Abstract
We aimed to determine the prevalence and co‐occurrence of tobacco smoking, alcohol consumption, and depressive symptoms among a sample of head and neck cancer (HNC) patients undergoing radiotherapy. A total of 307 HNC patients participated in a multi‐site stepped‐wedge randomized controlled trial (RCT) evaluating the effectiveness of a dietitian‐delivered health behavior intervention in patients with HNC undergoing radiotherapy. During week one of radiotherapy patients completed measures of smoking, alcohol consumption, and level of depression. Approximately one‐fifth (21%) of patients had two or more co‐occurring problems: current smoking, hazardous alcohol use, and/or likely presence of a major depressive episode (MDE). Approximately one‐third (34%) of the sample were current smokers, one‐third (31%) were drinking hazardously and almost one‐fifth (19%) had likely cases of depression. Comorbidity of smoking, hazardous alcohol use, and MDE is high in HNC patients, and interventions need to address this cluster of cancer risk factors.
Keywords: Alcohol, comorbidity, depression, head neck cancer, smoking
Introduction
Tobacco smoking, alcohol consumption, and depressive symptoms are important determinants and highly prevalent factors in the onset, prognosis, and recovery from HNC. Continued smoking in cancer patients has been associated with several adverse outcomes including an increased risk for other smoking‐related diseases, second primary tumors, disease recurrence, poorer response to radiotherapy, decreased survival, and increased toxicity, and side effects from radiotherapy 1, 2, 3, 4. Continued alcohol intake at problematic levels has been associated with secondary cancers, decreased survival rates 5, and lower quality of life scores 3 in HNC patients. Depression in cancer patients is associated with increased morbidity and possibly increased mortality 6, 7. In HNC specifically, depressive symptoms have been associated with poorer quality of life scores 8 and found to be predictive of malnutrition during treatment 9.
Given the importance of these risks factors, a number of studies have described patterns of tobacco or alcohol use or depressive symptoms among HNC patients undergoing treatment. Prevalence estimates of these risk factors vary considerably. For example, evidence from observational and intervention studies report between one‐third and 75% of HNC patients continue to smoke after diagnosis 1, 10, 11. Between 37% and 54% of HNC patients continue to consume alcohol after diagnosis, with up to 16% continuing to drink at a hazardous level 10, 12. Estimates of the prevalence of depression in HNC patients range from 15% to 57% 8, 10, 13, 14. Variability in the reported consumption of alcohol and tobacco use among HNC patients, and the prevalence of depression in this group may be attributable to differences in the underlying prevalence rates in the population where the studies were conducted, period effects, or differences in measurement of tobacco 15 or alcohol use, 10, 12 or differences in diagnostic measurements for depression 16.
Of particular concern for both the immediate clinical outcomes of HNC patients, and their longer‐term health is the co‐occurrence of these risk factors. The presence of multiple health risk factors markedly increases the likelihood of adverse treatment outcomes. Smoking, alcohol misuse, and depressive symptoms tend to cluster and their relationship is complex 17. Despite the high rates of smoking, alcohol consumption, and depression reported in HNC patients and their effect on patient outcomes, there is little research investigating the rates of comorbidity of these factors in HNC patients. Duffy et al. 3, 10 conducted a cross‐sectional study, and a subsequent cohort study to examine the prevalence and associations between smoking, problem drinking, depressive symptoms, and quality of life among HNC patients recruited from Veterans Affairs hospitals in the USA, which included patients at varying stages of treatment. In the cross‐sectional study of 80 HNC patients, 76% scored positive for one or more of smoking, at‐risk alcohol intake and significant depressive symptoms. The follow‐up cohort study with a convenience sample of 973 HNC patients at varying stages of treatment reported similar results. However, the authors did not specify the prevalence of those patients with two or more of these issues.
Given the clinical and public health salience of tobacco smoking, alcohol consumption, and depressive symptoms among HNC patients, and the limitations of previous studies, a more comprehensive assessment of risk factors, and their co‐occurrence among HNC patients is required. Such information is important for health service planning and to ensure that care is provided to HNC patients that maximizes the likelihood of a positive long‐term prognosis. In particular, identification of those HNC patients who have co‐occurrence of smoking, alcohol consumption, and depressive symptoms before undergoing radiation treatment, may assist in considering interventions in addition to radiotherapy.
