Abstract
Objective
To evaluate the clinical application of the minimally invasive modified pedicle screw–rod fixator for unstable pelvic ring injuries, including its feasibility, merits, and limitations.
Methods
Twenty-three patients (13 males, 10 females; average age, 36.3 years) with unstable pelvic ring injuries underwent anterior fixation using a modified pedicle screw–rod fixator with or without posterior fixation using a transiliac internal fixator. The clinical findings were assessed using Majeed scores. The quality of reduction was evaluated using the Matta criteria.
Results
Clinical results at 1 year postoperatively were excellent in 14 patients, good in 7, and fair in 2. The two patients with fair results had intermittent pain at the sacroiliac joint because of the posterior implant. One woman complained of persistent pain at the pubic tubercle during sexual intercourse. Iatrogenic neuropraxia of the unilateral lateral femoral cutaneous nerve occurred in three patients. Unilateral femoral nerve palsy occurred in one patient. The quality of fracture reduction was excellent in 12 patients, good in 8, and fair in 3. Heterotopic ossification occurred in eight patients; all were asymptomatic.
Conclusions
Minimally invasive modified pedicle screw-rod fixation is an effective alternative treatment for pelvic ring injuries.
Keywords: Pelvic ring, internal fixators, minimally invasive, anterior fixation, posterior fixation, modified INFIX
Introduction
Pelvic fractures represent a relatively rare injury, constituting only 0.3% to 6.0% of all fractures.1 However, high-energy pelvic ring injuries are often associated with high mortality and morbidity rates because of concomitant life-threatening damage and mechanical instability of the pelvic ring. Surgeons widely agree that for unstable pelvic ring injuries, proper reduction and fixation should be performed as early as possible to restore pelvic stability, promote functional rehabilitation, and reduce potential complications. However, some patients who undergo surgery for pelvic ring injuries develop postoperative pain, restricted activities of daily life, nonunion, and malunion. Successful treatment of unstable pelvic ring injuries is still a challenge for orthopedic surgeons.
Minimally invasive techniques are currently in widespread use. Compared with traditional open reduction and internal fixation, the potential benefits of minimally invasive techniques may include less blood loss, fewer soft tissue infections, better pain control, and faster rehabilitation. The pedicle screw–rod fixator is used in conjunction with a minimal-incision technique that was originally applied for posterior pelvic injuries. It is a type of transiliac internal fixator (TIFI) that bridges the sacroiliac joints and sacral area instead of widely exposing the fracture region.2,10,15 For anterior pelvic injuries, use of internal fixation (INFIX) and the pelvic bridge technique were recently described as minimally invasive. INFIX involves the use of a pedicle screw–rod fixator comprising two pedicle screws fixed into bilateral supra-acetabular bone and a curved rod interconnecting subcutaneously.3,18,25 Similarly, the pelvic bridge technique involves the use of a reconstruction plate or plate–rod system fixed subcutaneously from the ipsilateral iliac crest to the contralateral pubic tubercle.4,5,23 With reference to the two above-mentioned techniques, we modified routine INFIX for anterior ring fixation, and a TIFI was applied for posterior ring injuries if necessary. For patients with pubic symphysis separation, we applied symphyseal internal rod fixation (SYMFIX), which also involves the use of a modified screw–rod fixator.6 The purpose of this study was to evaluate the clinical application of this minimally invasive modified pedicle screw–rod fixator for unstable pelvic ring injuries, including the feasibility, merits, and limitations of the technique. The surgical technique and our initial clinical experience are presented in a series of 23 consecutive patients.
Patients and Methods
Preoperative evaluation
From January 2013 to October 2015, a total of 23 patients with unstable pelvic ring injuries underwent definitive anterior fixation using a modified pedicle screw–rod fixator with or without posterior fixation using a TIFI. Ethical review board approval was obtained before the start of this study. All patients provided written informed consent for participation in the study. The inclusion criterion was a rotationally unstable pelvic ring injury requiring anterior fixation. Such injuries included pubic ramus fractures and symphyseal disruption. The exclusion criteria were an age of <18 years, hemodynamic instability making patients unsuitable for surgery within 3 weeks, infection or soft tissue defects that prevented coverage of the fixation, acetabular or supra-acetabular fractures that impaired the stabilization of screw insertion, and a history of conservatively or surgically treated pelvic injuries. The indications for surgical repair of posterior ring injuries were sacroiliac displacement and sacral fractures. Comminuted sacral fractures were the best indications because no compression is possible for sacroiliac screws. Contraindications included sacroiliac joint disruptions combined with fractures of the dorsal ilium or sacral plexus injuries requiring open decompression.
All patients required preoperative anteroposterior and inlet and outlet pelvic radiographs to fully evaluate the displaced pelvic ring injuries. Three-dimensional pelvic computed tomography scans were performed as well. Preoperatively, we evaluated the severity of the injuries using the Injury Severity Score; the average score was 21.2 points (range, 9–50 points). According to the Advanced Trauma Life Support program guidelines, initial stabilization of vital signs was urgently performed. Six patients required aggressive fluid and blood transfusion and resuscitation in the intensive care unit because of hemodynamic instabilities. All patients were treated with preoperative ipsilateral skeletal or external traction to contribute to fracture reduction. Surgery was scheduled as early as possible when the patient’s physiological condition was stable. The average time from injury to surgery was 4.7 days (range, 1–12 days).
