Abstract
Schistosomiasis is a disease caused by a flatworm parasite that infects people in tropical and subtropical regions of Sub-Saharan Africa, South America, China, and Southeast Asia. The reliance on just one drug for current treatment emphasizes the need for new chemotherapeutic strategies. The aim of this study was to determine the phenotypic effects of extracts and fractions of leaf and stem bark of Erythrophleum ivorense (family Euphorbiaceae), a tree that grows in tropical parts of Africa, on two developmental stages of Schistosoma mansoni, namely, postinfective larvae (schistosomula or somules) and adults. Methanol leaf and stem bark extracts of E. ivorense were successively fractionated with acetone, petroleum ether, ethyl acetate, and methanol. These fractions were then incubated with somules at 0.3125 to 100 μg/mL and with adults at 1.25 μg/mL. The acetone fractions of both the methanol leaf and bark of E. ivorense were most active against the somules whereas the petroleum ether fractions showed least activity. For adult parasites, the acetone fraction of methanol bark extract also elicited phenotypic changes. The data arising provide the first step in the discovery of new treatments for an endemic infectious disease using locally sourced African medicinal plants.
1. Introduction
Schistosomiasis, also known as bilharzia, is a disease caused by blood flukes of the genus Schistosoma [1, 2]. There are five species of Schistosoma that infect humans: Schistosoma mansoni, Schistosoma haematobium, Schistosoma japonicum, Schistosoma intercalatum, and Schistosoma mekongi with the first three being the most common [3, 4]. The disease is chronic and morbid [5] and affects people in tropical and subtropical regions of Sub-Saharan Africa, South America, China, and Southeast Asia [6, 7]. It is estimated that about 200 million people are infected with another 400 million being at risk [8]. Schistosomiasis is a persistent public health issue in endemic countries.
In Ghana, schistosomiasis is caused mainly by S. haematobium and S. mansoni [9] and is known to affect mostly school-aged children from 10 years of age, particularly, boys. High prevalence of the disease (11 and 64%) has been reported in the Kumasi metropolitan area of Ghana [10]. Aryeetey et al. [11] also reported prevalence rates of 54.8 to 60.0% for urinary schistosomiasis (caused by S. haematobium) in the Akuapim South district of Eastern Ghana. Nkegbe [12] reported that about 50% of the people in the Volta and Greater Accra regions of Ghana suffer from schistosomiasis. Approximately 48% of school-aged children are infected with schistosomiasis in the rural north of Ghana [13].
Currently, the treatment of schistosomiasis relies upon just one drug, praziquantel, which is safe and reasonably effective [6, 7]. However, side effects, including headache, dizziness, stomach, joint and muscle pain, tiredness, weakness, skin rashes, and sweating, are common [7]. Also, the reliance on a single antimicrobial agent could precipitate resistance. It is, therefore, important to discover and develop new chemical molecules that could be used in place of or together with praziquantel.
Erythrophleum ivorense A. Chev. is a tree that grows in tropical parts of Africa, including Ghana, Congo, Cameroon, Gabon, Nigeria, and Liberia. It is a legume from the Euphorbiaceae family [14]. Phytochemical screening of methanol extracts of both leaves and bark reveals the presence of saponins and flavonoids. The methanol leaf extract contains condensed tannins and sterols whereas the methanol bark extract contains hydrolysable tannins and terpenoids [14]. E. ivorense contains alkaloids including erythrophleine [15]. Medicinally, it is used as an emetic, an analgesic, and a laxative in Sierra Leone [16]. It also possesses broad-spectrum antimicrobial activity [14]. The aim of this study was to phenotypically screen E. ivorense leaf and stem bark extracts and fractions against various developmental stages of S. mansoni.
2. Methods
2.1. Collection of Plant Material
The leaves and barks of E. ivorense were collected from the Botanic Garden, University of Ghana, in January 2015 by Mr. John Yaw Amponsah and authenticated by Professor Alex Asase of the Department of Botany of the University of Ghana, Legon. A voucher specimen (AA 45) of the plant material was kept in the Ghana Herbarium, University of Ghana, Legon, Accra, Ghana.
