Abstract
Cell-based therapies for acute and chronic liver diseases are under continuous progress. Mesenchymal stem/stromal cells (MSCs) are multipotent cells able to migrate selectively to damaged tissue and contribute to its healing and regeneration. The MSC pro-regenerative effect occurs due to their immunomodulatory capacity and their ability to produce factors that promote cell protection and survival. Likewise, it has been observed that part of their paracrine effect is mediated by MSC-derived extracellular vesicles (EVs). EVs contain proteins, lipids and nucleic acids (DNA, mRNA, miRNA, lncRNA) from the cell of origin, allowing for intercellular communication. Recently, different studies have demonstrated that MSC-derived EVs could reproduce, at least in part, the biological effects obtained by MSC-based therapies. Moreover, due to EVs’ stability for long periods of time and easy isolation methods they have become a therapeutic option to MSCs treatments. This review summarizes the latest results achieved in clinical trials using MSCs as cell therapy for liver regeneration, the role of EVs in liver physiopathology and the potential of MSCderived EVs as intercellular mediators and therapeutic tools in liver diseases.
Keywords: Mesenchymal stem cells, Extracellular vesicles, Cirrhosis, Liver, Acute damage, Regeneration
Core tip: Cell-based therapies for acute and chronic liver diseases are very attractive strategies. In particular, mesenchymal stem/stromal cells (MSCs) are multipotent cells able to induce protective and pro-regenerative effects in different liver diseases. The mechanism through which MSCs support tissue regeneration is via secretion of paracrine factors, and solid evidence supports that part of these effects is mediated by extracellular vesicles (EVs). Therefore, EVs have become an attractive option in the research for new treatments in liver diseases.
INTRODUCTION
A diverse set of toxic, metabolic, and inflammatory insults result in liver diseases and imply different degrees of inflammation, apoptosis, and necrosis of parenchymal cells[1-4]. For example, acute liver failure (ALF) is characterized by a massive and sudden death of hepatocytes that lead to abrupt hepatocellular and systemic dysfunction[3]. Similarly, in patients with chronic liver disease an important loss of viable parenchymal cells is observed[1,2,4]. Cirrhosis is caused by diverse chronic liver diseases, such as viral hepatitis and chronic alcoholism[1,2]. Moreover, increases in the prevalence of hypertriglyceridemia, obesity and diabetes in developed countries have resulted in an increase in the incidence of non-alcoholic fatty liver disease (NAFLD)[4,5]. This condition is characterized by a lipid accumulation in the liver that could lead to hepatocytes apoptosis and inflammation. Regardless the liver chronic disease origin, the apoptosis of hepatocytes results in extracellular matrix accumulation that will affect the liver histoarchitecture of liver and ultimately impair its function[4]. It is well known that mesenchymal stem/stromal cells (MSCs) migrate toward injured organs where they can provide tissue protection and promote liver regeneration[6-8]. These properties make MSCs interesting tools to carry therapeutic genes in modern cellular-based therapeutic strategies[6]. It is accepted that the main mechanism through which MSCs support tissue regeneration is via secretion of paracrine factors[7,9]. However, solid evidence supports that part of these effects are mediated by extracellular vesicles (EVs)[10]. In this review, we first provide an update on clinical trials using MSCs in different liver diseases; second, the mechanisms involved in the therapeutic effects of MSCs; third, general EVs characteristics and their role in liver diseases, and finally, the role of MSC-derived EVs as therapeutic tools for liver regeneration.
CLINICAL TRIALS INVOLVING THE USE OF MSCS IN LIVER DISEASES
Clinical investigations using MSCs to treat a broad spectrum of degenerative diseases, including liver diseases, are increasing steadily in recent years[11,12]. The first clinical trial using MSCs was started in 2005 and 52 trials are registered up to now (CinicalTrial.gov and reviewed by Tsuchiya 2017[13]). MSCs are obtained from bone marrow in most of the studies, but other sources such as umbilical cord, adipose tissue and menstrual blood has also been tested (Figure 1A). It should be noted that, allogeneic transplantation is more commonly used than autologous (Figure 1B). Between liver diseases, most of the trials are destined to the treatment of liver cirrhosis (Figure 1C) and only 2 of them are in phase II/III (CinicalTrial.gov). Unfortunately, only 22 of 52 registered clinical trials have published their results (Table 1). It is important to mention that MSCs were administered after culture in vitro between passages 3 to 6. Regarding the administration route, MSC transplant was performed by peripheral vein[14-28], hepatic artery[29-33], portal vein[15,27] or directly into the spleen[16,34,35]. One study performed on 12 patients showed similar therapeutic effects when MSCs were injected into the spleen or intravenously[17].
Figure 1.
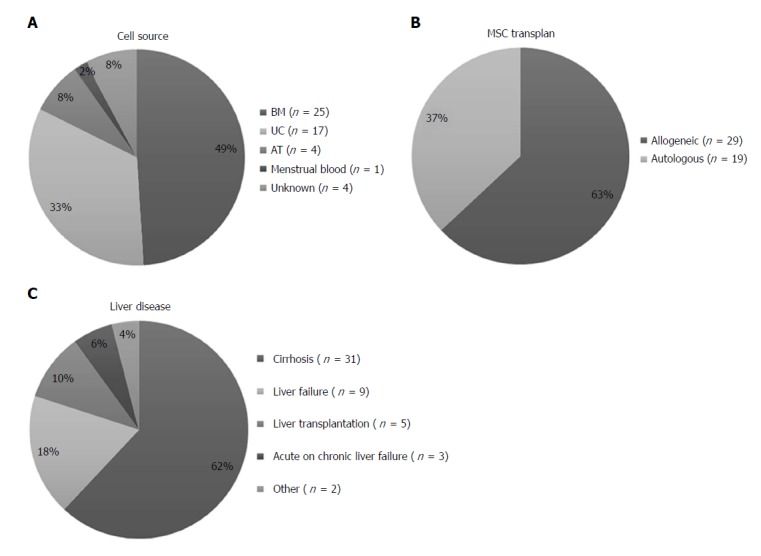
Mesenchymal stem/stromal cells clinical trials in liver disease. A: MSCs clinical trials classified by cell source; B: MSCs clinical trials classified by transplant type; C: MSCs clinical trials classified by liver disease treated. Data from http//www.clinicaltrial.gov. n: Number of clinical trials; MSCs: Mesenchymal stem/stromal cells; BM: Bone marrow; UC: Umbilical cord; AT: Adipose tissue.
Table 1.
Mesenchymal stem/stromal cells clinical trials for liver diseases
| Ref. | Etiology or disease | Cell source/origen | Study design (n; groups) |
Cell administration condition |
Phase | Results | Follow up | Side effects | ||
| Dose | P value | Route | ||||||||
| Mohamadnejad et al[14] 2007 | Cirrhosis | BM/Auto | n = 4 | 1, 0.6 × 107 | 2-4 | IV | I | MELD ↓ | 12 mo | None |
| Kharaziha et al[15] 2009 | Cirrhosis | BM/Auto | n = 8; 4 HBV 1 HCV 1 Alcohol 2 Control | 3.5 × 107 | 3-4 | PV/IV | I/II | MELD ↓ | 24 wk | None |
| El-Ansary et al[16] 2010 | Cirrhosis | BM/Auto | n = 12 | 10 × 106 | 1 | IS/IV | I | MELD ↓; no differences between IS vs IV | 6 mo | NA |
| Peng et al[29] 2011 | Cirrhosis (HBV) | BM/Auto | n = 158; 53 MSC 105 Control | 3.4-3.8 × 108 | 3 | HA | I/II | ALB ↑, MELD ↓ | 48 mo | None |
| Amer et al[34] 2011 | Cirrhosis (HCV) | BM/Auto | n = 40; 20 MSC 20 Control | 2 × 107 | NA | IS/IH | I/II | ALB ↑, C.P ↓, MELD ↓ | 6 mo | Fever (50%), transient shivering (15%) |
| El-Ansary et al[17] 2012 | Cirrhosis (HCV) | BM/Auto | n = 25; 9 MSC 6 Hep. Diff. 10 Control | 1 × 106/kg MSC or 40% HLCs and 60% MSCs | 5 | IV | II | ALB↑, MELD ↓, no differences between HLCs vs MSCs. | 6 mo | NA |
| Zhang et al[18] 2012 | Cirrhosis (HBV) | UC/Allo | n = 45; 30 MSC 15 Control | 0.5 × 106/kg every 4 wk, 3 times | 3-4 | IV | I/II | ALB ↑, MELD↓, ascites ↓ | 48 wk | None |
| Shi et al[19] 2012 | Acute-on-chronic Liver failure (HBV cirrhosis) | UC/Allo | n = 43; 34 MSC 9 Control | 0.5 × 106/kg every 4 wk, 3 times | 3-4 | IV | I/II | ALB ↑, PT ↑, MELD ↓, SR↑ | 72 wk | None |
| Mohamadnejad et al[20] 2013 | Cirrhosis | BM/Auto | n = 25; 14 MSC 11 Control | 2 × 108 | 3-4 | IV | II | No beneficial effect | 12 mo | None |
| Wang et al[21] 2013 | UDCA-resistant PBC | UC/Allo | n = 7 | 0.5 × 106/kg every 4 wk, 3 times | 4 | IV | I/II | ALP↓, γ-GT ↓, quality of life↑ (fatigue↓, pruritus↓) | 48 wk | None |
| Amin et al[35] 2013 | Cirrhosis | BM/Auto | n = 20 | 10 × 106 | 2 | IS | I/II | ALT ↓,AST ↓,BIL ↓, PT ↓, ALB↑, PT↑ | 24 wk | None |
| Jang et al[30] 2014 | Cirrhosis | BM/Auto | n = 11 | 5 × 107 every 4 wk, 2 times | 4-5 | HA | II | C.P ↓, TGF-β ↓, αSMA ↓, collagen1 ↓, fibrosis ↓, | 20 wk | None |
| Wang et al[18] 2014 | UDCA-resistant PBC | BM/Allo | n = 10 | 3-5 × 105/kg | 3-5 | IV | I/II | ALT ↓, AST ↓, γGT, BIL ↓, IgM ↓, Tregs ↑, IL-10↑, CD8+T cells↓ | 12 mo | None |
| Salama et al[23] 2014 | Cirrhosis | BM/Auto | n = 40; 20 MSC 20 Control | 1 × 106/kg | 0 | IV | II | ALT↓, AST↓,BIL↓, ALB↑, PT↑, C.P↓, ascites ↓ | 26 wk | NA |
| Xu et al[31] 2014 | Cirrhosis | BM/Auto | n = 56; 27 MSC 29 Control | 0.75 × 106/kg | NA | HA | II/III | ALB↑, MELD↓, ↑ Tregs/Th17 cell ratio, IL-17↓, TNFα↓, IL-6↓, TGF-β ↑ | 24 wk | NA |
