Highlights
-
•
Reactivation of Strongyloides after immunosuppression is an important consideration especially in endemic area with previous farming activity.
-
•
Provision of ivermectin can be limited in developing country.
-
•
Although albendazole is generally well tolerated, it may lead to fatal pancytopenia after prolonged exposure or with underlying liver disease.
Keywords: Strongyloidiasis, Immunocompromised, Albendazole, Adverse effect, Pancytopenia
Abstract
We report 7 cases of strongyloidiasis that had occurred from 2016 through 2017 in a tertiary hospital of southern China. Three of the 7 patients (age 66–77) with farming exposure many years ago developed symptomatic infection while receiving immunosuppressant for underlying medical conditions. The majority of them were treated with albendazole due to unavailability of ivermectin in mainland China. One of the 7 patients, with underlying IgG4 sclerosing cholangitis and secondary biliary cirrhosis was on immunosuppressives and developed severe pancytopenia 15 days after albendazole treatment. He ultimately died of polymicrobial sepsis. This was the second fatal case being reported in the literature as a consequence of albendazole-induced myelosuppression. We have undertaken a review of the literature regarding the use of albendazole for strongyloidiasis and its adverse effect with a focus on myelosuppression as a rare but potentially serious event.
Introduction
Strongyloides stercoralis infection remains endemic in developing countries in tropics asnd subtropics. The ability to complete lifecycle inside human body enables the helminth to persist for years after acquisition. Immunosuppression later in life suppresses the T-cell response in controlling the worm burden, and can result in hyperinfection and even dissemination. Patient may develop severe pneumonia and even meningitis at the most severe end of spectrum. Although prompt treatment with antihelminthics like albendazole and ivermectin can be life-saving, the side effects of them could be fatal in turn. We report a case series involving 7 patients with strongyloidiasis, in which one of them died due to myelosuppression from albendazole.
Case series
We prospectively followed up and collected data on all laboratory confirmed cases of Strongyloides stercoralis infections at The University of Hong Kong-Shenzhen Hospital in mainland China between July 2016 and November 2017.
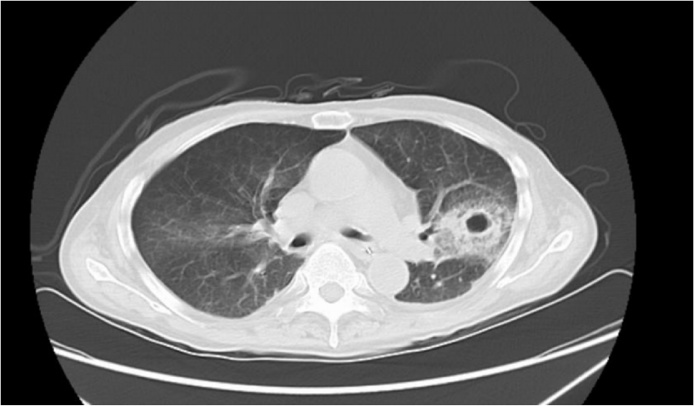
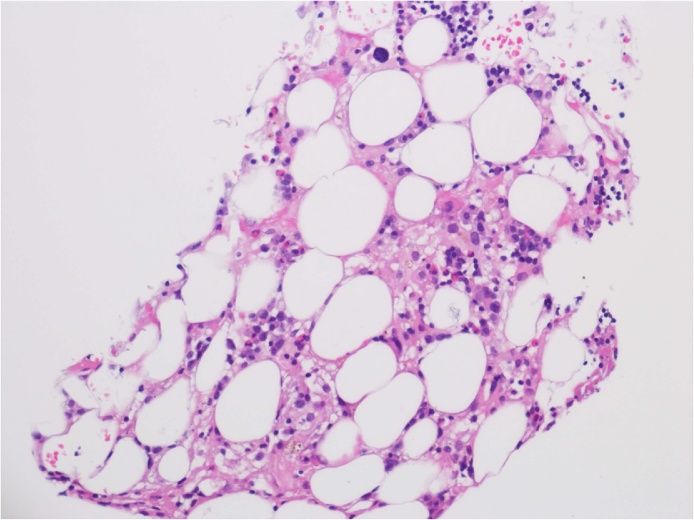
Seven cases of strongyloidiasis were encountered over the period of time. Details of the patients has been summarized in Table 1. Four of the seven patients were male. The age ranged from 49 to 83 years-old. Six of them had worked as farmers in paddy fields in the past. The two patients that presented with intestinal strongyloidiasis were not on any immunosuppressants, and both improved after 7 days of albendazole. The remaining five patients suffered from either hyperinfection or disseminated disease. Four of the five patients (80%) were taking corticosteroids for underlying autoimmune diseases. Three of those four patients with either hyperinfection or disseminated strongyloidiasis, were treated with albendazole alone achieving parasitological cure. Patient 5 with underlying IgG4 sclerosing cholangitis and