0001989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munstermann, L. E. (1980). Distinguishing geographic strains of the Aedes atropalpus group (Diptera: Culicidae) by analysis of enzyme variation. Annals of the Entomological Society of America, 73, 699–704. https://doi.org/10.1093/aesa/73.6.699 [Google Scholar]
- Munstermann, L. E. (1994). Unexpected genetic consequences of colonization and inbreeding: Allozyme tracking in Culicidae (Diptera). Annals of the Entomological Society of America, 87, 157–164. https://doi.org/10.1093/aesa/87.2.157 [Google Scholar]
- Murdock, C. C. , Blanford, S. , Hughes, G. L. , Rasgon, J. L. , & Thomas, M. B. (2014). Temperature alters Plasmodium blocking by Wolbachia . Scientific Reports, 4, 3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock, C. C. , Moller‐Jacobs, L. L. , & Thomas, M. B. (2013). Complex environmental drivers of immunity and resistance in malaria mosquitoes. Proceedings. Biological sciences/The Royal Society, 280, 20132030 https://doi.org/10.1098/rspb.2013.2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock, C. C. , Paaijmans, K. P. , Bell, A. S. , King, J. G. , Hillyer, J. F. , Read, A. F. , & Thomas, M. B. (2012). Complex effects of temperature on mosquito immune function. Proceedings. Biological sciences/The Royal Society, 279, 3357–3366. https://doi.org/10.1098/rspb.2012.0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock, C. C. , Paaijmans, K. P. , Cox‐Foster, D. , Read, A. F. , & Thomas, M. B. (2012). Rethinking vector immunology: The role of environmental temperature in shaping resistance. Nature Reviews Microbiology, 10, 869–876. https://doi.org/10.1038/nrmicro2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nethe, M. , Berkhout, B. , & van der Kuyl, A. C. (2005). Retroviral superinfection resistance. Retrovirology, 2, 52 https://doi.org/10.1186/1742-4690-2-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, C. M. , Cerutti, F. , Anderson, T. K. , Hamer, G. L. , Walker, E. D. , Kitron, U. D. , … Goldberg, T. L. (2011). Culex flavivirus and West Nile virus mosquito coinfection and positive ecological association in Chicago, United States. Vector‐Borne and Zoonotic Diseases, 11, 1099–1105. https://doi.org/10.1089/vbz.2010.0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne, S. E. , Iturbe‐Ormaetxe, I. , Brownlie, J. C. , O'Neill, S. L. , & Johnson, K. N. (2012). Antiviral protection and the importance of Wolbachia density and tissue tropism in Drosophila simulans . Applied and Environment Microbiology, 78, 6922–6929. https://doi.org/10.1128/AEM.01727-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, X. , Zhou, G. , Wu, J. , Bian, G. , Lu, P. , Raikhel, A. S. , & Xi, Z. (2012). Wolbachia induces reactive oxygen species (ROS)‐dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti . Proceedings of the National Academy of Sciences of the United States of America, 109, E23–E31. https://doi.org/10.1073/pnas.1116932108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesko, K. , & Mores, C. N. (2009). Effect of sequential exposure on infection and dissemination rates for West Nile and St. Louis encephalitis viruses in Culex quinquefasciatus . Vector‐Borne and Zoonotic Diseases, 9, 281–286. https://doi.org/10.1089/vbz.2007.0281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujhari, S. , & Rasgon, J. L. (2016). Zika virus: A newly emergent vector‐borne public health threat in the Americas. PeerJ Preprints, 4, e1781. [Google Scholar]
- Rainey, S. M. , Martinez, J. , McFarlane, M. , Juneja, P. , Sarkies, P. , Lulla, A. , … Jiggins, F. M. (2016). Wolbachia blocks viral genome replication early in infection without a transcriptional response by the endosymbiont or host small RNA pathways. PLoS Pathogens, 12, e1005536 https://doi.org/10.1371/journal.ppat.1005536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rances, E. , Ye, Y. H. , Woolfit, M. , McGraw, E. A. , & O'Neill, S. L. (2012). The relative importance of innate immune priming in Wolbachia‐mediated dengue interference. PLoS Pathogens, 8, e1002548 https://doi.org/10.1371/journal.ppat.1002548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, M. F. , Weinart, L. A. , Welch, J. J. , Linheiro, R. S. , Magwire, M. M. , Jiggins, F. M. , & Bergman, C. M. (2012). Population genomics of the Wolbachia endosymbiont in Drosophila melanogaster . PLoS Genetics, 8, e1003129 https://doi.org/10.1371/journal.pgen.1003129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, S. (2014). Rear and release: a new paradigm for dengue control. Austral Entomology, 53, 363–367. https://doi.org/10.1111/aen.12127 [Google Scholar]