| Suk et al[32] 2016 | Cirrhosis | BM/Auto | n = 55; 18 MSC (× 1 d) 19 MSC (× 2 d) 18 Control | 5 × 107 (1 mo post BM asp.) /5 × 107 (1 and 2 m post BM asp.) | 4-5 | HA | II | C.P ↓, fibrosis ↓ | 12 mo | None |
| Zhang et al[24] 2017 | Ischemic-type biliary lesions | UC/Allo | n = 82; 12 MSC 70 Control | 1 × 106/kg; week 1, 2, 4, 8, 12 and 16 | 4 | IV | II/III | BIL↓,ALP↓, γGT ↓, graft survival ↑ | 24 mo | None |
| Detry et al[25] 2017 | Liver transplantation | BM/Allo | n = 20; 10 MSC 10 Control | 1.5-3 × 106/kg; day 3 post-transplant | 2-3 | IV | I/II | No beneficial effect | 6 mo | None |
| Sakai et al[33] 2017 | Cirrhosis | AT/Auto | n = 4 | 6.6 × 105/kg | 0 | HA | I | ALB ↑, PT↓ | 1 mo | None |
| Shi et al[26] 2017 | Liver transplantation | UC/Allo | n = 20; 14 MSC 13 Control | 1 × 106/kg; every 4 wk, 3 times | 3-4 | IV | I | ALT↓, AST↓, BIL ↓, improve liver allograft histology, acute rejection↓ (↑ peripheral Tregs, ↑ Tregs/Th17 cell ratio). | 12 wk | None |
| Hartleif et al[27] 2017 | Pediatric liver transplantation | BM/Allo | n = 7 | 1 × 106/kg; day 0 and day 2 post-transplantation | 2-3 | PV/IV | I | NA | 24 mo | None |
| Lin et al[28] 2017 | Acute-on-chronic Cirrhosis (HBV) | BM/Allo | n = 110; 56 MSC 54 Control | 1-10 × 105 cells/kg; 1/wk, 4 wk | 5-6 | IV | I/II | MELD↓, SR↑, infections↓ | 24 wk | None |
P: Passage; BM: Bone Marrow; Allo: Allogeneic; Auto: Autologous; IV: Intravenous Infusion; MELD: Model for end-stage Liver Disease; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; IS: Intrasplenic; IH: Intrahepatic; NA: Not available; HA: Hepatic artery; CP: Child-Pugh score; HLC: Hepatocyte-like cells; UC: Umbilical cord; SR: Survival rate; Cr: Creatinine; BIL: Bilirubin; PBC: Primary biliary cirrhosis; UDCA: Ursodeoxycholic acid; Hep. Diff.: Hepatocyte differentiated; PT: Prothrombin time; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; γ-GT: γ-glutamyltranspeptidase; Asp: Aspiration.
In general, MSC administration in patients with different liver pathologies proved to be feasible and safe (Table 1). Regarding their efficacy, studies demonstrated that MSCs exert positive effects on liver function by increase in serum levels of albumin and improving coagulation or decreasing bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and γ-glutamyltransferase (γ-GT)[17-19,21-24,26,29,31,33-35]. In addition, the MELD (Model for End-stage Liver Disease) and Child-Pugh scores were improved after MSC treatment[14-19,23,28-32,34] (Table 1). Furthermore, in some studies it was demonstrated that the application of MSCs not only improved patient’s quality of life but also modulated the immune response of patients[22,26,31]. In this sense, a randomized clinical trial using autologous BM-derived MSCs in patients with hepatitis B virus-related liver cirrhosis resulted in an improvement of liver function, an increase in Treg cells, and a decrease in Th17 cells, serum levels of interleukin (IL)-17, tumor necrosis factor alpha (TNF-α) and IL-6; in addition, tumor growth factor beta (TGF-β) levels were increased in comparison with control group[31]. Similar results were reported in a phase I/II clinical trial in patients with primary biliary cirrhosis (PBC) transplanted with allogeneic MSCs that showed a reduction in the number of CD8+ T-cells and an increment of Treg cells and IL-10 serum levels[22].
Recently, Suk et al[32] reported a phase II clinical trial comparing the effects of one or two doses of autologous BM-derived MSC therapy with a control group in alcoholic patients. The authors observed that liver fibrosis quantification diminished after cell treatment, although no significant differences in fibrosis area were found between one and two doses. Importantly, no evidence of tumor formation was found during the follow-up in the MSC-treated groups[32].
On the other hand, MSCs were used to diminish hepatocellular damage in acute liver diseases. Recently, a phase II clinical trial in acute-on-chronic liver failure compared the standard medical treatment with one dose of allogeneic BM-derived MSC therapy[28]. In this study, MSC treatment demonstrated to be safe and able to improve bilirubin levels and MELD score. Interestingly, MSC transplant increased survival rate and decreased severe infection events[28].
Long-term immunosuppression is frequently associated with impairment in patients´ quality of life and increased risk of infection or cancer. Therefore, considering their immunomodulatory capacity, MSCs can be used to induce tolerance after liver transplantation. In this regard, Detry et al[25] reported that MSC infusion 3 d after liver transplant was feasible, safe and well tolerated. No opportunistic infections or de novo cancer were detected in 12 mo follow-up. However, no difference was observed in peripheral blood CD4+ T cell proportion and immunosuppression could not be withdrawn in MSC-treated patients. In addition, the administration of umbilical cord-derived MSCs as therapy to prolong graft-survival in patients with severe ischemic-type biliary lesions after liver transplantation was also evaluated[24]. Six doses of MSCs were infused intravenously every 2-4 wk, and patients were followed-up for 2 years and compared with a prospective cohort of patients treated with standard therapy. Remarkably, MSCs were safe, improved hepatic function, prolonged graft-survival and did not induce cancer or increase microbial infection events[24]. In conclusion, these results demonstrated that MSC therapy is a safe procedure, especially in the field of solid organ transplant where intense and prolonged immunosuppression is required. Moreover, MSC administration could prevent the development of opportunistic infections or de novo tumor formation. All considered, the promising information generated in the clinic opens the possibility for further studies to determine the optimal protocol conditions of MSC application needed to induce tolerance after liver transplant.
Application of MSCs for treatment of either acute or chronic liver diseases has a promising future in the clinic. In acute liver diseases, MSCs could have a role decreasing liver damage progression or as a bridge for liver transplant. In chronic liver diseases, MSCs could contribute to decrease liver damage, to ameliorate the degree of fibrosis, and even to avoid the need for transplant in some particular cases. On the other hand, in the post-transplant setting, MSC therapy could extend the graft survival and/or decrease the amount of immunosuppression. However, it is extremely important to understand that therapeutic potential of MSCs is an open question, and the information regarding source of MSCs, culture conditions, pre-condition protocols before cell transplantation, administration route, number of doses, and time of treatment are very heterogeneous and standardization is needed.
MECHANISM OF ACTION OF MSCS IN LIVER REGENERATION
As detailed above, is necessary to understand the mechanisms that mediate MSCs therapeutic effects prior to continuing with its clinical application. The understanding of MSC biology has grown considerably. In the last decade, many mechanisms involved in their regenerative potential have been identified. These mechanisms involve, at least in part, migration toward injured tissues, immunomodulatory properties, differentiation and/or secretion of regenerative factors, which induce cell survival and proliferation[7,9,36,37]. It has been described that MSCs can be recruited to inflamed tissue by classic mechanism of blood stream migration: Rolling, adherence to endothelium and transmigration[8,36]. The injured liver produces signals that induce migration and homing of different cell types[38,39]. Both, chronic and acute liver injury induce apoptosis and necrosis of hepatocytes and/or cholangiocytes, infiltration of leucocytes, monocytes and activation of Kupffer cells (KCs), liver sinusoidal endothelial cells (LSECs) and hepatic stellate cells (HeSCs)[2,3,40]. In this context, MSCs could be attracted by several chemokines, cytokines and factors secreted by the damaged liver microenvironment, such as IL-1β, IL-6, IL-8, monocyte chemoattractant protein-1 (MCP-1), growth-regulated protein (GRO), TNF-α, TGF-β1, platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and stromal cell-derived factor 1 (SDF-1) among others. Receptors for these chemoattractants were found to be expressed in MSCs allowing them to migrate to the injured liver[11,36].
