Abstract
Inorganic arsenic (iAs) exposure increases risk of several diseases, including cancer. Some nutrients such as flavonoids enhance glutathione activity, which in turn play a key role in iAs elimination. Our objective was to explore whether dietary non-soy flavonoids are associated with iAs metabolism. We hypothesized that the intake of flavonoids belonging to the following groups, flavan-3-ols, flavone, flavonol, flavanone, and anthocyanidin, is positively associated with urinary dimethylarsinic acid (DMA), which is the most soluble iAs metabolite excreted. We performed a cross-sectional study that included 1027 women living in an arsenic-contaminated area of northern Mexico. Flavonoid intake was estimated using a validated food frequency questionnaire. Concentration of urinary iAs and its metabolites (monomethylarsonic acid and DMA) were determined by high performance liquid chromatography ICP-MS. Results showed positive significant associations between DMA and the flavonoid groups flava-3-ols (β= 0.0112) and flavones (β= 0.0144), as well as the individual intake of apigenin (β= 0.0115), luteolin (β= 0.0138), and eriodictyol (β= 0.0026). Our findings suggest that certain non-soy flavonoids may improve iAs elimination; however, there is still very limited information available regarding the consumption of flavonoids and iAs metabolism.
Keywords: arsenic metabolism, flavonoids, Mexico, cross-sectional study
1. Introduction
Inorganic arsenic (iAs) is a metalloid naturally present in geological strata and contaminates drinking water sources in several areas of the world, including Argentina, Bangladesh, Chile, and Mexico. Cancer, development deficits, skin lesions, and diabetes, as well as respiratory, immunological, cardiovascular, and endocrine impairments are associated with iAs exposure [1].
Ingested iAs is biomethylated in the liver and eliminated primarily through urine. It is first reduced by glutathione (GSH) from its pentavalent (AsV) to its trivalent form (AsIII), then followed by oxidative methylation to form monomethylarsonic acid (MMAV) using S-adenosyl methionine (SAM). This reduction and methylation process is repeated to form dimethylarsinic acid (DMAV), the most hydrophilic metabolite. Proportions of iAs (0.20 to 0.25), MMA (0.15 to 0.25) and DMA (0.40 to 0.75) are found in urine. An increased proportion of MMA has been associated with arsenic-related disease risk [2]. Genetic, environmental, and dietary factors may contribute to the wide inter-individual variation in iAs elimination reported in the literature [3].
Flavonoids are a large group of natural polyphenolic compounds that are mainly derived from fruits and vegetables, which contributes to their color and flavor. There are six primary groups of flavonoids: flavan-3-ols, flavone, flavonol, flavanone, anthocyanidin, and isoflavone. Most flavonoids exert not only anti-inflammatory and anti-proliferative properties but also anti-oxidative activity [4]. Some flavonoids enhance γ-glutamylcysteine synthetase (GCSh) expression, increasing intracellular GSH [5], which may in turn improve iAs elimination. Dietary micronutrients, such as flavonoids, may modulate iAs elimination; however, available information is scarce. A recent study reported an increased intake of the soy isoflavones, genistein and daidzein, was associated with decreased urinary percentages of iAs and MMA respectively, and an increased percentage of DMA [6]. However, there is no information available on the intake of non-soy flavonoids.
Our objective was to evaluate a potential association between selected dietary non-soy flavonoids and iAs urinary metabolites in a group of Northern Mexican women exposed to iAs in drinking water. We hypothesized that the intake of non-soy flavonoids was positively associated with the proportion of DMA in urine.
