Abstract
The environmental toxin β-N-methylamino-L-alanine (BMAA) has been proposed to contribute to neurodegenerative diseases. We have previously shown that neonatal exposure to BMAA results in dose-dependent cognitive impairments, proteomic alterations and progressive neurodegeneration in the hippocampus of adult rats. A high BMAA dose (460 mg/kg) also induced intracellular fibril formation, increased protein ubiquitination and enrichment of proteins important for lipid transport and metabolism. The aim of this study was therefore to elucidate the role of neuronal lipids in BMAA-induced neurodegeneration. By using matrix assisted laser desorption/ionization imaging mass spectrometry (MALDI IMS), we characterized the spatial lipid profile in the hippocampus of six month-old rats that were treated neonatally (postnatal days 9–10) with 460 mg/kg BMAA. Multivariate statistical analysis revealed long-term changes in distinct ganglioside species (GM, GD, GT) in the dentate gyrus. These changes could be a consequence of direct effects on ganglioside biosynthesis through the b-series (GM3-GD3-GD2-GD1b-GT1b) and may be linked to astrogliosis. Complementary immunohistochemistry experiments towards GFAP and S100β further verified the role of increased astrocyte activity in BMAA-induced brain damage. This highlights the potential of imaging MS for probing chemical changes associated with neuropathological mechanisms in situ.
Keywords: Imaging mass spectrometry, Beta-N-methylamino-L-alanine (BMAA), Environmental toxin, Protein pathology, Gliosis, Glycosphingolipids
1. Introduction
The environmental toxin β-N-methylamino-L-alanine (BMAA) is suggested as a possible risk factor for neurodegenerative diseases [1–3]. BMAA is produced by cyanobacteria, diatoms and dinoflagellates in various aquatic, marine and terrestrial habitats. As BMAA may be present in mussels, crustaceans and fish, the sources of human exposure to BMAA may be more profuse than previously estimated [4,5]. The neurotoxin has also been found in the brains of Alzheimer’s disease and amyotrophic lateral sclerosis patients, while other researchers have failed to detect BMAA in human brain [6–9].
BMAA is a glutamate receptor agonist, which may induce neuronal degeneration via excitotoxic mechanisms. BMAA may also induce oxidative stress or noncanonical translation and lead to mis-incorporation of the beta-amino acid into protein sequences [10–12]. The uptake of BMAA in discrete brain regions of rodent fetuses and neonates is more efficient and selective than in the brain of adults [13–16]. Furthermore, neonatal exposure at postnatal day (PND) 9–10 results in dose-dependent learning and memory impairments, proteomic alterations and progressive neurodegeneration in the hippocampus of adult rats [16–18]. Developmental exposure to BMAA (150 mg/kg) induced decreased expression of proteins involved in energy metabolism and intracellular signaling in the adult hippocampus. Exposure to a higher dose (460 mg/kg) also induced altered expression of S100β, histones, calcium and calmodulin-binding proteins as well as guanine nucleotide-binding proteins. At this dose; neuronal degeneration, astrogliosis, intracellular fibril formation, and increased protein ubiquitination were evident in the hippocampus of some of the animals [17,19]. Laser microdissection of tissue samples from the affected hippocampal regions was performed and followed by LC–MS/MS based proteomic analysis. The results of those studies revealed an enrichment of proteins known to form protein inclusions in the cornus ammonis region 1 (CA1) of the hippocampus. In addition, the proteomic study demonstrated an enrichment of brain lipid binding protein (FABP7) and several annexins, which are essential for lipid transport and metabolism. These results suggests a prominent role of neuronal lipid species in BMAA neuropathology [17]. Hence, further studies of biochemical BMAA effects in the hippocampus are needed in order to understand the mechanisms of BMAA-induced hippocampal proteopathy and the associated neurobehavioral alterations.
Imaging mass spectrometry (IMS) is an emerging technique for probing the molecular architecture of biological tissues. The technique allows direct and comprehensive identification and spatial profiling of biomolecular species in situ, while maintaining high molecular specificity [20,21]. In particular, MALDI based IMS has been demonstrated to be a powerful approach for spatial profiling of neuropeptides [22], small proteins [23] and specifically lipids and glycolipids in complex biological tissues [24–26], which can be useful in toxicological research [27].
The aim of this study was to determine long-term changes in hippocampal lipid expression in adult rats neonatally exposed to BMAA. MALDI IMS was used in conjunction with multivariate statistical analysis tools for molecular histology-based segmentation of anatomical features and to identify regional alterations in neuronal lipid profiles in BMAA-induced neurodegeneration.
2. Materials and methods
2.1. Chemicals and reagents
β-N-Methylamino-L-alanine (L-BMAA) hydrochloride (≥97%) was obtained from Sigma-Aldrich (St. Louis, MO, USA). TissueTek optimial cutting tool (OCT) was purchased from Sakura Finetek (AJ Alphen aan den Rijn,The Netherlands). All other chemicals were of pro-analysis grade and purchased from Sigma Aldrich (St. Louis, MO, USA). Water was purified with a Milli-Q (Millipore, Bedford, MA, USA) purification system.
2.2. Experimental design
The experimental design and the BMAA dose examined were identical to those previously reported to induce a suit of short- and long-term changes including cognitive impairments in adult animals [18,28–31]. Briefly, pregnant outbred Wistar rats were obtained from Scanbur BK AB (Sollentuna, Sweden). The dams were housed alone in a standard macrolon cage containing wood-chip bedding and nesting material. The animals were maintained on standard pellet food (R36 Labfor; Lantmännen, Kimstad, Sweden) and water ad libitum, and were housed in a temperature- and humidity-controlled environment on a 12-h light/dark cycle. The litters were cross-fostered at the day of birth, PND 0. The male pups were given one daily sc injection (20 μl/g) of BMAA 460 mg/kg (corresponding to 600 mg/kg BMAA HCl) freshly dissolved in Hanks’ balanced salt solution, or vehicle, for 2 days on PND 9–10 (Suppl. Fig. S1). The animal experimental protocol was approved by the Uppsala Animal Ethical Committee and was consistent with the Swedish Legislation on Animal Experimentation (Animal Welfare Act SFS1998:56) and the European Union Directive on the Protection of Animals Used for Scientific Purposes (2010/63/EU).