The differentiation potential of MSCs has been considered one of the advantages for their regenerative application[7,41-43]. By definition, MSCs give rise to cells of mesodermal lineages including osteoblasts, adipoblasts and chondroblasts[44]. Furthermore, there are many reports demonstrating that MSCs might differentiate to ectodermal (neurons), endodermal (hepatocytes) as well as other mesodermal lineages (cardiomyocytes), but this field remains very controversial[7,41]. In this context, a putative mechanism postulated for liver regeneration was the MSC differentiation into hepatocyte-like cells[45]. A number of differentiation in vitro protocols were explored in MSCs obtained from bone marrow, adipose tissue or umbilical cord demonstrating that MSC-derived cells acquired characteristic markers and functions of immature and mature hepatocytes (reviewed in Fiore et al[7]). In fact, a clinical trial compared the effect of autologous BM-MSCs undifferentiated or differentiated into hepatocyte-like cells in patients with cirrhosis. Despite the improvement of liver function, there was no significant difference between undifferentiated or differentiated MSC-based treatment[17]. However, difficulties to study and reproduce the in vitro differentiation and in vivo tracking after their administration, makes the MSC differentiation to hepatocytes a controversial field. This difficulty is due, at least in part, to unspecificity and/or unreliability of methods/trackers/reagents frequently used for the identification of cells or the phenotype they might acquire after their transplantation[46,47]. It should be noted that track able MSCs by GFP expression is detectable in the liver up to 7 d after transplant and then the signal rapidly decreases in animal models[48]. Moreover, some evidence indicated that in spite of the MSC homing to the liver, the rate of differentiation to hepatocyte-like cells is very low (less to 1%)[49,50]. Together, this information demonstrates that the main regenerative effect of MSCs cannot be explained by the differentiation to hepatocyte-like cells.
Broad scientific consensus states that the regenerative effect of MSCs is due to paracrine mechanisms. The versatility of MSCs makes them able to differentially express factors depending on the surrounding microenvironment[51]. In the context of liver fibrosis, it was observed that MSCs produce high levels of anti-apoptotic growth factors such as SDF-1, VEGF, hepatocyte growth factor (HGF) and insulin-like growth factor (IGF)-I[9]. Also, the release of HGF, fibroblast growth factor (FGF), IL-6, fibrinogen and TGF-α can induce hepatocyte proliferation[51]. In addition, HGF and epidermal growth factor (EGF) can induce hepatic progenitor cell proliferation and differentiation[52] and VEGF increases angiogenesis, an important event for liver regeneration[53]. Moreover, IL-10, HGF and IGF-I produced by MSCs can reduce fibrogenesis by inhibition of HeSCs activation and proliferation[7,9]. MSCs also secrete factors involved in extracellular matrix remodeling and chemokines that attract immune cells which could modulate their function[51]. In this way, in vivo administration of conditioned medium (CM) obtained from MSC cultures can be effective to reduce liver injury. It was reported that the administration of MSC-derived CM significantly improved short-term survival in a D-galactosamine-induced rat model of fulminant hepatic failure[54,55]. Furthermore, MSC-CM therapy had great inhibitory effects on hepatocellular death reducing hepatocyte apoptosis in a 90%. In addition, an increase in liver regeneration programs and number of proliferating hepatocytes was observed[55]. Subsequently, it was demonstrated that EVs, present in CM, are partly responsible of the therapeutic effects of MSCs[10,56].
EXTRACELLULAR VESICLES
Although, EVs were described as intracellular mediators many years ago, they have recently generated great interest as therapeutic and diagnostic tools. Initially, “extracellular vesicle” was used to refer to all kind of vesicles released by cells. Nevertheless, the increased knowledge of their biology allowed to distinguish between different types of vesicles[57]. Exosomes (50-100 nm in diameter) are homogenous and the largest family of EVs and are different from microvesicles (100-1000 nm) and apoptotic bodies (500-2000 nm) in size and biogenesis[58]. While exosomes are originated from multivesicular bodies (MVBs), microvesicles are originated directly from plasma membrane and released to extracellular space[58]. At present, vesicles can only be fractioned according to their sizes and no specific markers have been described. Due to a large heterogeneity in methods of isolation and terminology in the past published results, in this review we will refer to exosomes and microvesicles as “EVs”. In order to define a minimal criteria for EV characterization, the International Society for Extracellular Vesicles (ISEV) suggests a semi-quantitative analysis for typical protein marker, such as CD9, CD63, CD81, Alix or TSG101, size analysis and morphology examination[59]. In addition to these specific proteins, EVs contain a large number of proteins (growth factors, cytokines, vesicles proteins), DNAs, mRNAs, microRNAs (miRNAs), long non-coding RNAs (lncRNAs)[57,59]. An interesting characteristic of EVs is that they can be charged with specific components of the cell of origin, and this “cargo” could be modified by different stimuli and microenvironment conditions[58]. As mentioned above, it has been demonstrated that EVs are involved in the paracrine effects of MSCs, but as EVs are implicated in many intercellular communications, we will first describe their implication in liver diseases.
EVS IN LIVER DISEASES
EVs have been implicated in a number of physiological and pathophysiological processes, such as immune response, angiogenesis, tissue regeneration, tumorigenesis/metastasis and neurodegenerative diseases[60-62]. In patients with primary biliary cirrhosis it was demonstrated that serum exosomes are taken up by peripheral monocytes and dendritic cells, resulting in the up regulation of co-stimulatory molecules[63]. Interestingly, serum circulating EVs present different miRNA composition in cirrhotic patients when compared with healthy controls[63,64]. Recent findings demonstrated that EVs are implicated in viral hepatitis, drug-induced injury, alcohol injury, non-alcoholic steatohepatitis and biliary injury[65,66].
EVs derived from parenchymal cells
Exosomes transport a variety of macromolecules that could act as signals between donor and recipient cells. In vitro studies demonstrated that liver parenchymal cells produce EVs that are involved in many physiological and pathophysiological processes[65,66]. For instance, it was demonstrated that there is an increase in the number of circulating exosomes with proliferative effect on hepatocytes after ischemia/reperfusion injury[67]. Nojima et al[67] reported that EVs derived from hepatocytes, but no other liver cells can induce hepatocyte proliferation in vitro. It should be noted that hepatocyte-derived EVs exert their effect in a dose-dependent manner. Furthermore, their administration in mice under ischemia/reperfusion liver injury or after 70% hepatectomy promotes hepatocyte proliferation and liver regeneration. Similarly, Herrera et al[68] showed that hepatic progenitor cell-derived EVs promote hepatocyte proliferation, suppress cell death, and accelerate liver regeneration in rats after hepatectomy. The author suggested that this effect is mediated by the delivery of RNA by EVs to target cells. It is worth mention, that current data demonstrated that hepatocyte-derived exosome properties are mediated by fusion with target hepatocytes transferring their cargos[67,69,70]. For example, hepatocyte-derived EVs transfer neutral ceramidase and sphingosine kinase 2 (SK2) within hepatocytes resulting in the induction of sphingosine-1-phosphate (S1P), a demonstrated promoter of cell proliferation[67] (Figure 2). Further studies of the same group showed that CXCR1 is required for packaging of SK2 into exosomes and CXCR2 regulates neutral sphingomyelinase activity and there by neutral ceramidase production[71].
Figure 2.