2.2. Preparation of Extracts and Fractions
The leaf and stem bark of E. ivorense were extracted using 99.8% v/v methanol (Sigma-Aldrich, MO, USA). Acetone (96% v/v), petroleum ether (96 v/v), ethyl acetate (98% v/v), and methanol (all solvents purchased from Sigma-Aldrich, MO, USA) were used for the successive extraction and fractionation of the methanol leaf and bark extracts using column chromatography. The extracts were prepared by the cold maceration of 300 g of powdered dry plant material in stoppered flasks containing 700 mL of solvent for 1 week at room temperature (28°C). After filtration, the solvent was evaporated under reduced pressure in a rotary evaporator at 40° C. The different extracts and fractions were conserved in tightly sealed glass vials and stored at 4°C.
2.3. Maintenance of S. Mansoni
A Puerto Rican isolate of Schistosoma mansoni was maintained by passage through Biomphalaria glabrata snails and 3-5-week-old female Golden Syrian hamsters as intermediate and definitive hosts, respectively [2, 17]. Infected snails were induced with light to shed infectious larvae (cercariae), as described by Colley and Wikel [18], and the cercariae were mechanically transformed to postinfective larvae (schistosomula or somules), as described Stefanic et al. [19]. Adults were harvested from euthanized hamsters at 6 weeks postinfection by reverse perfusion in RPMI 1640 medium [20].
2.4. Schistosoma mansoni Phenotypic Screening
For screening of somules and adults, transparent u-bottomed 96-well plates and flat-bottomed 24-well plates were used, respectively. The phenotypic screening was carried out as described [2, 17].
For first-pass screens with somules, extracts, and fractions were spotted into the wells to yield a final concentration of 100 μg/mL (1 μL of 20 mg/mL stock solution). The final concentration of dimethyl sulfoxide (DMSO; Sigma-Aldrich, MO, USA) was less than 0.5 % v/v. Somules were then added at a density of 30 to 40 parasites in 200 μL of Basch medium [21] supplemented with 2.5% v/v FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin (Gibco, Carlsbad, USA) [2]. Parasites were incubated in a humidified atmosphere of 5% CO2 at 37°C for 72 h. For dose-response tests, extracts, and fractions that elicited a phenotypic response were screened between 0.3125 and 100 μg/mL. Both first-pass and confirmatory dose-response assays were performed in duplicate. For screens with adult parasites, four to five worm pairs were incubated in the presence of 1.25 μg/mL extracts or fractions (final DMSO 0.5% v/v) in 2 mL complete Basch medium.
Phenotypic changes were visually recorded at 24 and 48 h for somules and at 3, 5, 24, and 48 h with adults, as described by Glaser et al. [22] and Fonseca et al. [23] using a Zeiss Axiovert A1 inverted microscope (10X magnification for the somules and 2.5X magnification for the adults; Carl Zeiss Microscopy, Thornwood, USA). As complex metazoans, schistosomes can exhibit a variety of dynamic phenotypic responses to drug insult as a function of time and doses. Accordingly, we use single word “descriptors” to record changes in somule movement, shape, translucence, surface integrity, and, for adults specifically, the ability to remain adhered (via oral and/or ventral suckers) to the bottom of the well (Table 1). We then convert these observations into an ordinal numeric output (a “severity score”) in order to allow for the relative comparison of compound effects. Specifically, each descriptor is awarded a value of 1 up to a maximum score of 4 (Table 1). When damage to the adult parasite's tegument (surface) is evident, the maximum score of four is awarded on the understanding that such damage is lethal, including in the mammalian host [24].
Table 1.
Descriptors and corresponding severity scores for the effect of extracts and fractions of E. ivorense on S. mansoni. Unless otherwise indicated, the descriptors employed are common to both somules and adults.
| Descriptor | Severity score |
|---|---|
| Round | 1 |
| On sides (adults: loss of ability to adhere to the well surface) | 1 |
| Uncoordinated (adults) | 1 |
| Shrunk (adults) | 1 |
| Dark | 1 |
| Slow | 1 |
| Overactive | 1 |
| Immobile | 1 |
| Partial death (% somules dead /well) | 1 |
| Degenerate | 4 |
| Dead | 4 |
| Tegumental damage (adults) | 4 |
Severity score: 1 (lowest) to 4 (highest).
