Abstract
Objective
To identify potential genes that may be involved in lipid metabolism in rats after treatment with aqueous extract of Arctium lappa L (burdock).
Methods
Rats were randomly divided into six groups: (i) control (standard diet); (ii) model group (high-fat diet only); (iii) high-fat diet and low-dose aqueous burdock root extract (2 g/kg); (iv) high-fat diet and moderate-dose aqueous burdock root extract (4 g/kg); (v) high-fat diet and high-dose aqueous burdock root extract (8 g/kg); and (vi) a positive control group exposed to a high-fat diet and simvastatin (10 mg/kg). Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis was performed to find the potential candidate genes involved in the modulation of blood lipids by treatment with aqueous burdock root extract.
Results
Burdock root extract reduced body weight and cholesterol levels in rats. KEGG analysis revealed 113 genes that were involved in metabolic pathways. Of these, 27 potential genes associated with blood lipid metabolism were identified.
Conclusions
Aqueous extract of burdock root reduced body weight and cholesterol in rats, possibly by modulating the differential expression of genes.
Keywords: Arctium lappa L., lipid metabolism, KEGG analysis, burdock
Introduction
Obesity is an epidemic disease that poses a severe threat to public health by increasing the incidence of type 2 diabetes mellitus, hypertension and heart disease.1 The prevalence of overweight and obesity is more than 60% in US adults, and the rate is rapidly increasing among children and adolescents.2 The underlying mechanism of obesity at the individual level is an imbalance of calorie intake and environmental influences.3 However, the exact molecular mechanism of obesity is still not well defined.
Glucose and lipid metabolism disorders have been considered risk factors for obesity. For example, excessive hepatic secretion of cholesterol, which results in supersaturated bile, was considered to be related to the pathogenesis of obesity.4 Curcumin plays a central role in the development of obesity and its complications through modulating lipid metabolism.5 To date, few genes have been identified to be responsible for the development of obesity as it is a complex disorder. Indeed, it is a challenge to identify which genes are responsible for obesity. With the emergence of differential gene expression techniques, it is possible to identify the genes that are expressed differentially in normal individuals compared with those who are obese. These lead us to investigate the roles of lipid metabolism in the onset of obesity at the molecular level.
Arctium lappa L. (greater burdock), a traditional Chinese medicine and an edible perennial plant of the Asteraceae family,6 has also been used for therapy in Europe, North America and Asia for hundreds of years. The burdock root has been used traditionally in herbal remedies to treat many diseases, including throat pain, arthritis, tonsillitis, rashes7 as well as cardiovascular diseases.8 It has been shown to attenuate the accumulation of cholesterol in the liver and serum, and it can lower blood lipids in those with atherosclerosis.9 To the best of our knowledge, few studies have been carried out to investigate the roles of burdock root in lipid metabolism. This study investigated differential gene expression after treatment with different concentrations of aqueous burdock root extract in rats in order to identify potential genes that may be involved in lipid metabolism.
Materials and methods
Animals
Healthy 6-week-old male specific pathogen-free Sprague–Dawley rats (weight range, 187–209 g) were purchased from the Animal Centre of the Chinese Academy of Medical Sciences (Beijing, China). The animals (n = 60) were housed in a 12-h light/12-h dark cycle, at a temperature of 20–24℃ and humidity of 40–70%. All of the animals had free access to food and water.
The animals were handled according to the guidelines from the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals. The study protocols were approved by the Ethical Committee of Qingdao University Medical College, Qingdao, Shandong Province, China. All measures were taken to minimize the suffering of the animals.
