Abstract
Acute otitis media affects 700 million people each year with children being disproportionately affected relative to adults. Group A streptococcus is a pathogen implicated in a broad array of human pathology. It is, however, a rare cause of acute otitis media and neuroinvasive disease in older adults with only 2–3 cases occurring per year in the United States. We describe two such cases from a single institution in Rhode Island in 2017. The clinical presentation, neuroimaging and management are reviewed. The mechanism of intracranial spread may have involved dehiscence of the bony tegmen of the roof of the middle ear cavity.
Keywords: Invasive Group A streptococcus, Complicated acute otitis media, Meningitis
Introduction
Acute otitis media (AOM) is a well-described infectious process affecting the middle ear cavity. AOM affects over 700 million people around the world each year; children tend to be disproportionately affected relative to adults with estimates of global incidence peaking at 61 percent in ages 1–4 and dropping to 1 percent in ages 35–44 [1]. The most commonly implicated organisms in adults are Streptococcus pneumoniae and nontypeable Haemophilus influenza [2]. Streptococcus pyogenes, the Group A streptococcus (GAS) is a rare cause of AOM in adults and only slightly more frequent in children, but when involved is more likely to present as a complicated infection relative to other bacteria [3]. Most episodes of AOM tend to be uncomplicated but may become complicated based on unique host and bacterial attributes. These complications can be subdivided by location into infratemporal and extratemporal; notable examples include mastoiditis and meningitis/central nervous system disease (the latter referred to as neuroinvasive disease) respectively [3]. In this case series we describe two rare presentations of GAS AOM complicated by neuroinvasive disease occurring in adults from Rhode Island in 2017. The clinical presentation, neuroimaging and management are reviewed.
Case 1
A 68-year-old previously healthy male presented to the emergency department with right ear pain/drainage and photophobia, first starting to feel unwell with malaise about one week prior to admission. The initial vitals upon arrival to emergency department were – temperature 99.7 F (few hours later 103.4 F), pulse 102, respiratory rate 18 and oxygen saturation 97% on ambient air. Physical examination revealed an ill-appearing elderly male. The right external auditory canal was noted to have serosanguinous drainage; this was suctioned after which the tympanic membrane (TM) was visualized to be draining purulent material concerning for perforation. Neck exam was remarkable for nuchal rigidity. Neurological exam was nonfocal apart from decreased hearing on the right. A diffuse macular erythematous rash was noted across the face, chest, back and extremities. Labs were notable for a while blood cell count of 11 200 cells/liter (90% segmented neutrophils). A computed tomography (CT) scan of the brain demonstrated opacification of the bilateral maxillary sinuses, right mastoid air cells and right middle ear. The right ear drainage was cultured and the patient was started on empiric treatment with intravenous (IV) vancomycin 15 mg/kg every 12 h, IV ceftriaxone 2 g every 12 h and IV clindamycin 900 mg every 8 h.
The patient underwent a lumbar puncture; cerebrospinal fluid (CSF) studies were notable for a nucleated cell count 9768 (96% polymorphonuclear leukocytes), glucose 26 mg/dL and protein 280 mg/dL. Gram stain was positive for gram positive cocci although CSF culture was negative. The right ear fluid culture eventually grew pansensitive beta hemolytic GAS after which antimicrobials were narrowed to IV penicillin 4 million units every 4 h. The patient continued to spike fevers prompting a brain MRI to search for intracranial complications of acute otitis media. This demonstrated enhancement of the right internal auditory canal (Fig. 1A), right mastoiditis with adjacent dural enhancement (Fig. 1B), and debris within occipital horns of bilateral lateral ventricles consistent with ventriculitis (Fig. 1C). The patient underwent microscopic ear cleaning where he was found to have pulsatile clear otorrhea concerning for a CSF leak. A dedicated temporal bone CT that demonstrated middle ear opacification consistent with otitis media (Fig. 1D) and a possible small dehiscence of the right tegmen (Fig. 1E). The pulsatile otorrhea continued necessitating placement of a lumbar drain for one week. The patient made a good recovery and was discharged home from the hospital on day 15 with a six-week course of IV penicillin G 4 million units every 4 h (to be administered by a home infusion company). The patient’s ear pain and drainage resolved and he remained neurologically nonfocal, except for sensineural hearing loss that persisted at his two-week follow-up.