This is the first study to examine: smoking status; alcohol consumption; the severity of depressive symptoms; and their co‐occurrence assessed during the first week of radiotherapy. Our primary objectives were to:
Report the rates and severity of tobacco smoking, alcohol consumption, depressive symptom severity, and likelihood of major depressive episode (MDE); and
Describe the pattern of co‐occurrence of these factors
Materials and Methods
Procedures
In this cross‐sectional study, 307 patients participated in a multi‐site stepped‐wedge randomized controlled trial (RCT; Trial registration no. ACTRN12613000320752) and completed baseline assessments. The trial evaluated the effectiveness of training dietitians in an intervention based on psychological strategies (motivational interviewing and cognitive behavior therapy) in order to reduce malnutrition in patients with HNC undergoing radiotherapy 18. Eligible patients were approached with information about the study (by their radiation oncologist and/or an independent data manager) and written informed consent taken.
Inclusion criteria
Patients eligible for inclusion in the trial met the following criteria:
Aged 18 years or older.
Pathologically confirmed diagnosis of HNC, involving the nasopharynx, oropharynx, oral cavity, larynx, or hypopharynx, requiring definitive or postoperative radiotherapy with curative intent.
Receiving radiotherapy to a dose of at least 60 Gy with regional nodal irradiation including as a minimum ipsilateral nodal levels II–III.
Available for follow‐up for at least 6 months post study initiation.
Capacity to provide written informed consent.
Exclusion criteria
Inability to communicate in English.
Presence of organic brain diseases (impairing ability to complete questionnaires satisfactorily).
Likely insignificant oral or pharyngeal mucositis as a complication of radiotherapy treatment.
The study received approval from the Hunter New England Human Research Ethics Committee (HREC) of Hunter New England Health (HREC/12/HNE/108; HNEHREC: 12/04/18/4.06).
Measures
Across five sites, during the first week of radiotherapy, an independent research officer administered assessment instruments. These included demographic information, patient clinical characteristics, measures of smoking and alcohol consumption, and related features (level of nicotine dependence, intentions to change smoking or alcohol consumption), and level of depression. The research officer also conducted chart reviews to extract cancer diagnosis, staging, and treatment data.
Demographic characteristics
Demographic information included age (years), gender (male/female), marital status, Aboriginal and Torres Strait Islander (ATSI) status, education, accommodation, and employment status.
Clinical characteristics
Clinical information included tumor site, tumor stage, proposed radiotherapy dose, proposed chemotherapy, surgery, and feeding tube status (prophylactic percutaneous endoscopic gastrostomy; PEG or nasogastric tube; NGT).
Smoking
Patients were asked about their smoking behavior (ever smoked, current smoker, most recent cigarette, number of cigarettes within the last 24 h, current nicotine replacement therapy; NRT use).
Expired carbon monoxide (CO) provided biochemical verification of smoking status. The Micro 11 Smokerlyser assessed breath levels of CO for all patients. A cut‐off of ≥4 CO parts per million (PPM) was used to classify abstinence from smoking, as has been suggested to increase specificity in determining smoking abstinence, particularly for those patient groups that might be more inclined to misrepresent their smoking status as has been found in HNC patients 19, 20, 21, 22, 23, 24 ,.
Nicotine dependence was measured via the Fagerstrom Test for Nicotine Dependence (FTND) – a six‐item, reliable, and valid self‐report questionnaire designed to assess the strength of nicotine dependence 25. Item scores are summed to produce a total score, with higher scores indicating higher levels of nicotine dependence (0–2 = very low; 3–4 = low; 5 = medium; 6–7 = high; 8–10 = very high dependence).
As a descriptive measure of chronicity and severity of smoking and alcohol consumption, intention to change was assessed using an adapted version of the measure developed by Etter and Sutton 26. For smoking, participants were asked to indicate the statement that best reflected their current plan to quit smoking:
I am not thinking about quitting in the near future, I intend to quit in the next 6 months, I intend to quit in the next 30 days, I have quit in the last 6 months, I have quit for 6 months or more, or Not applicable – Never smoked.
Alcohol consumption
The Alcohol Use Disorders Identification Test (AUDIT) 27 is a ten‐item self‐report measure developed by WHO to identify harmful patterns of alcohol use over the preceding 12 months. Items are summed to produce a total score, with scores >=8 indicating harmful or hazardous alcohol use, as well as possible alcohol dependence.
The AUDIT‐Consumption 27 consists of the first three items of the AUDIT and provides an index of alcohol use. It was employed to detect changes in quantity and/or type of alcohol consumed more recent to the start of treatment and referred to alcohol use in the preceding 2‐months. A score of ≥4 in men and a score ≥3 or more in women is considered positive for identifying hazardous drinking.