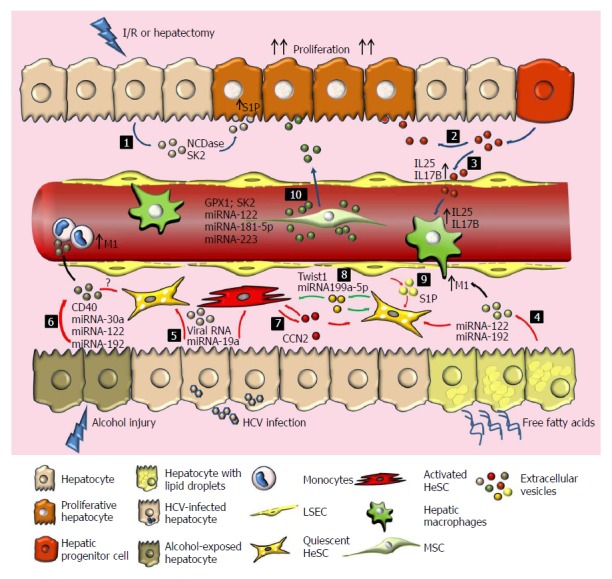
Extracellular vesicles as paracrine mediator in liver disease and therapeutics potential of mesenchymal stem/stromal cells. After ischemia reperfusion injury (I/R) or hepatectomy, hepatocytes (1) HPCs (2) release EVs with the ability to induce hepatocyte proliferation. (3) HPC-derived EVs stimulate LSECs and macrophages production of proliferative cytokines such as IL25 and IL17B. (4) On the other hand, free fatty acids induce the production of hepatocyte-derived EVs that result in the activation of quiescent HeSCs and pro-inflammatory macrophages (M1). (5) During chronic hepatitis C virus infection, EVs secreted by HCV-infected hepatocytes induce activation of HeSCs. (6) EVs secreted by hepatocytes after alcohol injury (containing CD40L and miRNAs) induce activation of monocytes and HeSCs. It seems to be a balance between EVs derived from active or quiescent HeSCs that promotes or inhibits fibrogenesis. Activated HeSC-derived EVs induce activation of quiescent HeSCs trough CCN2 (7) and quiescent HeSCs inhibit activated HeSCs transferring Twist1 or miRNA199a-5p (8). LSEC-derived EVs could also regulate HeSC activation (9). MSC-EVs induce hepatocyte proliferation, reduce oxidative stress and apoptosis, and modulate inflammatory response by carrying GPX1 or SK2 (10). Engineered MSC-EVs transferring miRNA-122, miRNA 181 5p and miRNA-223 have potential effects. The effects of MSC-EVs on HeSCs, hepatic macrophages, LSEC and infiltrated cells populations remain poorly explored. Green arrows: Inactivation of HeSCs; Red arrows: Activation of HeSCs; Blue arrow: Proliferative effect; Colors spots represent EVs from different cell origin; NCDase: Neutral ceramidase; SK2: Sphingosine kinase 2; S1P: Sphingosine-1-phosphate; IL: Interleukin; SK1: Sphingosine kinase 1; CCN2: Connective tissue growth factor; Twist1: Basic helix-loop-helix transcription factor; GPX1: Glutathione peroxidase 1; HCV: Hepatitis C virus; EVs: Extracellular vesicles.
On the other hand, hepatocyte-derived EVs in a nonalcoholic steatohepatitis (NASH) model interact with macrophages inducing an inflammatory phenotype[72] (Figure 2). Activation of the death receptor 5 (DR-5) expressed on hepatocytes by free fatty acid induces there lease of EVs that could stimulate IL1β and IL-6 expression on macrophages[72]. On the same line, EVs derived from palmitic acid (PA) stimulated hepatoma cells (Huh7 and HepG2) induce a profibrogenic phenotype on HeSCs[73]. Moreover, the activation of HeSCs seems to be related with the presence of miRNA-122 and miRNA-192 in the EVs[73] (Figure 2). Similarly, an increase in the number of circulating exosomes in mice is observed after chronic alcohol consumption[74]. Furthermore, in vitro incubation of hepatocytes with ethanol not only increases the release of exosomes but also allows monocyte activation through miRNA-122 horizontal transfer[74,75]. Remarkably, circulating EVs obtained from patients with alcoholic hepatitis show higher levels of miRNA-30a, miRNA-192 and, in particular, miRNA-122 than those obtained from heathy donors[74] (Figure 2).
EVs have also been implicated in horizontal transfer of information in chronic hepatitis C infection between hepatocytes and HeSCs. Devhare et al[76] demonstrated that EVs derived from HCV-infected hepatocytes transfer viral RNAs that increases the expression of profibrogenic markers on HeSCs. Interestingly, these EVs carry miR-19a that activates the STAT3 signaling, enhancing the expression of TGF-β and IL-6. Importantly, miR-19a up-regulation was observed in HCV-infected hepatocytes and in sera of chronic HCV patients with fibrosis[76,77] (Figure 2). Moreover, Seo et al[78] demonstrated that EVs are implicated in HeSCs activation via toll-like receptor 3 with the subsequent activation of γδ T cell population exacerbating liver fibrosis. Regarding other liver parenchymal cells, the role of cholangiocyte-derived EVs has been less studied. However, the presence of circulating EVs with altered miRNA composition and immunostimulatory functions on patients with PBC could suggest a role of cholangiocyte-derived EVs on this disease[63,64].
EVs derived from non-parenchymal cells
Non-parenchymal cells, including LSECs, Kupffer cells, lymphocytes, and HeSCs play a critical role in many liver diseases and use EVs for communication with neighbor cells during liver damage[65,66]. For instance, connective tissue growth factor (CCN2), a pro-fibrogenic mediator, is packaged into EVs produced by activated HeSCs. Then, exosome CCN2 can be delivered to other quiescent or activated HeSCs to induce trans-activation[79]. On the other hand, EVs derived from quiescent but not from activated HeSCs transport Twist 1 that suppress CCN2 on target cells through miR-214 induction[80]. Furthermore, Chen et al[81] showed that miR-199a-5p is loaded in quiescent HeSCs-derived EVs and also regulates CCN2 expression and activity on target cells (Figure 2). It seems to be a balance between EVs derived from active or quiescent HeSCs that promotes or inhibits liver damage. In addition, LSECs are known to maintain the HeSC quiescence through direct cell-to-cell contact and paracrine factors secretion[82]. Wang et al[83] demonstrated that LSEC-derived EVs have the ability to transfer Sphingosine kinase 1 (SK1) to regulate HeSC activation. In contrast, Ichinohe et al[84] demonstrated that progenitor hepatic cell-derived EVs stimulate LSECs and Kupffer cells to produce IL17B and IL25 resulting in proliferation of small progenitor hepatic cells (SPHCs) and liver regeneration (Figure 2). These findings evidence the importance of EVs derived from LSECs in affecting the activation state of neighboring cells. However, the mechanisms by which exosomes reach and attach target cells are not well understood. It has been reported that endocytosis of LSEC-derived EVs is mediated by the interaction between exosomal fibronectin and its ligand on the surface of HeSCs[83]. Additionally, integrin αvβ3 or α5β1 and heparan sulfate proteoglycan are ligand for EVs-HeSCs interaction and allow information transfer from endothelial cells[85].
Other important non-parenchymal cells involved in fibrogenesis are Kupffer cells and infiltrating macrophages. These cells are key players not only in fibrogenesis but also in fibrosis resolution and regeneration[86,87]. In alcoholic liver injury, hepatocyte-derived EVs enriched in miR122 sensitize macrophages to LPS and induce their production of pro-inflammatory cytokines[75]. Moreover, CD40L presence on hepatocyte-derived EVs during alcoholic hepatitis promotes macrophage activation and the switch to a pro-inflammatory profile[88,89]. In contrast, in vitro exposure of monocytes to alcohol increased their release of EVs, which in turn induce an anti-inflammatory M2 profile of naïve monocytes[90]. Interestingly, EVs derived from alcohol exposed monocytes contain high levels of miR-27a that is known to induce M2 polarization[90,91] (Figure 2).
In summary, it has been determined that EVs play a key role on the pathophysiological response to liver damage. EVs allow the interaction between parenchymal cells and non-parenchymal cells, mainly HeSCs, Kupffer cells and LSECs, mediating their anti/pro-fibrogenic state. The role of EVs in cell-to-cell communication during liver damage and the transfer of molecules, proteins and miRNAs is gaining importance in the field. Furthermore, this mechanism can be exploited for new therapeutic approaches or used as biomarkers in non-invasive methods. In line with this, it has recently been reported that MSCs release high levels of EVs that can mediate part of their therapeutic effects. Thus, considering the key role of EVs in liver cell communication, MSC-derived EVs (MSC-EVs) could be studied as a new therapeutic approach for hepatic regeneration strategies.
MSC-EVS AND THEIR POTENTIAL FOR LIVER REGENERATION
As described above, the main mechanism by which MSCs support the repair and regeneration of injured tissues is by releasing paracrine factors[7]. Recently, this paracrine mechanism was described to be partially mediated by EVs released by MSCs[10,56]. In vitro assays demonstrated that MSC-EVs induce hepatocyte proliferation and dedifferentiation into progenitor oval cells[92,93]. Therapeutic effects of MSC-EVs were demonstrated in pre-clinical models of acute kidney injury[94], and then in pathologies of heart, brain, kidney, muscle and liver[95].
Li et al[96] demonstrated that MSC-EVs reduced the degree of hepatic injury, collagen deposition and inflammation in mice with fibrosis induced by carbon tetrachloride (CCl4). The antifibrotic effect observed by MSC-EVs is mediated by the inactivation of TGF-β1/Smad signaling pathway. Moreover, a reversion of the epithelial-to-mesenchymal transition (EMT) both in vivo and in vitro was observed after the EV treatment[96] (Figure 2). Therefore, EVs derived from human MSCs were effective in mice demonstrating that they preserve at least part of the immunomodulatory properties of the cells of origin.
As for liver fibrosis, the therapeutic capacity of MSC-EVs was also assessed in acute models of liver injury. Cheng et al[97] studied the effect of EVs derived from menstrual blood MSCs in a galactosamine/LPS mice model. MSC-EVs were able to enhance animal survival and reversion of liver failure through hepatocyte apoptosis inhibition and systemic inflammation reduction. In addition, in vitro assays showed that EVs are taken up by AML12 cells (hepatocyte cell line) resulting in the inhibition of apoptosis induced by galactosamine/LPS[97]. Similar results were observed by Haga et al[98] using EVs derived from bone marrow MSCs in an animal model of acute liver failure. A relevant finding was the preservation of the biological activity of cryopreserved-EVs m up to 3 mo, indicating the stability of EVs[98].