Experimental design
The rats were randomly divided into the following six groups using a random number generator: (i) control group (n = 8), which was fed on a standard diet purchased from Shandong Experimental Animal Centre (Jinan, China); (ii) model group (n = 7), which was fed on a high-fat diet containing standard diet, cholesterol, pork fat, custard powder, and chocolate; (iii) low-dose group (n = 6), which was fed the high-fat diet and aqueous extract of burdock root (2 g/kg); (iv) moderate-dose group (n = 6), which was fed the high-fat diet and aqueous extract of burdock root (4 g/kg); (v) high-dose group (n = 6), which was fed the high-fat diet and aqueous extract of burdock root (8 g/kg); and (vi) positive control group (n = 7), which was fed the high-fat diet and simvastatin (10 mg/kg) via an intragastric injection. The administration of the aqueous extract of burdock root was also given via an intragastric injection. The control and model groups were given normal saline (10 ml/kg) via an intragastric injection. All six groups received intragastric injections once per day for 8 weeks.
Body weight measurements
The body weight was determined using a commercial balance before the treatment commenced (baseline before any change in diet) and immediately after the study, and at weeks 2, 6 and 8 after treatment. The interval between baseline and week 0 was approximately 5–7 days, during which rats were fed standard diet. Rats received either standard or high-fat diet throughout the 8-week treatment period. All weight determinations were performed at least in triplicate.
Determination of serum lipid profile
Fresh venous blood was collected from each animal following a 12-h fast during which water was provided freely. The samples were centrifuged at 1600 g in an Eppendorf centrifuge 5424 (Eppendorf, Mississauga, Ontario, Canada) for 10 min at room temperature to collect the supernatant, which was then stored at −80℃ until further analysis. Serum lipid levels, including total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and cholesterol (CHOL), were determined using commercial kits (Thermo Fisher Scientific, Rockford, IL, USA) according to the manufacturer’s instructions.
RNA sequencing
Venous blood was collected from the eye medial canthus of each animal following a 12-h fast during which water was provided freely. The blood was mixed with a 3-fold volume of TRIzol® Reagent (Invitrogen, Carlsbad, CA, USA) followed by storage at −80℃. Total RNA was extracted from the whole blood of rats using TRIzol® Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA library preparation was performed according to standard operating procedures (Directional mRNA-Seq Sample Preparation, Part # 15018460 Rev. A, October 2010; Illumina Inc., San Diego, CA, USA). RNA sequencing was performed as previously described.10 Raw short sequence fragments were accepted if they passed the quality filtering parameters.
KEGG analysis
Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis was performed by referring to the KEGG pathway database according to a previous study that used the KEGG Automatic Annotation Server (version 1.68 x).11 The analysis was used to determine the potential mechanisms that may be involved in blood lipid metabolism.
Statistical analyses
Data are presented as mean ± SD. All statistical analyses were performed using the SPSS® statistical package, version 18.0 (SPSS Inc., Chicago, IL, USA) for Windows®. Single factor analysis of variance and Q test were used for comparisons. A P-value < 0.05 was considered statistically significant.
Results
Regarding body weight, there were no significant differences between the six different groups at baseline (Table 1). However, the body weight of the rats fed with normal diet (control group) was significantly lower than the five groups fed with a high-fat diet at week 0 (P < 0.05 for all comparisons). This confirmed that a high-fat diet contributed to the increased body weight that was observed. Compared with the model group that only received a high-fat diet, there were significant decreases in the body weight in the groups treated with moderate-dose and high-dose burdock root extract at weeks 6 and 8 (P < 0.05 for all comparisons). In contrast, there were no significant differences in body weight in the group treated with low-dose burdock root extract compared with the model group at weeks 6 and 8.
Table 1.
Effects of aqueous burdock root extract on the body weight (g) of rats.
| Group | Baseline | Week 0 | Week 2 | Week 6 | Week 8 |
|---|---|---|---|---|---|
| Control group | 200 ± 5 | 231 ± 14 | 254 ± 31 | 281 ± 28 | 303 ± 28 |
| Model group | 202 ± 6 | 258 ± 27* | 288 ± 39 | 352 ± 40** | 381 ± 37** |
| Positive control group | 199 ± 5 | 255 ± 25* | 279 ± 36 | 310 ± 38 | 325 ± 26## |
| High-dose group | 198 ± 4 | 257 ± 20* | 281 ± 33 | 312 ± 35## | 328 ± 34## |
| Moderate-dose group | 203 ± 7 | 254 ± 22* | 280 ± 37 | 320 ± 33# | 335 ± 40## |
| Low-dose group | 200 ± 3 | 251 ± 23* | 283 ± 40 | 344 ± 37 | 371 ± 35 |
Data presented as mean ± SD.