secondary biliary cirrhosis on prednisolone 40 mg daily presented as hyperinfection. His sputum showed S. stercoralis that produced crawling tracks on agar. This patient developed severe pancytopenia after 15 days of albendazole, complicated by polymicrobial neutropenic sepsis. He developed bacteremia due to ESBL producing Klebsiella pneumoniae, as well as methicillin-resistant Staphylococcus aureus (MRSA) pneumonia and Cunninghamella pulmonary mucormycosis, finally succumbed with multiple cavitating lung lesions (Fig. 1). Although there was no evidence of strongyloidiasis from the respiratory specimen at the time of demise, parasitological clearance could not be declared as treatment was not completed and there were no documented negative specimen 2–4 weeks after albendazole. Bone marrow examination showed extreme hypocellularity and trilineage hypoplasia (Fig. 2). This is the second fatal case reported in the literature as a result of albendazole-induced myelosuppression.
Table 1.
Summary of the Clinical Features of the 7 Patients with Strongyloidiasis.
| Case | Sex/Age | Farming History | Underlying condition | Immunosuppressant | Presentation | Syndrome | Detection of Strongyloides | Treatment | Parasitological Cure | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/83y | Yes | Diabetes mellitus | Nil | Abdominal | Chronic intestinal | Stool | Albendazole | Yes | Clinical resolution |
| Hypertension | discomfort | infection | 400 mg BD x 7 days | |||||||
| 2 | M/64y | Yes | Hypertension | Nil | Abdominal | Chronic intestinal | Stool | Albendazole | Not documented | Clinical resolution |
| Alcoholism | discomfort | infection | 400 mg BD x 7 days | |||||||
| HBV related cirrhosis | ||||||||||
| 3 | F/67y | Yes | Henoch-Schönlein purpura | Methylprednisolone | Abdominal pain | Disseminated | Stool | Albendazole | Yes | Relapse after cessation of albendazole |
| Hypertension | 8 mg daily | Diarrhea | Endoscopic biopsy | 400 mg BD x 10 days; | ||||||
| Asthma | Skin rash | Ivermectin | ||||||||
| 200 μg/kg/day x 14 days | ||||||||||
| after symptomatic relapse | ||||||||||
| 4 | M/49y | No | Pemphigus vulgaris | Methylprednisolone | Vomiting | Disseminated | Endoscopic biopsy | Albendazole | Yes | Clinical resolution |
| 20 mg daily Azathioprine | Diarrhea | 400 mg BD x 21 days | ||||||||
| 50 mg daily | Skin rash | |||||||||
| 5 | M/77y | Yes | IgG4 sclerosing cholangitis | Prednisone | Community- | Hyperinfection | Sputum | Albendazole | Not documented | Death due to polymicrobial |
| 40 mg daily | acquired | Stool | 400 mg x 15 days | infections complicating | ||||||
| pneumonia | albendazole-induced | |||||||||
| pancytopenia | ||||||||||
| 6 | F/72y | Yes | Rheumatoid arthritis | Methylprednisolone | Abdominal pain | Disseminated | Stool | Albendazole | Yes | Clinical resolution |
| 8 mg daily | Cough | 400 mg mg x 2 days | ||||||||
| Methotrexate | Skin rash | Ivermectin | ||||||||
| 10 mg once a week | 200 μg/kg/day x 14 days | |||||||||
| after it is acquired | ||||||||||
| 7 | M/75y | Yes | HBV cirrhosis | Nil | Diarrhea | Disseminated | Stool | Ivermectin | Yes | Clinical resolution |
| Metastatic HCC | Skin rash | 200 μg/kg/day x 10 days | ||||||||
| Diabetes mellitus | ||||||||||
| Hypertension |
HBV: Hepatitis B Virus; HCC: hepatocellular carcinoma.