Surgical procedures
TIFI for posterior ring fixation
In patients with posterior instability, posterior fixation using a TIFI was typically performed first. The prone position was employed. The posterior superior iliac spine (PSIS) and dorsal iliac crest were marked preoperatively. Bilateral 3-cm incisions were made along the PSIS. The osseous entry point was located at the medial side of the dorsal iliac crest and 1 cm cranial to the PSIS. The pedicle finder was used to create a bony tunnel toward the anterior inferior iliac spine (AIIS). After ensuring that the tunnel did not penetrate the bony cortex, two 7-mm-diameter polyaxial pedicle screws with a length of 50 to 60 mm were inserted into the bilateral dorsal iliac crests. Partial resection of the PSIS at the entry point for settling the screw heads was preferred to minimize implant prominence and soft tissue irritation. A 6-mm-diameter titanium rod was maneuvered subfascially into the screw heads to connect the two screws. Compression, distraction, or even injured leg traction was applied to achieve reduction according to the characteristics of the posterior ring dislocation. The screw caps were tightened after the reduction, and the screw positions were checked with the anteroposterior, outlet, and inlet views of C-arm fluoroscopy.
Modified INFIX for anterior ring injuries
After stabilizing the posterior elements, the anterior pelvic ring was addressed. The patient was then placed on a radiolucent operative table in the supine position. The entire pelvis was sterilely prepped and draped from above the umbilicus to the proximal thigh. During routine INFIX, C-arm fluoroscopy was used to identify the AIIS as the osseous entry. The AIIS is not easily palpable in obese patients, but generally lies 3 to 4 cm distal and 2 cm medial to the anterior superior iliac spine. C-arm fluoroscopy was used to obtain an obturator outlet view and adjusted to obtain a “teardrop” appearance of the AIIS. A 3-cm longitudinal incision was then centered over the AIIS in line with the groin crease. Care was taken to protect the lateral femoral cutaneous nerve (LFCN), which often ran across the surgical field. Blunt dissection was performed between the sartorius and tensor fascia lata muscles to gain access to the AIIS. A starting pedicle awl was placed in the middle of the AIIS, which was the center of the “teardrop,” to open the cortex. Next, a pedicle finder was used to establish a bony tunnel toward the PSIS. The obturator inlet view was used to confirm that the tunnel had not penetrated the ilium. We also used the iliac oblique view to ensure that the pedicle finder was well clear of the hip joint and greater sciatic notch. A polyaxial pedicle screw was then inserted in the tunnel for adequate depth. Importantly, the screw head was fixed roughly 2 cm proud of the bone. In accordance with the Asian body habitus of the patients in this series, we used screws that were 60 to 80 mm in length and 6.5 or 7.0 mm in diameter. The same procedure was repeated for the contralateral hemipelvis. To modify the INFIX technique, we made an additional 3-cm Pfannenstiel incision 2 cm over the pubic symphysis. Blunt dissection was performed down to the level of the pubic tubercle. Either the ipsilateral or contralateral pubic tubercle was chosen to insert a polyaxial pedicle screw down into the inferior ramus. The pelvic inlet view was used to confirm the entry point of the pubic tubercle. The coronal orientation of the screw path was 45 to 60 degrees. Similarly, the screw head was left 2 cm proud of the pubic tubercle. We used screws that were 35 to 50 mm in length and 6.5 or 7.0 mm in diameter. After three screws had been positioned at the appropriate depth, a 6-mm-diameter long titanium rod was placed over all three screws to adjust its length and curve. The rod was often precontoured with an anterior and inferior bow to avoid potential compressive neurovascular complications. To develop a tunnel just under the superficial aspect of the fascia, long hemostats were inserted into the subcutaneous tissue from the Pfannenstiel incision to the incisions at the bilateral AIIS. The rod was then gently slid into the tunnel from the incision at the ipsilateral AIIS to the contralateral AIIS via the pubic tubercles. The rod was maneuvered into the screw heads to connect the three screws, and the screw caps were fixed loosely to retain the rod in place. Polyaxial screw heads greatly facilitated rod placement. At this point, the reduction tools were attached to the pedicle screws at the bilateral AIIS to manipulate both hemipelvises. Reduction of the anterior ring was achieved by lateral compression and, if necessary, leg traction and internal rotation. In patients with Tile type B2 fractures, the anterior ring was usually reduced without compression but with some distraction if necessary. As a final step, the screw caps were tightened definitively with a torque screwdriver to maintain the reduction. Importantly, the screws at the bilateral AIIS were tightened prior to tightening the screw at the pubic tubercle (Figure 1).
