Abstract
The Powassan Virus is the arthropod-borne vector responsible for Powassan neuroinvasive disease. The virus was first isolated in 1958 and has been responsible for approximately 100 cases of neuroinvasive disease. Rates of infection have been on the rise over the past decade with numerous states reporting their first confirmed case; New Jersey, New Hampshire and Connecticut all reported their first case within the last five years. We present here the first confirmed case of Powassan neuroinvasive disease in the nearby state of Rhode Island. A previously healthy 81-year-old female with known tick exposure presented with fever, altered sensorium, seizures and focal neurological deficits. After an extensive work-up that was largely unrevealing Powassan encephalitis was suspected. The diagnosis was confirmed with serological testing consisting of Powassan IgM enzyme-linked immunosorbent assay and Powassan plaque reduction neutralization testing. The case study provides evidence for the increasing spread of Powassan neuroinvasive disease and reinforces the importance of requesting focused testing for Powassan Virus in patients from an endemic area with a clinically compatible syndrome.
Abbreviations: CDC, center for disease control; CSF, cerebrospinal fluid; CT, computed tomography; ELISA, enzyme-linked immunosorbent assay; FLAIR, fluid attenuated inversion recovery; MRI, magnetic resonance imaging; POWV, Powassan Virus; PRNT, plague reduction neutralization testing
Keywords: Powassan encephalitis, Tick-borne disease, Viral encephalopathy
Background
The Powassan Virus (POWV) is the pathogen responsible for Powassan neuroinvasive disease. POWV is named after the town in which it was first isolated in 1958 – Powassan, Ontario [1]. POWV is part of the flaviviridae family and is composed of two distinct lineages; POWV lineage I is transmitted by Ixodes cookei which is mostly endemic in the Great Lakes region, and POWV lineage II is transmitted by Ixodes scapularis which is mostly endemic in the Northeast (Ixodes cookei has been known to occur in this area as well but to a lesser extent) [[1], [2], [3]]. Since isolation in 1958, POWV has been identified as the causative pathogen in approximately 100 cases of Powassan neuroinvasive disease in the United States (US), with the majority of cases occurring over the past decade in the aforementioned natural habitats for Ixodes cookei and Ixodes scapularis [1,4].
POWV transmission in the Northeast is closely linked to the life cycle of its arthropod vector. Ixodes scapularis has a two-year life cycle split into four stages [5]. The tick progresses from egg to larvae to nymph and finally to adult, with each transition requiring a blood meal for survival [5]. POWV is typically transmitted during these blood meals. The tick is equipped with numerous adaptations to ensure effective blood meals, including a feeding tube with barb to keep the tick firmly attached and saliva with anesthetic properties to prevent the host from feeling attachment [5]. This makes Ixodes scapularis an effective arthropod for the transmission of a wide range of human pathogens, including Borrelia burgdorferi, Babesia microti and Anaplasma phagocytophilum [5]. White-footed mice are the principal reservoir for these human pathogens, whereas white-tailed deer are the principal host for lxodes scapularis [6]. Medium-sized mammals that live near human dwellings such as skunks, woodchucks and squirrels may also serve as reservoirs for lxodes cookei [2]. Humans are considered incidental hosts, with the prior decade bearing witness to a rise in reported rates of POWV infection [7]. In this case study we present the first confirmed case of Powassan neuroinvasive disease in Rhode Island.
Case presentation
An 81-year-old Caucasian female presented in April 2016 to the emergency department after a fall. Previously the patient had been healthy, highly functional and independent; she lived at home by herself and could perform her activities of daily living without assistance. The patient enjoyed gardening, where she was frequently exposed to ticks. The patient lived in Rhode Island and had not recently traveled outside of the state prior to presentation.
On the day of admission, the patient had awoken at 3 AM to use the restroom but realized she was heading in the wrong direction. As she changed course, she tripped and fell onto her back and struck her head. There was no reported loss of consciousness. The patient laid on the floor for four hours (3 am −7 am) because she felt "stunned." She then got up, put on some clothing and headed to church. There she met her daughter who observed the patient to be oozing blood from the back of her head and to be mildly confused. The daughter grew concerned and brought the patient to the emergency department.