2. Methods and materials
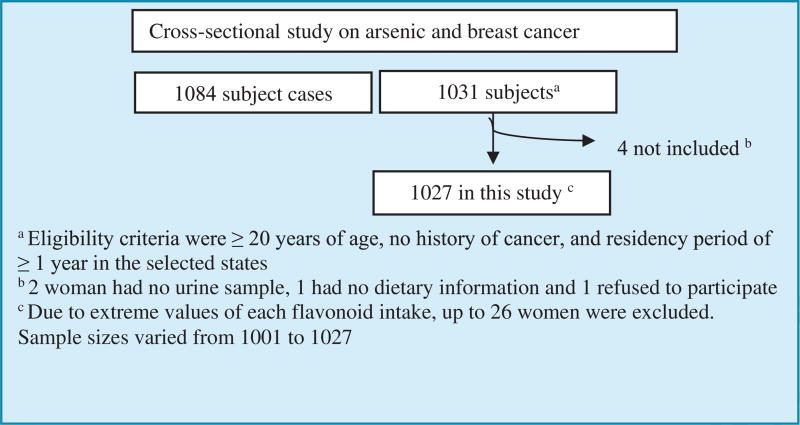
This cross-sectional study was comprised of 1,027 women that participated from 2007 to 2009 investigating arsenic exposure in relation to breast cancer risk (Figure 1) [7]. Participants were a probabilistic sample of residents living in five states in Northern Mexico. Eligibility criteria were ≥ 20 years of age, no history of cancer, and residency period of ≥ 1 year in the selected states. Women were identified through the master sample framework used for the National Health Surveys, from which a probabilistically selected list of housing addresses and an access sketch to facilitate their location was obtained [8]. In the houses where there was more than one eligible woman, only one participant was randomly chosen. Conversely, if no eligible woman was found in a household, or if she declined participation in the study, a new home was systematically located according to the standardized survey procedures. A grocery incentive was given to controls to increase the response rate.
Figure 1.
Selection of study population
Pending informed consent, women were interviewed face-to-face by trained interviewers in the homes of the women regarding dietary and sociodemographic characteristics. Body mass index (BMI) was obtained from anthropometric measurements. The response rate was 99%. The project was approved by the Institutional Review Board at the Mexico National Institute of Public Health [7].
2.1 Flavonoid intake
The daily consumption of 119 foods and 14 dishes of the previous year was estimated using a validated semi-quantitative food frequency questionnaire which included predetermined portions for each food, along with 10 response options ranging from "never" to "six or more times per day" [9]. Fruit and vegetable frequency of consumption was adjusted according to their availability throughout the year. For example, half the reported plum consumption was assumed since they are only available six months of the year. Based on the frequency of food consumption, we estimated the daily intake of total energy and flavonoids using the United States Department of Agriculture nutrient composition tables [10] [11]. For quince and Mexican hawthorn, two local foods, we used the tables provided by the Mexico National Institute of Nutrition Salvador Zubiran [12].
Food sources of flavonoid included the following fruits: banana, plum, peach, apple, orange, blackberry, strawberry, water melon, melon, mango, pear, papaya, fig, pineapple, quince, and avocado. In addition, the following vegetables, alliums, legumes, and other food items were included: cauliflower, squash flower, broccoli, lettuce, onions, garlic, broad beans, beans, green peas, nuts, vegetable oil, coffee, wine, and beer.
We estimated the intake of 21 flavonoids belonging to five groups: anthocyanidins (cyanidin, petunidin, delphinidin, malvidin, pelargonidin, and peonidin); flava-3-ols (catechin, epigallocatechin, epicatechin, epicatechin 3-gallate, epigallocatechin 3-gallate, and gallocatechin); flavanones (eriodictyol, hesperetin, and naringenin); flavones (apigenin and luteolin); and flavonols (isorhamnetin, kaempferol, myricetin, and quercetin). Food sources of specific compounds are shown in Supplemental Tables S1–S3. Isoflavone consumption was not included due to the low dietary intake of soy and its derivatives in the study region.
2.2 Urinary arsenic
Participants donated a first morning void urine sample, not necessarily on the same day of interview. Samples were collected in a sterile disposable polypropylene urine collection cup, maintained for at least two years at −70 °C until analysis. Concentrations (µg/L) of urinary arsenic metabolite species (iAsIII, iAsV, MMAV, DMAV, and arsenobetaine (AsB)) were determined by high performance liquid chromatography ICP-MS system, according to methodology previously described [13]. The limit of detection (LOD) for each species was iAsIII: 0.15 µg/L; iAsV: 0.41 µg/L; MMAV: 0.12 µg/L; DMAV: 0.12 µg/L; and AsB: 0.08 µg/L. Measurements below the LOD were given the corresponding concentrations of LOD divided by two (LOD/2), as suggested by Barr et al. [14]. The percentages of samples below the LOD were iAsIII: 19.28%; iAsV: 56.48%; MMAV: 1.95%; DMAV: 0.49%; and AsB: 24.15%. The urinary concentration of creatinine (mg/dL) was measured using an enzymatic method provided by the Randox kit (Randox, Antrim County, UK). Coefficients of variation in duplicate samples were: MMAV= 8%, DMAV= 9%, AsIII= 8% and creatinine= 2.76%.