2.3. Sample preparation
For the current study, brains from 4 BMAA exposed animals and 3 saline controls were collected. The animals were sacrificed by decapitation at 23 weeks of age, the brains were quickly frozen in dry ice and stored at −80 °C. Coronal cryosections were obtained at −18 °C on a cryostate microtome (Leica,CM1520, Leica Biosystems, Wetzlar, Germany) from the right hemisphere of all animals at the level of hippo-campus (−3.36 mm relative to the bregma). The brain sections (12 μm) were thaw mounted on cooled conductive glass slides suitable for MALDI IMS analysis (indium tin oxide, ITO, Bruker Daltonics, Bremen, Germany). The tissue sections were defrosted for 20 min under reduced pressure in a desiccator. Prior to matrix application, the sections were scanned in a Mirax desk slide scanner (Carl Zeiss, Jena, Germany). Using an automated nebulizer sprayer (ImagePrep, Bruker Daltonics, Bremen, Germany), a matrix solution with an initial concentration of 10 mg/ml 2,5 di-hydroxy benzoic acid (DHB, Sigma-Aldrich) dissolved in 50% acetonitrile (ACN) and 0.2% trifluoroacetic acid (TFA) was applied onto the tissue section. The DHB default program was used for matrix application with matrix thickness, incubation and wetness specifications set to default. The application program comprised 5 phases with a total of 50 spraying cycles and finished at a plateaued sensor signal that increased 1.9 V from offset, indicating total reflection and full matrix coverage, respectively. The corresponding final amount of deposited matrix was 220 μg/cm2.
2.4. MALDI imaging mass spectrometry
Data sequence preparation, data acquisition and visualization was performed using the FlexImaging software (v 3.0, Bruker Daltonics). Mass spectrometry data (mass range of 400–3000 Da) were acquired on an Ultraflextreme MALDI TOF/TOF instrument (Bruker Daltonics) in reflector negative mode. Five hundred laser shots per pixel were acquired at 1 kHz repetition rate with laser focus setting set to medium. The pixel to pixel distance, i.e. spatial resolution, was 70 μm.
2.5. Data analysis
All spectra were RMS (root mean square) normalized and calibrated externally using the batch-processing function in Flex Analysis (v 3.0, Bruker Daltonics). Calibration spectra were obtained from calibrant solution spots (Protein Calibration Mix 1, Bruker Daltonics) that were placed adjacent to the tissue slides.
Multivariate image analysis of the collected data was performed by Principal Component Analysis (PCA) and hierarchical clustering (bisecting k-means) based image segmentation for identifying anatomical regions based on their chemical profile (Fig. 1A–C). PCA and cluster analysis was done in SciLS (v2014, SciLS GmbH, Bremen, Germany). From the loading values, the most influential variables (i.e. m/z peaks) were used to outline the borders of the anatomical regions of the hippocampal formation, i.e. dentate gyrus (DG), CA1 and CA2/CA3 (CA2/3) (Fig. 1D–G). These were assigned using the implemented region of interest (ROI) feature in Flex Imaging. RMS normalized average spectra of the annotated ROIs were exported as *.csv files in FlexImaging. Binning analysis was performed in order to reduce the data significantly and to account for slight sifts in m/z values as typically observed over the whole tissue area. Here, an initial step comprised peak picking using the peak analyzer function in the origin software (v. 8.1 OriginLab, Northhampton, MA, USA). The ROI data from all animals were imported into Origin and peaks were detected on average spectra of each ROI (DG, CA1, CA2/3) for each sample. The determined bin borders for peak integration were exported and utilized for area under curve (AUC) peak integration within each bin (peak-bin) of all ROI average spectra using an in-house developed R script.
Fig. 1.
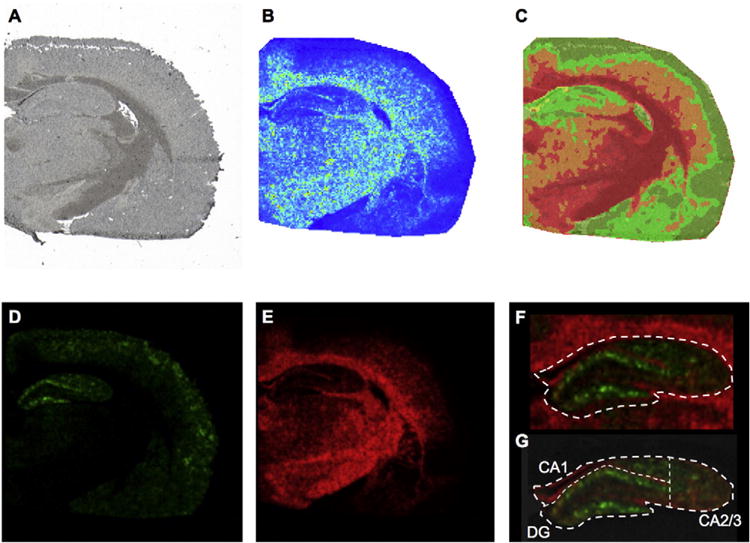
Multivariate Image analysis. (A) High resolution wide field micrograph showing an overview of a half coronal rat brain section used for MALDI IMS analysis. (B) Image of Principal Component 1 (PC1) obtained from Principal Component Analysis (PCA) of the MALDI IMS data. Principal components were used in order to outline the borders of anatomical regions, based on the highest variance in the data set. (C) Segmentation map obtained from multivariate image analysis of the same datadata. Bisecting k-means based clustering analysis identified pseudo-objects (clusters) of mass spectra signals representative of white matter, gray matter and inclusions in the CA1 molecular layer of hippocampus (yellow). (D, E) Correlation of the segmentation map MS data to the full MS revealed molecular ions localizing to the distinctive anatomical regions including (D) ganlioside GM1 (d18:1/20:0, green, m/z 1572.9) and (E) sulfatide ST(d18:1/24:1, red, m/z 888.5). (F) Based on the single ions, identified through segmentation and PCA, the hippocampus was outlined as grey matter region from the adjacent fiber tracts of the corpus callosum, as outlined by the sulfatide signal (ST d18:1/24:1, red). (G) Subregions in the hippocampus were annotated subsequently. Here, the DG ROI was outlined based on the molecular layer of the DG as highlighted by the GM1 d18:1/20:0 (m/z 1572.9) signal. The adjacent, CA1 that was in turn dorsally confined by the white matter and laterally defined by the molecular layer of the DG. The remaining lateral CA region was annotad as CA2 and CA3 ROI.