3. Results
3.1. Activity of Extracts and Their Fractions of E. ivorense against S. mansoni Somules
All of the extract fractions were active at 100 μg/mL and were subsequently tested in dose-response assays.
Focusing first on the methanol leaf extract for dose-response assays, the acetone fraction was potently antischistosomal with phenotypic alterations (degeneration and/or death, i.e., severity scores of 4) noted at both 24 and 48 h and at all dilutions (except 0.3125 μg/ml at 24 h; Figure 1(a); see also Figure 3(b) for a photo of the affected somules). By contrast, the methanol leaf extract elicited relative minor phenotypic alteration, only at 100 μg/mL (Figure 1(b)). At 24 h, the somules were rounded and overactive yielding a score of 2 but by 48 h both effects had disappeared and the worms had become darkened, thereby yielding a score of 1. There was no apparent activity below 100 μg/mL.
Figure 1.
Severity scores for fractions derived from E. ivorense methanol leaf extracts tested against somules. Scores associated with (a) the acetone fraction and (b) the methanol extract. Note that for (b), activity was only detected at 100 μg/mL.
Figure 3.
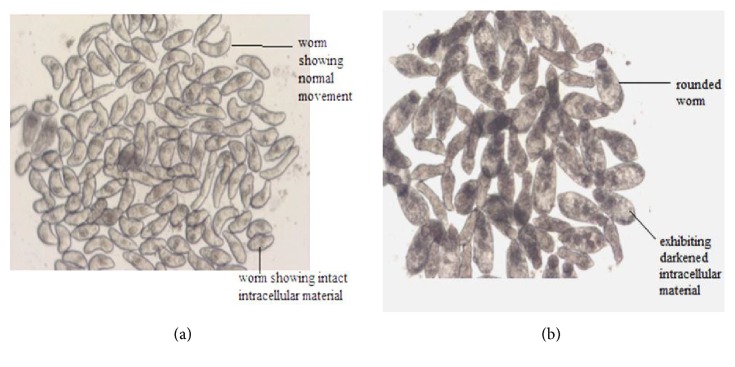
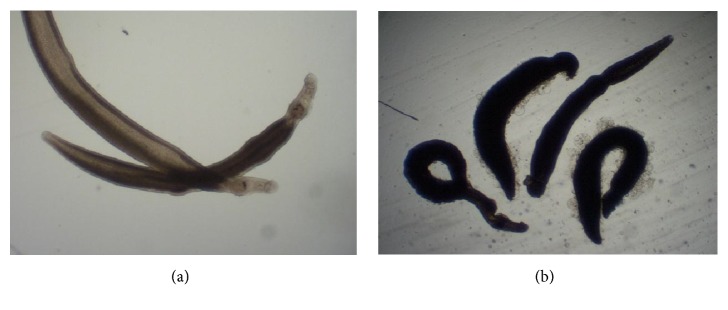
Examples of somules after exposure to E. ivorense extract fractions. (a) Control somules after 24 h. Each somule is approximately 200 μm in length. (b) Somules exposed to 2.5 μg/mL of the acetone fraction of the methanol leaf extract after 24 h. Note the rounding and severe degeneracy relative to control worms.