In order to evaluate urinary iAs, we calculated: 1) iAs concentration from the sum of iAsIII and iAsV; 2) total arsenic (TAs) as a result of the sum of iAs, MMAV (MMA), DMAV (DMA) and AsB; 3) proportions of iAs, MMA and DMA based on the total sum of these; and 4) methylation ratios: first= MMA/iAs; second= DMA/MMA; and total= DMA/iAs.
2.3 Statistical analyses
We described sociodemographic characteristics, urinary arsenic metabolite proportions and ratios, as well as flavonoid intake using medians, percentiles 10 and 90. Extreme values of flavonoid intake were excluded if they exceeded four standard deviations from means. In order to assess the relationship between proportions of iAs, MMA, and DMA, as the dependent variables and each flavonoid (log transformed) as independent variables, we used fractional multinomial logit models (FMLogit). The models use quasi-maximum likelihood to evaluate proportions, where each dependent variable is restricted to values between 0 and 1 and all sum to 1 for each observation [15] We performed sensitivity analyses excluding arsenic values below the LOD for each FMLogit model. In addition, we evaluated first, second, and total arsenic methylation ratios (log transformed) with multiple linear regression models and as a sensitivity analysis we censored arsenic values below the LOD with Tobit models [16].
The flavonoid intakes of interest were adjusted for energy, according to the residual method proposed by Willett et al. [17]. We considered as co-variables: births (tertile distribution), TAs-arsenobetaine (µg/L natural log transformed), BMI (kg/m2 natural log transformed), smoking status (non-smokers, ex-smokers, and current smokers), and age (years). To correct probability inflation due to multiple statistical modeling we applied a Bonferroni correction by considering p-values ≤ 0.002 (0.05/26, 26 = 21 flavonoids + 5 groups) statistically significant at a nominal p<0.05. Analyses were performed using Stata 14 (StataCorp, College Station, 2015).
3. Results
Participants in the study had medians of: 54 years of age, 6 years of education, 29.95 kg/m2 BMI, and 48 years of residence in selected states. Half of the women had a reproductive profile of 13 years of age at first menstruation, 4 births, 19 years old at first birth, and 36 months of total breastfeeding among parous (Table 1). Total urinary arsenic, excluding arsenobetaine, ranged from 0.57 to 303.29 µg/L (data not shown).
Table 1.
Characteristics of the study population (n=1027)
| Age (years) | 54.00 (37.00, 71.00) |
| BMI (kg/m2) | 29.95 (23.44, 38.54) |
| Education (years) | 6.00 (1.00, 11.00) |
| Residence in the study area (years) | 48.00 (30.00, 66.00) |
| Age at menarche (years) | 13.00 (11.00, 15.00) |
| Parity (number) | 4.00 (2.00, 10.00) |
| Age at first birth (years) | 19.00 (16.00, 26.00) |
| Total breastfeeding (months) † | 36.00 (4.00, 158.00) |
| Cigarettes per day (number) ‡ | 4.00 (1.00, 20.00) |
| Creatinine (mg/dl) | 63.99 (18.49, 161.50) |
| Arsenic compounds | |
| Total As (µg/g creatinine)§ | 25.90 (7.17, 152.94) |
| Total As-AsB (µg/g creatinine) | 19.96 (6.40, 98.41) |
| iAs | 1.95 (0.63, 9.96) |
| MMA | 1.82 (0.58, 9.37) |
| DMA | 15.62 (4.88, 75.22) |
| AsB | 0.85 (0.08, 37.74) |
| Arsenic proportionsı | |
| iAs | 0.10 (0.05, 0.19) |
| MMA | 0.10 (0.06, 0.15) |
| DMA | 0.80 (0.68, 0.87) |
| Arsenic ratios | |
| MMA/iAs | 1.00 (0.50, 1.79) |
| DMA/MMA | 8.00 (4.52, 14.88) |
| DMA/iAs | 7.84 (3.64, 16.12) |
Values are medians, P10 and P90
Among parous woman
Current smokers only
AsB (µg/g creatinine) in Total As=3.28%
Excluding AsB
The weekly median estimated intake of anthocyanidins was the highest amongst groups of flavonoids, in contrast to flavones that showed the lowest consumption. Out of 21 flavonoids, the weekly median consumptions of delphinidin, quercitin, petunidin, and hersperitin were the highest; whereas those of pelargonidin, gallocatechin, and eriodictyol were the lowest (Table 2).