Peak area values from all animals of the control- and 460 mg/kg BMAA-group, were evaluated for all three ROIs in a multivariate manner by Orthogonal Projection to Latent Structures by Partial Least Squares–Discriminant Analysis (OPLS-DA) using SIMCA (v. 14.0, Umetrics, Umeå, Sweden). OPLS-DA is an extension to the Partial Least Squares regression method that uses the group information coded in a binary matrix Y to decompose the systematic variation in X matrix into correlated to Y (predictive) between-class and uncorrelated to Y (orthogonal) within-class variations [32]. This separation between the predictive and orthogonal components facilitate a straightforward interpretation of the predictive loading vector and provides a direct measure of the influence each of the variables has in the model. The number of relevant components for the models was estimated through cross-validation (CV), by exclusion of one randomized sample for each CV round. The S-plot was used to visualize the variable influence in the predictive component of the OPLS-DA models. These plots combines the covariance (p[1]) and correlation (p(corr) [1]) loading profiles obtained in a projection-based model in a scatter plot. In essence, it displays the contribution and reliability each of the model variables has based on the component scores. The S-plot was further combined with the loading vector plot (p [1]) at 99% confidence intervals, in order to obtain additional information about the variability and significance of detected molecular species. Subsequent statistical analysis of individual lipid signals and comparisons between the groups were performed with unpaired, two tailed t-test (p < 0.05).
2.6. Immunohistochemistry
Double-antigen fluorescent immunohistochemistry was conducted on selected brain sections using rabbit anti-S100-β (1:200, Abcam, Cambridge, UK) in combination with mouse anti-GFAP (1:500, Millipore, Temecula, CA, USA) as primary antibodies. The secondary antibodies anti-rabbit-Alexa Fluor 488 (1:200, Invitrogen, Carlsbad, CA) and anti-mouse-Alexa Fluor 555 (1:200, Invitrogen, Carlsbad, CA) were used for visualization. Cell nuclei were stained by DAPI (4,6-diamidino-2-phenylindole). Sections incubated without primary antibody (only the antibody diluent, 2.5% horse serum in phosphate buffered saline, in the primary antibody step) served as negative controls.
3. Results and discussion
3.1. MALDI imaging reveals distinct localization of molecular species in the hippocampal formation
The acquired image data were investigated using unsupervised multivariate statistics in order to identify general clusters of lipids localizing to distinct anatomical regions (segmentation) and outline the anatomical regions. Both PCA and hierarchical clustering analysis (bisecting k-means) was employed [33,34]. PCA identified borders between main anatomical regions, including various features of hippocampus (Fig. 1B, Suppl. Fig. S2A–C). Inspection of the PCA loading data allowed to identify the variables (i.e. m/z peaks) that contributed most to the variance as observed in the corresponding score image (Fig. 1B). Sulfatide (ST) species (m/z 806–892) were found to contribute mostly to the high PC score values and outline the white matter (Suppl Fig. S2A–C). Variables that were not associated with white matter regions as expressed by a negative score in that component were in turn associated with the hippocampus and the molecular layer of the hippocampus, respectively. This included e.g. GM1 ganglioside species (m/z 1544–1575) (Suppl Fig. S2A). While PCA is characterized by superior contrast compared to clustering based image analysis, it does not allow for further segmentation of potential subregions. This was in turn facilitated by clustering analysis based image segmentation and revealed pseudo-objects corresponding to the grey and white matter as well as the hippocampus (Fig. 1C, Suppl. Fig. S2D) and its subregions. For that, the anatomical structures were assigned as individual regions of interest and correlated to the processed full MS data, in order to identify the associated chemical species that allowed image segmentation of the regions. Here molecular species that contributed most to variation and hence are characteristically localized to these anatomical regions could be identified [34]. In detail, ganglioside GM1 (d18:1/20:0, m/z 1572.9) outlining the dentate gyrus of the hippocampus and sulfatide ST(d18:1/24:1, m/z 888.5) localizing to the adjacent white matter regions allowed distinct annotation of the hippocampus (Fig. 1D–F). From that, subregions of the hippocampus, including the CA1, CA2/3 and DG could be precisely outlined (Fig. 1G). This facilitated subsequent extraction of the ROI spectral data for statistical comparison of the animal groups. These findings highlight the potential of this unbiased segmentation approach for elucidating the chemical architecture of complex biological tissues.
3.2. Multivariate statistical analysis identifies BMAA induced molecular changes in the hippocampus
For statistical analysis of imaging MS spectral data, we have employed a previously reported bottom up strategy based on data extracted from outlined anatomical regions of interest [23,35,36]. OPLS-DA was performed in order to examine neuronal lipid chemistry and metabolic differences between BMAA-exposed and control animals in the defined subregions of hippocampus, the CA1, CA2/3 and DG. All extracted spectral data were log2 transformed and Pareto scaled, generating a data matrix for each of the subregions. Models for each of the three hippocampal sub regions allowed separation of controls and BMAA-treated rats (RX2 cumulative CA1: 0,75; CA2/3: 0,97; DG: 0,94), with strong cross validation matrices (Q2 cumulative CA1:0,67; CA2/3:0,94; DG:0,91) as indicated in the corresponding score plots (Fig. 2A–C, Suppl. Fig. S3). The S-plot (Fig. 2D–F) and vector plot (Suppl. Fig. S4) for the loading values for each of the models revealed significant neurochemical changes associated with BMAA-exposure. Inspection of the loading data and subsequent univariate statistics revealed significant changes in distinct ganglioside species with various number of sialic acid residues in their head group, including monosialo- (GM), di-sialo-(GD) and tri-sialo-(GT) gangliosides (Fig. 3A–F, Suppl. Fig. S5).
