Abstract
Patients under consideration for lung transplantation as treatment for end-stage lung diseases such as idiopathic pulmonary fibrosis (IPF) often have risk factors such as a history of smoking or concomitant emphysema, both of which can predispose the patient to lung cancer. In fact, IPF itself increases the risk of lung cancer development by 6.8% to 20%. Solid organ malignancy (non-skin) is an established contraindication for lung transplantation. We encountered a clinical dilemma in a patient who presented with an IPF flare-up and underwent urgent evaluation for lung transplantation. After transplant, the patient's explanted lungs showed extensive adenocarcinoma in situ, with the foci of invasion and metastatic adenocarcinoma in N1-level lymph nodes, as well as usual interstitial pneumonia. Retrospectively, we saw no evidence to suggest malignancy in addition to the IPF flare-up. Clinical diagnostic dilemmas such as this emphasize the need for new noninvasive testing that would facilitate malignancy diagnosis in patients too sick to undergo invasive tissue biopsy for diagnosis. Careful pathological examination of explanted lungs in patients with IPF is critical, as it can majorly influence immunosuppressive regimens, surveillance imaging, and overall prognosis after lung transplant.
Keywords: Adenocarcinoma in situ, Lung transplant, Lung allocation score, Incidental tumors in lung explants, Lung explant pathology, Lung cancer in lung transplant recipients
Abbreviations
- AIS
adenocarcinoma in situ
- CT
computed tomography
- IPF
idiopathic pulmonary fibrosis
- ILD
interstitial lung disease
- LAS
Lung Allocation Score
- MRI
magnetic resonance imaging
- NSCLC
non–small cell lung cancer
- PET
positron emission tomography
- UIP
usual interstitial pneumonia
1. Introduction
Lung transplantation has gained acceptance as a modality for management of end-stage lung diseases such as idiopathic pulmonary fibrosis (IPF) and other interstitial lung diseases (ILDs), chronic obstructive pulmonary disease, cystic fibrosis, and pulmonary arterial hypertension [1]. The Lung Allocation Score (LAS), introduced in the United States in 2005, was instituted in an effort to identify patients in greater need for lung transplantation and to prioritize these patients for transplant [2]. The LAS system has decreased the average time patients spend on the waitlist pre-transplant; however, it has also resulted in sicker patients who have significantly increased supplemental oxygen needs undergoing transplant [2]. As a result, median age at the time of transplant has increased, and more patients are undergoing lung transplant for IPF and other ILDs [2].
Patients diagnosed with IPF and other ILDs frequently present in hypoxic respiratory failure with an acute disease flare-up, and most major transplant centers in the United States consider such patients for lung transplant [3]. However, imaging features associated with IPF/ILD occasionally make it difficult to differentiate between fibrotic foci and growing lung nodules. Ground-glass opacities, consolidation, or both further hinder clinicians' ability to detect underlying nodules or masses [4,5]. Patients on the transplant wait-list are often too sick to survive the recommended 2-year surveillance period for stability for indeterminate nodules, hence; clinicians often have a lower threshold to biopsy these patients. However, increased oxygen requirements and/or respiratory insufficiency often make invasive tissue diagnosis challenging due to the narrow window of transplantation and the risk of worsening respiratory failure from pneumothorax, which may make it impossible to oxygenate or ventilate patients [6].
Solid organ malignancy (non-skin) is a known contraindication to lung transplantation [7]. Several recent publications have described the incidence and management of unexpected neoplasms in explanted lungs between 0.8% and 2.2% [5,[8], [9], [10]]. Diagnosing an underlying malignancy is even more complicated in the setting of an IPF flare-up, given that IPF is a known risk factor for primary lung cancer, with a reported prevalence ranging from 6.8% to 20% (which is significantly higher than in the general population) [11]. We present an interesting clinical case in which extensive adenocarcinoma in situ (AIS) with foci of invasion on the explanted lungs was found in a lung transplant recipient who underwent transplant for a flare-up of IPF.