For intention to change assessment, participants were asked to indicate the statement that best reflected their current plan to cut down on drinking; I am not thinking about cutting down in the near future, I intend to cut down in the next 6 months, I intend to cut down in the next 30 days, I have cut down in the last 6 months, or I have cut down for 6 months or more. This was measured even in those who reported never having a drink in the last two months, as the statements include options for having cut down.
Depression
The PHQ‐9 28 is a self‐administered nine‐item questionnaire that can either be scored continuously to assess depressive symptoms (depressive severity), or scored categorically to assess the likely presence of a major depressive episode (MDE). Participants are asked to rate (on a scale of 0–3) the frequency of various MDE criteria over the previous 2 weeks. A cut‐off score of ≥8 has been suggested for identifying MDE in cancer patients 29, and the severity of the depression can be rated as 0–4 = minimal; 5–9 = mild; 10–14 = moderate; 15–19 = moderately severe; 20–27 = severe.
Statistical analysis
Descriptive statistics (means, SD, and frequencies) were conducted on all demographic and health variables, smoking, nicotine dependence, alcohol consumption, and depressive symptoms. Crosstab analyses were conducted to examine the co‐occurrence of current smoking (≥4 CO PPM), hazardous alcohol use (AUDIT‐C score ≥3 for women, ≥4 for men), and likely presence of MDE (PHQ‐9 score ≥8).
Results
Patient characteristics
The sample is described in Table 1. The mean age was 58 (SD 10) and most were male. Just over half (56%) had cancer of the oropharynx and most had stage IV (65%) cancer. All patients were scheduled to undergo radiotherapy; about a third had surgery prior to radiotherapy. Almost a quarter (23%) had a PEG feeding tube prior to starting radiotherapy and only 2% had a NGT.
Table 1.
Patient characteristics of head and neck cancer patients at week one of radiotherapy (N = 307)
| Variable | N/Mean | %/SD |
|---|---|---|
| Age (years) | 58 | 10.4 |
| Sex | ||
| Male | 244 | 80% |
| Female | 63 | 21% |
| Country | ||
| Australia | 198 | 65% |
| UK & Ireland | 38 | 12% |
| Other | 71 | 23% |
| Primary language | ||
| English | 285 | 93% |
| Other | 22 | 7% |
| ATSI | ||
| Yes | 6 | 2% |
| No | 300 | 98% |
| Marital status | ||
| Married | 156 | 51% |
| De‐facto/common law couples | 37 | 12% |
| Widowed | 12 | 4% |
| Separated/divorced | 57 | 18% |
| Single, never married | 40 | 13% |
| Other | 5 | 2% |
| Education level | ||
| 4 years of high school or less | 112 | 36% |
| 6 years of high school | 155 | 50% |
| University/Vocational College | 146 | 48% |
| Other | 1 | <1% |
| Accommodation (past year) | ||
| Private residence (own home, private rental) | 297 | 97% |
| Partially supported living (Department of housing, independent unit in retirement village/nursing home) | 9 | 3% |
| Other | 1 | <1% |
| Employment (past year) | ||
| No job | 19 | 6% |
| Full time | 152 | 50% |
| Part time/casual | 31 | 10% |
| Housework/stay at home parent | 7 | 2% |
| Studying | 2 | 1% |
| Retired/volunteer | 84 | 27% |
| Other | 12 | 4% |
| Tumor site | ||
| Nasopharynx | 23 | 8% |
| Oropharynx | 171 | 56% |
| Oral Cavity | 66 | 22% |
| Larynx | 29 | 9% |
| Hypopharynx | 11 | 4% |
| Unknown Primary | 7 | 2% |
| Tumor stage | ||
| I | 12 | 4% |
| II | 39 | 13% |
| III | 57 | 19% |
| IV | 199 | 65% |
| Radiotherapy | 307 | 100% |
| Surgery prior to radiotherapy | 97 | 32% |
| Concurrent chemotherapy | 247 | 81% |
| Prophylactic PEG | 71 | 23% |
| Prophylactic NGT | 7 | 2% |
| Hospital site | ||
| Site 1 | 23 | 8% |
| Site 2 | 100 | 33% |
| Site 3 | 83 | 27% |
| Site 4 | 101 | 33% |
Smoking, alcohol, and depression
Baseline smoking, alcohol consumption, and depressive symptoms are presented in Table 2.
Table 2.