Fig. 2.
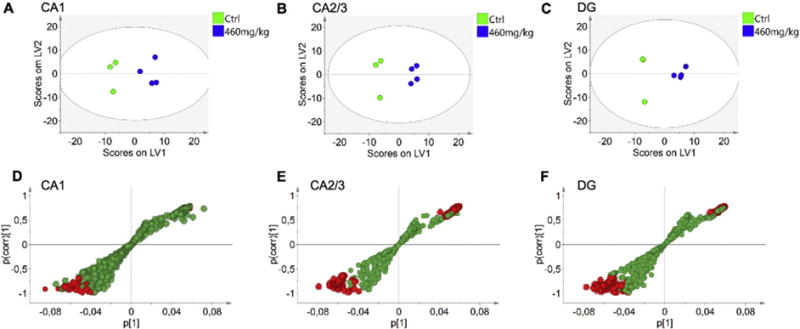
Multivariate analysis of spectral data reveals changes in hippocampus of BMAA exposed animals. OPLS-DA models were calculated for three regions of interest, CA1, CA2/3 and DG, using three control and four BMAA exposed animals. (A) For the CA1 region a 1 + 2 (1 predictive + 2 orthogonal components) was obtained. The model explained 75% of the variation in the data set (R2X cumulative) with predictive power of 0.67 (Q2 cumulative). (B) For CA2/3, a 1 + 2 model was calculated which explained 97% variation (R2X cumulative), with a predictive power of 0.94 (Q2 cumulative). The analysis of the DG resulted in a 1 + 2 model that explained 98% variation with a predictive power of 0.91 (C). Two of the animals in this region displayed similar score vectors (see Suppl. Fig. S3). S-plots of the OPLS-DA models allow for visualization of the magnitude (covariance, p [1]) and reliability (correlation, p(corr) [1]) of loading profiles with respect to the component scores in the models. In each of the regions, (D) CA1, (E) CA2/3, (F) DG, the red highlighted circles represent variables that display high magnitude and high reliability with respect to the predictive component scores. These can be identified as putative biomarkers of the two groups, based on 99% confidence interval (see Suppl. Fig. S4).
Fig. 3.
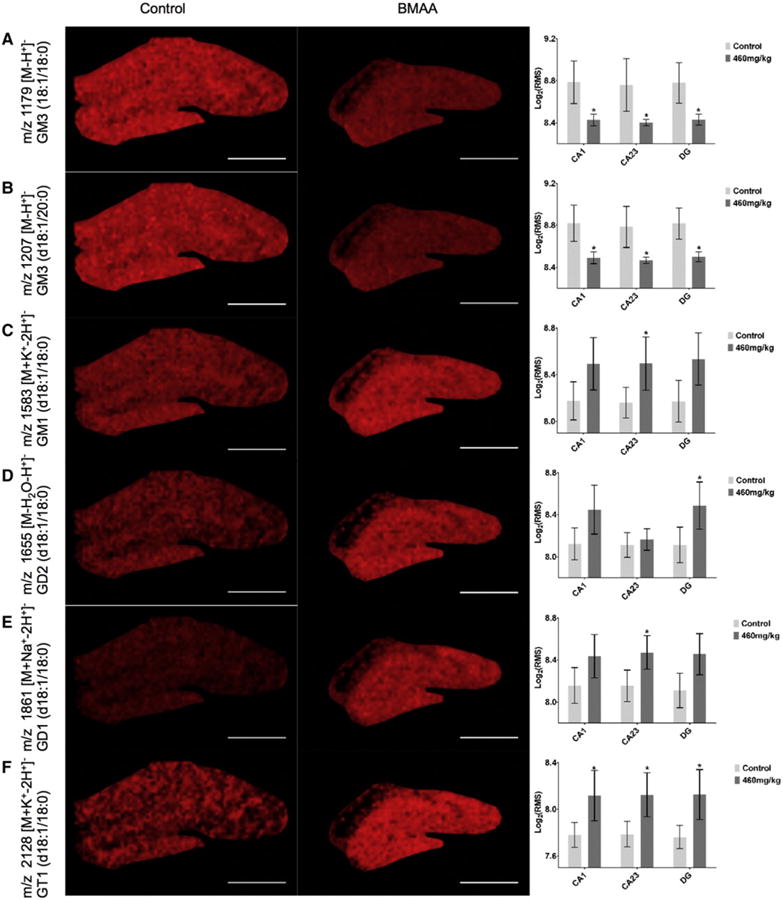
Alterations in lipid distribution as result of BMAA exposure. Discriminant analysis of differences between the control and 460 mg/kg BMAA exposed animals, revealed significant differences between the groups. Statistical analysis revealed m/z peaks that were significantly increased or decreased in the hippocampal regions as illustrated in the corresponding single ion images and boxplots, where the y-axis indicates log2 transformed bin intensity value retrieved from the RMS normalized m/z peak data. (A) GM3 (d18:1/18:0) m/z 1179 [M-H]− and (B) GM3 (d18:1/20:0) m/z 1207 [M-H]− were found to be significantly decreased in the entire hippocampus of BMAA exposed animals compared to controls. In contrast, GM1 (d18:1/18:0) m/z 1583 [M + K-2H]− (C), GD1 (d18:1/18:0) m/z 1861 [M + Na-2H]− (E) were significantly elevated in the CA2/3 -, and GD2 (d18:1/18:0) m/z 1655 [M-H2O-H]− in the DG-region (D). GT1 (d18:1/18:0) m/z 2128 [M + K-2H]− was elevated in all three ROI of the hippocampus (F). *p < 0.05, Scalebar 1 mm.