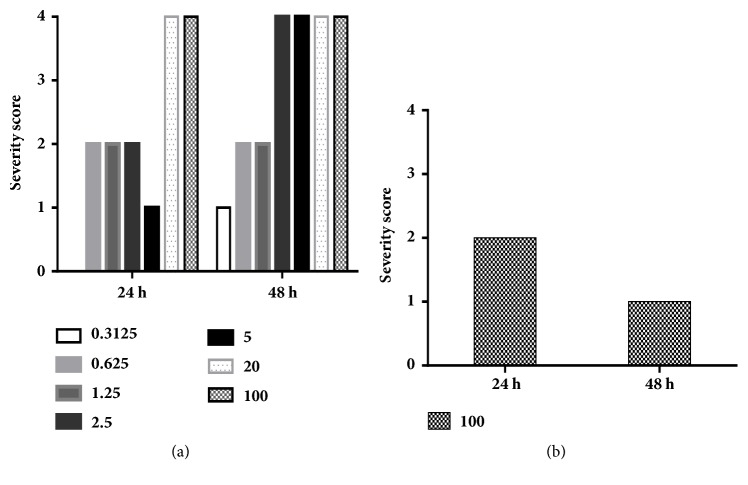
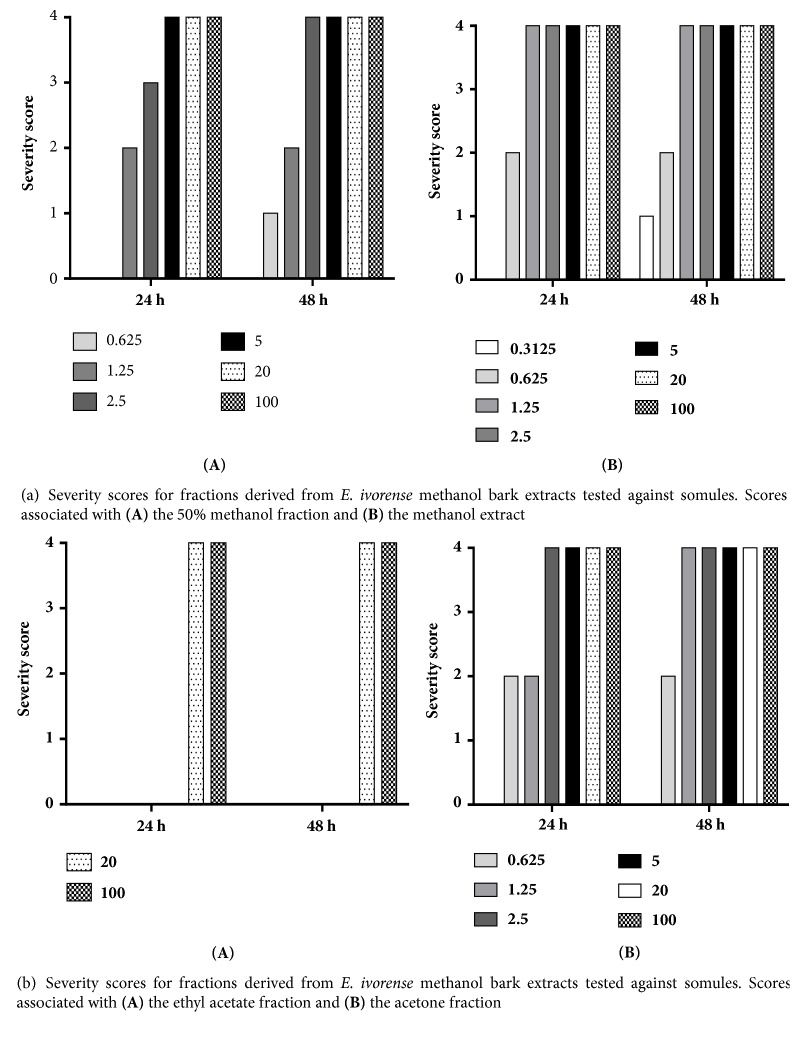
Focusing on the methanol stem bark extract, the 50% methanol fraction killed or caused degeneration in somules by 24 and 48 h (scores of 4) at 100, 20, 5, and 2.5 μg/mL (Figure 2(a)). The least concentration that exhibited a phenotypic effect was 0.625 μg/mL and then after 48 h about 50% of the worms had died giving a score of 1. No effect was observed at 24 h. At 0.3125 μg/ml, the fraction was inactive. For the methanol bark extract, the somules were dead or degenerated by 24 and 48 h at 100, 20, 5, 2.5, and 1.25 μg/mL (scores of 4). At a concentration of 0.625 μg/mL, the worms were darkened and rounded at 24 and 48 h, respectively (severity scores of 2 for each time point). At 0.3125 μg/mL after 48 h, the worms were rounded yielding a score of 1. No effect was observed after 24 h. The ethyl acetate fraction was relatively weak (Figure 2(b)). Thus, at 100 μg/mL somules were killed between 24 and 48 h of incubation, yielding a severity score of 4. At 20 μg/mL, the somules appeared rounded and degenerated by 24 h and were dead by 48 h (each a score of 4). Below 20 μg/mL, no effect was observed. Finally, powerful effects across time and dose were noted for the acetone fraction of the methanol stem bark extract (Figure 2(b)). Specifically, concentrations of 100, 20, 5, 2.5, and 1.25 μg/mL were lethal by 48 h (score of 4). At 0.625 μg/mL, the somules had become rounded and darkened yielding a score of 2. At the lowest concentration tested, 0.3125 μg/mL, no effects were observed (Figure 5).