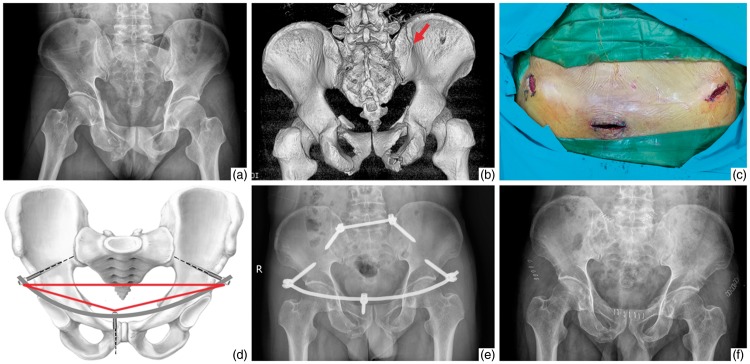
Figure 1.
A 35-year-old man with anterior and posterior pelvic ring injuries (Tile type C1) caused by a traffic accident. (a, b) Preoperative plain X-ray film and three-dimensional computed tomography image showed bilateral pubic ramus fractures combined with an avulsion fracture of the left posterior superior iliac spine. The red arrow shows the dorsal fracture. (c) Anterior surgical incisions for modified internal fixation. (d) Three screws at both the anterior inferior iliac spine and pubic tubercle constituted a geometric triangle. (e) Postoperative plain X-ray film showed good reduction after performance of anterior modified internal fixation using a posterior transiliac internal fixator. (f) One-year postoperative plain X-ray film showed fracture healing as evidenced by progressive callus formation.
SYMFIX for pubic symphysis separation
The patient was placed in the supine position. A Pfannenstiel incision was made over the pubic symphysis. Two polyaxial pedicle screws were fixed in the bilateral pubic tubercles, while no screw fixation was necessary for the bilateral AIIS. The pedicle screws were the same as those used in the pubic tubercle during the above-described modified INFIX procedure. A short straight titanium rod was used to connect the two screws. A compressor was attached to the two screw heads to close the pelvis anteriorly through the rod. Once compression was achieved, the screw caps were tightened (Figure 2).
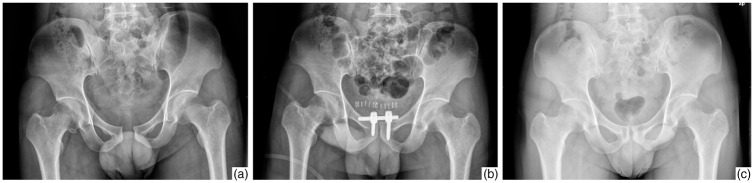
Figure 2.
A 30-year-old man with pubic symphysis separation (Tile type B1) caused by a crush injury. (a) Preoperative plain X-ray film showed pubic symphysis separation of >25 mm. (b) Postoperative plain X-ray film showed excellent reduction of the pubic symphysis with symphyseal internal rod fixation. (c) Nine-month postoperative plain X-ray film showed no displacement or heterotopic ossification.
Postoperative management and follow-up
Each patient’s postoperative rehabilitation protocol was dependent upon on the specific configuration of his or her pelvic ring injury and operative technique. All patients performed functional exercises of the lower limbs and joints in bed without weight bearing from postoperative day 1. The patients were allowed to sit on the bedside at 2 weeks postoperatively. Partial weight bearing with crutches was permitted at 4 weeks postoperatively for patients who underwent only anterior ring fixation and at 6 weeks postoperatively for those who underwent both anterior and posterior ring fixation. Finally, the patients were allowed to gradually begin walking with full weight bearing at 3 months postoperatively when postoperative radiographs demonstrated union as evidenced by progressive callus formation. The implant was removed at an average of 10 months postoperatively (range, 4–12 months). Follow-up visits were arranged at 4 weeks, 6 weeks, 3 months, 6 months, 9 months, 12 months, and 15 months postoperatively for clinical and radiological examinations. The clinical findings before removal of the implant were assessed using Majeed scores, which included five functional indicators (pain, sitting, standing, sexual intercourse, and work).8 The quality of pelvic ring reduction was evaluated according to the Matta criteria, which involve grading of the maximal displacement measured on anteroposterior and inlet and outlet pelvic radiographs: excellent (0–4 mm), good (5–10 mm), fair (11–20 mm), or poor (>20 mm).9
Results
The patients comprised 13 males and 10 females with an average age of 36.3 years (range, 20–57 years). According to the Tile classification, there were 18 type B fractures (3 type B1, 7 type B2, and 8 type B3) and 5 type C fractures (5 type C1).7 The fractures were caused by traffic accidents in 10 patients, falls from a height in 7, and crush injuries in 6. Concomitant injuries included six lower extremity fractures, eight upper limb fractures, two lumbar fractures, five chest injuries, three urinary injuries, and two craniocerebral injuries (Table 1). In this study, 7 patients underwent only anterior pelvic ring fixation and 16 patients underwent both anterior and posterior ring fixation. The operation for anterior ring fixation took an average of 26.6 min (range, 15–36 min), with a mean intraoperative blood loss volume of 29.1 ml (range, 20–37 ml). The operation for both anterior and posterior ring fixation took an average of 50.6 min (range, 43–69 min), with a mean intraoperative blood loss volume of 56.8 ml (range, 39–75 ml). For both anterior and posterior ring fixation, the time spent turning the patient from the prone to supine position was not included in the operation time. All patients were followed up for an average of 15 months (range, 13–20 months), and no patients died