3.3. BMAA induces changes in hippocampal ganglioside levels
Although the study was limited to seven, unevenly distributed (3 controls and 4 BMAA 460 mg/kg) animals the discriminant analysis as well as univariate statistics (t-test, p < 0.05) revealed significant differences of peak signals between the two groups. GM3 ((18:1/18:0) [M-H]− 1179) (Fig. 3A) and GM3 ((d18:1/20:0) [M-H]− 1207) (Fig. 3B) were found to be significantly decreased in all ROI of the hippocampus (CA1, CA2/3, DG) in the BMAA exposed group as compared to controls. In contrast, GM3 metabolites including GM1 ((d18:1/18:0) [M + K-2H]− 1583) (Fig. 3C), GD1 ((d18:1/18:0) [M + Na-2H]− 1861) (Fig. 3E) were significantly elevated in the CA2/3 region, and GD2 ((d18:1/18:0) [M-H2O-H]− 1655) in the DG (Fig. 3D). GT1 ((d18:1/18:0) [M + K-2H]− 2128) was elevated in all three ROI of the hippocampus (Fig. 3F)
Gangliosides are glycosphingolipids consisting of mono- to polysialylated oligosaccharide chains linked to a ceramide unit. They are most abundant in the central nervous system (CNS) and typically localized in the outer layer of the plasma membrane with the ceramide moiety acting as an anchor and with the oligosaccharide chains exposed to the external environment [37]. Gangliosides are the main glycolipids of neuronal surfaces and their surface expression patterns are regulated by processes involving biosynthetic/catabolic steps and intracellular trafficking [38,39]. The ceramide residues, are synthesized in the endoplasmic reticulum, while glycosylation of these residues takes place in the Golgi and trans-Golgi network [38]. The observed effects on hippocampal ganglioside levels in rats neonatally treated with BMAA can be a consequence of direct effects on the ganglioside biosynthesis through the b-series (GM3-GD3-GD2-GD1b-GT1b) as well as through the a-series GM3-GM2-GM1 (Suppl. Fig. S5) [39]. Gangliosides are involved in numerous cellular functions, such as signal transduction, cell proliferation and differentiation, cell death, cell-cell recognition and adhesion [40]. They are particularly involved in brain development and maturation [41,42] and are thought to play a role in processes such as neuritogenesis [43], axon generation and stability [44], synaptic transmission [45], memory formation [46], and neurodegeneration [47–49]. The current findings illustrate the power of imaging MS for elucidating the biological importance of ganglioside expression. Few methods exist for the study of ganglioside distribution in biological samples. Some antibodies are available to visualize gangliosides with different constituent of oligosaccharides, but immunological methods cannot detect differences in the hidden ceramide structure [25]. IMS in turn has the capability to analyse several ganglioside species simultaneously. The key strength of IMS compared to common imaging techniques is the ability to provide spatial localization of chemical species together with quantitative estimates as particularly illustrated previously for ganglioside species in the brain [24–26]. Similarly, the distribution of various gangliosides in rodent brain has been reported in other recent studies [50–52]. Here, a characteristic increase of cortical GM2 and GM3 levels was reported in a Hunter’s disease (HD) mouse model [53]. HD is however a lysosomal storage disorder, similar to Tay-Sach’s-, Sandhoff’s- and Nickman Pick’s disease, which differs quite significantly in brain pathology and is characterized by impaired ganglioside degradation and intraneuronal accumulation, which explains the discrepancy to our findings.
3.4. Immunohistochemistry confirms astroglial involvement in BMAA-induced neurodegeneration
In addition to the diverse biological functions mentioned above, gangliosides are involved in the modulation of the immune system [40]. They are suggested to play an important role in astroglial cell differentiation and activation [54–56]. In an in vitro study, gangliosides have been shown to stimulate astroglial cell proliferation, where GT1b was identified to be the most potent species [55], which is well in line with the results obtained in the present study (Fig. 3). Indeed, complementally immunohistochemistry experiments targeting GFAP and S100β revealed a marked upregulation of these proteins not only in the histopathologically altered CA1 but also in DG, confirming an ongoing astrogliosis in the hippocampus of BMAA-exposed animals (Fig. 4). Taken together these data suggest that increased ganglioside metabolism as indicated by increased downstream products is a consequence of neurotoxin induced proteopathy, neurodegeneration and gliosis, respectively.
Fig. 4.
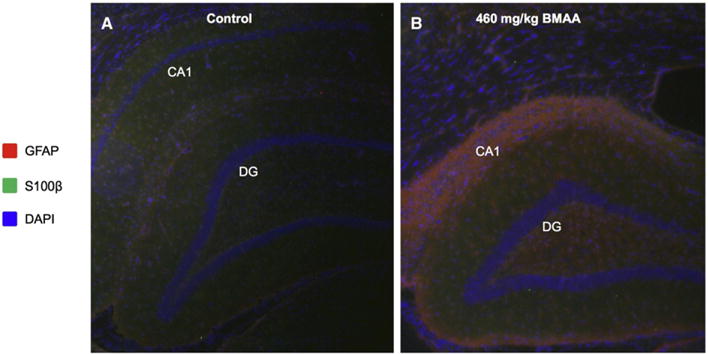
Immunohistochemistry reveals glial activation in the adult hippocampus upon neonatal BMAA exposure. Double antigen staining was performed against GFAP and S100β on hippocampal sections of control (A) and BMAA exposed animals (B). The data show massive gliosis and reactive astrocyte species localizing to the histopathologically altered region in the hippocampus of BMAA treated rats. This was indicated by increased GFAP immune-reactivity (red) in the CA1 and DG of BMAA exposed animals (B) compared to controls (A). GFAP positive cells were also found to stain positively for S100β serving as additional marker for astrocyte activation (green, B). Magnification: lens = ×10.