Figure 2.
Figure 5.
Adult S. mansoni severity scores after exposure to 1.25 μg/mL of fractions derived from the methanol bark extract of E. ivorense. (a) 50% methanol fraction, (b) methanol bark extract, and (c) acetone fraction.
3.2. Activity of Extracts and Their Fractions of E. ivorense against Adult S. mansoni Adults
Upon completion of the somule screens, extracts and fractions that exhibited phenotypic changes at the lower concentrations (from 5 to 0.3126 μg/mL) were selected and screened at 1.25 μg/mL against adults (Figure 4).
Figure 4.
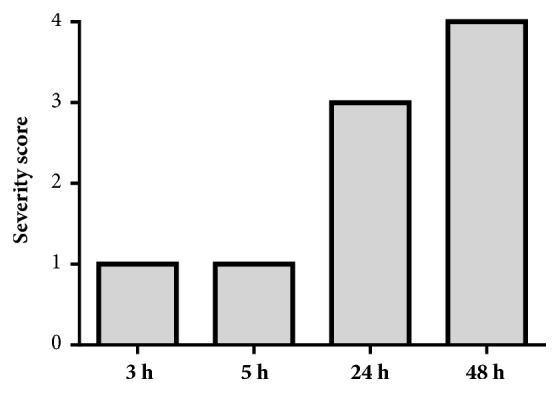
Adult S. mansoni severity scores after exposure to 1.25 μg/mL of the acetone fraction derived from E. ivorense methanol leaf extracts.
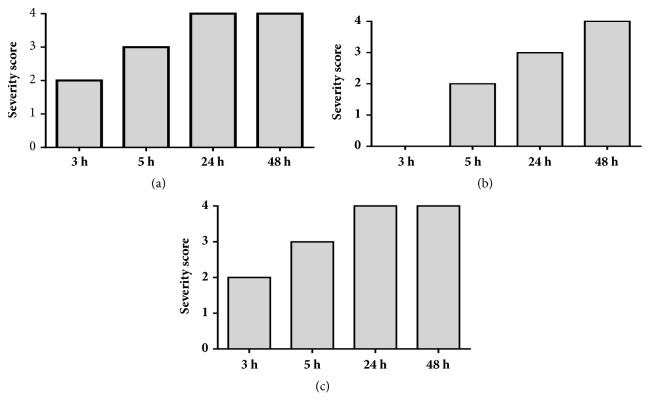
The acetone fraction of the methanol bark extract of E. ivorense caused the adult worms to become uncoordinated by 3 and 5 h, giving a score of 1. After 24 h, the worms remained uncoordinated and, in addition, had become dark with an inability to adhere to the floor of the well, yielding a score of 3. By the end of 48 h incubation period, the worms were dead and exhibited tegumental (surface) damage (a score of 4) (Figures 5 and 6).
Figure 6.
Examples of adult S. mansoni males after exposure to 1.25 μg/mL of E. ivorense methanol bark extract fractions. (a) Control worms (each worm is approximately 500 to 700 μm long); (b) damaged worms in the presence of the 50% v/v methanol fraction after 24 h. Note the marked darkening and the damage (blebbing) to the tegumental surface.
Turning to the methanol bark extracts, it was observed after 3 h that in the presence of the 50% v/v methanol fraction, the worms had become uncoordinated with an inability to adhere to the base of the well (yielding a score of 2). By 5 h, the worms had become slightly shrunk in addition to the previous effects, thus increasing the score to 3. By 24 h, the worms exhibited tegumental damage and markedly slowed motility (score of 4). After 48 h, all of the worms were dead. The methanol bark extract caused no effects after 3 h. At 5 h, however, the worms were uncoordinated and not adhering to the well floor (a score of 2). At 24 h, the worms were damaged in tegumental manner and by 48 h were dead (in each case a score of 4). Finally, in the presence of the acetone fraction after 3 h, adult worms were uncoordinated and not adhering to the well floor, yielding a score of 2. By 5 h, the worms had also shrunk (a score of 3). After 24 h, the worms were dead with tegumental damage (score of 4) (Figure 6).