Chronic intestinal infection: detection of Strongyloides stercoralis and symptoms confined to intestinal tract; Hyperinfection: the total parasitic load increases while the detection of Strongyloides stercoralis and the clinical manifestations are confined to the usual migration route; Disseminated: detection of Strongyloides stercoralis with symptomatic organ involvement outside the usual migration route.
Fig. 1.
High-resolution computerized tomography scan of patient 5 with cavitating lung lesions due to albendazole-induced pancytopenia over left upper lobe with surrounding halo sign.
Fig. 2.
Bone marrow biopsy of patient 5 showing hypocellular marrow with trilineage hypoplasia.
Discussion
About 55–100 million people are infected by S. stercoralis worldwide each year [1]. The prevalence of S. stercoralis infection in China had been reported as 0.12% [2]. There was a regional variation with a higher prevalence noted in the southern and southwestern provinces such as Yunnan (11.7%) [3] and a lower prevalence in eastern part of China such as Shandong (1.29%) [4]; and in the more arid Western China (0.04%) [5]. Six of our 7 patients had past history of farming and had come from Guangdong province of South China. It was most likely that they had acquired the S. stercoralis infection from the paddy field by exposing the bare skin of their legs and feet to the soil. The two of patients that developed intestinal symptoms only were not on any immunosuppressants whereas 4 of the remaining 5 patients with hyperinfection or disseminated disease were.
Both ivermectin and albendazole have been used for intestinal strongyloidiasis without hyperinfection or dissemination. However, ivermectin has not been available in China for a number of years as the sole manufacturer for ivermectin in China has discontinued its production. A review article was published in 2016 comparing the efficacy of albendazle and ivermectinin the treatment of uncomplicated strongyloidiasis (i.e. no evidence of hyperinfection or dissemination) [6]. It was found that a single dose or two doses of ivermectin (each dose 200 μg/kg) was more efficacious in achieving a parasitological cure in each of the 4 published trials compared with 3–7 days of albendazole (daily dose of 400–800 mg). The pooled risk ratio is 1.79 (95% confidence interval 1.55-2.08). The rate of parasitological cure for ivermectin was 82.7%–95%; whereas it was 37.5–63.3% for albendazole [6]. Historically, thiabendazole had been another drug of choice for treating uncomplicated strongyloidiasis. Even though it had been shown to demonstrate similar efficacy in parasitological cure compared with ivermectin (risk ratio 1.07, 95% confidence interval 0.96–1.20), it was not favored due to more adverse events [6]. As expected, our two patients with uncomplicated strongyloidiasis were successfully treated with 7 days of oral albendazole.
To date, no trials have been reported that compared the efficacy between ivermectin and albendazole in the treatment of complicated strongyloidiasis (i.e. hyperinfection or disseminated diseases due to S. stercoralis). The existing experience and recommendations have been based on case reports. Buonfrate et al. summarized the clinical features, treatment and outcome of 133 cases of complicated strongyloidiasis receiving single agent for treatment between 1991 and 2011 [7]. The mortality of patients receiving ivermectin only was 47% (18 out of 38 patients), whereas mortality of patients receiving albendazole only was 73% (25 out of 34 patients). No further statistical analysis was performed in this systematic review. In our series, 4 out of the 5 patients with hyperinfection or disseminated strongyloidiasis were treated with albendazole (patients 3–6), with or without ivermectin. Patient 4 achieved parasitological cure after 21 days of albendazole given at 400 mg twice daily. Patient 3 had symptomatic recurrence despite parasitological cure, required additional of ivermectin. Patient 6 also achieved parasitological cure after 2 days of albendazole. Patient 5 was treated with a course of albendazole but died without any documentation of parasitological clearance, as it requires negative sputum and stool exams performing 2–4 weeks after treatment. Therefore, albendazole achieved parasitological cure in 3 out of 4 patients while the remaining patient without documented clearance eventually succumbed.