Table 2.
Dietary intake of flavonoids (mg/week)
| Anthocyanidins | 96.31 (30.52, 188.73) | |
| Cyanidin | 1.71 (0.36, 6.84) | |
| Petunidin | 26.41 (8.71, 61.04) | |
| Delphinidin | 40.51 (14.48, 82.27) | |
| Malvidin | 18.19 (6.00, 42.03) | |
| Pelargonidin | 0.00 (0.00, 4.12) | |
| Peonidin | 0.01 (0.00, 0.08) | |
| Flava-3-ols | 15.02 (5.68, 45.59) | |
| Catechin | 7.35 (1.79, 23.80) | |
| Epigallocatechin | 0.63 (0.19, 1.45) | |
| Epicatechin | 5.92 (1.14, 14.02) | |
| Epicatechin 3-gallate | 0.01 (0.00, 0.07) | |
| Epigallocatechin 3-gallate | 0.28 (0.08, 0.80) | |
| Gallocatechin | 0.00 (0.00, 0.03) | |
| Flavanones | 31.61 (0.77, 169.27) | |
| Eriodictyol | 0.00 (0.00, 0.42) | |
| Hesperetin | 19.84 (0.00, 110.50) | |
| Naringenin | 11.12 (0.77, 61.46) | |
| Flavones | 1.84 (0.86, 4.31) | |
| Apigenin | 0.06 (0.03, 0.20) | |
| Luteolin | 1.75 (0.79, 4.11) | |
| Flavonols | 37.14 (21.73, 67.04) | |
| Isorhamnetin | 2.90 (0.85, 5.41) | |
| Kaempferol | 2.36 (1.27, 5.11) | |
| Myricetin | 1.05 (0.49, 1.94) | |
| Quercetin | 30.04 (17.78, 56.06) |
Values are medians, P10 and P90
We found that increases in consumption of the groups of flavan-3-ols and flavones; and individually the flavones apigenin and luteolin, as well as the flavanone eriodictyol; were significantly associated with increased DMA and reduced iAs proportions. Each 100% increase in the consumption of flavan-3-ols, flavones, apigenin, luteolin, and eriodictyol decreased (respectively) in 0.0102, 0.0125, 0.0104, 0.0119, and 0.0020 the proportion of urinary iAs; and increased in 0.0112, 0.0144, 0.0115, 0.0138, and 0.0026 the proportion of DMA, respectively (Table 3).
Table 3.
Arsenic proportions and flavonoid intake
| Flavonoid | iAs | MMA | DMA | |||
|---|---|---|---|---|---|---|
| Anthocyanidins | 0.0003 | (0.933) | 0.0001 | (0.970) | −0.0004 | (0.931) |
| Cyanidin | −0.0036 | (0.150) | −0.0008 | (0.391) | 0.0043 | (0.117) |
| Petunidin | 0.0019 | (0.569) | 0.0002 | (0.883) | −0.0021 | (0.595) |
| Delphinidin | −0.0023 | (0.544) | −0.0005 | (0.762) | 0.0028 | (0.527) |
| Malvidin | 0.0019 | (0.562) | 0.0003 | (0.855) | −0.0022 | (0.580) |
| Pelargonidin | −0.0004 | (0.457) | −0.0002 | (0.493) | 0.0006 | (0.371) |
| Peonidin | −0.0009 | (0.372) | −0.0002 | (0.658) | 0.0011 | (0.348) |
| Flava-3-ols | −0.0102 | (0.002) | −0.0010 | (0.477) | 0.0112 | (0.0 02) |
| Catechin | −0.0080 | (0.005) | −0.0009 | (0.473) | 0.0089 | (0.006) |
| Epigallocatechin | −0.0036 | (0.142) | −0.0026 | (0.033) | 0.0063 | (0.035) |
| Epicatechin | −0.0046 | (0.010) | −0.0011 | (0.233) | 0.0057 | (0.008) |
| Epicatechin 3-gallate | −0.0014 | (0.198) | −0.0001 | (0.813) | 0.0015 | (0.245) |
| Epigallocatechin 3-gallate | −0.0063 | (0.021) | 0.0000 | (0.995) | 0.0063 | (0.048) |
| Gallocatechin | 0.0001 | (0.939) | 0.0003 | (0.459) | −0.0004 | (0.732) |
| Flavanones | −0.0032 | (0.010) | −0.0004 | (0.560) | 0.0035 | (0.0 20) |
| Eriodictyol | −0.0020 | (0.001) | −0.0006 | (0.027) | 0.0026 | (0.000) |
| Hesperetin | −0.0008 | (0.088) | −0.0002 | (0.458) | 0.0010 | (0.096) |
| Naringenin | −0.0037 | (0.012) | −0.0003 | (0.684) | 0.0040 | (0.027) |
| Flavones | −0.0125 | (0.001) | −0.0019 | (0.247) | 0.0144 | (0.0 01) |
| Apigenin | −0.0104 | (0.000) | −0.0012 | (0.370) | 0.0115 | (0.001) |
| Luteolin | −0.0119 | (0.002) | −0.0019 | (0.258) | 0.0138 | (0.002) |
| Flavonols | −0.0091 | (0.142) | 0.0030 | (0.251) | 0.0061 | (0.3 84) |