4. Discussion
For S. mansoni somules, the phenotypic effects, and corresponding severity scores, were generally observed to be dose-dependent. de Moraes et al. [25] and Long et al. [17] similarly reported dose-dependent activity against S. mansoni for piplartine and polo-like kinase inhibitors, respectively. It was also observed that, for both developmental stages, the phenotypic effects were dynamic and time-dependent (severity scores can increase or decrease or the qualitative nature of the effect may change), although, in general, the longer the time of contact, the more pronounced the effects and the higher the severity score. For example, this was observed for somules with the acetone fraction of methanol bark extract of E. ivorense. At a concentration of 0.625 μg/mL after 24 h, the severity score was 2, but after 48 h the score had increased to 4. Similar observations were made with the ethyl acetate fractions and acetone fraction of the methanol leaf extract of the same plant.
The current antischistosomal drug, praziquantel, causes alterations to the tegumental surface of adult worms which makes the parasite vulnerable to host immune killing [26–28]. Methanol fractions and extract of E. ivorense bark and leaf also caused tegumental damage which is encouraging in light of the need for new antischistosomal agents, especially as many of the fractions and extracts also exhibited activity against somules, against which praziquantel is less active in vivo [24]. Any new drug molecule of interest should be active against all developmental stages of the parasite [7].
Secondary metabolites such as alkaloids, tannins, flavonoids, saponins, and sterols are responsible for the antimicrobial, analgesic, antiinflammatory, and antioxidant properties of medicinal plants [29, 30]. The activity exhibited by the algae extracts against the parasites could be as a result of the phytochemical constituents present in them. Our screening data suggest that the acetone fractions of both the bark and leaf exhibited the stronger activity. Secondary metabolites such as alkaloids, tannins, terpenoids, and flavonoids are found in E. ivorense as reported by Adu-Amoah et al. [14] and the activities observed may be attributable to these constituents. According to Molgaard et al. [31], extracts of Abrus precatorius, which exhibited significant activity against juvenile worms of S. mansoni, contain these phytochemical constituents. It is, therefore, possible that these secondary metabolites present in these extracts are responsible for the observed activity: the metabolites could be working synergistically or eliciting individual effects. Our future studies will include the bioactivity-guided isolation and characterization of the discrete compounds responsible for the current antischistosomal activities.
Finally, Adu-Amoah et al. [14] have reported that methanol leaf and bark extracts of E. ivorense (100, 300, and 1000 mg/kg) show no toxicity in vitro against HaCaT keratinocytes and in vivo in studies in male Wistar rats. Hence it is conceivable that the 1.25–100 μg/mL concentrations that elicited activity in both parasite stages will not induce significant toxicity.
5. Conclusion
Methanol leaf and stem bark extracts and fractions of E. ivorense exhibited activity against both S. mansoni somules and adults. Of all the solvents used for the extraction and fractionation, the methanol extract of the stem bark, acetone fractions of the leaf and stem bark extracts, and the 50% methanol fraction of the stem bark extract showed the greatest activities against both stages. Considering the schistosomicidal effects against somules and adults of S. mansoni, the medicinal plant E. ivorense may offer a step forward in the search for novel antischistosomal agents, due to the urgent need for new drugs.