Smoking, alcohol consumption, and depressive symptoms at baseline
| Variable | N (%) |
|---|---|
| Smoking | |
| Current smoker (self‐report) (n = 304) | 40 (13%) |
| Number of cigarettes within last 24 h (n = 38) | |
| 0–9 | 28 (74%) |
| 10–20 | 9 (24%) |
| 21–30 | 1 (3%) |
| Ever smoked (n = 305) | 232 (76%) |
| Currently using NRT (n = 232) | 18 (9%) |
| Most recent cigarette (n = 230) | |
| <24 h | 38 (17%) |
| <2 weeks | 11 (5%) |
| <1 month | 11 (5%) |
| <6 months | 46 (20%) |
| <1 year | 9 (4%) |
| <5 years | 16 (7%) |
| >5 years | 99 (43%) |
| Nicotine dependence; FTND (patients who had smoked in the last month) (n = 53) | |
| Very low | 19 (36%) |
| Low | 19 (36%) |
| Medium | 6 (11%) |
| High | 9 (17%) |
| Very high | 0 |
| CO confirmed current smokers (n = 280) | |
| CO PPM ≥4 | 94 (34%) |
| Intentions to change (smoking) (n = 295) | |
| I am not thinking about quitting in the near future | 15 (5%) |
| I intend to quit in the next 6 months | 15 (5%) |
| I intend to quit in the next 30 days | 16 (5%) |
| I have quit in the last 6 months | 67 (23%) |
| I have quit for 6 months or more | 113 (38%) |
| Not applicable – Never smoked | 71 (24%) |
| Alcohol consumption | |
| AUDIT (past year) | |
| Frequency of use (how often do you have a drink containing alcohol?) (n = 303) | |
| Never | 46 (15%) |
| Monthly or less | 56 (19%) |
| 2 to 4 times a month | 34 (11%) |
| 2 to 3 times a week | 57 (19%) |
| 4 or more times a week | 109 (36%) |
| Typical consumption (alcohol drinks on a typical day when drinking?) (n = 257) | |
| 1–2 | 129 (50%) |
| 3–4 | 66 (26%) |
| 5–6 | 43 (17%) |
| 7–9 | 5 (2%) |
| 10 or more | 14 (5%) |
| Frequency of 6 or more standard drinks on one occasion (n = 257) | |
| Never | 111 (43%) |
| Less than monthly | 54 (21%) |
| Monthly | 40 (16%) |
| Weekly | 30 (12%) |
| Daily or almost daily | 22 (9%) |
| Harmful/hazardous use (AUDIT ≥8) (n = 294) | 77 (30%) |
| AUDIT‐C (past 2 months) | |
| Frequency of use (how often do you have a drink containing alcohol?) (n = 306) | |
| Never | 114 (37%) |
| Monthly or less | 47 (15%) |
| 2 to 4 times a month | 38 (12%) |
| 2 to 3 times a week | 47 (15%) |
| 4 or more times a week | 60 (20%) |
| Typical consumption (alcohol drinks on a typical day when drinking?) (n = 192) | |
| 1–2 | 119 (62%) |
| 3–4 | 39 (20%) |
| 5–6 | 23 (12%) |
| 7–9 | 2 (1%) |
| 10 or more | 9 (5%) |
| Frequency of 6 or more standard drinks on one occasion (n = 192) | |
| Never | 133 (70%) |
| Less than monthly | 18 (9%) |
| Monthly | 14 (7%) |
| Weekly | 14 (7%) |
| Daily or almost daily | 13 (7%) |
| Hazardous drinking (AUDIT‐C ≥ 3 for women, ≥4 for men) (n = 306) | 94 (31%) |
| Intentions to change (alcohol use) (n = 301) | |
| I am not thinking about cutting down in the near future | 140 (47%) |
| I intend to cut down in the next 6 months | 7 (2%) |
| I intend to cut down in the next 30 days | 14 (5%) |
| I have cut down in the last 6 months | 105 (35%) |
| I have cut down for 6 months or more | 35 (12%) |
| Depressive symptoms (PHQ‐9) (n = 303) | |
| Minimal | 197 (65%) |
| Mild | 70 (23%) |
| Moderate | 22 (7%) |
| Moderately severe | 11 (4%) |
| Severe | 3 (1%) |
| Likely presence of MDE (PHQ‐9 score ≥8) | 58 (19%) |
Comorbidity
Of 276 patients with complete data for all three outcomes, 21% scored positive for two or more of the following problems: current smoking (≥4 CO PPM), hazardous alcohol use (AUDIT‐C score ≥3 for women, ≥4 for men), and likely presence of MDE (PHQ‐9 score ≥8) (Fig. 1). For those patients who had ever smoked and reported reducing their alcohol intake (from 4 or more times per week in the 12 months before baseline to less than that in the 2 months before baseline), 32% (n = 13/41) also reported quitting smoking recently (i.e., their last cigarette was between 2 weeks and 6 months prior to baseline).