Initial vitals upon arrival to the emergency department were as follows: temperature 103.6 F, pulse 126, blood pressure 150/75, respiratory rate 16, oxygen saturation 98% on room air. Physical examination revealed a woman in no acute distress who was alert, oriented to person, place and time, and neurologically intact. Patient was noted to have a 4 cm occipital laceration that was not actively oozing blood. There was also bruising along the left shoulder, elbow and wrist although no significant tenderness to palpation and normal range of motion. A chest radiograph and computed tomography (CT) scan of the head and cervical spine were unremarkable. The patient was admitted to the medicine service.
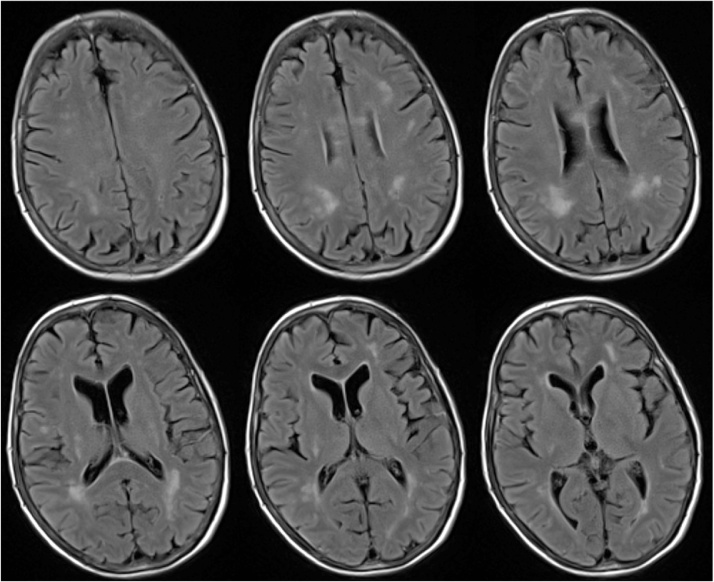
On hospital days 1–4 the patient continued to spike fevers reaching 106.1 F. She received extensive laboratory and microbiological testing to identify the cause of her fevers that were ultimately found to be unrevealing (results displayed in Table 1). Imaging studies consisting of a CT chest/abdomen/pelvis were also found to be unrevealing. On hospital day six the patient became somnolent and confused. She became unable to recognize her daughter. She was noted to have neck stiffness, diffuse motor weakness, increased tone and cogwheel rigidity. A lumbar puncture was performed after which the patient was started on empiric treatment for bacterial meningitis and herpes encephalitis. Numerous cerebral spinal fluid studies were sent off over the proceeding days (results displayed in Table 1). Magnetic resonance imaging of the brain demonstrated multiple foci of nonspecific white matter T2/FLAIR hyperintensities in the superficial and deep white matter as well as corpus callosum (image displayed in Fig. 1). On hospital day eight electroencephalograph was obtained which demonstrated an epileptogenic process involving bifrontal and bitemporal lobes felt to represent nonconvulsive status epilepticus. This was successfully treated with anti-epileptics. On hospital day 11 a repeat lumbar puncture was performed for further CSF studies (results displayed in Table 1). On hospital day 13 the patient's mental status gradually improved with titration of her anti-epileptics. She became alert, oriented to person, place and time and neurologically nonfocal. Eventually the patient's serum Powassan IgM enzyme-linked immunosorbent assay (ELISA) came back positive, serum Powassan plaque reduction neutralization (PRNT) 2660 (positive control >320), CSF Powassan IgM ELISA positive and CSF Powassan PRNT 64 (positive control >320). Additional testing for co-infections was negative for Borrelia burgdorferi, Babesia microti and Anaplasma phagocytophilum. Additional testing for other endemic arboviruses was also negative for Eastern equine encephalitis virus, Western equine encephalitis virus and Saint Louis encephalitis virus.
Table 1.