2. Case report
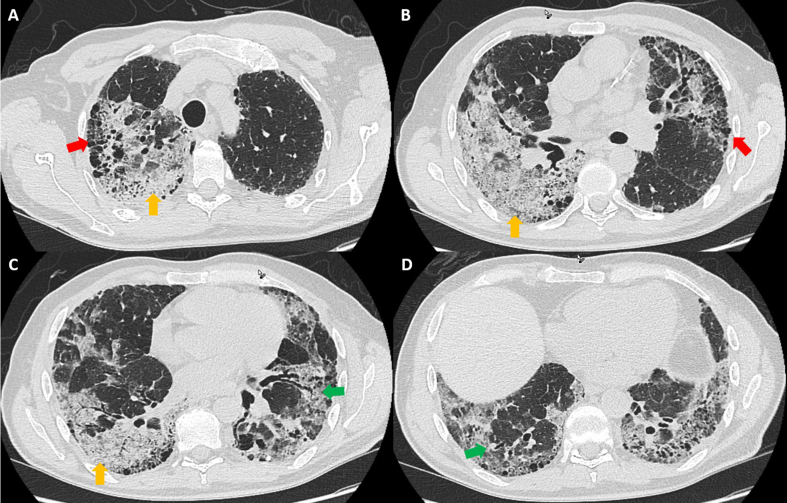
A 69-year-old white man diagnosed with IPF 5 years earlier was referred to our institution for consideration for lung transplant. Previous evaluation for autoimmune diseases was negative. He had a remote smoking history of 12 pack-years, and had quit smoking more than 20 years earlier. He was in robust clinical shape, with typical clinical stigmata of IPF, with a restrictive lung defect on pulmonary function tests (forced vital capacity: 39% predicted); severely reduced diffusion capacity (25% predicted); clubbed fingers; and coarse, leathery crackles. Desaturations were noted to 70% on his 6-minute walk test on 30 L of supplemental high-flow oxygen via nasal cannula. Computed tomography (CT) of the chest showed an interstitial lung process with an apical-to-basal gradient, with peripheral reticulation and honeycomb formation (Fig. 1), consistent with a usual interstitial pneumonia (UIP) pattern.
Fig. 1.
Computed tomogram of the chest in lung windows shows honeycombing (red arrows), subpleural reticulation (green arrows), and extensive consolidation (yellow arrows) that pathologically demonstrated organizing pneumonia with adenocarcinoma in situ with invasion on explant pathology.
The patient's chest CT also showed a bilateral, dense consolidative process involving both lower lobes of the lungs with imaging features consistent with superimposed pneumonia on the background UIP or a UIP flare-up (Fig. 1). He was admitted to the inpatient lung transplant service for evaluation for potential lung transplant candidacy. Evaluation for concurrent pneumonia, including blood and sputum cultures, urine Streptococcus pneumoniae and legionella antigens, serum mycoplasma serology, and viral respiratory polymerase chain reaction from nasopharyngeal swab were negative. Empiric antibiotics, which were originally initiated due to imaging features that triggered concern for a superimposed consolidative process, were de-escalated.
The patient ultimately underwent bilateral sequential lung transplantation off cardiopulmonary bypass (cytomegalovirus: donor +, recipient+) 2 weeks after initial presentation. Induction immunosuppression was initiated, with basiliximab on days 1 and 4. Post-transplant immunosuppression included tacrolimus, mycophenolate mofetil, and prednisone.
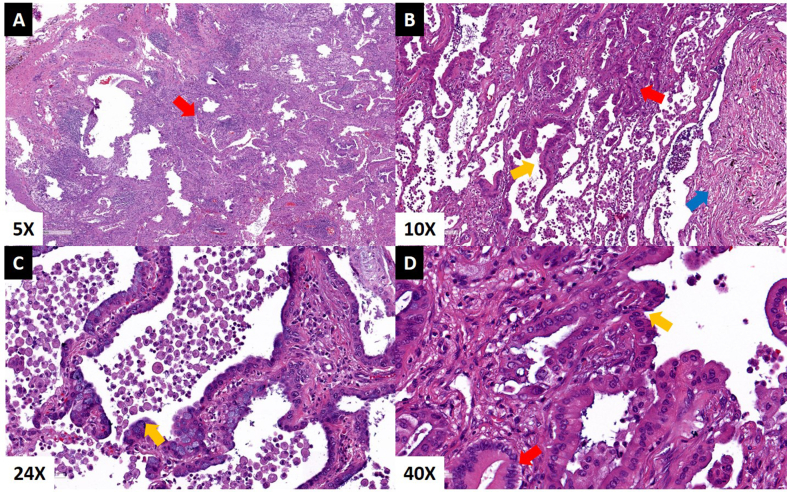
On day 3, the explanted lungs showed pathological evidence of UIP with subpleural fibrosis, foci of honeycomb formation, and organizing pneumonia. The lungs also showed a diffuse, non-mucinous, lepidic-predominant AIS with foci of microinvasion diffusely involving all lobes, measuring 19 cm on the right lung with negative resection margins and no evidence of tumor in resected lymph nodes (Fig. 2). The left lung showed a 13-cm non-mucinous AIS with multiple foci of invasion (largest invasive focus was 0.8 cm), with negative resection margins. Two of twelve left-sided lymph nodes removed at the time of transplant were positive for metastatic adenocarcinoma (N1 lymph nodes; largest metastatic focus was 2 mm). Although systematic staging of the mediastinum is not performed at the time of transplant, the most likely pathological stage based on available data consistent with bilateral T4 tumors (independent primaries) [12] with N1 disease on the left side was IIIA [12]. This clinical picture suggested two separate T4 lung adenocarcinomas in the background of diffuse AIS.