Figure 1.
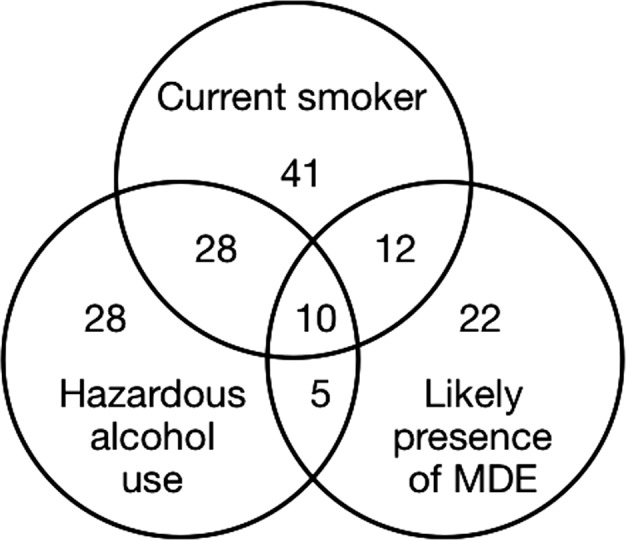
Comorbidity at baseline.
Discussion
Comorbidity
This is the first study to examine: smoking status; alcohol consumption; the severity of depressive symptoms; and their co‐occurrence assessed during the first week of radiotherapy. Approximately one‐fifth of the sample (n = 59/276; 21%) scored positive for two or more problems; smoking, hazardous alcohol consumption, and probable depression. Interestingly, of patients who reported i) having smoked in their lifetime; and ii) reducing their alcohol intake prior to baseline, approximately one‐third reported quitting smoking relatively recently. This is in line with previous research that suggests smoking cessation can enhance sobriety from alcohol, as opposed to impede alcohol abstinence 30.
In an oncology setting, HNC patients may feel overwhelmed by recent diagnosis, treatment schedules, and side effects. Health professionals’ focus may be primarily on treating the malignancy and resources and time are limited. In such circumstances, it would be valuable to treat comorbid problems together rather than separately. There is little research investigating effective interventions for such comorbidity in this population. The co‐occurrence of reduced alcohol intake and smoking in our participants prior to baseline, demonstrates the potential for concurrent reductions in smoking and alcohol use in the HNC population. For those HNC patients who continue to smoke, drink alcohol at hazardous levels or experience depressive symptoms during treatment and particularly those with co‐occurrence of these issues, a multicomponent, intensive treatment may be beneficial 10.
Smoking
Consistent with previous research, approximately one‐third (34%) of the sample were current smokers (CO PPM) at the beginning of radiotherapy 1, 10. Coupled with the potential for those who had recently quit to relapse over the course of treatment, assessment of smoking status, and the development of cessation interventions in this group warrants attention. A recent review 31 found that very few smoking cessation trials have been conducted with the HNC population but that a multicomponent approach (i.e., pharmacotherapy and evidence‐based psychosocial therapies) may be beneficial, also addressing co‐occurring risk factors.
Self‐reported smoking status was also much lower than reported in previous studies with HNC patients 1, 10. Given the research that suggests patients may minimise their smoking status, particularly in smoking‐related cancers, it may be that some patients misrepresent their smoking status 22. There is evidence that for some cancer patients, particularly those with smoking‐related cancers such as HNC, diagnosis is sufficient to produce abstinence 11. However, there is also a considerable rate of relapse for HNC patients who quit smoking; as high as between 13 and 90% depending on follow‐up period 21, 32, 33. Given the evidence that demonstrates the negative effects of tobacco use on treatment outcomes and survival, smoking status should be measured and biochemically confirmed at diagnosis, throughout treatment and at follow‐up in this population 33, with a view to offering assistance with smoking cessation interventions.
Alcohol consumption
Compared to smoking, less research has been conducted on alcohol consumption in HNC patients and the results of our study help to characterize this health behavior in this cancer population. The rate of alcohol consumption (last 12 months 85%; last 2 months 63%) in our sample was comparable to that of current drinkers (75%) in a sample (n = 107) of newly diagnosed HNC patients 34. Further, about one‐third of our sample scored positive for hazardous drinking, relative to the past 2 months (AUDIT‐C). This finding combined with those who were found to be at risk of alcohol‐related disorders (30%; AUDIT; past 12 months) is similar to rates described in previous studies 3, 12, 35. Given the association between problem drinking and secondary cancers, decreased survival rates and poorer quality of life 3, 5, this degree of problem drinking in HNC patients is concerning.