P < 0.05 versus control group; **P < 0.01 versus control group; #P < 0.05 versus model group; ##P < 0.01 versus model group; analysis of variance and Q test.
Regarding blood lipid levels, at 2 weeks after treatment, there were no significant differences in the TG, LDL-C, HDL-C and CHOL among the six groups (Table 2). At week 6, the level of CHOL was significantly elevated in the model group compared with the control group (P < 0.01); but there were no significant differences in the TG, LDL-C and HDL-C levels between these two groups. At 8 weeks after treatment, the levels of CHOL in the three groups treated with burdock root extract were significantly lower compared with the model group (P < 0.05 for all comparisons), but there were no other significant differences observed in TG, LDL-C and HDL-C levels.
Table 2.
Effects of aqueous burdock root extract on the blood lipid levels of rats.
| Group | n | Week 2 |
Week 6 |
Week 8 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TG, mM | LDL-C, mM | HDL-C, mM | CHOL, mM | TG, mM | LDL-C, mM | HDL-C, mM | CHOL, mM | TG, mM | LDL-C, mM | HDL-C, mM | CHOL, mM | ||
| Control group | 8 | 0.63 ± 0.17 | 0.24 ± 0.08 | 0.96 ± 0.18 | 1.50 ± 0.33 | 0.60 ± 0.16 | 0.23 ± 0.06 | 0.96 ± 0.15 | 1.72 ± 0.27 | 0.66 ± 0.20 | 0.20 ± 0.11 | 1.20 ± 0.28 | 1.30 ± 0.23 |
| Model group | 7 | 0.69 ± 0.18 | 0.30 ± 0.07 | 1.04 ± 0.09 | 1.63 ± 0.27 | 0.59 ± 0.17 | 0.20 ± 0.07 | 1.35 ± 0.22 | 2.42 ± 0.34* | 0.79 ± 0.18 | 0.31 ± 0.07 | 1.44 ± 0.29 | 2.33 ± 0.21* |
| Positive control group | 7 | 0.64 ± 0.12 | 0.26 ± 0.05 | 1.01 ± 0.15 | 1.52 ± 0.24 | 0.52 ± 0.18 | 0.24 ± 0.11 | 1.24 ± 0.22 | 2.00 ± 0.31 | 0.63 ± 0.20 | 0.25 ± 0.04 | 1.30 ± 0.23 | 1.92 ± 0.14## |
| High-dose group | 6 | 0.78 ± 0.26 | 0.24 ± 0.06 | 0.99 ± 0.14 | 1.61 ± 0.25 | 0.70 ± 0.24 | 0.24 ± 0.16 | 1.38 ± 0.37 | 2.23 ± 0.57 | 0.88 ± 0.36 | 0.22 ± 0.08 | 1.19 ± 0.19 | 1.81 ± 0.20## |
| Moderate- dose group | 6 | 0.71 ± 0.19 | 0.29 ± 0.07 | 1.03 ± 0.21 | 1.66 ± 0.29 | 0.72 ± 0.23 | 0.25 ± 0.08 | 1.35 ± 0.22 | 2.21 ± 0.36 | 0.81 ± 0.18 | 0.34 ± 0.08 | 1.33 ± 0.31 | 2.04 ± 0.26# |
| Low-dose group | 6 | 0.68 ± 0.22 | 0.28 ± 0.09 | 0.95 ± 0.16 | 1.58 ± 0.37 | 0.51 ± 0.11 | 0.26 ± 0.11 | 1.37 ± 0.28 | 2.33 ± 0.37 | 0.78 ± 0.27 | 0.28 ± 0.13 | 1.35 ± 0.26 | 1.95 ± 0.27## |
Data presented as mean ± SD.