Selected Laboratory Values.
| Test | Results |
|---|---|
| Cerebrospinal Fluid Studies | |
| Color/Appearance | Colorless, clear |
| Red Blood Cell Count | Tube 1 429 (Tube 4 69) |
| Nucleated Cell Count | 197 (Differential: 1% Polymorphonuclear cells, 81% Lymphocytes, 9% Monocytes, 9% Other (plasma cells) |
| Glucose | 60 |
| Protein | 121 |
| Gram Stain and Culture | No organisms on gram stain and no growth at 48 hours on culture |
| Enterovirus PCR | No DNA sequences detected |
| Herpes Simplex Virus I PCR | No DNA sequences detected |
| Herpes Simplex Virus II PCR | No DNA sequences detected |
| Varicella Zoster Virus PCR | No DNA sequences detected |
| Epstein-Barr Virus PCR | No DNA sequences detected |
| Cytomegalovirus PCR | No DNA sequences detected |
| Lyme IgM, IgG Western Blot | No bands detected |
| Cryptococcus Antigen | Negative |
| VDRL | Non Reactive |
| Powassan IgM Capture ELISA | Positive |
| Powassan PRNT | 64 (POS Control > 320) |
| Serum West Nile IgM and IgG | Ab: <0.90, <1.30 (negative) |
| Fungal Smear and Culture | Calcofluor White Stain without yeast or fungal elements identified; culture with no fungus isolated at 4 weeks |
| AFB Smear and Culture | No AFB isolated at 6 weeks |
| Pathology Review | “Heterogeneous population of lymphoid cells, plasmacytoid lymphocytes and plasma cells which may be reactive in nature, but couldn't exclude a lymphoproliferative process” |
| Eastern Equine Encephalitis Virus | Eastern Equine IgG <1:4 (Antibody Not Detected) |
| Antibody Panel, IFA | Eastern Equine IgM <1:4 (Antibody Not Detected) |
| Western Equine Encephalitis Virus | Western Equine IgG <1:4 (Antibody Not Detected) |
| Antibody Panel, IFA | Western Equine IgM <1:4 (Antibody Not Detected) |
| California Encephalitis Virus Antibody | California IgG <1:4 (Antibody Not Detected) |
| Panel, IFA | California IgM <1:4 (Antibody Not Detected) |
| St. Louis Encephalitis Virus Antibody | St. Louis IgG <1:4 (Antibody Not Detected) |
| Panel, IFA | St. Louis IgM <1:4 (Antibody Not Detected) |
| Serum Studies | |
| Powassan IgM Capture ELISA | Positive |
| Powassan PRNT | 2660 (POS Control > 320) |
| Lyme Reflex | 0.65 Index (negative) |
| Babesia microti IgM, IgG | <1:20, <1:64 (negative) |
| Blood Parasite Prep, Thick Smear for Blood Parasite | None seen |
| Anaplasma Phagocytophilium PCR | Not Detected |
| Anaplasma Phagocytophilium IgM, IgG | <1:20, <1:64 (negative) |
| Mycoplasma pneumonia IgM | 0.43 (negative) |
| Mycoplasma pneumonia IgG | 0.20 (negative) |
| Urine Legionella Antigen | Negative |
| Quantiferon Gold | Nil 0.094 (no preexisting immune response) |
| Mitogen Nil 0.790 (healthy immune response) | |
| Ag Nil -0.041 (negative for TB) | |
| Plasma Herpes I and II PCR | Negative |
| VZV PCR | Negative |
Abbreviations: PCR Polymerase Chain Reaction; CSF = Cerebral Spinal Fluid; VDRL = Venereal Disease Research Laboratory; ELISA = enzyme-linked immunosorbent assay; PRNT = Plaque Reduction Neutralization Test; IFA = Immunofluorescence Assay.
Fig. 1.
MRI Brain: T2/FLAIR white matter hyperintensity involving the deep and superficial periventricular white matter as well as the corpus callosum.
Over the next week the patient’s hospital course became complicated by deep vein thrombosis, pulmonary embolism and urinary tract infection prolonging her stay. On hospital day 19 the patient was transferred to the hospital’s acute inpatient rehabilitation unit. The patient underwent two weeks of intensive physical and occupational therapy and made excellent progress. She was discharged home under the care of her daughter.