Albendazole belongs to the benzimidazole class of drugs and is used to treat a wide range of parasitic diseases. It is generally well tolerated with low incidences of gastrointestinal discomfort. Rarely, albendazole is associated with more severe side effects such as leukopenia (0.044%), anemia (0.004%), and raised liver enzymes (0.035%) [8]. One of our patients (patient 5) was noted to have pancytopenia 15 days after albendazole treatment. He went on and developed Klebsiella pneumoniae bacteremia, MRSA pneumonia, and pulmonary mucormycosis due to Cunninghamella spp. and died of multiorgan failure soon afterwards. On review of the literature, there was one other fatal case of albendazole-induced pancytopenia, being reported by Opatrny et al. and which was similar to our patient 5 [9]. In Opatrny’s report, the patient was started on albendazole for hydatid cyst disease and developed pancytopenia 16 days after the treatment. It was complicated by Klebsiella pneumoniae bacteremia, and the patient died of esophageal variceal bleeding due to severe thrombocytopenia. In 2014, there were 2 cases of bilineage cytopenia reported separately [10,11], both following a month of albendazole treatment for hydatid cyst disease. The case reported by Fredj [10] developed leukopenia and thrombocytopenia, while the other case reported by Açıkgöz [11] developed thrombocytopenia and anemia. It is noteworthy that severe bone marrow suppression in these cases had developed after a prolonged duration of albendazole of over 10 days, used to treat extra-intestinal Angiostrongylus infection and hydatid cyst disease respectively. Albendazole kills parasites by inhibiting tubulin polymerization, resulting in the loss of cytoplasmic microtubules [12]. It is postulated that the inhibition of tubulin might impair the cell turnover of actively dividing cells of human e.g. hematopoietic cells, thus leading to bone marrow suppression after prolonged exposure. The effect might be exacerbated in patients with preexisting liver failure as albendazole is metabolized in liver to less potent metabolites such as albendazole sulfoxide [9]. Our patient is the second fatal case being reported in the literature as a result of albendazole-induced myelosupression. We recommend careful monitoring of full blood count in cases where albendazole is being used, especially in prolonged course (>10 days), or where there is pre-existing liver dysfunction.
In view of the potential fatal outcome of complicated strongyloidiasis and treatment limitation, screening of patient at risk might provide an important step in preventing its development. Risk factors for hyperinfection and disseminated strongyloidiasis include the use of immunosuppressive drugs including corticosteroids, the presence of human T-lymphotropic virus-1 (HTLV-1) infection, recipients of solid organ or hematological stem cell transplant, patients with hematological malignancy, hypogammaglobulinemia, uremia, severe malnutrition, or diabetes mellitus [13]. It may be prudent to screen patients for intestinal carriage of Strongyloides before starting immunosuppression where there are also a past history of farming, residence or travel to endemic area. The sensitivity of single stool examination is only 75.9% for intestinal carriage of S. stercoralis, but increases to 92% with 3 stool specimens [14]. However, the negative predictive value varies with local disease prevalence. In a low prevalence setting, the use of serological testing is preferable. In the past, immunofluoresence antibody testing against Strongyloides had been used predominantly. More recently, the use of enzyme-linked immunosorbent assay (ELISA) and luciferase immunoprecipitation system (LIPS) had increased the sensitivity up to 97%, and the negative predictive value is nearly 100% in low prevalence setting [15]. Stool molecular testing is only used in a few reference laboratories and the sensitivity is unclear at this stage. Southern China has relatively high prevalence of Strongyloides stercoralis. Hence, we recommend screening with multiple stool microscopy examination and a peripheral blood examination for eosinophil count before commencement of immunosuppressive therapy.
Conclusion
In summary, we have reported 7 cases of strongyloidiasis and its course of treatment that had occurred in an endemic part of China. Immunosuppression appeared to be an important risk factor for the development of complicated strongyloidiasis. Albendazole induced myelosuppression is a rare but serious concern and may lead to death through neutropenic sepsis. Hence, the use of albendazole should be accompanied by careful and close monitoring of full blood count especially during prolonged treatment.
Financial support
This work was partly supported by the donations of The Hong Kong Hainan Commercial Association South China Microbiology Research Fund and the Hui Hoy and Chow Sin Lan Charity Fund Limited, and funding from the Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, the Ministry of Education of China. The sponsors had no role in the design and conduct of the study, in the collection, analysis and interpretation of data, or in the preparation, review or approval of the manuscript.