or were lost to follow-up. No patients developed hemorrhagic shock, deep venous thrombosis, or wound infection postoperatively. According to the Majeed scores, the clinical results before removal of the implant were excellent in 14 patients, good in 7, and fair in 2 (Table 2). The two patients with fair results had intermittent pain at the sacroiliac joint because of the posterior implant. The pain was gradually relieved after removal of the TIFI. All patients with anterior implants could sit, stand, squat, lie prone, and lie on their side normally. The anterior screw caps and the titanium rod could be palpated without sharp pain except in one lean woman who complained of persistent pain at the pubic tubercle during sexual intercourse. Her pain was also relieved after anterior implant removal. Iatrogenic neuropraxia of the unilateral LFCN occurred in three patients and resolved spontaneously at 1 month postoperatively. One patient developed unilateral femoral nerve palsy on postoperative day 1. The patient underwent urgent reoperation for adjustment of the anterior ring fixation. The palsy was gradually relieved thereafter, and the patient fully recovered within 2 months after anterior implant removal. All pelvic fractures achieved bone union without nonunion or malunion. Postoperative radiographs showed no secondary displacement, loosening, or fracture of the implants. Heterotopic ossification at the anterior screw heads was noted in eight patients, all of whom were clinically asymptomatic. According to the Matta criteria, the quality of fracture reduction was excellent in 12 patients, good in 8, and fair in 3.
Table 1.
Patients’ characteristics.
| No. | Sex | Age (y) | Weight (kg) | Tile type of fracture | Cause of injury | Concomitant injuries | Time from injury to surgery (d) | Surgical procedures | Operation time (min) | Blood loss (ml) | Fracture reduction |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 23 | 79 | C1 | Traffic injury | Left humeral fracture, bladder rupture | 6 | Modified INFIX + TIFI | 61 | 75 | Good |
| 2 | Male | 34 | 69 | B2 | Fall injury | None | 4 | Modified INFIX | 30 | 34 | Good |
| 3 | Female | 29 | 61 | B1 | Traffic injury | Right ankle fracture | 5 | Modified INFIX | 27 | 33 | Excellent |
| 4 | Male | 57 | 69 | C1 | Fall injury | Epidural hematoma, right distal radial fracture, right calcaneal fracture | 10 | Modified INFIX + TIFI | 69 | 70 | Excellent |
| 5 | Female | 33 | 65 | B2 | Traffic injury | None | 3 | Modified INFIX + TIFI | 49 | 66 | Good |
| 6 | Female | 37 | 58 | B3 | Crush injury | Left radial fracture, multiple rib fractures | 5 | Modified INFIX + TIFI | 52 | 53 | Fair |
| 7 | Male | 45 | 72 | B2 | Traffic injury | None | 2 | Modified INFIX | 36 | 37 | Excellent |
| 8 | Female | 20 | 53 | B3 | Fall injury | Left and right calcaneal fractures, L1 compression fracture | 9 | Modified INFIX + TIFI | 59 | 55 | Excellent |
| 9 | Male | 38 | 71 | B1 | Crush injury | None | 1 | Modified INFIX | 24 | 29 | Excellent |
| 10 | Male | 42 | 75 | B2 | Crush injury | Right ulnar fracture | 3 | Modified INFIX + TIFI | 43 | 42 | Excellent |
| 11 | Male | 53 | 68 | B3 | Fall injury | Left intertrochanteric fracture, rib fracture | 5 | Modified INFIX + TIFI | 52 | 47 | Fair |
| 12 | Female | 29 | 63 | B3 | Traffic injury | None | 3 | Modified INFIX + TIFI | 53 | 61 | Good |
| 13 | Female | 31 | 57 | B3 | Fall injury | L2 compression fracture, right femoral fracture | 5 | Modified INFIX + TIFI | 49 | 41 | Excellent |
| 14 | Male | 49 | 67 | C1 | Crush injury | Left distal radial fracture, multiple rib fractures | 6 | Modified INFIX + TIFI | 58 | 58 | Good |
| 15 | Female | 43 | 62 | B3 | Traffic injury | Rib fracture | 4 | Modified INFIX + TIFI | 47 | 39 | Fair |
| 16 | Male | 33 | 77 | B2 | Crush injury | None | 4 | Modified INFIX | 31 | 25 | Excellent |
| 17 | Female | 36 | 59 | C1 | Traffic injury | Left olecranon fracture | 4 | Modified INFIX + TIFI | 53 | 67 | Good |
| 18 | Male | 35 | 80 | B3 | Traffic injury | Subdural hematoma, right tibiofibular fracture, multiple rib fractures | 12 | Modified INFIX + TIFI | 64 | 69 | Excellent |
| 19 | Male | 43 | 72 | C1 | Fall injury | None | 3 | Modified INFIX + TIFI | 47 | 45 | Good |
| 20 | Female | 45 | 65 | B2 | Traffic injury | Left distal radiual fracture, urethral rupture | 4 | Modified INFIX + TIFI | 57 | 63 | Excellent |
| 21 | Male | 30 | 67 | B1 | Crush injury | None | 2 | SYMFIX | 15 | 20 | Excellent |
| 22 | Female | 25 | 46 | B2 | Fall injury | None | 3 | Modified INFIX | 23 | 26 | Good |
| 23 | Male | 24 | 78 | B3 | Traffic injury | Right humeral fracture, urethral rupture | 5 | Modified INFIX + TIFI | 55 | 58 | Excellent |
INFIX, internal fixation; TIFI, transiliac internal fixator; SYMFIX, symphyseal internal rod fixation
Table 2.