| Isorhamnetin | 0.0049 | (0.138) | 0.0032 | (0.029) | −0.0081 | (0.038) |
| Kaempferol | −0.0125 | (0.005) | 0.0014 | (0.496) | 0.0111 | (0.030) |
| Myricetin | −0.0107 | (0.017) | −0.0028 | (0.186) | 0.0135 | (0.011) |
| Quercetin | −0.0103 | (0.089) | 0.0023 | (0.380) | 0.0080 | (0.242) |
Values are β regression coefficients adjusted by total arsenic, age, BMI, parity and smoking. P values are presented in brackets
Total methylation capacity significantly increased by 0.0892 and 0.9888 with each 100% increased consumption of the flavan-3-ols and flavones, respectively. Specifically, each 100% increased consumption of catechin, eriodictyol, apigenin, and luteolin was related to an increase in total methylation by 0.0758, 0.0192, 0.0913, and 0.0947 respectively. The increased consumption of eriodictyol was also associated with a 0.0121 increased second methylation (Table 4).
Table 4.
Arsenic methylation ratios and flavonoid intake
| Flavonoid | Methylation ratios | |||||
|---|---|---|---|---|---|---|
| First | Second | Total | ||||
| Anthocyanidins | −0.0073 | (0.770) | 0.0018 | (0.937) | −0.0055 | (0.868) |
| Cyanidin | 0.0198 | (0.187) | 0.0192 | (0.137) | 0.0389 | (0.043) |
| Petunidin | −0.0160 | (0.444) | −0.0022 | (0.915) | −0.0181 | (0.524) |
| Delphinidin | 0.0048 | (0.841) | 0.0123 | (0.586) | 0.0171 | (0.592) |
| Malvidin | −0.0159 | (0.447) | −0.0027 | (0.896) | −0.0185 | (0.515) |
| Pelargonidin | 0.0014 | (0.726) | 0.0042 | (0.270) | 0.0056 | (0.268) |
| Peonidin | 0.0029 | (0.720) | 0.0066 | (0.330) | 0.0095 | (0.333) |
| Flava-3-ols | 0.0586 | (0.007) | 0.0306 | (0.085) | 0.0892 | (0.001) |
| Catechin | 0.0507 | (0.007) | 0.0250 | (0.124) | 0.0758 | (0.001) |
| Epigallocatechin | −0.0022 | (0.901) | 0.0401 | (0.008) | 0.0379 | (0.083) |
| Epicatechin | 0.0211 | (0.121) | 0.0232 | (0.039) | 0.0443 | (0.006) |
| Epicatechin 3-gallate | 0.0077 | (0.368) | 0.0065 | (0.375) | 0.0141 | (0.179) |
| Epigallocatechin 3-gallate | 0.0487 | (0.009) | 0.0136 | (0.401) | 0.0624 | (0.006) |
| Gallocatechin | 0.0017 | (0.799) | −0.0017 | (0.793) | −0.0000 | (0.999) |
| Flavanones | 0.0161 | (0.074) | 0.0131 | (0.115) | 0.0292 | (0.009) |
| Eriodictyol | 0.0071 | (0.081) | 0.0121 | (0.002) | 0.0192 | (0.000) |
| Hesperetin | 0.0044 | (0.228) | 0.0041 | (0.217) | 0.0086 | (0.063) |
| Naringenin | 0.0183 | (0.094) | 0.0148 | (0.142) | 0.0330 | (0.014) |
| Flavones | 0.0472 | (0.062) | 0.0515 | (0.019) | 0.0988 | (0.002) |
| Apigenin | 0.0495 | (0.011) | 0.0419 | (0.019) | 0.0913 | (0.000) |
| Luteolin | 0.0459 | (0.063) | 0.0488 | (0.024) | 0.0947 | (0.002) |
| Flavonols | 0.0701 | (0.098) | −0.0108 | (0.754) | 0.0593 | (0.235) |
| Isorhamnetin | 0.0069 | (0.748) | −0.0479 | (0.017) | −0.0410 | (0.131) |
| Kaempferol | 0.0655 | (0.050) | 0.0184 | (0.506) | 0.0839 | (0.028) |
| Myricetin | 0.0378 | (0.233) | 0.0611 | (0.032) | 0.0989 | (0.010) |
| Quercetin | 0.0728 | (0.076) | −0.0010 | (0.977) | 0.0718 | (0.141) |
Values are β regression coefficients adjusted by total arsenic, age, BMI, parity and smoking. P values are presented in brackets
4. Discussion
We accepted the hypothesis that the intakes of apigenin, luteolin, and eriodictyol non-soy flavonoids, as well as the flavan-3-ols and flavones groups, are positively associated with the proportion of DMA. These findings are congruent with the Ilmiawati study reporting that the consumption of soy-isoflavones increased percentage of DMA and decreased percentages of iAs and MMA [6].