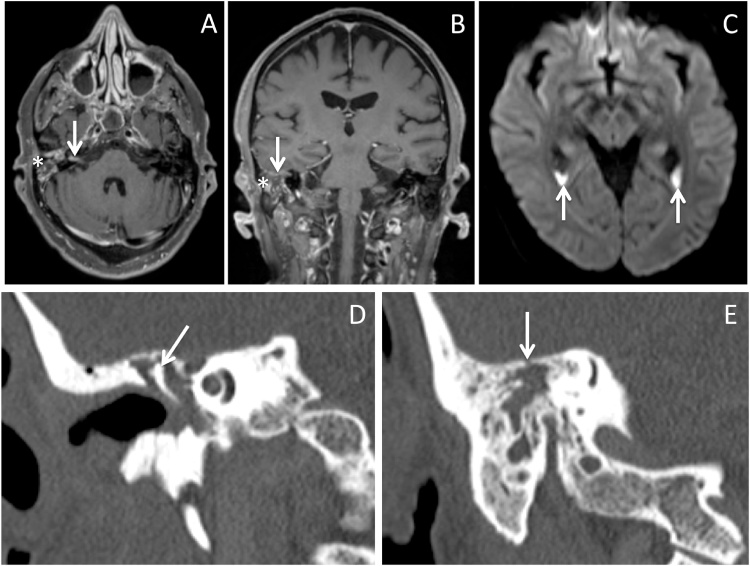
Fig. 1.
A) Enhancement of right mastoid air cell complex consistent with mastoiditis (asterisk), with enhancement in right internal auditory canal consistent with meningitis (arrow). B) Enhancement of right mastoid air cell complex consistent with mastoiditis (asterisk), with dural enhancement at floor of right middle cranial fossa consistent with meningitis (arrow). C) Layering pus with diffusion restriction in lateral ventricle occipital horns (arrows). D) Opacification of right middle ear cavity, surrounding the ossicular chain (arrow). E) Small focus of tegmen dehiscence (arrow).
Case 2
A 65-year-old male presented to the emergency department with right ear pain/drainage, headache, nausea/vomiting and subjective fever. The patient first started to feel unwell two weeks prior to admission. The patient’s past medical history was notable for hypertension, hyperlipidemia and non-insulin-dependent diabetes. The initial vitals upon arrival to the emergency department were – temperature 97.7 F, pulse 98, respiratory rate 20, blood pressure 167/75 and oxygen saturation 98% on ambient air. Physical exam revealed a male who was alert, oriented and not in acute distress. The right ear was noted to have purulent drainage. There were no meningeal signs. Neurological exam was nonfocal.
Initial labs were remarkable for a while blood cell count of 21 400 cells/liter (91% segmented neutrophils). CT brain was notable for right mastoid and middle ear opacification. The patient received morphine after which he rapidly deteriorated and became somnolent and disoriented. A lumbar puncture was performed; CSF studies were notable for a nucleated cell count 6150 (96 percent polymorphonuclear leukocytes), glucose <10 mg/dL and protein 658 mg/dL. The patient was empirically started on IV vancomycin 15 mg/kg every 8 h, IV ceftriaxone 2 g every 12 h and IV ampicillin 2 g every 4 h as well as IV dexamethasone 10 mg every 6 h. The patient was intubated for airway protection. The suspicion for intracranial complications remained high and so a brain MRI was obtained. This demonstrated right mastoiditis (Fig. 2A), tegmen dehiscence (Fig. 2B), adjacent focus of cerebritis (Fig. 2A and C) and dependent layering of pyogenic fluid within the lateral ventricles compatible with ventriculitis (Fig. 2D). The patient was given mannitol and a ventriculostomy was placed. The patient was admitted to the intensive care unit.