Majeed scores before implant removal.
| No. | Pain | Sitting | Standing | Sexual intercourse | Work | Total | Grade | Complications |
|---|---|---|---|---|---|---|---|---|
| 1 | 20 | 8 | 30 | 4 | 12 | 74 | Good | LFCN irritation, heterotopic ossification |
| 2 | 25 | 10 | 34 | 4 | 20 | 93 | Excellent | Heterotopic ossification |
| 3 | 30 | 10 | 34 | 4 | 16 | 94 | Excellent | |
| 4 | 25 | 10 | 32 | 4 | 16 | 87 | Excellent | Heterotopic ossification |
| 5 | 25 | 8 | 30 | 3 | 16 | 82 | Good | |
| 6 | 20 | 8 | 28 | 4 | 16 | 76 | Good | Heterotopic ossification |
| 7 | 30 | 8 | 36 | 4 | 16 | 94 | Excellent | |
| 8 | 25 | 10 | 32 | 4 | 20 | 91 | Excellent | |
| 9 | 25 | 10 | 36 | 4 | 20 | 95 | Excellent | |
| 10 | 25 | 10 | 30 | 4 | 20 | 89 | Excellent | |
| 11 | 15 | 6 | 28 | 3 | 12 | 64 | Fair | Heterotopic ossification |
| 12 | 25 | 8 | 30 | 4 | 12 | 79 | Good | LFCN irritation |
| 13 | 25 | 8 | 36 | 4 | 16 | 89 | Excellent | |
| 14 | 20 | 8 | 28 | 3 | 16 | 75 | Good | Heterotopic ossification |
| 15 | 15 | 6 | 30 | 3 | 12 | 66 | Fair | Femoral nerve palsy |
| 16 | 30 | 10 | 32 | 4 | 16 | 92 | Excellent | |
| 17 | 25 | 8 | 30 | 4 | 20 | 87 | Excellent | Heterotopic ossification |
| 18 | 25 | 10 | 34 | 4 | 20 | 93 | Excellent | |
| 19 | 20 | 8 | 30 | 3 | 16 | 77 | Good | Heterotopic ossification |
| 20 | 30 | 10 | 30 | 4 | 20 | 94 | Excellent | LFCN irritation |
| 21 | 30 | 10 | 32 | 4 | 20 | 96 | Excellent | |
| 22 | 25 | 8 | 28 | 2 | 16 | 79 | Good | Pain during sexual intercourse |
| 23 | 25 | 8 | 32 | 4 | 20 | 89 | Excellent |
LFCN, lateral femoral cutaneous nerve
Discussion
The pelvic ring comprises the hip bone, sacrum, and several ligaments. The anterior pelvic ring includes the pubic symphysis, bilateral pubic ramus, and ventral ilium, providing 30% of pelvic stability. The posterior ring mainly comprises the sacrum, dorsal ilium, and sacroiliac joint complex, providing 70% of pelvic stability.10 During the past several decades, various techniques have been employed for both anterior and posterior pelvic ring injuries. Common methods of anterior pelvic fixation include open plating, external fixation, and intramedullary screw fixation. Fixation of posterior pelvic injuries involves percutaneous sacroiliac screws, reconstruction plates, and pedicle screw-rod fixators. Each technique has its own unique set of advantages and drawbacks. The optimal fixation technique for pelvic ring injuries remains controversial. Anterior external fixation is helpful to achieve initial hemodynamic stabilization with a shorter operating time and less blood loss than open reduction and internal fixation. However, it fails to maintain sufficient stability for vertically unstable injuries. Its complications include pin tract infection, aseptic loosening, restricted activities, and nerve damage. There has been an increasing interest in minimally invasive plate osteosynthesis for anterior pelvic fixation with less trauma and better stability.11,17 Nevertheless, neurovascular injuries may occur because of difficulty in dissection and a prolonged learning time. Additionally, the ligaments and muscle are partially excised during exposure of the medial window, which may cause hernias.11 For posterior plate osteosynthesis, suitable bending of the plates intraoperatively is technically demanding and sometimes difficult to achieve. The plate may also easily irritate local soft tissue and cause discomfort in the supine position.10 Percutaneous sacroiliac screw fixation has the advantage of secure stability and minimal blood loss, but it requires an experienced surgeon and high-quality intraoperative fluoroscopy. Nerve root damage might occur if the direction of the screw deviates from 4 degrees.12
Spinal instruments were originally applied with the Galveston technique for treatment of lumbosacral junction fractures. Lumbopelvic distraction spondylodesis was subsequently introduced for sacral fractures, but this technique restricts mobility of the lumbar segment and renders no rotational stability.13 In 1998, Schildhauer et al.14 reported that triangular osteosynthesis provides rotational stability. Triangular osteosynthesis involves lumbopelvic distraction and transverse fixation with wide exposure and more trauma. In the present study, a TIFI was applied to stabilize the posterior pelvic ring; this technique was first reported by Korovessis et al.15 in 2000. It is a percutaneous technique involving less exposure and avoidance of the lumbar spine. The fixator functions as a suspension bridge structure similar to the sacroiliac joint complex, partly maintaining the integrity of the pelvic ring. The bilateral iliac pedicle screws are directed obliquely, creating a strong torque to withstand both sagittal plane rotations. Because vertical displacement lacks a reduction capability, injured leg traction is necessary for vertical reduction of sacroiliac joint disruptions. In their biomechanical study, Vigdorchik et al.16 