The intake of flavan-3-ols, flavanones, flavones, and flavonols in our study sample was lower than that reported in two other studies in Europe and the United States of America [18,19]. Fruits and vegetables, such as dried oregano, citrus fruits, fresh parsley, fresh capers, sorrel, and radish are the main sources of these compounds [20]. Low intake of fruits and vegetables in the Northern regions of Mexico has been reported by the National Nutrition Survey, confirming our intake estimations [21]. In addition, we found that consumption of anthocyanidins is similar to that reported across Europe, but higher than that reported in the U.S.A. [18,19]. Food sources of anthocyanidins include grapes, which are highly produced in some of the northern states of Mexico [20,22].
Depending on their chemical structure, flavonoids may modulate the activity of GSH. In general, those lacking the catechol group in the B-ring show antagonistic behavior with GSH intracellular levels; whereas flavonoids with a catechol group, such as quercetin and eriodictyol, have opposite actions, as observed in COS-1 kidney and ARPE-19 retinal cells [23–25]. Experimentally increased GSH levels in the gastrocnemius muscle were reported in mice fed with polyphenol-berries [5].
In our sample, 17.33 % of women exceeded the occupational health guideline limit of 35 µg/L of total iAs in urine [26]. It may be possible that even in this highly exposed group, flavonoid intake improved iAs elimination since we found an increased proportion of DMA after adjusting by total iAs concentration in our statistical analysis. Further studies are needed to determine the intake of food sources of flavonoids that would be needed to reduce the burden of disease related to iAs exposures.
One limitation of our study was that the assessment of iAs exposure was based on a single urine sample, reflecting the metabolic profile of the previous 10 hours [27]. Thus, the underlying assumption to interpret our results is that an average water arsenic intake did not significantly change on a daily basis over the study period. Additionally, varying dilution of urine samples may also distort the results. The concentration of creatinine, an indicator of dilution, has been associated with the efficiency of iAs elimination [28]. However, the use of creatinine to control for potential concentration/dilution issues is a matter of debate. Some authors suggest dividing urinary concentrations of the metabolite of interest by the respective concentration of creatinine, whereas others recommend its inclusion as an adjustment variable in multivariate models [28,29]. In this study, by using proportions and ratios of arsenic metabolites in the present study, the value of creatinine concentration was mathematically eliminated. Furthermore, because arsenic concentration was highly correlated with several important covariates, it was not included in the final models. Another limitation was that due to co-linearity among nutrients related to iAs metabolism, we did not include all of them in the models; however, we adjusted our models by confounders previously established in the literature [30]. In addition, since this was a secondary analysis of a previous study, sample power calculations were not performed.