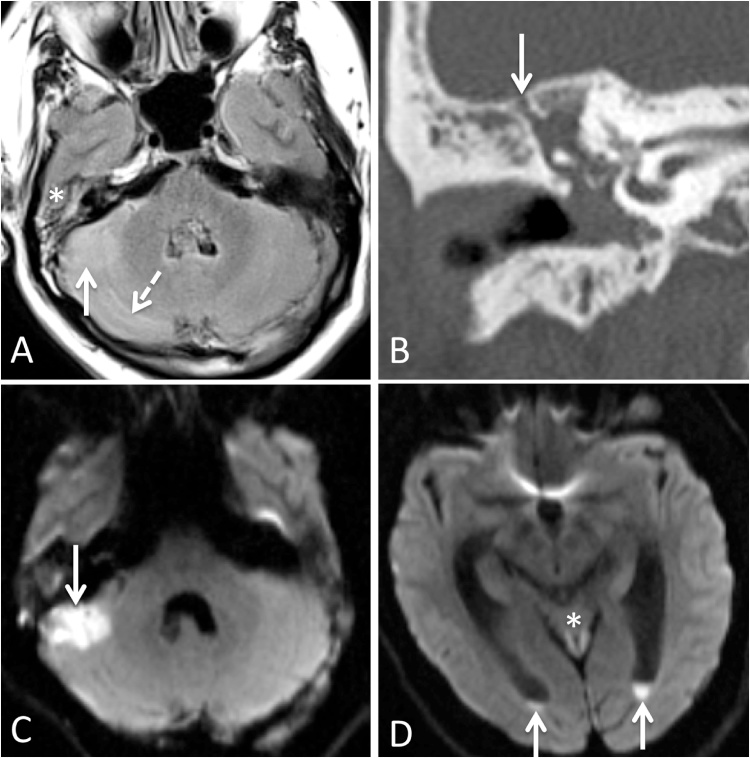
Fig. 2.
A) FLAIR hyperintense signal in right mastoid air cells (asterisk) consistent with mastoiditis and in lateral cerebellum (arrow) consistent with encephalitis, with sulcal FLAIR hyperintensity consistent with meningitis (dashed arrow). B) Opacification of right middle ear cavity, surrounding the ossicular chain with a small focus of tegmen dehiscence (arrow). C) Diffusion restriction in right lateral cerebellum (arrow) consistent with encephalitis. D) Layering pus with diffusion restriction in lateral ventricle occipital horns (arrows), with diffusion restriction in superior cerebellar vermis consistent with encephalitis (asterisk).
The patient’s admission CSF and blood cultures grew beta-hemolytic GAS. Antimicrobials were deescalated to IV penicillin G 4 million units every 4 h and ofloxacin ear drops were also started. A dedicated CT temporal bone was obtained that demonstrated right mastoid air cell opacification and right middle ear opacification with focal dehiscence of the bony tegmen of the roof of the right middle ear cavity (Fig. 2A). A right-sided myringotomy tube was placed for decompression. The patient was eventually extubated. The patient’s mental status had improved; he was noted to be alert, oriented and neurologically nonfocal. The extraventricular drain was removed after a successful clamp trial (previously had been draining 220–380 mL/day). The patient made a good recovery and was discharged from the hospital on day 11 to a skill nursing facility with a six-week course of IV penicillin G 4 million units every 4 h. He was briefly readmitted for vertical diplopia felt to be from natural progression of his cerebritis and ventriculitis, and then readmitted again for pyelonephritis felt to be from urinary retention. The patient was noted to be doing well at his two-month follow-up.
Discussion
We report here two cases of AOM from GAS progressing to neuroinvasive disease occurring in older adults. GAS is a well-described pathogen implicated in a broad array of human disease, however it is a rare cause of AOM and neuroinvasive disease. A study by Beek et al reported cases of acute bacterial meningitis in adults between 1987 and 2000 in the Netherlands and found the incidence of GAS meningitis to be at 0.03 cases per 100,000 population [4]. A more recent study by Nelson et al detailing the epidemiology of invasive GAS infections in the United States between 2005–2012 found a total 18 cases of GAS meningitis/CNS disease in adults ≥65 [5]. This translates to only 2–3 cases per year, clarifying the rarity of this bacterium in the pathogenesis of neuroinvasive disease among older adults [5].