Discussion and conclusions
During 2006–2015 a median of seven cases of Powassan neuroinvasive disease have been reported annually with numerous states reporting their first confirmed case; New Jersey [8], New Hampshire [9] and Connecticut [3] all reported their first case within the last five years. This patient represents the first confirmed case of Powassan neuroinvasive disease in the nearby state of Rhode Island. Perhaps the most important question raised by this development is what may account for the dramatic increase in Powassan neuroinvasive disease. There are several potential explanations for this phenomenon. One possibility would be increasing recognition and testing of POWV. Another possibility would be increasing prevalence of POWV among its reservoir – the white-tailed deer. A study published by Nofchissey et al. [10] provides convincing evidence for the latter; the prevalence of POWV serum antibodies in white-tailed deer was demonstrated to have increased from 25 to 63 percent in 2001–2004 to 82–91 percent in 2005–2008. This suggests an increase in the spread of POWV among its reservoir and by extension, an increase in transmissibility through its arthropod vector to humans.
The increase in reported rates of this emerging pathogen demands attention, as standard arbovirus panels do not test for POWV, and therefore it is the responsibility of the clinician to be able to identify the virus in patients from an endemic area with a clinically compatible syndrome. Our patient’s presentation bears a strong resemblance to previous case reports [1,4,11] published in the literature. For example, the patient resided in an endemic area, reported tick exposure and presented with fever, altered sensorium, seizures and focal neurological deficits. The patient’s serum Powassan IgM and Powassan PRNT fulfilled the diagnostic criteria for Powassan neuroinvasive disease as set forth by the CDC [[12], [13], [14]]; although the CSF was not positive serology alone is considered sufficient to make the diagnosis. The patient’s brain MRI demonstrated nonspecific T2/FLAIR hyperintensities involving the superficial and deep white matter, and actually differed from the majority of prior case studies which demonstrated involvement of the deep gray matter [1,4,11]. Finally, the patient’s CSF studies were noted to have lymphocytic pleocytosis, elevated protein and normal glucose [1,4,11]. Overall, while there were no distinguishing features to suggest a diagnosis of Powassan neuroinvasive disease specifically, there were certainly enough distinguishing features to suggest a diagnosis of a viral encephalitis. This highlights the importance of assessing for POWV in all such patients from an endemic area. [4]
Like most other viral encephalitides there is no effective treatment for Powassan neuroinvasive disease. Prior case studies have attempted treatment with steroids [4], intravenous immunoglobulin [4] and ribavirin/interferon [14] although with mixed results. Among the various pathogens transmitted by Ixodes scapularis, POWV is unique in that it requires as little as 15 minutes of tick attachment to its host for successful transmission [15]. On the contrary, Borrelia burgdorferi, Babesia microti and Anaplasma phagocytophilum require 12–48 h [15]. Such rapid transmission reinforces the importance of prevention when journeying to endemic areas. Travelers should be encouraged to use permethrin-treated clothing and/or adapt the habit of showering to remove nonattached ticks [1].
Conclusion
The past decade has seen an increase in the reported cases of Powassan neuroinvasive disease. Over the same period there has been a notable increase in seroconversion among POWV’s white tailed deer reservoir, potentially explaining at least a part of this difference. The increase in incidence of Powassan neuroinvasive disease in number and across state lines underscores the importance of requesting focused testing for POWV beyond that offered by standard arbovirus panels in suggestive patients. Once infection is underway, treatment is largely supportive, highlighting the importance of prevention.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and
accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of
this journal on request.
Availability of data & material
All relevant data is published in Table 1.
Competing interest
All authors report no conflict of interest with this case report.
Funding
There were no sources of funding for this case report.
Authors’ contributions
Dr. Kavin Patel is the primary author and was responsible for drafting the manuscript. Dr. Kavin Patel, Dr. Jennie Johnson, Dr. Ioannis Zacharioudakis, Dr. Jerrold Boxerman, Dr. Timothy Flanigan and Dr. Rebecca Reece all made substantial contributions to the analysis and interpretation of data as well as revision of the intellectual content.
Acknowledgments
We would like to thank Diane Brady, Michael Gosciminski and Daniela Quilliam from the Center for Acute Infectious Diseases and Epidemiology at the Rhode Island Department of Health for their contributions.