It is known that induction of oxidative stress in the liver results in severe hepatic diseases by inducing cell apoptosis. A protective and proliferative effect of MSC-EVs in a lethal mice model induced by a single dose of CCl4 has been reported[99]. In addition, MSC-EVs inhibited hepatocyte apoptosis induced by acetaminophen and H2O2 and increased cell viability in vitro[99]. In this line, Yan et al[100] demonstrated that EVs derived from umbilical cord MSCs induced an antioxidant effect on hepatocytes. Glutathione peroxidase 1 (GPX1) delivered on MSC-EVs reduces the reactive oxygen species (ROS) intracellular levels and inhibits the oxidative stress-induced apoptosis in vitro and in vivo. Remarkably, authors demonstrated that a low dose of EVs (16 mg/kg of body weight) resulted in similar effects either by tail vein administration or oral gavage. In addition, Nong et al[101] tested the effect of EVs generated from MSC-derived Induced pluripotent stem cells (iPSCs) (MSC-iPSC-EVs) in hepatic ischemia-reperfusion (I-R) injury models. In vivo administration of MSC-iPSC-EVs in I-R injury mice model resulted in a decrease of oxidative stress response and apoptosis, and an increase of hepatocyte proliferation (Figure 2).Consistently, an amelioration of hepatic damage and inflammatory response was observed after EV treatment. It should be noted that MSC-iPSC-EVs keep the characteristics of EVs usually obtained from tissue-derived MSCs (bone marrow, adipose tissue and umbilical cord)[101]. In addition, it has been reported that MSC-iPSC-EVs could directly fuse with hepatocytes increasing the activity of sphingosine kinase (SK1). Moreover, the increase in SK activity will in turn increase the sphingosine-1-phosphate (S1P) levels affecting hepatocyte proliferation[93] (Table 2).
Table 2.
Mesenchymal stem/stromal cells-derived extracellular vesicles in experimental models of liver disease
| Ref. | EVs Isolation/characteristics | Experimental model | Protocol | Biological effects |
| Haga et al[98] 2016 | Ultracentrifugation Size: 46-116 nm Alix+ CD9+, CD81+ | C57Bl mice. ALF, i.p. 20 mg/body DGalNAc + 0,3 mg/body TNF-α | 2 × 108 to 2 × 1010 i.p./i.v. | ↑ Survival, ↑ F4/80, ↑ inhibitor MMP-1 and IL-6, ↓ inflammation and apoptosis, ↓ ALT/AST, ↓ ALP, ↓ EGF, SCF, IFN-γ, IP-10, IL-1α, MIP3, MCP-1/3 |
| Yan et al[100] 2016 | Ultracentrifugation Size: 30-100 nm CD9+, CD61+, CD63+ | BALB/c-nu/nu mice, i.p. CCL4induced ALF, 0.15-0.35 mL/kg | 8, 16, and 32 mg/kg i.v./oral | ↓ Oxidative stress and apoptosis Induces ERK1/2 phosphorylation and Bcl2 expression Inhibits IKKB/NFκB/casp9/3 pathway |
| Tan et al[99] 2014 | TFF, 100.kDa MWCO filter Size: 55-100 nm | C57BL/6 mice. CCL4-induced ALF, i.p. 0.05 mL CCL4/kg | 0.4 μg (100 μL) i.s. | ↑ Cell viability: TAMH, THLE-2, and Huh-7 ↑ Hepatocytes proliferation ↓ ALT/AST ↓ Casp 3/7 ↑ antiapoptoticBcl-xL |
| Chen et al[97] 2017 | Centrifugation and Exoquick-TC Size: 30-100 nm CD63+ and tsg101+ | C57BL/6 mice. ALF, i.p. D-GalNAc 800 mg/kg and LPS 50 μg/kg | 1 μg/μL i.v. | ↑ Liver function and survival ↓ Apoptosis ↓ TNF-α, IL-6 and IL-1 ↓ Casp-3 ↓ Necrosis and inflammation |
| Nong et al[101] 2016 | Ultracentrifugation and ultrafiltration Size: 50-60 nm CD9+, CD63+ and CD81+ | Rats I/R injury | 600 μg suspended in 400 μL of PBS i.v. | ↑ GSH, GSH-PX and SOD ↓ AST/ALT ↓ TNF-α, IL-6 and HMGB1 ↓ Casp-3 and Bax |
| Du et al[93] 2017 | Centrifugation and filtered by 0, 45-μm PVDF filter ExoQuick Size: 100-200 nm Alix+, CD63+ and CD81+ | C57 mice I/R injury | 2.5 × 1012 particles in 500 μL of PBS i.v. | ↑ Hepatocytes proliferation ↑ SK activity and S1P formation. Hepatoprotective and proliferative effect abolished by the inhibition of SK or S1P receptor 1 |
i.p.: Intraperitoneal injection; i.v.: Intravenous injection; i.s.: Intrasplenic; MMP: Metalloproteinases; CCL4: Corbon tetrachloride; D-GalNAc: N Acetylgalactosamine; IL: Interleukins; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; EGF: Epidermal growth factor; SCF: Stem cell factor; TNF: Tumor necrosis factor; IFN: Interferon; IP: Inducible protein; MIP: Macrophage inflammatory protein; MCP: Monocyte chemotactic protein; ERK: Extracellular signal-regulated kinase; Bcl: B-cell lymphoma; TFF: Tangential flow filtration; MWCO: Molecular weight cut-off; ALF: Acute liver failure; TAMH: Transgenic mouse hepatocyte; THLE: T-antigen immortalized human liver epithelial; Casp: Caspase; GSH: Glutathione; GSH-PX: Glutathione peroxidase; SOD: Superoxide dismutase; HMGB: High mobility group box; PBS: Phosphate-buffered saline; SK: Sphingosine kinase; S1P: Sphingosine 1-phosphate.
Although there is a great therapeutic potential of MSC-EVs in liver protection and regeneration, it is mandatory to understand the mechanisms involved in their biological effects. One key point of research is to know the bio distribution of EVs after systemic administration in vivo. In vitro assays showed that EVs are taken up via integrin mediated endocytosis by target liver cells[67,85,90,97]. In addition, MSC-EV biodistribution after intravenous (IV) administration shows EVs uptake as fast as 3 to 6 h after injection in liver, spleen and lung cells[97]. Furthermore, Haga et al[98] reported that liver, spleen and lung from mice with fulminant hepatitis exert a higher uptake of MSC-EVs in comparison with organs from healthy mice. However, since these experiments have been carried out using lipophilic tracers, confirmation of these results with specific markers is necessary. The MSC-EV survival in circulation after being administered and the recognition pathways used by the target cells need to be studied in depth. Moreover, this knowledge could help to define the better scheme of doses, the time of treatment and the route of administration depending of the type of liver damage. In addition, conformation details of proteins and nucleic acids loaded in the EVs is required.
ENGINEERING MSC-EVS FOR LIVER REGENERATION
Recently, an increased interest has been shown by the research community to improve the efficiency and potency of EVs loading specific cargos[102]. As described above, the EVs produced by MSCs have useful properties that would allow them to be used as therapies in different liver pathologies[62], and advances in nanomedicine would allow to improve the technology to generate more effective EVs[102,103]. Thereby, engineered EVs can be generated with strategies based on covalent surface chemistry, hydrophobic insertions or membrane permeabilization. Moreover, modification of the parental cells through metabolic labeling, genetic modification or by insertion of exogenous material could also produce modified EVs. These engineered EVs could carry specific DNAs, mRNAs or non-coding RNAs to the specific cell[102].
Lou et al[104] demonstrated that miRNA-122 expression in MSCs by lentiviral infection increased their therapeutic effect in a fibrosis model in mice. MiR-122 positively regulates proliferation and trans differentiation of HeSCs having an important role in liver fibrogenesis[105]. Authors demonstrated that in vitro miRNA-122 is transferred to HeSCs through MSC-EVs resulting in the regulation of genes involved in collagen maturation and cell proliferation[104]. Another strategy uses EVs derived from MSCs with transient expression of miRNA-181-5p as a therapeutic option in a liver fibrosis model[106]. As for miRNA-122, miRNA-181-5p was delivered by MSC-EVs reducing the fibrosis and the inflammatory state of fibrotic mice[106]. Moreover, in vitro experiments show that after reaching the target cell, miRNA-181-5p binds to3´-UTR of STAT3 and Bcl-2, which in turn down regulates TGF-β1 expression and induces autophagy in HeSCs[106]. Finally, in a model of autoimmune hepatitis, a cytoprotective effect of MSC-EVs engineered to charge miRNA-223 was observed. The in vitro experiments demonstrated that miRNA-223 levels were increased in hepatocytes after their incubation with the engineered EVs[107]. Similar results were observed in vivo with an increase of this miRNA in the liver and a reduction of its target gene NLRP3, and therefore a decrease in hepatocyte apoptosis[107] (Figure 2).
In summary, more information is needed not only to develop more efficient therapies for liver diseases based on MSC-EVs but also to engineer them to increase their efficacy and potency.
