Abstract
Purpose
To report a case of rapid “epiretinal membrane” (“ERM”) development following intravitreal bevacizumab for juvenile Coats' disease.
Observations
A 7-year old boy was followed for four years with asymptomatic stage 2 Coats' disease in his left eye. At age 11, he developed symptomatic cystoid macular edema. Argon laser photocoagulation to the leaking aneurysms failed to improve his vision, which had symptomatically declined to 20/30. Four-months after laser, a single injection of intravitreal bevacizumab was given. Rapid development of an “ERM” was noticed on his first post-injection follow-up at 4 weeks. By 8-weeks post-injection the visual acuity had deteriorated to 20/400. 25 + gauge pars plana vitrectomy with “ERM” peeling was performed, with recovery of vision to 20/30 at the 4 months post-operative visit.
Conclusions and importance
Intravitreal bevacizumab may induce rapidly progressive “ERM” in patients with juvenile Coats' disease.
Keywords: Coats disease, Epiretinal membrane, Intravitreal bevacizumab
1. Introduction
Coats' disease is a sporadic, typically monocular disorder characterised by retinal telangiectasias (Stage 1) which can progress to lipid exudation (Stage 2) and retinal detachment (Stages 3–5) in late disease.1, 2, 3 It has a strong male predominance (3:1) and although it may be recognised at any age, most cases present within the first decade of life.2,3
Management depends on disease severity. Laser photocoagulation and/or cryotherapy to the telangiectatic vessels is recommended when lipid exudation threatens the macula. In recent times, anti-vascular endothelial growth factor (VEGF) agents have also been used as an adjuvant to treat Coats' disease. This is based on the observation that VEGF is known to be markedly elevated in eyes affected by Coats' disease.1,3 We describe a potential adverse effect - rapid development of “epiretinal membrane” (“ERM”) following a single injection of intravitreal bevacizumab in a patient with Stage 2 juvenile Coats' disease. Informed consent for this report was provided by the patient and his parents.
2. Case report
A 7-year-old boy with good general health was referred with telangiectatic vessels, aneurysms and intraretinal lipid temporal to his left fovea confirmed on optical coherence tomography scans (OCT, Fig. 1A). The rest of the ocular examination including the right eye was normal and given the male gender, age and clinical findings a diagnosis of Stage 2 Coats' disease was made. Since he was asymptomatic and the visual acuity was 20/20, the patient and his parents opted for close observation.
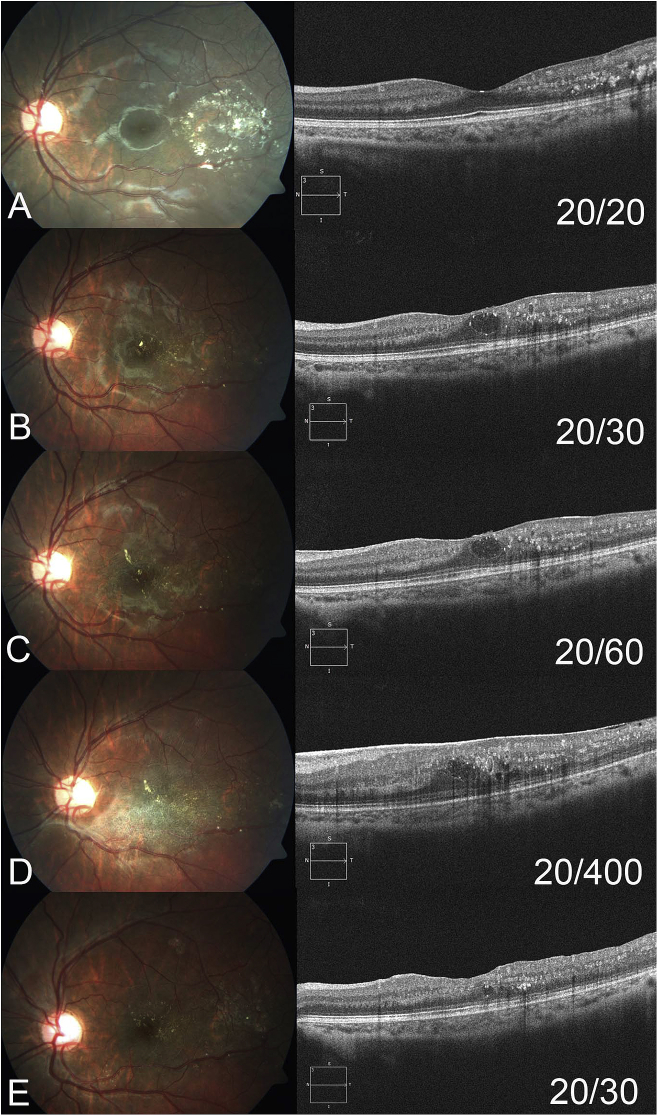
Fig. 1.