To reduce differential measurement error, laboratory analyses of urinary arsenic metabolites were blinded to the flavonoid intake status; nevertheless, random error should not be ruled out and would result in a sub-estimation of our associations. In addition, to reduce the possibility of false positive results due to multiple comparisons, we utilized Bonferroni adjustment. Furthermore, we performed sensitivity analyses excluding women with iAs metabolites below the LOD, which yielded similar results.
In conclusion, our findings suggest that certain non-soy flavonoid intake may improve iAs elimination. Nevertheless, there is still very limited information available regarding the consumption of flavonoids and iAs metabolism and further research is warranted.
Supplementary Material
Acknowledgments
This study was supported by CONACYT Fondo Sectorial de Investigación en Salud y Seguridad Social 2005-2-14373, 2009-1-111384, 2010-1-140962, POCPN 2013-01-215464 and FOSISS 272632; as well as by grant MD 001452 from the National Center on Minority Health and Health Disparities of the National Institutes of Health, Luz Claudio, Principal Investigator.
Abbreviations
- iAs
inorganic arsenic
- GSH
glutathione
- MMAV
monomethylarsonic acid
- SAM
S-adenosyl methionine
- DMAV
dimethylarsinic acid
- GCSh
γ-glutamylcysteine synthetase
- AsB
arsenobetaine
- LOD
limit of detection
- FMLogit
fractional multinomial logit models
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare they have no actual or potential conflict of interest.
References
- 1.Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Suk WA. Broad Scope of Health Effects from Chronic Arsenic Exposure : Update on a Worldwide Public Health Problem. Env Heal Perspect. 2013;121:295–303. doi: 10.1289/ehp.1205875. doi: http://dx.doi.org/10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agency for Toxic Substances and Disease Registry. Toxicological Profile for Arsenic. U.S. Department of Health and Human Services. Public Health Service; 2007. [PubMed] [Google Scholar]
- 3.Shen H, Niu Q, Xu M, Rui D, Xu S, Feng G, et al. Factors Affecting Arsenic Methylation in Arsenic-Exposed Humans : A Systematic Review and Meta-Analysis. Int J Environ Res Public Heal. 2016;13:205. doi: 10.3390/ijerph13020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S, Pandey AK. Chemistry and Biological Activities of Flavonoids : An Overview. Sci World J. 2013;2013:1–16. doi: 10.1155/2013/162750. doi:doi.org/10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moskaug JØ, Carlsen H, Myhrstad MCW, Blomhoff R. Polyphenols and glutathione synthesis regulation 1 – 4. Am J Clin Nutr. 2005;81(suppl):277–83. doi: 10.1093/ajcn/81.1.277S. [DOI] [PubMed] [Google Scholar]
- 6.Ilmiawati C, Ogawa M, Sasaki S, Watanabe T, Kayama F. Urinar y arsenic excretion profiles and associated dietar y factors in Japanese women from a coastal area in Chiba Prefecture. Jichi Med Univ J. 2013;36:1–11. [Google Scholar]
- 7.López-Carrillo L, Hernández-Ramírez RU, Gandol AJ, Ornelas-Aguirre JM, Torres-Sánchez L, Cebrian ME. Arsenic methylation capacity is associated with breast cancer in northern Mexico. Toxicol Appl Pharmacol. 2014;280:53–9. doi: 10.1016/j.taap.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Tapia-Conyer R, Gutierrez G, Sepulveda J. Methodology of the National Seroepidemiologic Survey, Mexico. Salud Publica Mex. 1992;34(2):124–35. [PubMed] [Google Scholar]
- 9.Galván-Portillo M, Torres-Sánchez L, Hernández-Ramírez RU, Anaya-Loyola MA. Cuestionario de frecuencia de consumo de alimentos para estimación de ingestión de folato en México. Salud Publica Mex. 2011;53:237–46. doi: 10.1590/s0036-36342011000300008. [DOI] [PubMed] [Google Scholar]
- 10.United States Department of Agriculture. National Nutrient Database for Standard Reference Release 20. Agricultural Research Service; 2007. [Google Scholar]
- 11.United States Department of Agriculture. Database for the Flavonoid Content of Selected Foods Release 3.1. 2014 [Google Scholar]
- 12.Instituto Nacional de la Nutrición Salvador Zubirán. Comisión Nacional de Alimentos. México: 1992. Tablas de uso práctico del valor nutritivo de los alimentos de mayor consumo en México. [Google Scholar]