CONCLUSION
MSCs-based therapy has emerged as a potent and innovative treatment for acute and chronic liver diseases. The safety and feasibility observed in the early clinical trials using MSCs have increased the interest to translate the use of these cells to the clinic. Moreover, pro-regenerative results and an improvement in the life quality of patients were observed. In ALF, MSCs could have a role decreasing liver damage progression due their immunomodulatory properties. In chronic liver diseases, MSCs could contribute to decrease liver damage and to ameliorate the degree of fibrosis. Even more, in both case MSC treatment could not only delay the transplant but also to avoid it in some particular cases. In addition, in the post-transplant setting, MSC therapy could extend the graft survival and/or decrease the amount of immunosuppression required. Although the main mechanism by which MSCs support the repair and regeneration of injured livers is by releasing paracrine factors, strong evidences demonstrated that this paracrine mechanism is mediated by EVs released by MSCs. Therefore, due to EVs’ stability for long periods of time and easy isolation methods they have become a therapeutic option to MSCs treatments in liver diseases. At present, EVs are strongly explored for therapeutic or diagnostic application, and more information is needed to develop more efficient tools for liver diseases based on MSC-EVs. However, it is important to understand that therapeutic potential of MSCs or its EVs is still a matter of debate. In addition, standardization of source of MSCs, culture conditions, pre-condition protocols for cell transplantation, administration route, doses and time of treatment is required. Nevertheless, considering that development of new therapeutic approaches for liver diseases is urgent, MSCs emerge as potent innovation. Thus, take advantage of the therapeutic potential of MSCs as promising tool for liver regeneration could attend to an important worldwide human health problem.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Argentina
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
Conflict-of-interest statement: The authors indicate no potential conflicts of interest.
Peer-review started: April 13, 2018
First decision: April 27, 2018
Article in press: June 2, 2018
P- Reviewer: Miloso M, Musumeci G, Scarfì S S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
Contributor Information
Esteban Juan Fiore, Laboratory of Gene Therapy, Instituto de Investigaciones en Medicina Traslacional, CONICET-Universidad Austral, Buenos Aires 999071, Argentina.
Luciana María Domínguez, Laboratory of Gene Therapy, Instituto de Investigaciones en Medicina Traslacional, CONICET-Universidad Austral, Buenos Aires 999071, Argentina.
Juan Bayo, Laboratory of Gene Therapy, Instituto de Investigaciones en Medicina Traslacional, CONICET-Universidad Austral, Buenos Aires 999071, Argentina.
Mariana Gabriela García, Laboratory of Gene Therapy, Instituto de Investigaciones en Medicina Traslacional, CONICET-Universidad Austral, Buenos Aires 999071, Argentina.
Guillermo Daniel Mazzolini, Laboratory of Gene Therapy, Instituto de Investigaciones en Medicina Traslacional, CONICET-Universidad Austral, Buenos Aires 999071, Argentina. gmazzoli@austral.edu.ar.
References
- 1.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830–841. doi: 10.1136/gutjnl-2014-306842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stravitz RT, Kramer DJ. Management of acute liver failure. Nat Rev Gastroenterol Hepatol. 2009;6:542–553. doi: 10.1038/nrgastro.2009.127. [DOI] [PubMed] [Google Scholar]
- 4.Trovato FM, Catalano D, Musumeci G, Trovato GM. 4Ps medicine of the fatty liver: the research model of predictive, preventive, personalized and participatory medicine-recommendations for facing obesity, fatty liver and fibrosis epidemics. EPMA J. 2014;5:21. doi: 10.1186/1878-5085-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trovato FM, Martines GF, Brischetto D, Catalano D, Musumeci G, Trovato GM. Fatty liver disease and lifestyle in youngsters: diet, food intake frequency, exercise, sleep shortage and fashion. Liver Int. 2016;36:427–433. doi: 10.1111/liv.12957. [DOI] [PubMed] [Google Scholar]
- 6.Aquino JB, Bolontrade MF, García MG, Podhajcer OL, Mazzolini G. Mesenchymal stem cells as therapeutic tools and gene carriers in liver fibrosis and hepatocellular carcinoma. Gene Ther. 2010;17:692–708. doi: 10.1038/gt.2010.10. [DOI] [PubMed] [Google Scholar]
- 7.Fiore EJ, Mazzolini G, Aquino JB. Mesenchymal Stem/Stromal Cells in Liver Fibrosis: Recent Findings, Old/New Caveats and Future Perspectives. Stem Cell Rev. 2015;11:586–597. doi: 10.1007/s12015-015-9585-9. [DOI] [PubMed] [Google Scholar]
- 8.Garcia MG, Bayo J, Bolontrade MF, Sganga L, Malvicini M, Alaniz L, Aquino JB, Fiore E, Rizzo MM, Rodriguez A, et al. Hepatocellular carcinoma cells and their fibrotic microenvironment modulate bone marrow-derived mesenchymal stromal cell migration in vitro and in vivo. Mol Pharm. 2011;8:1538–1548. doi: 10.1021/mp200137c. [DOI] [PubMed] [Google Scholar]
- 9.Volarevic V, Nurkovic J, Arsenijevic N, Stojkovic M. Concise review: Therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells. 2014;32:2818–2823. doi: 10.1002/stem.1818. [DOI] [PubMed] [Google Scholar]
- 10.Camussi G, Deregibus MC, Cantaluppi V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem Soc Trans. 2013;41:283–287. doi: 10.1042/BST20120192. [DOI] [PubMed] [Google Scholar]
- 11.Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 12.Mobasheri A, Kalamegam G, Musumeci G, Batt ME. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas. 2014;78:188–198. doi: 10.1016/j.maturitas.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Tsuchiya A, Kojima Y, Ikarashi S, Seino S, Watanabe Y, Kawata Y, Terai S. Clinical trials using mesenchymal stem cells in liver diseases and inflammatory bowel diseases. Inflamm Regen. 2017;37:16. doi: 10.1186/s41232-017-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, Bagheri M, Bashtar M, Ghanaati H, Baharvand H, Ghavamzadeh A, Malekzadeh R. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007;10:459–466. [PubMed] [Google Scholar]
- 15.Kharaziha P, Hellström PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F, Telkabadi M, Atashi A, Honardoost M, Zali MR, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21:1199–1205. doi: 10.1097/MEG.0b013e32832a1f6c. [DOI] [PubMed] [Google Scholar]
- 16.El-Ansary M MS, Abdel-Aziz I, Abdel-Hamid S. Phase I Trial: Mesenchymal Stem Cells Transplantation in End Stage Liver Disease. Journal of American Science. 2010;6:1. [Google Scholar]
- 17.El-Ansary M, Abdel-Aziz I, Mogawer S, Abdel-Hamid S, Hammam O, Teaema S, Wahdan M. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev. 2012;8:972–981. doi: 10.1007/s12015-011-9322-y. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Lin H, Shi M, Xu R, Fu J, Lv J, Chen L, Lv S, Li Y, Yu S, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27 Suppl 2:112–120. doi: 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- 19.Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z, Zhang A, Shi J, Chen L, Lv S, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1:725–731. doi: 10.5966/sctm.2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohamadnejad M, Alimoghaddam K, Bagheri M, Ashrafi M, Abdollahzadeh L, Akhlaghpoor S, Bashtar M, Ghavamzadeh A, Malekzadeh R. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013;33:1490–1496. doi: 10.1111/liv.12228. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Li J, Liu H, Li Y, Fu J, Sun Y, Xu R, Lin H, Wang S, Lv S, et al. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013;28 Suppl 1:85–92. doi: 10.1111/jgh.12029. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Han Q, Chen H, Wang K, Shan GL, Kong F, Yang YJ, Li YZ, Zhang X, Dong F, et al. Allogeneic bone marrow mesenchymal stem cell transplantation in patients with UDCA-resistant primary biliary cirrhosis. Stem Cells Dev. 2014;23:2482–2489. doi: 10.1089/scd.2013.0500. [DOI] [PubMed] [Google Scholar]
- 23.Salama H, Zekri AR, Medhat E, Al Alim SA, Ahmed OS, Bahnassy AA, Lotfy MM, Ahmed R, Musa S. Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res Ther. 2014;5:70. doi: 10.1186/scrt459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang YC, Liu W, Fu BS, Wang GY, Li HB, Yi HM, Jiang N, Wang G, Zhang J, Yi SH, et al. Therapeutic potentials of umbilical cord-derived mesenchymal stromal cells for ischemic-type biliary lesions following liver transplantation. Cytotherapy. 2017;19:194–199. doi: 10.1016/j.jcyt.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Detry O, Vandermeulen M, Delbouille MH, Somja J, Bletard N, Briquet A, Lechanteur C, Giet O, Baudoux E, Hannon M, et al. Infusion of mesenchymal stromal cells after deceased liver transplantation: A phase I-II, open-label, clinical study. J Hepatol. 2017;67:47–55. doi: 10.1016/j.jhep.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Shi M, Liu Z, Wang Y, Xu R, Sun Y, Zhang M, Yu X, Wang H, Meng L, Su H, et al. A Pilot Study of Mesenchymal Stem Cell Therapy for Acute Liver Allograft Rejection. Stem Cells Transl Med. 2017;6:2053–2061. doi: 10.1002/sctm.17-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartleif S, Schumm M, Döring M, Mezger M, Lang P, Dahlke MH, Riethmüller J, Königsrainer A, Handgretinger R, Nadalin S, et al. Safety and Tolerance of Donor-Derived Mesenchymal Stem Cells in Pediatric Living-Donor Liver Transplantation: The MYSTEP1 Study. Stem Cells Int. 2017;2017:2352954. doi: 10.1155/2017/2352954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY, Chen XY, Liu QL, Peng L, Li JG, Mei YY, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology. 2017;66:209–219. doi: 10.1002/hep.29189. [DOI] [PubMed] [Google Scholar]