Potential conflicts of interest
J.F.-W.C. has received travel grants from Pfizer Corporation Hong Kong and Astellas Pharma Hong Kong Corporation Limited, and was an invited speaker for Luminex Corporation and Gilead Sciences Hong Kong Limited. The other authors declared no conflict of interests. The funding sources had no role in study design, data collection, analysis or interpretation or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. We thank Simon Lo and Ricky Lau for their technical support.
Acknowledgements
We are grateful to the staff at the Department of Clinical Microbiology and Infection Control, The University of Hong Kong-Shenzhen Hospital, and Department of Microbiology, The University of Hong Kong for facilitation of the study.
References
- 1.Concha R., Harrington W., Jr., Rogers Al. Intestinal strongyloidiasis: recognition, management, and determinants of outcome. J Clin Gastroenterol. 2005;39(March (3)):203–211. doi: 10.1097/01.mcg.0000152779.68900.33. [DOI] [PubMed] [Google Scholar]
- 2.Wang C., Xu J., Zhou X. Strongyloidiasis: an emerging infectious disease in China. Am J Trop Med Hyg. 2013;88(3):420–425. doi: 10.4269/ajtmh.12-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinmann P., Zhou X.-N., Du Z.-W. Occurrence of Strongyloides stercoralis in Yunnan Province, China, and comparison of diagnostic methods. PLoS Negl Trop Dis. 2007;1(1):e75. doi: 10.1371/journal.pntd.0000075. Cappello M., ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo J.D., Zhao L.L. Strongyloides stercoralis investigation among population at Yellow River flood area in the lower Yellow River [in Chinese] Chin J Parasitic Dis Contr. 2005;18:2–4. [Google Scholar]
- 5.Hu J.Y., Tang Z.M. A case report of Strongyloides stercoralis infection [in Chinese] Chin J Parasitic Dis Contr. 2003;16:228. [Google Scholar]
- 6.Henriquez-Camacho C., Gotuzzo E., Echevarria J. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev. 2016;1:1–50. doi: 10.1002/14651858.CD007745.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buonfrate D., Requena-Mendez A., Angheben A. Severe strongyloidiasis: a systematic review of case reports. BMC Infect Dis. 2013;13:78. doi: 10.1186/1471-2334-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horton J. Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitology. 2000;121(S1):S113–S132. doi: 10.1017/s0031182000007290. [DOI] [PubMed] [Google Scholar]
- 9.Opatrny L., Prichard R., Snell L., Maclean J.D. Death related to albendazole-related pancytopenia. Am J Trop Med Hyg. 2005;72(3):291–294. [PubMed] [Google Scholar]
- 10.Ben Fredj N., Chaabane A., Chadly Z., Ben Fadhel N., Boughattas N.A., Aouam K. Albendazole-induced associated acute hepatitis and bicytopenia. Scand J Infect Dis. 2014;46(February (2)):149–151. doi: 10.3109/00365548.2013.835068. [DOI] [PubMed] [Google Scholar]
- 11.Açıkgöz P., Türkbeyler İH., Pehlivan Y. Thrombocytopenia caused by albendazole in a patient with Sjögren’s syndrome: a case report. Eur J Rheumatol. 2014;1(1):44–45. doi: 10.5152/eurjrheum.2014.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luvira V., Watthanakulpanich D., Pittisuttithum P. Management of Strongyloides stercoralis: a puzzling parasite. Int Health. 2014;6(December (4)):273–281. doi: 10.1093/inthealth/ihu058. [DOI] [PubMed] [Google Scholar]
- 13.Meija R., Nutman T.B. Screening, prevention and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Curr Opin Infect Dis. 2012;25:458–463. doi: 10.1097/QCO.0b013e3283551dbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cartwright C.P. Utility of multiple-stool-specimen ova and parasite examinations in a high-prevalence setting. J Clin Microbiol. 1999;37:2408–2411. doi: 10.1128/jcm.37.8.2408-2411.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buonfronte D., Formenti F., Perandin F., Bisoffi Z. New approaches to the diagnosis of Strongyloides stercoralis infection. Clin Microbiol Infect. 2015;21:543–552. doi: 10.1016/j.cmi.2015.04.001. [DOI] [PubMed] [Google Scholar]