4. Conclusions
The present study demonstrates the potential of MALDI imaging for interrogating the biochemical architecture of CNS tissue and cells. It also shows that MALDI Imaging is a promising technique for spatial studies of molecular alterations underlying neurotoxin induced brain pathology. The long-term changes in ganglioside expression patterns in the adult hippocampus of animals neonatally treated with BMAA may indicate the mechanism underlying the observed cognitive impairments and neuropathology.
Supplementary Material
Acknowledgments
The Swedish research council VR (#2014-6447, JH), FORMAS (OK), the Royal Society of Arts and Sciences in Gothenburg (KVVS, JH), Carl Tryggers Stiftelse (OK), Alzheimerfonden (JH), Demensfonden (JH), Jeanssons Stiftelsen (JH), Ahlén Stiftelsen (JH), Stiftelsen Gamla Tjänarinnor (JH), Stohnes Stiftelse (JH) and Stiftelsen Wilhelm and Martina Lundgrens Vetenskapsfond (JH) are acknowledged for financial support.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bbapap.2016.12.004.
Footnotes
This article is part of a Special Issue entitled: MALDI Imaging, edited by Dr. Corinna Henkel and Prof. Peter Hoffmann.
The authors declare no conflict of interest.
References
- 1.Banack SA, Cox PA. Biomagnification of cycad neurotoxins in flying foxes: implications for ALS-PDC in Guam. Neurology. 2003;61:387–389. doi: 10.1212/01.wnl.0000078320.18564.9f. [DOI] [PubMed] [Google Scholar]
- 2.Spencer PS, Nunn PB, Hugon J, Ludolph AC, Ross SM, Roy DN, Robertson RC. Guam amyotrophic lateral sclerosis-parkinsonism-dementia linked to a plant excitant neurotoxin. Science. 1987;237:517–522. doi: 10.1126/science.3603037. [DOI] [PubMed] [Google Scholar]
- 3.Cox PA, Davis DA, Mash DC, Metcalf JS, Banack SA. Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proc Biol Sci. 2016;283 doi: 10.1098/rspb.2015.2397. http://dx.doi.org/10.1098/rspb.2015.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang L, Kiselova N, Rosen J, Ilag LL. Quantification of neurotoxin BMAA (beta-N-methylamino-L-alanine) in seafood from Swedish markets. Sci Rep. 2014;4:6931. doi: 10.1038/srep06931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lage S, Annadotter H, Rasmussen U, Rydberg S. Biotransfer of beta-N-methylamino-L-alanine (BMAA) in a eutrophicated freshwater lake. Mar Drugs. 2015;13:1185–1201. doi: 10.3390/md13031185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murch SJ, Cox PA, Banack SA. A mechanism for slow release of biomagnified cyanobacterial neurotoxins and neurodegenerative disease in Guam. Proc Natl Acad Sci U S A. 2004;101:12228–12231. doi: 10.1073/pnas.0404926101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pablo J, Banack SA, Cox PA, Johnson TE, Papapetropoulos S, Bradley WG, Buck A, Mash DC. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer’s disease. Acta Neurol Scand. 2009:216–225. doi: 10.1111/j.1600-0404.2008.01150.x. [DOI] [PubMed] [Google Scholar]
- 8.Snyder LR, Cruz-Aguado R, Sadilek M, Galasko D, Shaw CA, Montine TJ. Lack of cerebral bmaa in human cerebral cortex. Neurology. 2009;72:1360–1361. doi: 10.1212/WNL.0b013e3181a0fed1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montine TJ, Li K, Perl DP, Galasko D. Lack of beta-methylamino-l-alanine in brain from controls, AD, or Chamorros with PDC. Neurology. 2005;65:768–769. doi: 10.1212/01.wnl.0000174523.62022.52. [DOI] [PubMed] [Google Scholar]
- 10.Copani A, Canonico PL, Catania MV, Aronica E, Bruno V, Ratti E, van Amsterdam FT, Gaviraghi G, Nicoletti F. Interaction between beta-N-methylamino-L-alanine and excitatory amino acid receptors in brain slices and neuronal cultures. Brain Res. 1991;558:79–86. doi: 10.1016/0006-8993(91)90716-9. [DOI] [PubMed] [Google Scholar]
- 11.Lobner D, Piana PM, Salous AK, Peoples RW. Beta-N-methylamino-L-alanine enhances neurotoxicity through multiple mechanisms. Neurobiol Dis. 2007;25:360–366. doi: 10.1016/j.nbd.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao SD, Banack SA, Cox PA, Weiss JH. BMAA selectively injures motor neurons via AMPA/kainate receptor activation. Exp Neurol. 2006;201:244–252. doi: 10.1016/j.expneurol.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Smith QR, Nagura H, Takada Y, Duncan MW. Facilitated transport of the neurotoxin, beta-N-methylamino-L-alanine, across the blood-brain barrier. J Neurochem. 1992;58:1330–1337. doi: 10.1111/j.1471-4159.1992.tb11346.x. [DOI] [PubMed] [Google Scholar]
- 14.Karlsson O, Berg C, Brittebo EB, Lindquist NG. Retention of the cyanobacterial neurotoxin beta-N-methylamino-l-alanine in melanin and neuromelanin-containing cells—a possible link between Parkinson-dementia complex and pigmentary retinopathy. Pigment Cell Melanoma Res. 2009;22:120–130. doi: 10.1111/j.1755-148X.2008.00508.x. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson O, Jiang L, Ersson L, Malmstrom T, Ilag LL, Brittebo EB. Environmental neurotoxin interaction with proteins: dose-dependent increase of free and protein-associated BMAA (beta-N-methylamino-L-alanine) in neonatal rat brain. Sci Rep. 2015;5:15570. doi: 10.1038/srep15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlsson O, Lindquist NG, Brittebo EB, Roman E. Selective brain uptake and behavioral effects of the cyanobacterial toxin BMAA (beta-N-methylamino-L-alanine) following neonatal administration to rodents. Toxicol Sci. 2009;109:286–295. doi: 10.1093/toxsci/kfp062. [DOI] [PubMed] [Google Scholar]