- 29.Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, Zheng YB, Gao ZL. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820–828. doi: 10.1002/hep.24434. [DOI] [PubMed] [Google Scholar]
- 30.Jang YO, Kim YJ, Baik SK, Kim MY, Eom YW, Cho MY, Park HJ, Park SY, Kim BR, Kim JW, et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int. 2014;34:33–41. doi: 10.1111/liv.12218. [DOI] [PubMed] [Google Scholar]
- 31.Xu L, Gong Y, Wang B, Shi K, Hou Y, Wang L, Lin Z, Han Y, Lu L, Chen D, et al. Randomized trial of autologous bone marrow mesenchymal stem cells transplantation for hepatitis B virus cirrhosis: regulation of Treg/Th17 cells. J Gastroenterol Hepatol. 2014;29:1620–1628. doi: 10.1111/jgh.12653. [DOI] [PubMed] [Google Scholar]
- 32.Suk KT, Yoon JH, Kim MY, Kim CW, Kim JK, Park H, Hwang SG, Kim DJ, Lee BS, Lee SH, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology. 2016;64:2185–2197. doi: 10.1002/hep.28693. [DOI] [PubMed] [Google Scholar]
- 33.Sakai Y TM, Seki A, Sunagozaka H, Terashima T, Komura T, Yamato M, Miyazawa M, Kawaguchi K, Nasti A, Mochida H, et al. Phase I clinical study of liver regenerative therapy for cirrhosis by intrahepatic arterial infusion of freshly isolated autologous adipose tissue-derived stromal/stem (regenerative) cell Reg. Ther. 2017;6:52–64. doi: 10.1016/j.reth.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amer ME, El-Sayed SZ, El-Kheir WA, Gabr H, Gomaa AA, El-Noomani N, Hegazy M. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol. 2011;23:936–941. doi: 10.1097/MEG.0b013e3283488b00. [DOI] [PubMed] [Google Scholar]
- 35.Amin MA, Sabry D, Rashed LA, Aref WM, el-Ghobary MA, Farhan MS, Fouad HA, Youssef YA. Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin Transplant. 2013;27:607–612. doi: 10.1111/ctr.12179. [DOI] [PubMed] [Google Scholar]
- 36.Bayo J, Marrodán M, Aquino JB, Silva M, García MG, Mazzolini G. The therapeutic potential of bone marrow-derived mesenchymal stromal cells on hepatocellular carcinoma. Liver Int. 2014;34:330–342. doi: 10.1111/liv.12338. [DOI] [PubMed] [Google Scholar]
- 37.Usunier B, Benderitter M, Tamarat R, Chapel A. Management of fibrosis: the mesenchymal stromal cells breakthrough. Stem Cells Int. 2014;2014:340257. doi: 10.1155/2014/340257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bayo J, Fiore E, Aquino JB, Malvicini M, Rizzo M, Peixoto E, Andriani O, Alaniz L, Piccioni F, Bolontrade M, et al. Increased migration of human mesenchymal stromal cells by autocrine motility factor (AMF) resulted in enhanced recruitment towards hepatocellular carcinoma. PLoS One. 2014;9:e95171. doi: 10.1371/journal.pone.0095171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bayo J, Real A, Fiore EJ, Malvicini M, Sganga L, Bolontrade M, Andriani O, Bizama C, Fresno C, Podhajcer O, et al. IL-8, GRO and MCP-1 produced by hepatocellular carcinoma microenvironment determine the migratory capacity of human bone marrow-derived mesenchymal stromal cells without affecting tumor aggressiveness. Oncotarget. 2016;8:80235–80248. doi: 10.18632/oncotarget.10288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuroda Y, Kitada M, Wakao S, Dezawa M. Bone marrow mesenchymal cells: how do they contribute to tissue repair and are they really stem cells? Arch Immunol Ther Exp (Warsz) 2011;59:369–378. doi: 10.1007/s00005-011-0139-9. [DOI] [PubMed] [Google Scholar]
- 42.Gardner OFW, Musumeci G, Neumann AJ, Eglin D, Archer CW, Alini M, Stoddart MJ. Asymmetrical seeding of MSCs into fibrin-poly(ester-urethane) scaffolds and its effect on mechanically induced chondrogenesis. J Tissue Eng Regen Med. 2017;11:2912–2921. doi: 10.1002/term.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Musumeci G, Lo Furno D, Loreto C, Giuffrida R, Caggia S, Leonardi R, Cardile V. Mesenchymal stem cells from adipose tissue which have been differentiated into chondrocytes in three-dimensional culture express lubricin. Exp Biol Med (Maywood) 2011;236:1333–1341. doi: 10.1258/ebm.2011.011183. [DOI] [PubMed] [Google Scholar]
- 44.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 45.Meier RP, Müller YD, Morel P, Gonelle-Gispert C, Bühler LH. Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence? Stem Cell Res. 2013;11:1348–1364. doi: 10.1016/j.scr.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 46.Lin CS, Xin ZC, Dai J, Lue TF. Commonly used mesenchymal stem cell markers and tracking labels: Limitations and challenges. Histol Histopathol. 2013;28:1109–1116. doi: 10.14670/hh-28.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reagan MR, Kaplan DL. Concise review: Mesenchymal stem cell tumor-homing: detection methods in disease model systems. Stem Cells. 2011;29:920–927. doi: 10.1002/stem.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fiore EJ, Bayo JM, Garcia MG, Malvicini M, Lloyd R, Piccioni F, Rizzo M, Peixoto E, Sola MB, Atorrasagasti C, et al. Mesenchymal stromal cells engineered to produce IGF-I by recombinant adenovirus ameliorate liver fibrosis in mice. Stem Cells Dev. 2015;24:791–801. doi: 10.1089/scd.2014.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai LJ, Li HY, Guan LX, Ritchie G, Zhou JX. The therapeutic potential of bone marrow-derived mesenchymal stem cells on hepatic cirrhosis. Stem Cell Res. 2009;2:16–25. doi: 10.1016/j.scr.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M, et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756–763. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- 51.Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Li WL, Su J, Yao YC, Tao XR, Yan YB, Yu HY, Wang XM, Li JX, Yang YJ, Lau JT, et al. Isolation and characterization of bipotent liver progenitor cells from adult mouse. Stem Cells. 2006;24:322–332. doi: 10.1634/stemcells.2005-0108. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, Wang X, Wang L, Chiu JD, van de Ven G, Gaarde WA, Deleve LD. Hepatic vascular endothelial growth factor regulates recruitment of rat liver sinusoidal endothelial cell progenitor cells. Gastroenterology. 2012;143:1555–1563.e2. doi: 10.1053/j.gastro.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47:1634–1643. doi: 10.1002/hep.22236. [DOI] [PubMed] [Google Scholar]
- 56.Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142–4157. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6:287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 59.Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maumus M, Jorgensen C, Noël D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie. 2013;95:2229–2234. doi: 10.1016/j.biochi.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 61.Huang L, Ma W, Ma Y, Feng D, Chen H, Cai B. Exosomes in mesenchymal stem cells, a new therapeutic strategy for cardiovascular diseases? Int J Biol Sci. 2015;11:238–245. doi: 10.7150/ijbs.10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Börger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, Giebel B. Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tomiyama T, Yang GX, Zhao M, Zhang W, Tanaka H, Wang J, Leung PS, Okazaki K, He XS, Lu Q, et al. The modulation of co-stimulatory molecules by circulating exosomes in primary biliary cirrhosis. Cell Mol Immunol. 2017;14:276–284. doi: 10.1038/cmi.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fornari F, Ferracin M, Trerè D, Milazzo M, Marinelli S, Galassi M, Venerandi L, Pollutri D, Patrizi C, Borghi A, et al. Circulating microRNAs, miR-939, miR-595, miR-519d and miR-494, Identify Cirrhotic Patients with HCC. PLoS One. 2015;10:e0141448. doi: 10.1371/journal.pone.0141448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maji S, Matsuda A, Yan IK, Parasramka M, Patel T. Extracellular vesicles in liver diseases. Am J Physiol Gastrointest Liver Physiol. 2017;312:G194–G200. doi: 10.1152/ajpgi.00216.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2017;14:455–466. doi: 10.1038/nrgastro.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nojima H, Freeman CM, Schuster RM, Japtok L, Kleuser B, Edwards MJ, Gulbins E, Lentsch AB. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol. 2016;64:60–68. doi: 10.1016/j.jhep.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, Calogero R, Bussolati B, Tetta C, Camussi G. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010;14:1605–1618. doi: 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fatima F, Ekstrom K, Nazarenko I, Maugeri M, Valadi H, Hill AF, Camussi G, Nawaz M. Non-coding RNAs in Mesenchymal Stem Cell-Derived Extracellular Vesicles: Deciphering Regulatory Roles in Stem Cell Potency, Inflammatory Resolve, and Tissue Regeneration. Front Genet. 2017;8:161. doi: 10.3389/fgene.2017.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim KM, Abdelmohsen K, Mustapic M, Kapogiannis D, Gorospe M. RNA in extracellular vesicles. Wiley Interdiscip Rev RNA. 2017:8. doi: 10.1002/wrna.