It has been suggested that the high rate of continued drinking in this population may be in part explained by lack of patient awareness of the association between alcohol and HNC 12. Indeed, almost half of our sample endorsed “I am not thinking about cutting down (alcohol use) in the near future.” Health care personnel across numerous specialties have reported that they do not deem discussing alcohol acceptable 36. However, physicians involved in the treatment of HNC patients are well placed to provide information about the hazards of continued drinking and studies of primary care patients have demonstrated that most are open to advice from physicians about their alcohol use 37. This opportunity for intervention is especially important in HNC patients where alcohol consumption in combination with smoking is responsible for the majority of these cancers 38 and continued use increases the risk of a secondary cancer 5.
Despite the high rate of current drinking at baseline, a proportion of patients had cut down on drinking four or more times per week from 36% in the last 12 months to 20% in the last 2 months. More patients were also drinking in the lower range of drinking on a typical day in the past 2 months as compared to the past 12 months. It may be that as for smoking, the symptoms or diagnosis of cancer is sufficient to change alcohol use for some, whilst others who continue to drink at harmful levels despite a cancer diagnosis need additional support to cut down.
Depression
Almost one‐fifth (19%) of our patients were identified as having likely cases of depression using the PHQ‐9. This is consistent with the lower range of rates reported in the HNC literature 8, 10, 13, 14. Identifying the prevalence of depression in HNC patients is complicated by the use of varying screening and diagnostic tools, unclear reporting of depression diagnoses versus depressive symptoms and time of measurement (e.g., pre or post cancer treatment). However, even conservative estimates of depressive symptoms and likely cases of depression in this cancer population at the pretreatment stage warrants attention. The importance of screening for depression and offering referral for psychosocial support has been highlighted in the numerous evidence‐based cancer guidelines that recommend this delivery of care.
Limitations
A limitation of the study is that although a valid self‐report tool was used to measure the likelihood of meeting criteria for a major depressive disorder, this was not confirmed by a diagnostic assessment and may have resulted in an overestimation of patients with depression. The patients in our sample were undergoing treatment with curative intent. Consequently, our findings are limited to this population.
Conclusions
The occurrence of smoking, alcohol consumption, and depressive symptoms was considerable. For a sizeable group of patients, these problems were co‐occurring. Screening and assessment of these behaviors and conditions should be conducted prior to treatment in order to provide intervention for those who continue to smoke or for recent quitters, consume alcohol, or experience depression. Additional support may be necessary for a subgroup with comorbid issues. Treating smoking, hazardous alcohol use, and/or depressive symptoms is likely to be associated with improved treatment outcomes and greater survival in HNC patients.
Conflict of Interest
This work was supported by the National Health and Medical Research Council (APP1021018; 2011/3654). This funder had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Cancer Medicine 2018; 7(6):2382–2390
Trial registry: http://www.anzctr.org.au
Registration no. ACTRN12613000320752
Research involving Human Participants and/or Animals: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1. Browman, G. P. , Wong G., Hodson I., Sathya J., Russell R., McAlpine L., et al. 1993. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N. Engl. J. Med. 328:159–163. [DOI] [PubMed] [Google Scholar]
- 2. Chen, A. M. , Chen L. M., Vaughan A., Sreeraman R., Farwell D. G., Luu Q., et al. 2011. Tobacco smoking during radiation therapy for head‐and‐neck cancer is associated with unfavorable outcome. Int. J. Radiat. Oncol. Biol. Phys. 79:414–419. [DOI] [PubMed] [Google Scholar]