preferred to use a TIFI for sacral fractures, not sacroiliac joint disruptions. We found no clinical difference in the stability between the two posterior injuries. For comminuted sacral fractures, Zhu et al.17 fixed two screws in each ilium to strengthen the solidity. We used one larger-diameter screw in each ilium to withstand the pullout force and achieved satisfactory radiological outcomes. In our experience, the soft tissue attached to the dorsal ilium was not stripped off to avoid iatrogenic neurovascular injury, a narrow angle in the sagittal plane was preferred for screw insertion to reduce soft tissue irritation, and the screw heads were settled in the iliac cortex at a level not higher than the PSIS. Our study involving use of a TIFI showed that no skin infection occurred.
Surgical use of a pedicle screw–rod fixator for treatment of anterior pelvic ring fractures was first reported by Kuttner et al.18 in 2009. This INFIX technique has gained popularity as an alternative to external fixation because it avoids issues with pin sites and long-term discomfort, especially in morbidly obese patients. Compared with external fixation, INFIX has similar translational and superior rotational stiffness of in vitro mechanical stability.19 In one study involving a single-stance pelvic fracture model, INFIX was stiffer than external fixation at both the pubic symphysis and sacroiliac joint.20 The type of pedicle screw determines the performance of INFIX, with monoaxial screws providing significantly more stiffness than polyaxial screws. Some authors have stated that polyaxial INFIX is not stiffer than external fixation.21 However, polyaxial screws have been suggested to reduce the difficulty of rod manipulation. Failure of INFIX has been reported in morbidly obese patients because of extreme force. Owen et al.22 described the use of two interconnected INFIX devices as a salvage technique for this problem. We designed a modified INFIX inspired by the pelvic bridge. In all previously published reports, INFIX involves two polyaxial screws fixed in both AIIS. By means of the Pfannenstiel incision used in the pelvic bridge, we fixed an additional polyaxial screw in either pubic tubercle. Three screws at both AIIS and the pubic tubercle constituted a geometric triangle, which added the conjunctive points between the screw and the curved rod. The curvature of the rod was more analogous to the anatomy of the anterior pelvic ring. Hence, this modified INFIX using three-point fixation is thought to be more stable than routine INFIX using two-point fixation for anterior pelvic ring fractures, although biomechanical testing is required for verification. Like the pelvic bridge, no fascia or muscle trauma occurs in the Pfannenstiel incision because the screws are positioned directly over the tubercles where the rectus tendons attach.23 This minimizes the risk of ilioinguinal nerve and iliohypogastric nerve neuropathy. An additional Pfannenstiel incision has also been used to create a subcutaneous tunnel to the bilateral AIIS; this is similar to the pelvic bridge technique for bilateral pubic ramus fractures. For routine INFIX, the subcutaneous tunnel is created directly from one side of the AIIS to the other, increasing the risk of neurovascular injury and abdominal perforation. In contrast to routine INFIX, the rod is not placed under the bikini line location, but still within the bikini area. One study showed that the bikini area stayed in a relatively stable position from sitting to standing in all individuals.24 Our Majeed scores verified no interference with sitting, standing, or squatting. No sharp pain developed in association with the anterior screws and rod, with the exception of persistent pain at the pubic tubercle during sexual intercourse in a lean woman. In this case, the soft tissue over the pubic tubercle was so thin that it became irritated by the screw in the pubic tubercle. Her pain was relieved after removal of the pubic tubercle screw. Based on both the clinical and radiological results, there was no selective difference in the additional screw position on either side of the pubic tubercle, especially for bilateral pubic ramus fractures (butterfly fractures). According to our experience, the screws at the bilateral AIIS should be tightened prior to ensure that the strain is concentrated in the supra-acetabular region, which is composed of dense cancellous bone that provides excellent purchase for fixation. In contrast, the pubic tubercle has relatively sparse bone quality. The pubic tubercle screw assists in pullout strain. Compared with previous reports of minimally invasive plate osteosynthesis,11,17 our modified INFIX for anterior fixation had a relatively lower quality of fracture reduction but comparable clinical outcomes as indicated by the Majeed scores. This might have occurred because the quality of fracture reduction is not always positively associated with the clinical outcome.