Contributor Information
Kavin M. Patel, Email: kavin.patel@lifespan.org.
Jennie Johnson, Email: Jennie.Johnson@lifespan.org.
Ioannis M. Zacharioudakis, Email: Ioannis.Zacharioudakis@lifespan.org.
Jerrold L. Boxerman, Email: JBoxerman@lifespan.org.
Timothy P. Flanigan, Email: TFlanigan@lifespan.org.
Rebecca M. Reece, Email: RReece@lifespan.org.
References
- 1.Hermance M.E., Thangamani S. Powassan virus: an emerging arbovirus of public health concern in North America. Vector-Borne Zoonotic Dis. 2017;17(7):453–462. doi: 10.1089/vbz.2017.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC Outbreak of Powassan encephalitis—Maine and Vermont, 1999–2001. Morb Mortal Wkly Rep. 2018 https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5035a4.htm . Published 2001 [Accessed 21 March 2018] [PubMed] [Google Scholar]
- 3.Tutolo J.W., Staples J.E., Sosa L., Bennett N. Notes from the field: Powassan virus disease in an infant—Connecticut, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(15):408. doi: 10.15585/mmwr.mm6615a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piantadosi A., Rubin D.B., McQuillen D.P., Hsu L., Lederer P.A., Ashbaugh C.D. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis. 2015;62(6):707–713. doi: 10.1093/cid/civ1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Center for Disease Control . 2018. Lifecycle of blacklegged ticks.https://www.cdc.gov/lyme/transmission/blacklegged.html . Published 2017 [Accessed 21 March 2018] [Google Scholar]
- 6.Stafford K.C., Williams S.C. 2014. Deer, ticks and lyme disease – deer management as a strategy for the reduction of lyme disease.http://www.ct.gov/caes/lib/caes/documents/publications/fact_sheets/entomology/deer_&_ticks_fact_sheet.pdf [Google Scholar]
- 7.CDC . 2018. Statistics & maps.https://www.cdc.gov/powassan/statistics.html . Published 2017 [Accessed 21 March 2018] [Google Scholar]
- 8.New Jersey Deptartment of Health . 2017. Powassan virus.http://www.state.nj.us/health/cd/documents/faq/powassan_faq.pdf [Accessed 21 March 2018] [Google Scholar]
- 9.Division of Public Health Services . 2018. NH department of health and human services urges residents to take precautions against multiple diseases carried by ticks.https://www.dhhs.nh.gov/media/pr/2017/05162017-tick-season.htm . Published 2017 [Accessed 21 March 2018] [Google Scholar]
- 10.Nofchissey R.A., Deardorff E.R., Blevins T.M., Anishchenko M., Bosco-Lauth A., Berl E. Seroprevalence of Powassan virus in New England deer, 1979–2010. Am J Trop Med Hyg. 2013;88(6):1159–1162. doi: 10.4269/ajtmh.12-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raval M., Singhal M., Guerrero D., Alonto A. Powassan virus infection: case series and literature review from a single institution. BMC Res Notes. 2012;5(1):1. doi: 10.1186/1756-0500-5-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC . 2018. Clinical & lab evaluations.https://www.cdc.gov/powassan/clinicallabeval.html . Published 2015 [Accessed 21 March 2018] [Google Scholar]
- 13.CDC . CDC–U.S. Dept of Health and Human Services; 2018. Tickborne illnesses of the united states.https://www.cdc.gov/ticks/tickbornediseases/index.html . Published 2017 [Accessed 21 March 2018] [Google Scholar]
- 14.El Khoury M.Y., Camargo J.F., White J.L., Backenson B.P., Dupuis A.P., II, Escuyer K.L. Potential role of deer tick virus in powassan encephalitis cases in lyme disease-endemic areas of New York, U.S.A. Emerg Infect Dis. 2013;19(12):1926–1933. doi: 10.3201/eid1912.130903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebel G.D., Kramer L.D. Short report: duration of tick attachment required for transmission of powassan virus by deer ticks. Am J Trop Med Hyg. 2004;71(3):268–271. [PubMed] [Google Scholar]