P < 0.01 versus control group; #P < 0.05 versus model group; ##P < 0.01 versus model group; analysis of variance and Q test.
TG, triglyceride; LDL-C low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; CHOL, cholesterol.
When the control group was compared with the model group, the number of genes that were up-regulated and down-regulated was 10 and 273, respectively (Table 3). When the control group was compared with the low-dose group, the number of genes that were up-regulated and down-regulated was 55 and 605, respectively. When the control group was compared with the high-dose group, the number of genes that were up-regulated and down-regulated was 199 and 799, respectively. When the control group was compared with the positive control group, the number of genes that were up-regulated and down-regulated was 115 and 675, respectively. When the control group was compared with the moderate-dose group, the number of genes that were up-regulated and down-regulated was 122 and 633, respectively. In addition, the differential expression of genes in the model group compared with the three groups treated with burdock root extract and the group treated with simvastatin was determined, which revealed the total number of genes upregulated and downregulated were 665 and 699, respectively.
Table 3.
Differential expression of genes among the groups of rats treated with or without aqueous burdock root extract.
| Comparison | Number of up-regulated genes | Number of down-regulated genes | Total number of genes |
|---|---|---|---|
| Control group versus model group | 10 | 273 | 283 |
| Control group versus low-dose group | 55 | 605 | 660 |
| Control group versus high-dose group | 199 | 799 | 998 |
| Control group versus positive control group | 115 | 675 | 790 |
| Control group versus moderate-dose group | 122 | 633 | 755 |
| Model control group versus low-dose group | 82 | 73 | 155 |
| Model control group versus high-dose group | 136 | 232 | 368 |
| Model control group versus positive control group | 256 | 250 | 506 |
| Model control group versus moderate-dose group | 191 | 144 | 335 |
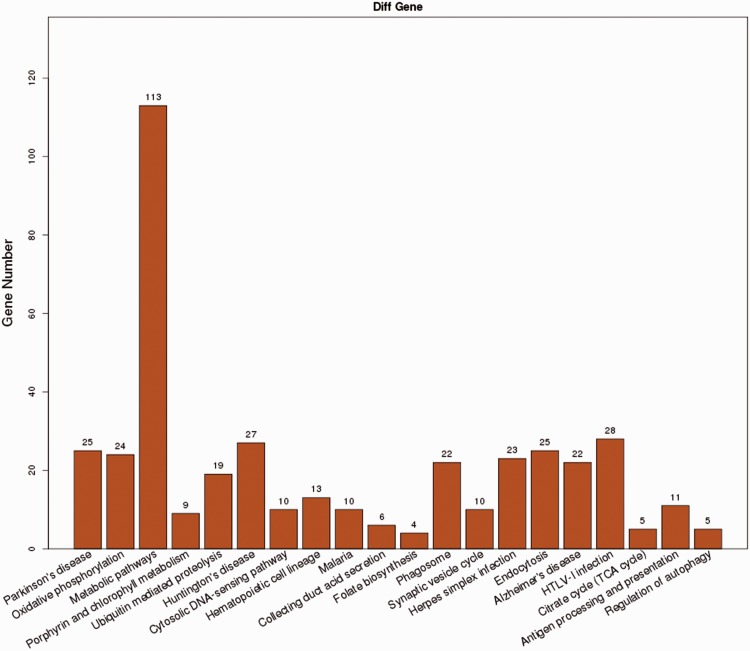
Among the genes included in the KEGG analysis, the majority of the genes were closely related to metabolic pathways (Figure 1), followed by those involved in human T-cell lymphotropic virus type 1 infection, Huntington’s disease, Parkinson’s disease and endocytosis. To investigate which signalling pathway may be involved in the modulation of blood lipids, Kobas software was used for the KEGG pathway analysis. Pathways with a P-value < 0.05 and false discovery rate <0.05 were considered to be closely involved in the metabolism of blood lipids. A total of 27 potential genes associated with the metabolism of blood lipids were identified (Table 4).