Colour fundus photographs (left) and optical coherence tomography scans with corresponding horizontal rasters through the fovea (right) at different time points. A) At baseline there is a patch of circinate hard exudate and mild edema temporal to the fovea with surrounding telangiectasias. Optical coherence tomography demonstrates hyper-reflective hard exudate temporal to, but not involving the fovea. Vision is 20/20. B) Four years after baseline the patient is noticing blurring of his vision and the acuity has declined to 20/30. There is lipid deposition migrating towards the fovea and optical coherence tomography demonstrates cystoid macular edema. Focal argon laser photocoagulation is applied to telangiectasic vessels temporal to the fovea. C) Four months after focal argon laser photocoagulation, there has been no improvement in the cystoid macular edema and the vision has declined further to 20/60. A trace thickening of the internal limiting membrane/”epiretinal membrane” is just visible on optical coherence tomography. D) Four weeks following intravitreal bevacizumab injection, there has been marked growth of a dense “epiretinal membrane” over the macula. There is thickening of the retina and loss of the foveal dip on optical coherence tomography scans. Vision is 20/150 and worsened to 20/400 by 8 weeks following intravitreal bevacizumab injection. E) Four months following pars plana vitrectomy and “epiretinal membrane” peeling, there has been marked reduction in the macular edema and vision has partially recovered to 20/30. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The patient was followed twice a year for three and a half years, during which time his disease remained stable. However, at four years he started to notice blurring of his vision which had dropped to 20/30 due to development of cystoid macular edema (Fig. 1B). Fundus fluorescein angiography was attempted but aborted due to difficulty with cannulation. Argon laser photocoagulation was applied to the temporal aneurysms and affected retina but four months later following this there was still no resolution of the edema and his vision had worsened to 20/60 (Fig. 1C). An OCT raster through the fovea showed trace thickening of the internal limiting membrane/”ERM”. A single injection of intravitreal bevacizumab (Avastin; Genentech, San Francisco, CA, 1.25mg/0.05mL) was given under topical and subconjunctival anaesthesia.
At the 4-week post-injection follow-up visit the vision had dropped to 20/150 and a dense “ERM” was noted at the macula (Fig. 1D). At 8 weeks post-injection the vision had deteriorated to 20/400 with further progression of the “ERM”. The central foveal thickness had increased from 333μm at the time of the bevacizumab injection to 579μm. The patient underwent 25-gauge pars plana vitrectomy with “ERM” and internal limiting membrane (ILM) peeling using trypan blue dye (0.06%). The posterior hyaloid was found to be extremely thickened. Supplemental endolaser was applied to the temporal and inferior aneursysms, telangiectasias and retina. Interestingly, histopathological analysis demonstrated highly folded, paucicellular membrane favouring ILM. Four months post-operatively the macular edema had largely resolved with improvement of vision back to 20/30 and reduction in central foveal thickness to 270μm (Fig. 1E).