Acknowledgments
The authors are grateful to the World Intellectual Property Organization-Re:Search (WIPO-Re:Search), for the fellowship to Gertrude Kyere-Davies, and to Mr. Thomas Bombelles, Head of Global Health in the Global Challenges Division of WIPO, for his strong support during this project. Screening activities at the CDIPD were supported in part by the NIH-NIAID Award R21AI107390.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Savioli L., Albonico M., Engels D., Montresor A. Progress in the prevention and control of schistosomiasis and soil-transmitted helminthiasis. Parasitology International. 2004;53(2):103–113. doi: 10.1016/j.parint.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Abdulla M.-H., Ruelas D. S., Wolff B., et al. Drug discovery for schistosomiasis: Hit and lead compounds identified in a library of known drugs by medium-throughput phenotypic screening. PLOS Neglected Tropical Diseases. 2009;3(7, article no. e478) doi: 10.1371/journal.pntd.0000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gryseels B., Polman K., Clerinx J., Kestens L. Human schistosomiasis. The Lancet. 2006;368(9541):1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 4.Davis A. Schistosomiasis. In: Cook G. C., Zumla A. I., editors. Manson’s Tropical Diseases. Saunders Elsevier; 2009. pp. 1425–1460. [Google Scholar]
- 5.King C. H. Parasites and poverty: the case of schistosomiasis. Acta Tropica. 2010;113(2):95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Utzinger J., Bergquist R., Olveda R., Zhou X.-N. Important helminth infections in southeast asia. diversity, potential for control and prospects for elimination. Advances in Parasitology. 2010;72(C):1–30. doi: 10.1016/S0065-308X(10)72001-7. [DOI] [PubMed] [Google Scholar]
- 7.Caffrey C. R., Secor W. E. Schistosomiasis: From drug deployment to drug development. Current Opinion in Infectious Diseases. 2011;24(5):410–417. doi: 10.1097/QCO.0b013e328349156f. [DOI] [PubMed] [Google Scholar]
- 8.Steinmann P., Keiser J., Bos R., Tanner M., Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. The Lancet Infectious Diseases. 2006;6(7):411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 9.Bosompem K. M., Bentum I. A., Otchere J., et al. Infant schistosomiasis in Ghana: a survey in an irrigation community. Tropical Medicine & International Health. 2004;9(8):917–922. doi: 10.1111/j.1365-3156.2004.01282.x. [DOI] [PubMed] [Google Scholar]
- 10.Tay S. C., Amankwa R., Gbedema S. Y. Prevalence of schistosoma haematobium infection in ghana: a retrospective case study in kumasi. International Journal of Parasitology Research. 2011;3(2):48–52. doi: 10.9735/0975-3702.3.2.48-52. [DOI] [Google Scholar]
- 11.Aryeetey M. E., Wagatsuma Y., Yeboah G., et al. Urinary schistosomiasis in southern Ghana: 1. Prevalence and morbidity assessment in three (defined) rural areas drained by the Densu river. Parasitology International. 2000;49(2):155–163. doi: 10.1016/S1383-5769(00)00044-1. [DOI] [PubMed] [Google Scholar]
- 12.Nkegbe E. Sex prevalence of schistosomiasis among school children in five communities in the lower river volta basin of South Eastern Ghana. African Journal of Biomedical Research. 2010;13(1):87–88. [Google Scholar]
- 13.Anto F., Asoala V., Adjuik M. Water contact activities and prevalence of schistosomiasis infection among school-age children in communities along an irrigation scheme in Rural Northern Ghana. Journal of Bacteriology & Parasitology. 2013;4, article 177 doi: 10.4172/2155-9597.1000177. [DOI] [Google Scholar]
- 14.Adu-Amoah L., Agyare C., Kisseih E., Ayande P. G., Mensah K. B. Toxicity assessment of Erythrophleum ivorense and Parquetina nigrescens. Toxicology Reports. 2014;1:411–420. doi: 10.1016/j.toxrep.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosch C. H. Ressources végétales de l"Afrique tropicale Wageningen. Netherlands: 2016. Erythrophleumivorense(A. Chev). Plant resources of tropical Africa. http://www.database.prota.org/search.htm. [Google Scholar]
- 16.Loder J., Culvenor C., Nearn R., Russell G., Stanton D. Tumor inhibitory plants: New Alkaloids from the bark of Erythrophleum chlorostachys (Leguminoseae) Australian Journal of Chemistry. 1974;27(1):179–185. doi: 10.1071/CH9740179. [DOI] [Google Scholar]