- 3. Duffy, S. A. , Terrell J. E., Valenstein M., Ronis D. L., Copeland L. A., and Connors M.. 2002. Effect of smoking, alcohol, and depression on the quality of life of head and neck cancer patients. Gen. Hosp. Psychiatry 24:140–147. [DOI] [PubMed] [Google Scholar]
- 4. Sitas, F. , Weber M. F., Egger S., Yap S., Chiew M., and O'Connell D.. 2014. Smoking cessation after cancer. J. Clin. Oncol. 32:3593–3595. [DOI] [PubMed] [Google Scholar]
- 5. Mayne, S. T. , Cartmel B., Kirsh V., and Goodwin W. J.. 2009. Alcohol and tobacco use pre‐ and post‐diagnosis and survival in a cohort of patients with early stage cancers of the oral cavity, pharynx and larynx. Cancer Epidemiol. Biomarkers Prev. 18:3368–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goodwin, J. S. , Zhang D. D., and Ostir G. V.. 2004. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. J. Am. Geriatr. Soc. 52:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lawrence, D. , D'Arcy C., Holman J., Jablensky A. V., Threfall T. J., and Fuller S. A.. 2000. Excess cancer mortality in Western Australian psychiatric patients due to higher case fatality rates. Acta Psychiatr. Scand. 101:382–388. [DOI] [PubMed] [Google Scholar]
- 8. de Graeff, A. , de Leeuw J. R. J., Ros W. J. G., Hordijk G.‐J., Blijham G. H., and Winnubst J. A. M.. 2000. Pretreatment factors predicting quality of life after treatment for head and neck cancer. Head Neck 22:398–407. [DOI] [PubMed] [Google Scholar]
- 9. Britton, B. , Clover K., Bateman L., Odelli C., Wenham K., Zeman A., et al. 2012. Baseline depression predicts malnutrition in head and neck cancer patients undergoing radiotherapy. Support. Care Cancer 20:335–342. [DOI] [PubMed] [Google Scholar]
- 10. Duffy, S. A. , Ronis D. L., Valenstein M., Lambert M. T., Fowler K. E., Gregory L., et al. 2006. A tailored smoking, alcohol, and depression intervention for head and neck cancer patients. Cancer Epidemiol. Biomarkers Prev. 15:2203–2208. [DOI] [PubMed] [Google Scholar]
- 11. Ostroff, J. S. , Jacobsen P. B., Moadel A. B., Spiro R. H., Shah J. P., Strong E. W., et al. 1995. Prevalence and predictors of continued tobacco use after treatment of patients with head and neck cancer. Cancer 75:569–576. [DOI] [PubMed] [Google Scholar]
- 12. Potash, A. E. , Karnell L. H., Christensen A. J., Vander Weg M. W., and Funk G. F.. 2010. Continued alcohol use in patients with head and neck cancer. Head Neck 32:905–912. [DOI] [PubMed] [Google Scholar]
- 13. Duffy, S. A. , Ronis D. L., Valenstein M., Fowler K. E., Lambert M. T., Bishop C., et al. 2007. Depressive symptoms, smoking, drinking, and quality of life among head and neck cancer patients. Psychosomatics 48:142–148. [DOI] [PubMed] [Google Scholar]
- 14. Archer, J. , Hutchison I., and Korszun A.. 2008. Mood and malignancy: head and neck cancer and depression. J. Oral Pathol. Med. 37:255–270. [DOI] [PubMed] [Google Scholar]
- 15. Burris, J. L. , Studts J. L., DeRosa A. P., and Ostroff J. S.. 2015. Systematic review of tobacco use after lung or head/neck cancer diagnosis: results and recommendations for future research. Cancer Epidemiol. Biomarkers Prev. 24:1450–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lambert, S. D. , Clover K., Pallant J. F., Britton B., King M. T., Mitchell A. J., et al. 2015. Making sense of variations in prevalence estimates of depression in cancer: a co‐calibration of commonly used depression scales using Rasch analysis. J. Natl. Compr. Canc. Netw. 13:1203–1211. [DOI] [PubMed] [Google Scholar]
- 17. Bonevski, B. , Regan T., Paul C., Baker A. L., and Bisquera A.. 2014. Associations between alcohol, smoking, socioeconomic status and comorbidities: evidence from the 45 and up study. Drug Alcohol Rev. 33:169–176. [DOI] [PubMed] [Google Scholar]
- 18. Britton, B. , McCarter K., Baker A., Wolfenden L., Wratten C., Bauer J., et al. 2015. Eating As Treatment (EAT) study protocol: a stepped‐wedge, randomised controlled trial of a health behaviour change intervention provided by dietitians to improve nutrition in patients with head and neck cancer undergoing radiotherapy. BMJ Open 5:e008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cropsey, K. L. , Trent L. R., Clark C. B., Stevens E. N., Lahti A. C., and Hendricks P. S.. 2014. How low should you go? Determining the optimal cutoff for exhaled carbon monoxide to confirm smoking abstinence when using cotinine as reference. Nicotine Tob. Res. 16:1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perkins, K. A. , Karelitz J. L., and Jao N. C.. 2013. Optimal carbon monoxide criteria to confirm 24‐hr smoking abstinence. Nicotine Tob. Res. 15:978–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharp, L. , Johansson H., FagerstrÖM K., and Rutqvist L. E.. 2008. Smoking cessation among patients with head and neck cancer: cancer as a ‘teachable moment’. Eur. J. Cancer Care 17:114–119. [DOI] [PubMed] [Google Scholar]