3. Discussion
ERM occurs in 2.5–4.4% of patients with Coats' disease.4,5 It may be precipitated by laser photocoagulation or cryotherapy, but can also occur in untreated patients.6,7 The presumed pathogenesis involves chronic leakage from diseased vessels leading to reactive glial proliferation and ERM formation. ERMs may lead to traction on the retina and further accumulation of fluid in the subretinal space.6 The growth and contraction of ERM is usually a slow process, but in our case, after a four-year history of relatively stable Coats' disease, an “ERM” developed rapidly following a single dose of intravitreal bevacizumab. This time course suggests a likely cause-effect relationship. It is unlikely that the initial laser photocoagulation was the main cause since this had been performed 4 months prior to intravitreal bevacizumab, and only trace thickening of the ILM/“ERM” had been identified at the time of injection.
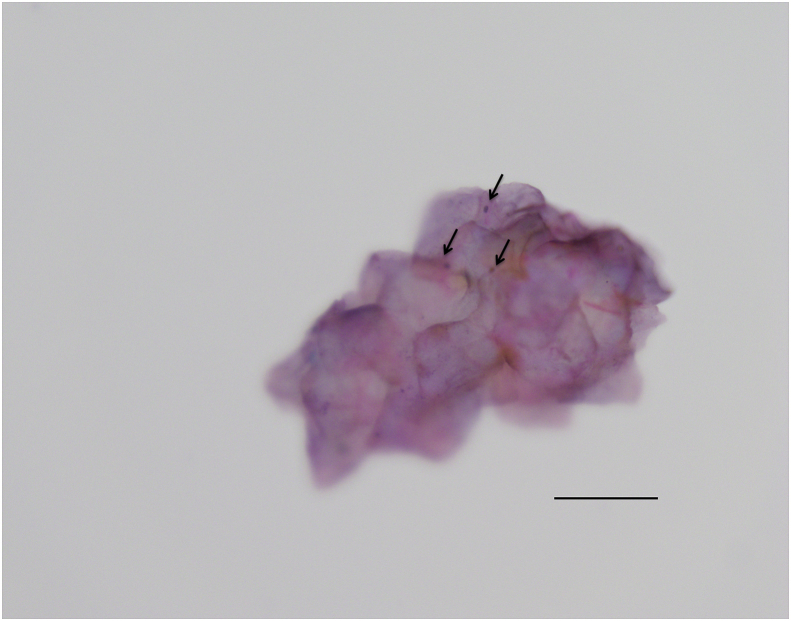
Large population studies have not found intravitreal anti-VEGF therapy to be associated with ERM development and progression.8 However, some small case series have shown rapid progression of ERM in patients being treated with anti-VEGF agents for retinal vein occlusion9 or proliferative diabetic retinopathy.10 It is thought that anti-VEGF agents may accelerate the fibrosis of pre-existing fibrovascular elements.10, 11, 12 Anti-VEGF agents may alter the relation, expression, and activity of numerous cytokines, growth factors, and mediators present in retinal tissue. These include transforming growth factor β and connective tissue growth factor, important mediators of retinal fibrosis and ERM development.9, 10, 11 Although vitreoretinal fibrosis leading to tractional retinal detachment has been described in patients with Coats disease treated with intravitreal bevacizumab,1 our case is the first to our knowledge, of rapid macular “ERM” development. To our surprise, the histopathology was more suggestive of ILM rather than typical ERM elements (Fig. 2). This suggests that the membrane may not in fact have been a “true” ERM (as described in idiopathic ERMs), but fibrous metaplasia of the ILM. Alternatively, ERM elements may have been lost during tissue processing or the “epiretinal membrane” may have represented thickening of the posterior hyaloid.
Fig. 2.
Histopathology showed fragmented, folded, paucicellular membrane material favouring internal limiting membrane. A few oval nuclei (arrows) could be seen. No convincing neurosensory retinal elements or epiretinal membrane elements were seen attached to the membrane in the sample. It is possible that the “epiretinal membrane” represented fibrous metaplasia of the internal limiting membrane, was lost during processing, or was actually thickening of the posterior hyaloid and therefore never collected. x60 objective (Olympus BX53 microscope, Olympus Corporation, Shinjuku, Japan). Scale bar approximately 50 μm.