- 17.Long T., Neitz R. J., Beasley R., et al. Structure-Bioactivity Relationship for Benzimidazole Thiophene Inhibitors of Polo-Like Kinase 1 (PLK1), a Potential Drug Target in Schistosoma mansoni. PLOS Neglected Tropical Diseases. 2016;10(1) doi: 10.1371/journal.pntd.0004356.e0004356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colley D. G., Wikel S. K. Schistosoma mansoni: Simplified method for the production of schistosomules. Experimental Parasitology emphasizes. 1974;35(1):44–51. doi: 10.1016/0014-4894(74)90005-8. [DOI] [PubMed] [Google Scholar]
- 19.Štefanic S., Dvořák J., Horn M., et al. RNA interference in Schistosoma mansoni schistosomula: selectivity, sensitivity and operation for larger-scale screening. PLOS Neglected Tropical Diseases. 2010;4(10, article e850) doi: 10.1371/journal.pntd.0000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdulla M.-H., Lim K.-C., Sajid M., McKerrow J. H., Caffrey C. R. Schistosomiasis mansoni: Novel chemotherapy using a cysteine protease inhibitor. PLoS Medicine. 2007;4(1):0130–0138. doi: 10.1371/journal.pmed.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basch P. F. Cultivation of Schistosoma mansoni In vitro. I. Establishment of Cultures from Cercariae and Development until Pairing. The Journal of Parasitology . 1981;67(2):p. 179. doi: 10.2307/3280632. [DOI] [PubMed] [Google Scholar]
- 22.Glaser J., Schurigt U., Suzuki B. M., Caffrey C. R., Holzgrabe U. Anti-schistosomal activity of cinnamic acid esters: Eugenyl and thymyl cinnamate induce cytoplasmic vacuoles and death in schistosomula of Schistosoma mansoni. Molecules. 2015;20(6):10873–10883. doi: 10.3390/molecules200610873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fonseca N. C., Da Cruz L. F., Da Silva Villela F., et al. Synthesis of a sugar-based thiosemicarbazone series and structure-activity relationship versus the parasite cysteine proteases rhodesain, cruzain, and Schistosoma mansoni cathepsin B1. Antimicrobial Agents and Chemotherapy. 2015;59(5):2666–2677. doi: 10.1128/AAC.04601-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrews P., Thomas H., Pohlke R., Seubert J. Praziquantel. Medicinal Research Reviews. 1983;3(2):147–200. doi: 10.1002/med.2610030204. [DOI] [PubMed] [Google Scholar]
- 25.Moraes J. D., Nascimento C., Lopes P. O. M. V., et al. Schistosoma mansoni: in vitro schistosomicidal activity of piplartine. Experimental Parasitology emphasizes. 2011;127(2):357–364. doi: 10.1016/j.exppara.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Pax R., Bennett J. L., Fetterer R. A benzodiazepine derivative and praziquantel: Effects on musculature of Schistosoma mansoni and Schistosoma japonicum. Naunyn-Schmiedeberg's Archives of Pharmacology. 1978;304(3):309–315. doi: 10.1007/BF00507974. [DOI] [PubMed] [Google Scholar]
- 27.Fetterer R. H., Pax R. A., Bennett J. L. Praziquantel, potassium and 2,4-dinitrophenol: Analysis of their action on the musculature of Schistosoma mansoni. European Journal of Pharmacology. 1980;64(1):31–38. doi: 10.1016/0014-2999(80)90366-0. [DOI] [PubMed] [Google Scholar]
- 28.Doenhoff M. J., Sabah A. A. A., Fletcher C., Webbe G., Bain J. Evidence for an immune-dependent action of praziquantel on Schistosoma mansoni in mice. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1987;81(6):947–951. doi: 10.1016/0035-9203(87)90360-9. [DOI] [PubMed] [Google Scholar]
- 29.Murugan T., Wins J. A., Murugan M. Antimicrobial activity and phytochemical constituents of leaf extracts of cassia auriculata. Indian Journal of Pharmaceutical Sciences. 2013;75(1):p. 122. doi: 10.4103/0250-474X.113546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGaw L. J., Jäger A. K., Van Staden J. Antibacterial, anthelmintic and anti-amoebic activity in South African medicinal plants. Journal of Ethnopharmacology. 2000;72(1-2):247–263. doi: 10.1016/S0378-8741(00)00269-5. [DOI] [PubMed] [Google Scholar]
- 31.Mølgaard P., Nielsen S. B., Rasmussen D. E., Drummond R. B., Makaza N., Andreassen J. Anthelmintic screening of Zimbabwean plants traditionally used against schistosomiasis. Journal of Ethnopharmacology. 2001;74(3):257–264. doi: 10.1016/S0378-8741(00)00377-9. [DOI] [PubMed] [Google Scholar]