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nojima H, Konishi T, Freeman CM, Schuster RM, Japtok L, Kleuser B, Edwards MJ, Gulbins E, Lentsch AB. Chemokine Receptors, CXCR1 and CXCR2, Differentially Regulate Exosome Release in Hepatocytes. PLoS One. 2016;11:e0161443. doi: 10.1371/journal.pone.0161443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, Charlton MR, Shah VH, Malhi H, Gores GJ. Lipid-Induced Signaling Causes Release of Inflammatory Extracellular Vesicles From Hepatocytes. Gastroenterology. 2016;150:956–967. doi: 10.1053/j.gastro.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee YS, Kim SY, Ko E, Lee JH, Yi HS, Yoo YJ, Je J, Suh SJ, Jung YK, Kim JH, et al. Exosomes derived from palmitic acid-treated hepatocytes induce fibrotic activation of hepatic stellate cells. Sci Rep. 2017;7:3710. doi: 10.1038/s41598-017-03389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Momen-Heravi F, Saha B, Kodys K, Catalano D, Satishchandran A, Szabo G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J Transl Med. 2015;13:261. doi: 10.1186/s12967-015-0623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Momen-Heravi F, Bala S, Kodys K, Szabo G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci Rep. 2015;5:9991. doi: 10.1038/srep09991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Devhare PB, Sasaki R, Shrivastava S, Di Bisceglie AM, Ray R, Ray RB. Exosome-Mediated Intercellular Communication between Hepatitis C Virus-Infected Hepatocytes and Hepatic Stellate Cells. J Virol. 2017:91. doi: 10.1128/JVI.02225-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bukong TN, Momen-Heravi F, Kodys K, Bala S, Szabo G. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS Pathog. 2014;10:e1004424. doi: 10.1371/journal.ppat.1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seo W, Eun HS, Kim SY, Yi HS, Lee YS, Park SH, Jang MJ, Jo E, Kim SC, Han YM, et al. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by γδ T cells in liver fibrosis. Hepatology. 2016;64:616–631. doi: 10.1002/hep.28644. [DOI] [PubMed] [Google Scholar]
- 79.Charrier A, Chen R, Chen L, Kemper S, Hattori T, Takigawa M, Brigstock DR. Exosomes mediate intercellular transfer of pro-fibrogenic connective tissue growth factor (CCN2) between hepatic stellate cells, the principal fibrotic cells in the liver. Surgery. 2014;156:548–555. doi: 10.1016/j.surg.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen L, Chen R, Kemper S, Charrier A, Brigstock DR. Suppression of fibrogenic signaling in hepatic stellate cells by Twist1-dependent microRNA-214 expression: Role of exosomes in horizontal transfer of Twist1. Am J Physiol Gastrointest Liver Physiol. 2015;309:G491–G499. doi: 10.1152/ajpgi.00140.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen L, Chen R, Velazquez VM, Brigstock DR. Fibrogenic Signaling Is Suppressed in Hepatic Stellate Cells through Targeting of Connective Tissue Growth Factor (CCN2) by Cellular or Exosomal MicroRNA-199a-5p. Am J Pathol. 2016;186:2921–2933. doi: 10.1016/j.ajpath.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DeLeve LD. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology. 2015;61:1740–1746. doi: 10.1002/hep.27376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang R, Ding Q, Yaqoob U, de Assuncao TM, Verma VK, Hirsova P, Cao S, Mukhopadhyay D, Huebert RC, Shah VH. Exosome Adherence and Internalization by Hepatic Stellate Cells Triggers Sphingosine 1-Phosphate-dependent Migration. J Biol Chem. 2015;290:30684–30696. doi: 10.1074/jbc.M115.671735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ichinohe N, Ishii M, Tanimizu N, Kon J, Yoshioka Y, Ochiya T, Mizuguchi T, Hirata K, Mitaka T. Transplantation of Thy1+ Cells Accelerates Liver Regeneration by Enhancing the Growth of Small Hepatocyte-Like Progenitor Cells via IL17RB Signaling. Stem Cells. 2017;35:920–931. doi: 10.1002/stem.2548. [DOI] [PubMed] [Google Scholar]
- 85.Chen L, Brigstock DR. Integrins and heparan sulfate proteoglycans on hepatic stellate cells (HSC) are novel receptors for HSC-derived exosomes. FEBS Lett. 2016;590:4263–4274. doi: 10.1002/1873-3468.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fiore E, Malvicini M, Bayo J, Peixoto E, Atorrasagasti C, Sierra R, Rodríguez M, Gómez Bustillo S, García MG, Aquino JB, et al. Involvement of hepatic macrophages in the antifibrotic effect of IGF-I-overexpressing mesenchymal stromal cells. Stem Cell Res Ther. 2016;7:172. doi: 10.1186/s13287-016-0424-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramachandran P, Iredale JP. Macrophages: central regulators of hepatic fibrogenesis and fibrosis resolution. J Hepatol. 2012;56:1417–1419. doi: 10.1016/j.jhep.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 88.Saha B, Momen-Heravi F, Furi I, Kodys K, Catalano D, Gangopadhyay A, Haraszti R, Satishchandran A, Iracheta-Vellve A, Adejumo A, et al. Extracellular vesicles from mice with alcoholic liver disease carry a distinct protein cargo and induce macrophage activation through heat shock protein 90. Hepatology. 2018;67:1986–2000. doi: 10.1002/hep.29732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, Cao S, Contreras PC, Malhi H, Kamath PS, et al. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol. 2016;64:651–660. doi: 10.1016/j.jhep.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saha B, Momen-Heravi F, Kodys K, Szabo G. MicroRNA Cargo of Extracellular Vesicles from Alcohol-exposed Monocytes Signals Naive Monocytes to Differentiate into M2 Macrophages. J Biol Chem. 2016;291:149–159. doi: 10.1074/jbc.M115.694133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saha B, Bruneau JC, Kodys K, Szabo G. Alcohol-induced miR-27a regulates differentiation and M2 macrophage polarization of normal human monocytes. J Immunol. 2015;194:3079–3087. doi: 10.4049/jimmunol.1402190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu HH, Lee OK. Exosomes from mesenchymal stem cells induce the conversion of hepatocytes into progenitor oval cells. Stem Cell Res Ther. 2017;8:117. doi: 10.1186/s13287-017-0560-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Du Y, Li D, Han C, Wu H, Xu L, Zhang M, Zhang J, Chen X. Exosomes from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells (hiPSC-MSCs) Protect Liver against Hepatic Ischemia/ Reperfusion Injury via Activating Sphingosine Kinase and Sphingosine-1-Phosphate Signaling Pathway. Cell Physiol Biochem. 2017;43:611–625. doi: 10.1159/000480533. [DOI] [PubMed] [Google Scholar]
- 94.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Akyurekli C, Le Y, Richardson RB, Fergusson D, Tay J, Allan DS. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Rev. 2015;11:150–160. doi: 10.1007/s12015-014-9545-9. [DOI] [PubMed] [Google Scholar]
- 96.Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen L, Xiang B, Wang X, Xiang C. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res Ther. 2017;8:9. doi: 10.1186/s13287-016-0453-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haga H, Yan IK, Takahashi K, Matsuda A, Patel T. Extracellular Vesicles from Bone Marrow-Derived Mesenchymal Stem Cells Improve Survival from Lethal Hepatic Failure in Mice. Stem Cells Transl Med. 2017;6:1262–1272. doi: 10.1002/sctm.16-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tan CY, Lai RC, Wong W, Dan YY, Lim SK, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5:76. doi: 10.1186/scrt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yan Y, Jiang W, Tan Y, Zou S, Zhang H, Mao F, Gong A, Qian H, Xu W. hucMSC Exosome-Derived GPX1 Is Required for the Recovery of Hepatic Oxidant Injury. Mol Ther. 2017;25:465–479. doi: 10.1016/j.ymthe.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nong K, Wang W, Niu X, Hu B, Ma C, Bai Y, Wu B, Wang Y, Ai K. Hepatoprotective effect of exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats. Cytotherapy. 2016;18:1548–1559. doi: 10.1016/j.jcyt.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 102.Armstrong JP, Holme MN, Stevens MM. Re-Engineering Extracellular Vesicles as Smart Nanoscale Therapeutics. ACS Nano. 2017;11:69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dilnawaz F, Acharya S, Sahoo SK. Recent trends of nanomedicinal approaches in clinics. Int J Pharm. 2018;538:263–278. doi: 10.1016/j.ijpharm.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 104.Lou G, Yang Y, Liu F, Ye B, Chen Z, Zheng M, Liu Y. MiR-122 modification enhances the therapeutic efficacy of adipose tissue-derived mesenchymal stem cells against liver fibrosis. J Cell Mol Med. 2017;21:2963–2973. doi: 10.1111/jcmm.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zeng C, Wang YL, Xie C, Sang Y, Li TJ, Zhang M, Wang R, Zhang Q, Zheng L, Zhuang SM. Identification of a novel TGF-β-miR-122-fibronectin 1/serum response factor signaling cascade and its implication in hepatic fibrogenesis. Oncotarget. 2015;6:12224–12233. doi: 10.18632/oncotarget.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M, Lu L. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21:2491–2502. doi: 10.1111/jcmm.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen L, Lu FB, Chen DZ, Wu JL, Hu ED, Xu LM, Zheng MH, Li H, Huang Y, Jin XY, et al. BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol Immunol. 2018;93:38–46. doi: 10.1016/j.molimm.2017.11.008. [DOI] [PubMed] [Google Scholar]