Figure 1.
Number of genes involved in various biological processes as identified by Kyoto Encyclopedia of Genes and Genomes analysis. HTLV-1, human T-cell lymphotropic virus type 1; TCA, tricarboxylic acid.
Table 4.
Identification of genes involved in the metabolic pathways using Kyoto Encyclopedia of Genes and Genomes analysis.
| ID | Sample number | Gene ID | Gene name |
|---|---|---|---|
| rno00062 | 4 | 54398|29411|170670|171155 | Ppt2||Ppt1||Hadha||Hadhb |
| rno00120 | 2 | 246211|170588 | Hsd3b7||Acot8 |
| rno00140 | 2 | 361802|24800 | Hsd17b8||Sts |
| rno00561 | 4 | 29254|294324|316737|311821 | Mgll||Ptdss2||Lpin2||Agpat2 |
| rno00564 | 5 | 294324|293620|316737|311821|287644 | Agpat3||Ptdss2||Lpin2||Agpat2||Phospho1 |
| rno00565 | 1 | 114113 | Pafah1b3 |
| rno00600 | 4 | 313339|65196|170897|83537 | Acer2||B4galt6||Sphk1||Smpd2 |
| rno00590 | 7 | 24404|287454|25290| 311865|24693|81639|24886 | Gpx1||Alox12||Alox5||Ptges2||Ptgs1|| Alox15||Tbxas1 |
| rno00591 | 1 | 81639 | Alox15 |
| rno00592 | 1 | 83512 | Fads2 |
Discussion
The KEGG project consists of a reference pathway database. The resulting projection of the reference pathways onto organisms with sequenced genomes has been widely used to predict the metabolic pathways present in an organism from the genome.12 This present study aimed to screen the genes associated with the metabolism of blood lipids in rats in order to identify the potential mechanisms responsible for changes in blood lipid levels. The current results demonstrated that burdock root extract could reduce body weight in a dose-dependent manner in rats fed a high-fat diet and it reduced cholesterol levels.
Arctium lappa L. (greater burdock), widely used in China for hundreds of years, has been commonly used as a medicine for reducing heat and detoxification.6 Generally, the burdock root is recommended as a food, and is accepted for the treatment of various disorders such as gout, hypertension, as well as inflammation.6 Previously, most of the studies on burdock root focused on the hepatoprotective and anti-inflammatory effects, as well as the anti-oxidant properties. For example, a previous study demonstrated that burdock root had a neuro-protective role against glutamate-induced oxidative stress by inhibiting the phosphorylation of p38, JNK and ERK signalling pathways.13 In addition, in a TNBS colitis model, burdock root showed anti-inflammatory intestinal activity in vivo.14 Moreover, burdock root showed protective effects on oxidation of low-density lipoprotein and oxidative stress in RAW 264.7 macrophages.15 Few studies have been performed to investigate the role of burdock root in lipid metabolism. In this present study, the levels of cholesterol were significantly lower in the groups treated with burdock root extract compared with the model group at 8 weeks after treatment. These findings suggest that burdock root extract could attenuate the cholesterol levels effectively.