- 17.Karlsson O, Berg AL, Hanrieder J, Arnerup G, Lindstrom AK, Brittebo EB. Intracellular fibril formation, calcification, and enrichment of chaperones, cytoskeletal, and intermediate filament proteins in the adult hippocampus CA1 following neonatal exposure to the nonprotein amino acid BMAA. Arch Toxicol. 2015;89:423–436. doi: 10.1007/s00204-014-1262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlsson O, Roman E, Berg AL, Brittebo EB. Early hippocampal cell death, and late learning and memory deficits in rats exposed to the environmental toxin BMAA (beta-N-methylamino-L-alanine) during the neonatal period. Behav Brain Res. 2011;219:310–320. doi: 10.1016/j.bbr.2011.01.056. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson O, Berg AL, Lindstrom AK, Hanrieder J, Arnerup G, Roman E, Bergquist J, Lindquist NG, Brittebo EB, Andersson M. Neonatal exposure to the cyanobacterial toxin BMAA induces changes in protein expression and neurodegeneration in adult hippocampus. Toxicol Sci. 2012;130:391–404. doi: 10.1093/toxsci/kfs241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caprioli RM, Farmer TB, Gile J. Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Anal Chem. 1997;69:4751–4760. doi: 10.1021/ac970888i. [DOI] [PubMed] [Google Scholar]
- 21.Hanrieder J, Phan NT, Kurczy ME, Ewing AG. Imaging mass spectrometry in neuroscience. ACS Chem Neurosci. 2013;4:666–679. doi: 10.1021/cn400053c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanrieder J, Ljungdahl A, Falth M, Mammo SE, Bergquist J, Andersson M. L-DOPA-induced dyskinesia is associated with regional increase of striatal dynorphin peptides as elucidated by imaging mass spectrometry. Mol Cell Proteomics. 2011;10:M111 009308. doi: 10.1074/mcp.M111.009308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanrieder J, Ekegren T, Andersson M, Bergquist J. MALDI imaging of post-mortem human spinal cord in amyotrophic lateral sclerosis. J Neurochem. 2013;124:695–707. doi: 10.1111/jnc.12019. [DOI] [PubMed] [Google Scholar]
- 24.Colsch B, Jackson SN, Dutta S, Woods AS. Molecular microscopy of brain gangliosides: illustrating their distribution in hippocampal cell layers. ACS Chem Neurosci. 2011;2:213–222. doi: 10.1021/cn100096h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colsch B, Woods AS. Localization and imaging of sialylated glycosphingolipids in brain tissue sections by MALDI mass spectrometry. Glycobiology. 2010;20:661–667. doi: 10.1093/glycob/cwq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods AS, Colsch B, Jackson SN, Post J, Baldwin K, Roux A, Hoffer B, Cox BM, Hoffer M, Rubovitch V, Pick CG, Schultz JA, Balaban C. Gangliosides and ceramides change in a mouse model of blast induced traumatic brain injury. ACS Chem Neurosci. 2013;4:594–600. doi: 10.1021/cn300216h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karlsson O, Hanrieder J. Imaging mass spectrometry in drug development and toxicology. Arch Toxicol. 2016 doi: 10.1007/s00204-016-1905-6. http://dx.doi.org/10.1007/s00204-016-1905-6. [DOI] [PMC free article] [PubMed]
- 28.Karlsson O, Roman E, Brittebo EB. Long-term cognitive impairments in adult rats treated neonatally with beta-N-Methylamino-L-Alanine. Toxicol Sci. 2009;112:185–195. doi: 10.1093/toxsci/kfp196. [DOI] [PubMed] [Google Scholar]
- 29.Engskog MK, Karlsson O, Haglof J, Elmsjo A, Brittebo E, Arvidsson T, Pettersson C. The cyanobacterial amino acid beta-N-methylamino-l-alanine perturbs the intermediary metabolism in neonatal rats. Toxicology. 2013;312:6–11. doi: 10.1016/j.tox.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Karlsson O, Jiang L, Andersson M, Ilag LL, Brittebo EB. Protein association of the neurotoxin and non-protein amino acid BMAA (beta-N-methylamino-L-alanine) in the liver and brain following neonatal administration in rats. Toxicol Lett. 2014;226:1–5. doi: 10.1016/j.toxlet.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 31.Karlsson O, Kultima K, Wadensten H, Nilsson A, Roman E, Andren PE, Brittebo EB. Neurotoxin-induced neuropeptide perturbations in striatum of neonatal rats. J Proteome Res. 2013;12:1678–1690. doi: 10.1021/pr3010265. [DOI] [PubMed] [Google Scholar]
- 32.Bylesjö M, Rantalainen M, Cloarec O, Nicholson JK, Holmes E, Trygg J. OPLS discriminant analysis: combining the strengths of PLS-DA and SIMCA classification. J Chemom. 2007;20:341–351. [Google Scholar]
- 33.Jones EA, Deininger SO, Hogendoorn PCW, Deelder AM, McDonnell LA. Imaging mass spectrometry statistical analysis. J Proteomics. 2012;75:4962–4989. doi: 10.1016/j.jprot.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Deininger SO, Ebert MP, Fuetterer A, Gerhard M, Roecken C. M.A.L.D.I. Imaging, Combined with hierarchical clustering as a new tool for the interpretation of complex human cancers. J Proteome Res. 2008;7:5230–5236. doi: 10.1021/pr8005777. [DOI] [PubMed] [Google Scholar]