Fig. 2.
Hematoxylin and eosin stain of adenocarcinoma from explanted lungs. A] Invasive focus of well-differentiated adenocarcinoma (red arrow) B] Interface of adenocarcinoma in situ (yellow arrow) and invasive adenocarcinoma (red arrow) with evidence of fibrotic changes (blue arrow) C] Alveolar walls lined by neoplastic cells with focal cytoplasmic mucin without invasion consistent with adenocarcinoma in situ (yellow arrow) D] High-power view of the interface of invasive adenocarcinoma (red arrow) and adenocarcinoma in situ (yellow arrow).
The patient had an uneventful post-transplant course and was discharged home on postoperative day 9. Given the risk of metastatic spread of tumor foci due to ongoing immunosuppression, mycophenolate mofetil was discontinued and the patient was maintained on tacrolimus and prednisone-based immunosuppression. Staging magnetic resonance imaging (MRI) of the brain and positron emission tomography (PET) revealed no evidence of metastatic disease elsewhere in the body, and his clinical stage was therefore determined to be IIIA for the cancer on both sides. Further pathological testing for targeted therapy mutations revealed no evidence of mutations for EGFR, ALK, ROS-1, RET, BRAF and <1% expression of PD-L1. His case was discussed at Tumor Board, and serial CT/PET surveillance was recommended given the lack of active malignant disease and the lack of tumor mutations amenable to targeted chemotherapy. Retrospective imaging and pathology correlation revealed the presence of AIS/invasive adenocarcinoma in the consolidative processes in bilateral lungs in addition to IPF flare-up.
Surveillance imaging at 3 months revealed no fluorodeoxyglucose-avid lesions, and he was continued on tacrolimus- and prednisone-based immunosuppression. Five months after transplant, however, he presented to the emergency department with severe back pain. MRI of the spine and subsequent PET showed extensive metastasis to the spine and to the liver. He underwent palliative radiation to the spine and kyphoplasty, but due to severe uncontrolled pain, he elected to transition to hospice, and died 7 days later.
3. Discussion
The introduction of the LAS has resulted in more patients undergoing transplant for IPF and other ILDs [2], and the use of extracorporeal membrane oxygenation support for patients in respiratory failure expands the window during which patients with acute or chronic lung failure may undergo transplant [13]. Because of the LAS, lungs are preferentially allocated to those with a higher chance of imminent death while on the waitlist. Patients diagnosed with IPF or other ILDs are more likely to develop acute lung failure than patients with other lung diseases because patients with IPF or other ILDs are often diagnosed later in the course of the disease. This delay in diagnosis frequently stems from clinicians' inadequate understanding of the appropriate diagnostic workup and from the increased probability of end-stage acute or chronic lung failure secondary to a disease flare-up [3]. The median survival of patients diagnosed with IPF is 3 years [2], and this decreases to 13 months when patients with IPF have concomitant lung cancer [11]. Moreover, coexistence of a solid organ tumor (non-skin) is a contraindication for lung transplantation [7].
The most common histologic type of lung cancer in patients with IPF is non–small cell lung cancer (NSCLC)—predominantly adenocarcinoma, followed by squamous cell carcinoma [5,14,15]. Most of these tumors are found in the periphery of the lungs and the lower lobes [15]. The more common background CT patterns noted in such patients are subpleural fibrosis, reticular pattern, and honeycombing [15]. Furthermore, most lung cancers in patients with IPF are found within fibrotic regions, and some invade areas of honeycombing [14]. Although tissue sampling is of critical importance for diagnosis and staging of concomitant lung cancer, such sampling (whether percutaneous needle CT-guided biopsy, transbronchial needle aspiration or biopsy, or video-assisted thoracoscopic biopsy) can increase the risk of acute ILD exacerbation, significantly increasing mortality [15].