To identify the potential molecular mechanisms involved, the present study investigated the differential expression of the genes that may be involved. Differential expression of genes in the model group revealed the total number of genes upregulated and downregulated were 665 and 699, respectively, compared with the three groups treated with burdock root and the group treated with simvastatin. Among these genes, 113 genes were associated with metabolic pathways. To identify the potential genes that may be involved in the metabolic pathways, KEGG analysis was performed. The KEGG analysis identified 27 genes (Ppt2, Ppt1, Hadha, Hadhb, Hsd3b7, Acot8, Hsd17b8, Sts, Mgll, Ptdss2, Lpin2, Agpat2, Agpat3, Phospho1, Pafah1b3, Acer2, B4galt6, Sphk1, Smpd2, Gpx1, Alox12, Alox5, Ptges2, Ptgs1, Alox15, Tbxas1, Fads2) involved in 10 metabolic pathways. Among these genes, several have been reported to be closely related to the metabolism of blood lipids such as Ptges2 and Agpat3. For example, the protein encoded by Ptges2 is a membrane-associated prostaglandin E synthase,16 which catalyses the conversion of prostaglandin H2 to prostaglandin E2 (PGE2). Membrane-associated prostaglandin E synthase (mPGE synthase) was previously purified to apparent homogeneity from the microsomal fraction of bovine heart.17 This protein has also been shown to activate the transcription regulated by a gamma-interferon-activated transcription element.17 To the best of our knowledge, Ptges2 gene polymorphisms were associated with fatty acid metabolism pathway and preterm delivery in a US urban black population.18 In addition, mice with a deletion of mPtges2 showed important effects on the eicosanoid and fatty acid profile.19 In particular, in lipopolysaccharide-induced peritoneal macrophages from mPGES-1 knock-out (mPGES-1 -/-, KO) mice, PGE2 production was markedly attenuated, whereas levels of prostaglandin D2 metabolites (15-deoxy-Δ(12,14) prostaglandin J2 and 15-deoxy-Δ(12,14) PGD2) were increased compared with wild type mice.19 The levels of oxidized fatty acid 13-hydroxyoctadecadienoic acid were also significantly up-regulated in KO macrophages.19 Significant differences in the total lipid fatty acid (FA) composition (decrease in monounsaturated FA and increase in eicosadienoic acid) were detected in the spleens of KO and WT mice.19 For the Agpat3 gene, the encoded product was reported to convert lysophosphatidic acid into phosphatidic acid.20 Previous research showed AGPAT3 and AGPAT5 prefer different lysophospholipids as acyl acceptors in the presence of different fatty acids, which provides solid evidence for the roles of mitochondria in promoting glycerophospholipids biosynthesis.21 In addition, the membrane topology of the human AGPAT3 gene has been reported to be involved in many reactions that produce phospholipids and triglycerides.22 Moreover, Agpat8 could accommodate fatty acyl chains of both substrates in an orientation where the NHX(4)D motif participates in catalysis.23 This present study demonstrated that the Ptges2 and Agpat8 genes were differentially expressed after burdock root extract treatment in rats. Despite the lack of direct evidence, we could speculate that these may be two of the targets for the modulation of blood lipids in vivo. Future studies will focus on screening the potential gene candidate(s) responsible for modulating lipid metabolism in rats.
The main limitation in this study was that although 27 potential genes associated with the metabolism of blood lipids were identified, it was not possible to determine the exact mechanisms involved during this current research study. In future, research will focus on the knock-down of gene expression in order to identify which gene(s) play crucial roles in these biological processes.
In conclusion, this present study showed that aqueous extract of burdock root could attenuate the body weight of rats in a dose-dependent manner following administration of a high-fat diet. In addition, it could also reduce cholesterol levels in the same rats. In order to achieve these changes, burdock root extract appears to induce differential expression of genes in rats.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Scientific Foundation (no. 81173593).