LFCN irritation is the most prevalent iatrogenic neurovascular complication. The LFCN may be injured during dissection, rod placement, or even rod removal. The length of the rod end lateral to the screw has a direct relationship with the LFCN. The rod should be trimmed as short as possible to reduce LFCN irritation. Temporary neuropraxia of the unilateral LFCN was observed in 13% (3 of 23) of patients in the present study, which is lower than the rate of 30% reported by Vaidya et al.3 All symptoms were transient and did not recur after rod removal.25 Femoral nerve palsy is considered a rare and devastating complication of INFIX. A likely cause is the limited space available for the psoas and femoral nerve.26 Sinking down the screws or undercontouring the rod can lead to compression of the femoral nerve and artery. The screws are suggested to be placed 15 to 40 mm proud of the bone depending on the patient’s body habitus. The surgeon’s inexperience may also be a risk factor. In a cadaveric study, the femoral nerve was found to be the structure most at risk of compression by the rod.27 Hesse et al.26 reported that resolution of femoral nerve palsy was variable and incomplete despite removal of the implants. In the present study, only one case of unilateral femoral nerve palsy was noted. The anterior implant was urgently revised and the palsy gradually resolved. The patient recovered fully after removal of the anterior implant. We consider that early diagnosis and adjustment without delay played a significant role in the nerve recovery in this patient. Heterotopic ossification at the screw heads was an asymptomatic finding in 34.8% (8 of 23) of patients in our study, which is comparable with the rate of 35% in routine INFIX.25 Thorough lavage of the surgical site is recommended for prevention of this complication. We could conclude that despite the additional incision and screw, complications of modified INFIX did not increase significantly.
This was an initial clinical series of our experience using modified pedicle screw–rod fixation for unstable pelvic ring injuries. Several limitations of our study should be acknowledged. First, because of the small sample size and lack of long-term follow up, the final evaluation needs further investigation. Second, we used a clinical case-based analysis to predict the stability of the fixation methods. A randomized case-control study is necessary to obtain validated results. Third, our conclusion was an indirect deduction and lacked direct support from biomechanical evidence. In particular, whether the injured side of the pubic tubercle differed from the uninjured for the screw position in the biomechanical stability is unclear. Biomechanical tests and finite element analysis are needed to provide a theoretical basis.
Conclusions
Pelvic ring disruptions are challenging injuries to treat. Based on the good clinical and radiological outcomes obtained in the present study, our minimally invasive modified pedicle screw–rod fixation provided satisfactory efficacy for unstable pelvic ring injuries. We believe that our method is an effective alternative for treatment of pelvic ring injuries. Future multi-institutional, prospective randomized studies are needed for further evaluation.
Authors’ contributions
X.T.W. and Z.Q.L. conceived the study. Z.Q.L. and W.Q.F. performed the surgery. S.Z. collected and analyzed the data. X.T.W. drafted the manuscript. All authors read and approved the final manuscript.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Gansslen A, Pohlemann T, Paul C, et al. Epidemiology of pelvic ring injuries. Injury 1996; 27(Suppl 1): S-A13–20. [PubMed] [Google Scholar]
- 2.Dienstknecht T, Berner A, Lenich A, et al. A minimally invasive stabilizing system for dorsal pelvic ring injuries. Clin Orthop Relat Res 2011; 469: 3209–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaidya R, Colen R, Vigdorchik J, et al. Treatment of unstable pelvic ring injuries with an internal anterior fixator and posterior fixation: initial clinical series. J Orthop Trauma 2012; 26: 1–8. [DOI] [PubMed] [Google Scholar]
- 4.Cole PA, Gauger EM, Anavian J, et al. Anterior pelvic external fixator versus subcutaneous internal fixator in the treatment of anterior ring pelvic fractures. J Orthop Trauma 2012; 26: 269–277. [DOI] [PubMed] [Google Scholar]
- 5.Hiesterman TG, Hill BW, Cole PA. Surgical technique: a percutaneous method of subcutaneous fixation for the anterior pelvic ring: the pelvic bridge. Clin Orthop Relat Res 2012; 470: 2116–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osterhoff G, Tiziani S, Hafner C, et al. Symphyseal internal rod fixation versus standard plate fixation for open book pelvic ring injuries: a biomechanical study. Eur J Trauma Emerg Surg 2016; 42: 197–202. [DOI] [PubMed] [Google Scholar]