In our case, following surgical intervention, the patient's vision recovered to 20/30 after 4 months. Although management of ERM via vitrectomy and membrane peeling in the setting of Coats' disease has been reported rarely,6,7 it was felt that expedient management would likely prevent the permanent macular changes that may be seen if ERMs are left untreated for longer periods.6,7 This timely intervention is likely to have prevented more severe visual acuity deterioration in our patient.
In summary, we report a case of rapid macular “ERM” development following intravitreal bevacizumab for juvenile Coats' disease. Caution is advised when considering anti-VEGF agents for the management of this disease.
Patient consent
The patient and his parents provided written informed consent for the report.
Funding
No funding or grant support.
Conflicts of interest
The following authors have no financial disclosures: AWK, MH, SC, ATF.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ajoc.2018.06.002.
Contributor Information
Andrew W. Kam, Email: andrew.kam@hotmail.com.
Adrian T. Fung, Email: adrianfungi@yahoo.com.au.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Ramasubramanian A., Shields C.L. Bevacizumab for Coats' disease with exudative retinal detachment and risk of vitreoretinal traction. Br J Ophthalmol. 2012;96:356–359. doi: 10.1136/bjophthalmol-2011-300141. [DOI] [PubMed] [Google Scholar]
- 2.Shields J.A., Shields C.L. Review: coats disease: the 2001 LuEsther T. Mertz lecture. Retina. 2002;22:80–91. doi: 10.1097/00006982-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Sigler E.J., Randolph J.C., Calzada J.I., Wilson M.W., Haik B.G. Current management of Coats disease. Surv Ophthalmol. 2014;59:30–46. doi: 10.1016/j.survophthal.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Rishi P., Rishi E., Uparkar M. Coats' disease: an Indian perspective. Indian J Ophthalmol. 2010;58:119–124. doi: 10.4103/0301-4738.60081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shields J.A., Shields C.L., Honavar S.G., Demirci H., Cater J. Classification and management of coats disease: the 2000 proctor lecture. Am J Ophthalmol. 2001;131:572–583. doi: 10.1016/s0002-9394(01)00896-0. [DOI] [PubMed] [Google Scholar]
- 6.Kumar P., Kumar V. Vitrectomy for epiretinal membrane in adult-onset Coats' disease. Indian J Ophthalmol. 2017;65:1046–1048. doi: 10.4103/ijo.IJO_322_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shukla D.M.S., Chakraborty S.M.S., Behera U.C.M.S., Kim R.D.N.B. Vitrectomy for epimacular membrane secondary to adult-onset coats' disease. Ophthamic Surg Las Im. 2008;39:239–241. doi: 10.3928/15428877-20080501-15. [DOI] [PubMed] [Google Scholar]
- 8.Ghasemi Falavarjani K., Nguyen Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye (Lond) 2013;27:787–794. doi: 10.1038/eye.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marticorena J., Romano M.R., Heimann H. Intravitreal bevacizumab for retinal vein occlusion and early growth of epiretinal membrane: a possible secondary effect? Br J Ophthalmol. 2011;95:391–395. doi: 10.1136/bjo.2009.177287. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q., Qi Y., Chen L. The relationship between anti-vascular endothelial growth factor and fibrosis in proliferative retinopathy: clinical and laboratory evidence. Br J Ophthalmol. 2016;100:1443–1450. doi: 10.1136/bjophthalmol-2015-308199. [DOI] [PubMed] [Google Scholar]
- 11.Chu S.-J., Zhang Z.-H., Wang M., Xu H.-F. Effect of bevacizumab on the expression of fibrosis-related inflammatory mediators in ARPE-19 cells. Int J Ophthalmol. 2017;10:366–371. doi: 10.18240/ijo.2017.03.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang M., Chu S., Zeng F., Xu H. Bevacizumab modulates the process of fibrosis in vitro. Clin Exp Ophthalmol. 2015;43:173–179. doi: 10.1111/ceo.12374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.