- Rognes, T. , Flouri, T. , Nichols, B. , Quince, C. , & Mahe, F. (2016). VSEARCH: A versatile open source tool for metagenomics. PeerJ, 4, e2584 https://doi.org/10.7717/peerj.2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Seco, M. P. , Rosario, D. , Domingo, C. , Hernández, L. , Valdes, K. , Guzmán, M. G. , & Tenorio, A. (2005). Generic RT‐nested‐PCR for detection of flaviviruses using degenerated primers and internal control followed by sequencing for specific identification. Journal of Virological Methods, 126, 101–109. https://doi.org/10.1016/j.jviromet.2005.01.025 [DOI] [PubMed] [Google Scholar]
- Schnettler, E. , Sreenu, V. B. , Mottram, T. , & McFarlane, M. (2016). Wolbachia restricts insect‐specific flavivirus infection in Aedes aegypti cells. Journal of General Virology, 97, 3024–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbus, L. R. , Casper‐Lindley, C. , Landmann, F. , & Sullivan, W. (2008). The genetics and cell biology of Wolbachia‐host interactions. Annual Review of Genetics, 42, 683–707. https://doi.org/10.1146/annurev.genet.41.110306.130354 [DOI] [PubMed] [Google Scholar]
- Shaw, W. R. , Marcenac, P. , Childs, L. M. , Buckee, C. O. , Baldini, F. , Sawadogo, S. P. , … Catteruccia, F. (2016). Wolbachia infections in natural Anopheles populations affect egg laying and negatively correlate with Plasmodium development. Nature Communications, 7, 11772 https://doi.org/10.1038/ncomms11772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, M. , Lin, X. D. , Tian, J. H. , Chen, L. J. , Chen, X. , Li, C. X. , … Buchmann, J. (2016). Redefining the invertebrate RNA virosphere. Nature, 540, 539 https://doi.org/10.1038/nature20167 [DOI] [PubMed] [Google Scholar]
- Stollar, V. , & Thomas, V. L. (1975). An agent in the Aedes aegypti cell line (Peleg) which causes fusion of Aedes albopictus cells. Virology, 64, 367–377. https://doi.org/10.1016/0042-6822(75)90113-0 [DOI] [PubMed] [Google Scholar]
- Teixeira, L. , Ferreira, A. , & Ashburner, M. (2008). The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster . PLoS Biology, 6, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terradas, G. , & McGraw, E. A. (2017). Wolbachia‐mediated virus blocking in the mosquito vector Aedes aegypti . Current Opinion in Insect Science, 22, 37–44. https://doi.org/10.1016/j.cois.2017.05.005 [DOI] [PubMed] [Google Scholar]
- Thellin, O. , Zorzi, W. , Lakaye, B. , De Borman, B. , Coumans, B. , Hennen, G. , … Heinen, E. (1999). Housekeeping genes as internal standards: Use and limits. Journal of Biotechnology, 75, 291–295. https://doi.org/10.1016/S0168-1656(99)00163-7 [DOI] [PubMed] [Google Scholar]
- Walker, T. , Johnson, P. H. , Moreira, L. A. , Iturbe‐Ormaetxe, I. , Frentiu, F. D. , McMeniman, C. J. , … Lloyd, A. L. (2011). The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature, 476, 450–453. https://doi.org/10.1038/nature10355 [DOI] [PubMed] [Google Scholar]
- Webster, C. L. , Waldron, F. M. , Robertson, S. , Crowson, D. , Ferrari, G. , Quintana, J. F. , … Lazzaro, B. P. (2015). The discovery, distribution, and evolution of viruses associated with Drosophila melanogaster . PLoS Biology, 13, e1002210 https://doi.org/10.1371/journal.pbio.1002210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinart, L. A. , Arauju‐Jnr, E. V. , Ahamed, M. Z. , & Welch, J. J. (2015). The incidence of bacterial endosymbionts in terrestrial arthropods. Proceedings. Biological sciences/The Royal Society, 282, 20150249 https://doi.org/10.1098/rspb.2015.0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, P. M. , Serbus, L. R. , Debec, A. , Codina, A. , Bray, W. , Guichet, A. , … Sullivan, W. (2017). Reliance of Wolbachia on high rates of host proteolysis revealed by a genome‐wide RNAi screen of Drosophila Cells. Genetics, 205, 1473–1488. https://doi.org/10.1534/genetics.116.198903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi, Z. , Dean, J. L. , Khoo, C. , & Dobson, S. L. (2005). Generation of a novel Wolbachia infection in Aedes albopictus (Asian tiger mosquito) via embryonic microinjection. Insect Biochemistry and Molecular Biology, 35, 903–910. https://doi.org/10.1016/j.ibmb.2005.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger, D. S. (2016). Epidemiology of Zika Virus. Neurologic Clinics, 34, 1049–1056. https://doi.org/10.1016/j.ncl.2016.06.008 [DOI] [PubMed] [Google Scholar]
- Zele, F. , Nicot, A. , Berthomieu, A. , Weill, M. , Duron, O. , & Rivero, A. (2014). Wolbachia increases susceptibility to Plasmodium infection in a natural system. Proceedings. Biological sciences/The Royal Society, 281, 20132837 https://doi.org/10.1098/rspb.2013.2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, G. , Etebari, K. , & Asgari, S. (2016). Wolbachia suppresses cell fusing agent virus in mosquito cells. Journal of General Virology, 97, 3427–3432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available at Figshare https://doi.org/10.4225/03/5aa1a8b0af9f3.