Although we can never be completely sure why the two patients in this case study with AOM went onto develop neuroinvasive disease, we postulate the etiology is likely multifactorial. Firstly, there was a delay between onset of symptomatology and the initiation of antibiotics – about one week in the first patient and about two weeks in the second patient. This may have allowed the infection to propagate locally. Secondly, and more importantly, the CT temporal bone scan of both patients showed dehiscence of the bony tegmen of roof of the middle ear cavity. This bony defect may have paved way for the infection to spread from the middle ear and into the intracranial compartment, leading to neuroinvasive disease. Of course, there are other mechanisms of entry as well that would not require the existence of either of these predisposing factors. For example the middle ear infection may have also spread into the CNS through retrograde thrombophlebitis or extension along preformed anatomical pathways such as the oval and/or round windows [6].
It is important to be mindful of the potential for complications in AOM. Expert consultation was pursued as soon as it became apparent based upon imaging that the patients had both infratemporal and extratemporal complications. These were best addressed by otolaryngology and neurosurgery respectively. For example, in our second patient otolaryngology placed a tympanostomy tube for middle ear decompression and neurosurgery placed an extraventricular drain for CSF decompression. This multidisciplinary approach served well for the patients, both of whom were noted to be making good recoveries at their follow-up appointments, with sensineural hearing loss and vertical diplopia the only deficits registered. This highlights the importance of getting the appropriate consultants involved early on when complicated AOM is suspected.
Conclusion
GAS is a rare cause of AOM and neuroinvasive disease; only 2–3 cases are reported per year in the older adult population in the United States. The mechanism of spread may involve dehiscence of the bony tegmen of the roof of the middle ear cavity. Neuroimaging is useful to assess for infratemporal and extratemporal complications, and may serve as a guide on the appropriateness of expert consultation with otolaryngology and neurosurgery respectively. Prompt recognition of AOM complications and early involvement of a multidisciplinary care team may be beneficial in the overall care of these patients.
Funding
There were no sources of funding for this review article.
Consent
Written consent was obtained from both patients and is available upon request.
Conflict of interest
None of the authors report a conflict of interest with this study.
Authors’ contributions
Dr. Kavin Patel is the primary author and is responsible for drafting the manuscript. Dr. Gerard Nau is the principal investigator and made contributions to the analysis and interpretation of data. Dr. Jennie Johnson assisted with the overall direction of the project and was directly involved in the patient’s care. Dr. Jerold Boxerman made contributions to the analysis and interpretation of the radiology findings. All authors made contributions to the revision of the intellectual content.
Acknowledgements
We would like to thank Dr. Rebecca Reece as well as Bridget Teevan, Daniela Quilliam, Utpala Bandy and Karen Luther from the Rhode Island Department of Health for their contributions.
Contributor Information
Kavin M. Patel, Email: kavin.patel@lifespan.org.
Jennie E. Johnson, Email: jennie.johnson@lifespan.org.
Jerrold L. Boxerman, Email: JBoxerman@lifespan.org.
Gerard J. Nau, Email: Gerard_nau@brown.edu.
References
- 1.Monasta L., Ronfani L., Marchetti F., Montico M., Brumatti L., Bavcar A. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0036226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celin S.E., Bluestone C.D., Stephenson J., Yilmaz H.M., Collins J.J. Bacteriology of acute otitis media in adults. JAMA J Am Med Assoc. 1991;266(16):2249–2252. [PubMed] [Google Scholar]
- 3.Ramakrishnan K., Sparks R.A., Berryhill W.E. Diagnosis and treatment of otitis media. Am Fam Phys. 2007;76(11):1650–1658. [PubMed] [Google Scholar]
- 4.van de Beek D., De GJ Spanjaard L., Sela S., Vermeulen M., Dankert J. Group a streptococcal meningitis in adults: report of 41 cases and a review of the literature. Clin Infect Dis. 2002;34(9):e32–e36. doi: 10.1086/339941. doi:CID011000 [pii];10.1086/339941 [doi] [DOI] [PubMed] [Google Scholar]
- 5.Nelson G., Pondo T., Toews K., Farley M., Lindegren M., Lynfield R. Epidemiology of invasive group a streptococcal infections in the United States, 2005–2012. Clin Infect Dis. 2016;63(4):478–486. doi: 10.1093/cid/ciw248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geyik M.F., Kokoglu O.F., Hosoglu S., Ayaz C. Acute bacterial meningitis as a complication of otitis media and related mortality factors. Yonsei Med J. 2002;43(5):573–578. doi: 10.3349/ymj.2002.43.5.573. [DOI] [PubMed] [Google Scholar]