References
- 1.Wyatt SB, Winters KP, Dubbert PM. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Am J Med Sci 2006; 331: 166–174. [DOI] [PubMed] [Google Scholar]
- 2.Hurt RT, Kulisek C, Buchanan LA, et al. The obesity epidemic: challenges, health initiatives, and implications for gastroenterologists. Gastroenterol Hepatol (NY) 2010; 6: 780–792. [PMC free article] [PubMed] [Google Scholar]
- 3.Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet 2010; 375: 1737–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennion LJ, Grundy SM. Effects of obesity and caloric intake on biliary lipid metabolism in man. J Clin Invest 1975; 56: 996–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alappat L, Awad AB. Curcumin and obesity: evidence and mechanisms. Nutr Rev 2010; 68: 729–738. [DOI] [PubMed] [Google Scholar]
- 6.Cao J, Zhang P, Xu C, et al. Effect of aqueous extract of Arctium lappa L. (burdock) roots on the sexual behavior of male rats. BMC Complement Altern Med 2012; 12: 8–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan YS, Cheng LN, Wu JH, et al. A review of the pharmacological effects of Arctium lappa (burdock). Inflammopharmacology 2011; 19: 245–254. [DOI] [PubMed] [Google Scholar]
- 8.Best MM, Duncan CH. Effects of thiouracil and sitosterol on diet-induced hypercholesterolemia and lipomatous arterial lesions in the rat. Am Heart J 1959; 58: 214–220. [DOI] [PubMed] [Google Scholar]
- 9.Best MM, Duncan CH, Van Loon EJ, et al. The effects of sitosterol on serum lipids. Am J Med 1955; 19: 61–70. [DOI] [PubMed] [Google Scholar]
- 10.Ho DW, Yang ZF, Yi K, et al. Gene expression profiling of liver cancer stem cells by RNA-sequencing. PloS One 2012; 7: e37159–e37159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christenson MK, Trease AJ, Potluri LP, et al. De novo Assembly and Analysis of the Northern Leopard Frog Rana pipiens Transcriptome. J Genomics 2014; 2: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 2000; 28: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sólyomváry A, Mervai Z, Tóth G, et al. A simple and effective enrichment process of the antiproliferative lignan arctigenin based on the endogenous enzymatic hydrolysis of Serratula tinctoria and Arctium lappa fruits. Process Biochem 2015; 50: 2281–2288. [Google Scholar]
- 14.de Almeida AB, Sánchez-Hidalgo M, Martín AR, et al. Anti-inflammatory intestinal activity of Arctium lappa L.(Asteraceae) in TNBS colitis model. J Ethnopharmacol 2013; 146: 300–310. [DOI] [PubMed] [Google Scholar]
- 15.Wang BS, Yen GC, Chang LW, et al. Protective effects of burdock (Arctium lappa Linne) on oxidation of low-density lipoprotein and oxidative stress in RAW 264.7 macrophages. Food Chem 2007; 101: 729–738. [Google Scholar]
- 16.Chaudhry U, Zhuang H, Doré S. Microsomal prostaglandin-E synthase-2: cellular distribution and expression in Alzheimer’s disease. Exp Neurol 2010; 223: 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer A, Grallert H, Böhme M, et al. Association analysis between the prostaglandin E synthase 2 R298H polymorphism and body mass index in 8079 participants of the KORA study cohort. Genet Test Mol Biomarkers 2009; 13: 223–226. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Wang G, Hong X, et al. Associations between gene polymorphisms in fatty acid metabolism pathway and preterm delivery in a US urban black population. Hum Genet 2012; 131: 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Idborg H, Olsson P, Leclerc P, et al. Effects of mPGES-1 deletion on eicosanoid and fatty acid profiles in mice. Prostaglandins Other Lipid Mediat 2013; 107: 18–25. [DOI] [PubMed] [Google Scholar]
- 20.Leung DW. The structure and functions of human lysophosphatidic acid acyltransferases. Front Biosci 2001; 6: D944–D953. [DOI] [PubMed] [Google Scholar]
- 21.Prasad SS, Garg A, Agarwal AK. Enzymatic activities of the human AGPAT isoform 3 and isoform 5: localization of AGPAT5 to mitochondria. J Lipid Res 2011; 52: 451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt JA, Yvone GM, Brown WJ. Membrane topology of human AGPAT3 (LPAAT3). Biochem Biophys Res Commun 2010; 397: 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal AK, Barnes RI, Garg A. Functional characterization of human 1-acylglycerol-3-phosphate acyltransferase isoform 8: cloning, tissue distribution, gene structure, and enzymatic activity. Arch Biochem Biophys 2006; 449: 64–76. [DOI] [PubMed] [Google Scholar]