Until recently, opinions differed regarding whether patients with AIS (formerly referred to as bronchoalveolar cell carcinoma) should be considered for lung transplantation. de Perrot et al. [9] demonstrated that 5-year survival in lung transplant recipients with explanted lungs positive for AIS was correlated with the tumor stage (ie, 51% for stage I carcinoma, 14% for stage II and III carcinoma, and 23% for incidental multifocal AIS) [9]. Notably, 88% of patients with multifocal AIS in that study had tumor recurrence limited to the transplanted lung [9]. Similar 5-year survival in transplant recipients with stage I AIS has been reported by others [16]. Although respiratory failure secondary to AIS is considered to be an indication for lung transplant, a major challenge of diagnosing AIS is that the entire specimen of the tumor must be reviewed to ensure that no tissue invasion has occurred [17,18]. Ground-glass lesions are the typical presentation of AIS [19], but AIS is an unintuitive diagnosis in a patient with UIP/IPF because consolidation or extensive ground-glass opacities in these patients are commonly attributed to disease flare-up.
Adenocarcinoma in the explanted lungs drastically changed the way we managed this patient. Immunosuppression has been thought to predispose patients to early tumor recurrence, especially patients with ILD/IPF [5]. Post-transplant immunosuppressive medications such as calcineurin inhibitors have been linked to tumor progression, possibly due to various mechanisms, including disruption of apoptosis and DNA repair [20,21]. In this case, mycophenolate mofetil was discontinued and the patient was maintained on tacrolimus- and prednisone-based immunosuppression, with the intention of initiating an mTOR inhibitor such as everolimus 3 months post-transplant. mTOR inhibitors (eg, sirolimus and everolimus) have been found to decrease cancer progression in transplant patients [22,23].
This case offers some noteworthy learning points. The increased risk of primary lung malignancy in patients being considered for lung transplant must be acknowledged. NSCLC presenting as enlarged nodules or masses should be serially followed or biopsied, depending on the advancement of the ILD and the urgency for transplant. Patients who present with an ILD flare-up should undergo bronchoscopic lavage for cytology. However, the entire clinical picture may not always suggest ongoing malignancy—a fact illustrated by the present case, in which the patient had only a remote smoking history and had no ongoing weight loss or other signs that would suggest a concurrent malignant process. Although noninvasive blood-based tests have been approved to test tumor mutations for targeted therapy in NSCLC (especially adenocarcinoma), no noninvasive tests are available to facilitate diagnosis of malignancy. Further research into such blood-based and inhaled-breath testing would certainly aid in such challenging clinical situations, especially given the poor clinical outcomes in lung transplant recipients who develop recurrent cancer.
Disclosure statement
The authors declare no conflicts of interest regarding the publication of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Lund L.H., Khush K.K., Cherikh W.S. The registry of the international society for heart and lung transplantation: thirty-fourth adult heart transplantation Report-2017; focus theme: allograft ischemic time. J. Heart Lung Transplant. 2017;36(10):1037–1046. doi: 10.1016/j.healun.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Valapour M., Skeans M.A., Smith J.M. OPTN/SRTR 2015 annual data report: lung. Am. J. Transplant. 2017;17(Suppl 1):357–424. doi: 10.1111/ajt.14129. [DOI] [PubMed] [Google Scholar]
- 3.Travis W.D., Costabel U., Hansell D.M. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lidegran M.K., Ringertz H.G., Frenckner B.P., Linden V.B. Chest and abdominal CT during extracorporeal membrane oxygenation: clinical benefits in diagnosis and treatment. Acad. Radiol. 2005;12(3):276–285. doi: 10.1016/j.acra.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Panchabhai T.S., Arrossi A.V., Patil P.D. Unexpected neoplasms in lungs explanted from lung transplant recipients: a single-center experience and review of literature. Transplant. Proc. 2018;50(1):234–240. doi: 10.1016/j.transproceed.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Hutchinson J.P., Fogarty A.W., McKeever T.M., Hubbard R.B. In-hospital mortality after surgical lung biopsy for interstitial lung disease in the United States. 2000 to 2011. Am. J. Respir. Crit. Care Med. 2016;193(10):1161–1167. doi: 10.1164/rccm.201508-1632OC. [DOI] [PubMed] [Google Scholar]