- 13.Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ, et al. Rice consumption contributes to arsenic exposure in US women. Proc Natl Acad Sci United States Am. 2011;108:20656–60. doi: 10.1073/pnas.1109127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barr DB, Landsittel D, Nishioka M, Thomas K, Curwin B, Raymer J, et al. A survey of laboratory and statistical issues related to farmworker exposure studies. Environ Health Perspect. 2006;114:961–8. doi: 10.1289/ehp.8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papke LE, Wooldridge JM. Econometric Methods for Fractional Response Variables with an Application to 401 (K) plan Participation Rates. J Appl Econom. 1996;11:619–32. [Google Scholar]
- 16.Tobin J. Estimation of Relationships for Limited Dependent Variables. Econometrica. 2007;26:24–36. [Google Scholar]
- 17.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–8S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 18.Chun OK, Chung SJ, Song WO. Estimated Dietary Flavonoid Intake and Major Food Sources of U. S. Adults. 2007;1137:1244–52. doi: 10.1093/jn/137.5.1244. 2. [DOI] [PubMed] [Google Scholar]
- 19.Vogiatzoglou A, Mulligan AA, Lentjes MAH, Luben RN, Spencer JPE, Schroeter H, et al. Flavonoid Intake in European Adults (18 to 64 Years) PLoSONE. 2015;10(5):1–22. doi: 10.1371/journal.pone.0128132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozłowska A, Szostak-Węgierek D. Flavonoids-food sources and health benefits. Rocz Panstw Zakl Hig. 2014;65(2):79–85. [PubMed] [Google Scholar]
- 21.Ramírez-Silva I, Rivera JA, Ponce X, Hernández-Ávila M. Fruit and vegetable intake in the Mexican population : Results from the Mexican National Health and Nutrition Survey 2006. Salud Publica Mex. 2009;51(supl):4, S574–85. [PubMed] [Google Scholar]
- 22.SAGARPA. Estudio de demanda de uva de mesa mexicana en tres países miembros de la unión europea, y de exploración del mercado de Nueva Zelandia. Asociación agrícola local de productores de uva. 2009 [Google Scholar]
- 23.Johnson J, Maher P, Hanneken A. The Flavonoid, Eriodictyol, Induces Long-term. Protection in ARPE-19 Cells through Its Effects on Nrf2 Activation and Phase 2 Gene Expression. Invest Ophthalmol Vis Sci. 2009;50(5):2398–406. doi: 10.1167/iovs.08-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myhrstad MC, Carlsen H, Nordstrom O, Blomhoff R, Moskaug JO. Flavonoids increase the intracellular glutathione level by transactivation of the y-glutamylcysteine synthetase catalytical subunit promoter. Free Biol Med. 2002;32(5):386–93. doi: 10.1016/s0891-5849(01)00812-7. [DOI] [PubMed] [Google Scholar]
- 25.Pereira RB, Sousa C, Costa A, Andrade PB, Valentão P. Glutathione and the Antioxidant Potential of Binary Mixtures with Flavonoids: Synergisms and Antagonisms. Molecules. 2013;18:8858–72. doi: 10.3390/molecules18088858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ACGIH. Biological exposure guidelines; American Conference of Governmental Industrial Hygienist and Occupational Safety and Health Administration expanded standards; 2012. [Google Scholar]
- 27.Agency for Toxic Substances and Disease Registry. Arsenic Toxicity. U.S. Department of Health and Human Services. Division of Toxicology and Human Health Sciences Environmental Medicine Branch; 2013. [Google Scholar]
- 28.Basu A, Mitra S, Chung J, Mazumder DNG, Ghosh N, Kalman D, et al. Creatinine, Diet, Micronutrients, and Arsenic Methylation in West Bengal, India. Environ Health Perspect. 2011;119:1308–13. doi: 10.1289/ehp.1003393. http://dx.doi.org/10.1289/ehp.1003393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary Creatinine Concentrations in the U. S. Population : Implications for Urinary Biologic Monitoring Measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.López-Carrillo L, Gamboa-Loira B, Becerra W, Hernández-Alcaraz C, Hernández-Ramírez RU, Gandol AJ, et al. Dietary micronutrient intake and its relationship with arsenic metabolism in Mexican women. Environ Res. 2016;151:445–50. doi: 10.1016/j.envres.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.