- 22. Warren, G. W. , Arnold S. M., Valentino J. P., Gal T. J., Hyland A. J., Singh A. K., et al. 2012. Accuracy of self‐reported tobacco assessments in a head and neck cancer treatment population. Radiother. Oncol. 103:45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Javors, M. A. , Hatch J. P., and Lamb R. J.. 2005. Cut‐off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction 100:159–167. [DOI] [PubMed] [Google Scholar]
- 24. Lamb, R. J. , Kirby K. C., Morral A. R., Galbicka G., and Iguchi M. Y.. 2010. Shaping smoking cessation in hard‐to‐treat smokers. J. Consult. Clin. Psychol. 78:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heatherton, T. F. , Kozlowski L. T., Frecker R. C., and Fagerstrom K.‐O.. 1991. The Fagerström test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br. J. Addict. 86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 26. Etter, J.‐F. , and Sutton S.. 2002. Assessing ‘stage of change’ in current and former smokers. Addiction 97:1171–1182. [DOI] [PubMed] [Google Scholar]
- 27. Bush, K. , Kivlahan D. R., McDonell M. B., Fihn S. D., Bradley K. A., and for the Ambulatory Care Quality Improvement Project . 1998. The audit alcohol consumption questions (audit‐c): an effective brief screening test for problem drinking. Arch. Intern. Med. 158:1789–1795. [DOI] [PubMed] [Google Scholar]
- 28. Kroenke, K. , and Spitzer R. L.. 2002. The PHQ‐9: a new depression diagnostic and severity measure. Psychiatr. Ann. 32:509–515. [Google Scholar]
- 29. Thekkumpurath, P. , Walker J., Butcher I., Hodges L., Kleiboer A., O'Connor M., et al. 2011. Screening for major depression in cancer outpatients: the diagnostic accuracy of the 9‐item patient health questionnaire. Cancer 117:218–227. [DOI] [PubMed] [Google Scholar]
- 30. Prochaska, J. J. , Delucchi K., and Hall S. M.. 2004. A meta‐analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J. Consult. Clin. Psychol. 72:1144–1156. [DOI] [PubMed] [Google Scholar]
- 31. McCarter, K. , Martínez Ú., Britton B., Baker A., Bonevski B., Carter G., et al. 2016. Smoking cessation care among patients with head and neck cancer: a systematic review. BMJ Open 6:e012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gritz, E. R. , Schacherer C., Koehly L., Nielsen I. R., and Abemayor E.. 1999. Smoking withdrawal and relapse in head and neck cancer patients. Head Neck 21:420–427. [DOI] [PubMed] [Google Scholar]
- 33. Simmons, V. N. , Litvin E. B., Jacobsen P. B., Patel R. D., McCaffrey J. C., Oliver J. A., et al. 2013. Predictors of smoking relapse in patients with thoracic cancer or head and neck cancer. Cancer 119:1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kugaya, A. , Akechi T., Okuyama T., Nakano T., Mikami I., Okamura H., et al. 2000. Prevalence, predictive factors, and screening for psychologic distress in patients with newly diagnosed head and neck cancer. Cancer 88:2817–2823. [DOI] [PubMed] [Google Scholar]
- 35. Duffy, S. A. , Khan M. J., Ronis D. L., Fowler K. E., Gruber S. B., Wolf G. T., et al. 2008. Health behaviors of head and neck cancer patients the first year after diagnosis. Head Neck 30:93–102. [DOI] [PubMed] [Google Scholar]
- 36. Kääriäinen, J. , Sillanaukee P., Poutanen P., and K. Seppä . 2001. Opinions on alcohol‐related issues among professionals in primary, occupational, and specialized health care. Alcohol Alcohol. 36:141–146. [DOI] [PubMed] [Google Scholar]
- 37. Miller, P. M. , Thomas S. E., and Mallin R.. 2006. Patient attitudes towards self‐report and biomarker alcohol screening by primary care physicians. Alcohol Alcohol. 41:306–310. [DOI] [PubMed] [Google Scholar]
- 38. Adelstein, D. J. , Ridge J. A., Gillison M. L., Chaturvedi A. K., D'Souza G., Gravitt P. E., et al. 2009. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting, November 9–10, 2008, Washington, DC. Head Neck 31:1393–1422. [DOI] [PubMed] [Google Scholar]