- 7.Weill D., Benden C., Corris P.A. A consensus document for the selection of lung transplant candidates: 2014–an update from the pulmonary transplantation council of the international society for heart and lung transplantation. J. Heart Lung Transplant. 2015;34(1):1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Belli E.V., Landolfo K., Keller C., Thomas M., Odell J. Lung cancer following lung transplant: single institution 10 year experience. Lung Cancer. 2013;81(3):451–454. doi: 10.1016/j.lungcan.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 9.de Perrot M., Chernenko S., Waddell T.K. Role of lung transplantation in the treatment of bronchogenic carcinomas for patients with end-stage pulmonary disease. J. Clin. Oncol. 2004;22(21):4351–4356. doi: 10.1200/JCO.2004.12.188. [DOI] [PubMed] [Google Scholar]
- 10.Strollo D.C., Dacic S., Ocak I., Pilewski J., Bermudez C., Crespo M.M. Malignancies incidentally detected at lung transplantation: radiologic and pathologic features. AJR Am. J. Roentgenol. 2013;201(1):108–116. doi: 10.2214/AJR.12.9374. [DOI] [PubMed] [Google Scholar]
- 11.Ozawa Y., Suda T., Naito T. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology. 2009;14(5):723–728. doi: 10.1111/j.1440-1843.2009.01547.x. [DOI] [PubMed] [Google Scholar]
- 12.In Edge S.B., Byrd D.R., Carducci M.A., Compton C.C. seventh ed. Springer; New York: 2009. AJCC Cancer Staging Manual. [Google Scholar]
- 13.Todd E.M., Biswas Roy S., Hashimi A.S. Extracorporeal membrane oxygenation as a bridge to lung transplantation: a single-center experience in the present era. J. Thorac. Cardiovasc. Surg. 2017;154(5):1798–1809. doi: 10.1016/j.jtcvs.2017.06.063. [DOI] [PubMed] [Google Scholar]
- 14.Khan K.A., Kennedy M.P., Moore E. Radiological characteristics, histological features and clinical outcomes of lung cancer patients with coexistent idiopathic pulmonary fibrosis. Lung. 2015;193(1):71–77. doi: 10.1007/s00408-014-9664-8. [DOI] [PubMed] [Google Scholar]
- 15.Tomassetti S., Gurioli C., Ryu J.H. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest. 2015;147(1):157–164. doi: 10.1378/chest.14-0359. [DOI] [PubMed] [Google Scholar]
- 16.Yusen R.D., Edwards L.B., Dipchand A.I. The registry of the international society for heart and lung transplantation: thirty-third adult lung and heart-lung transplant Report-2016; focus theme: primary diagnostic indications for transplant. J. Heart Lung Transplant. 2016;35(10):1170–1184. doi: 10.1016/j.healun.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Behera M., Owonikoko T.K., Gal A.A. Lung adenocarcinoma staging using the 2011 IASLC/ATS/ERS classification: a pooled analysis of adenocarcinoma in situ and minimally invasive adenocarcinoma. Clin. Lung Cancer. 2016;17(5):e57–e64. doi: 10.1016/j.cllc.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y.P., Heuvelmans M.A., Zhang H., Oudkerk M., Zhang G.X., Xie X.Q. Changes in quantitative CT image features of ground-glass nodules in differentiating invasive pulmonary adenocarcinoma from benign and in situ lesions: histopathological comparisons. Clin. Radiol. 2018 May;73(5):504.e9–504.e16. doi: 10.1016/j.crad.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Yanagawa M., Kusumoto M., Johkoh T. Radiologic-Pathologic correlation of solid portions on thin-section CT images in lung adenocarcinoma: a multicenter study. Clin. Lung Cancer. 2018 May;19(3):e303–e312. doi: 10.1016/j.cllc.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Ahlers C., Kreideweiss S., Nordheim A., Ruhlmann A. Cyclosporin A inhibits Ca2+-mediated upregulation of the DNA repair enzyme DNA polymerase beta in human peripheral blood mononuclear cells. Eur. J. Biochem. 1999;264(3):952–959. doi: 10.1046/j.1432-1327.1999.00700.x. [DOI] [PubMed] [Google Scholar]
- 21.Pritchard D.E., Singh J., Carlisle D.L., Patierno S.R. Cyclosporin A inhibits chromium(VI)-induced apoptosis and mitochondrial cytochrome c release and restores clonogenic survival in CHO cells. Carcinogenesis. 2000;21(11) doi: 10.1093/carcin/21.11.2027. 2027-33. [DOI] [PubMed] [Google Scholar]
- 22.Chiurchiu C., Carreno C.A., Schiavelli R. Results of the conversion to everolimus in renal transplant recipients with posttransplantation malignancies. Transplant. Proc. 2010;42(1):277–279. doi: 10.1016/j.transproceed.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez A., Marcen R., Pascual J. Conversion from calcineurin inhibitors to everolimus in kidney transplant recipients with malignant neoplasia. Transplant. Proc. 2006;38(8):2453–2455. doi: 10.1016/j.transproceed.2006.08.016. [DOI] [PubMed] [Google Scholar]