- 7.Tile M. Pelvic ring fractures: should they be fixed? J Bone Joint Surg Br 1988; 70: 1–12. [DOI] [PubMed] [Google Scholar]
- 8.Majeed SA. Grading the outcome of pelvic fractures. J Bone Joint Surg Br 1989; 71: 304–306. [DOI] [PubMed] [Google Scholar]
- 9.Matta JM. Indications for anterior fixation of pelvic fractures. Clin Orthop Relat Res 1996; 329: 88–96. [DOI] [PubMed] [Google Scholar]
- 10.Bi C, Wang Q, Nagelli C, et al. Treatment of unstable posterior pelvic ring fracture with pedicle screw-rod fixator versus locking compression plate: a comparative study. Med Sci Monit 2016; 22: 3764–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu X, Tang M, Zhou Z, et al. Minimally invasive treatment for pubic ramus fractures combined with a sacroiliac joint complex injury. Int Orthop 2013; 37: 1547–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sagi HC, Lindvall EM. Inadvertent intraforaminal iliosacral screw placement despite apparent appropriate positioning on intraoperative fluoroscopy. J Orthop Trauma 2005; 19: 130–133. [DOI] [PubMed] [Google Scholar]
- 13.Käch K, Trentz O. Distractionsspondylodese des Sacrums bei “Vertical-Shear-Laesionen” des Beckens. Unfallchirurg 1994; 97: 28–38. [in German, English Abstract]. [PubMed] [Google Scholar]
- 14.Schildhauer TA, Josten C, Muhr G. Triangular osteosynthesis of vertically unstable sacrum fractures: a new concept allowing early weight-bearing. J Orthop Trauma 1998; 12: 307–314. [DOI] [PubMed] [Google Scholar]
- 15.Korovessis P, Stamatakis M, Baikousis A. Posterior stabilization of unstable sacroiliac injuries with the Texas Scottish Rite Hospital spinal instrumentation. Orthopedics 2000; 23: 323–327. [DOI] [PubMed] [Google Scholar]
- 16.Vigdorchik JM, Jin X, Sethi A, et al. A biomechanical study of standard posterior pelvic ring fixation versus a posterior pedicle screw construct. Injury 2015; 227: 1491–1496. [DOI] [PubMed] [Google Scholar]
- 17.Zhu L, Wang L, Shen D, et al. Treatment of pelvic fractures through a less invasive ilioinguinal approach combined with a minimally invasive posterior approach. BMC Musculoskeletal Disorders 2015; 16: 167–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuttner M, Klaiber A, Lorenz T, et al. The pelvic subcutaneous cross-over internal fixator. Unfallchirurg 2009; 112: 661–669. [DOI] [PubMed] [Google Scholar]
- 19.Osterhoff G, Tiziani S, Ferguson SJ, et al. Mechanical testing of a device for subcutaneous internal anterior pelvic ring fixation versus external pelvic ring fixation. BMC Musculoskeletal Disorders 2014; 15: 111–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vigdorchik JM, Esquivel AO, Jin X, et al. Biomechanical stability of a supra-acetabular pedicle screw Internal fixation device (INFIX) vs external fixation and plates for vertically unstable pelvic fractures. J Orthop Surg Res 2012; 7: 31–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eagan M, Kim H, Manson TT, et al. Internal anterior fixators for pelvic ring injuries: do monaxial pedicle screws provide more stiffness than polyaxial pedicle screws? Injury 2015; 46: 996–1000. [DOI] [PubMed] [Google Scholar]
- 22.Owen MT, Tinkler B, Stewart R. Failure and salvage of “INFIX” instrumentation for pelvic ring disruption in a morbidly obese patient. J Orthop Trauma 2013; 27: e243–e246. [DOI] [PubMed] [Google Scholar]
- 23.Moazzam C, Heddings A, Moodie P, et al. Anterior pelvic subcutaneous internal fixator application: an anatomic study. J Orthop Trauma 2012; 26: 263–268. [DOI] [PubMed] [Google Scholar]
- 24.Vaidya R, Oliphant B, Jain R, et al. The bikini area and bikini line as a location for anterior subcutaneous pelvic fixation: an anatomic and clinical investigation. Clin Anat 2013; 26: 392–399. [DOI] [PubMed] [Google Scholar]
- 25.Vaidya R, Kubiak EN, Bergin PF, et al. Complications of anterior subcutaneous internal fixation for unstable pelvis fractures: a multicenter study. Clin Orthop Relat Res 2012; 470: 2124–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hesse D, Kandmir U, Solberg B, et al. Femoral nerve palsy after pelvic fracture treated with INFIX: a case series. J Orthop Trauma 2015; 29: 138–143. [DOI] [PubMed] [Google Scholar]
- 27.Apivatthakakul T, Rujiwattanapong N. “Anterior subcutaneous pelvic internal fixator (INFIX), Is it safe?”A cadaveric study. Injury 2016; 47: 2077–2080. [DOI] [PubMed] [Google Scholar]