- 35.Hanrieder J, Gerber L, Sandelius A Persson, Brittebo EB, Ewing AG, Karlsson O. High resolution metabolite imaging in the hippocampus following neonatal exposure to the environmental toxin BMAA using ToF-SIMS. ACS Chem Neurosci. 2014;5:568–575. doi: 10.1021/cn500039b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanrieder J, Malmberg P, Lindberg OR, Fletcher JS, Ewing AG. Time-of-flight secondary ion mass spectrometry based molecular histology of human spinal cord tissue and motor neurons. Anal Chem. 2013;85:8741–8748. doi: 10.1021/ac401830m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Echten G, Sandhoff K. Ganglioside metabolism. Enzymology, topology, and regulation. J Biol Chem. 1993;268:5341–5344. [PubMed] [Google Scholar]
- 38.Sandhoff K, Harzer K. Gangliosides and gangliosidoses: principles of molecular and metabolic pathogenesis. J Neurosci Off J Soc Neurosci. 2013;33:10195–10208. doi: 10.1523/JNEUROSCI.0822-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu RK, Tsai YT, Ariga T, Yanagisawa M. Structures, biosynthesis, and functions of gangliosides—an overview. J Oleo Sci. 2011;60:537–544. doi: 10.5650/jos.60.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potapenko M, Shurin GV, de Leon J. Gangliosides as immunomodulators. Adv Exp Med Biol. 2007;601:195–203. doi: 10.1007/978-0-387-72005-0_20. [DOI] [PubMed] [Google Scholar]
- 41.Yu RK, Macala LJ, Taki T, Weinfield HM, Yu FS. Developmental changes in ganglioside composition and synthesis in embryonic rat brain. J Neurochem. 1988;50:1825–1829. doi: 10.1111/j.1471-4159.1988.tb02484.x. [DOI] [PubMed] [Google Scholar]
- 42.Kotani M, Terashima T, Tai T. Developmental changes of ganglioside expressions in postnatal rat cerebellar cortex. Brain Res. 1995;700:40–58. doi: 10.1016/0006-8993(95)00923-e. [DOI] [PubMed] [Google Scholar]
- 43.Prinetti A, Iwabuchi K, Hakomori S. Glycosphingolipid-enriched signaling domain in mouse neuroblastoma neuro2a cells. Mechanism of ganglioside-dependent neuritogenesis. J Biol Chem. 1999;274:20916–20924. doi: 10.1074/jbc.274.30.20916. [DOI] [PubMed] [Google Scholar]
- 44.Schnaar RL. Brain gangliosides in axon-myelin stability and axon regeneration. FEBS Lett. 2010;584:1741–1747. doi: 10.1016/j.febslet.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramirez OA, Gomez RA, Carrer HF. Gangliosides improve synaptic transmission in dentate gyrus of hippocampal rat slices. Brain Res. 1990;506:291–293. doi: 10.1016/0006-8993(90)91264-h. [DOI] [PubMed] [Google Scholar]
- 46.Rahmann H. Brain gangliosides and memory formation. Behav Brain Res. 1995;66:105–116. doi: 10.1016/0166-4328(94)00131-x. [DOI] [PubMed] [Google Scholar]
- 47.Haughey NJ, Bandaru VV, Bae M, Mattson MP. Roles for dysfunctional sphingolipid metabolism in Alzheimer’s disease neuropathogenesis. Biochim Biophys Acta. 2010;1801:878–886. doi: 10.1016/j.bbalip.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohmi Y, Tajima O, Ohkawa Y, Mori A, Sugiura Y, Furukawa K. Gangliosides play pivotal roles in the regulation of complement systems and in the maintenance of integrity in nerve tissues. Proc Natl Acad Sci U S A. 2009;106:22405–22410. doi: 10.1073/pnas.0912336106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Posse de Chaves E, Sipione S. Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS Lett. 2010;584:1748–1759. doi: 10.1016/j.febslet.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 50.Weishaupt N, Caughlin S, Yeung KK, Whitehead SN. Differential anatomical expression of ganglioside GM1 species containing d18:1 or d20:1 sphingosine detected by MALDI imaging mass spectrometry in mature rat brain. Front Neuroanat. 2015;9:155. doi: 10.3389/fnana.2015.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Wang J, Liu J, Han J, Xiong S, Yong W, Zhao Z. Combination of ESI and MALDI mass spectrometry for qualitative, semi-quantitative and in situ analysis of gangliosides in brain. Sci Rep. 2016;6:25289. doi: 10.1038/srep25289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kitakaze K, Mizutani Y, Sugiyama E, Tasaki C, Tsuji D, Maita N, Hirokawa T, Asanuma D, Kamiya M, Sato K, Setou M, Urano Y, Togawa T, Otaka A, Sakuraba H, Itoh K. Protease-resistant modified human beta-hexosaminidase B ameliorates symptoms in GM2 gangliosidosis model. J Clin Invest. 2016;126:1691–1703. doi: 10.1172/JCI85300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dufresne M, Guneysu D, Patterson NH, Marcinkiewicz MM, Regina A, Demeule M, Chaurand P. Multimodal detection of GM2 and GM3 lipid species in the brain of mucopolysaccharidosis type II mouse by serial imaging mass spectrometry and immunohistochemistry. Anal Bioanal Chem. 2016 doi: 10.1007/s00216-016-0076-x. http://dx.doi.org/10.1007/s00216-016-0076-x. [DOI] [PubMed]
- 54.Facci L, Skaper SD, Favaron M, Leon A. A role for gangliosides in astroglial cell differentiation in vitro. J Cell Biol. 1988;106:821–828. doi: 10.1083/jcb.106.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katoh-Semba R, Facci L, Skaper SD, Varon S. Gangliosides stimulate astroglial cell proliferation in the absence of serum. J Cell Physiol. 1986;126:147–153. doi: 10.1002/jcp.1041260120. [DOI] [PubMed] [Google Scholar]
- 56.Skaper SD, Facci L, Rudge J, Katoh-Semba R, Manthorpe M, Varon S. Morphological modulation of cultured rat brain astroglial cells: antagonism by ganglioside GM1. Brain Res. 1986;390:21–31. doi: 10.1016/0165-3806(86)90148-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


