Abstract
Objective
Similar to perfusion studies after acute ischemic stroke, measuring cerebral blood volume (CBV) via C-arm computed tomography before and after therapeutic interventions may help gauge subsequent revascularization. We tested serial dilutions of intra-arterial injectable contrast medium (CM) to determine the optimal CM concentration for quantifying parenchymal blood volume by flat-panel detector imaging (FD-PBV).
Methods
CM was diluted via saline power injector, instituting time delays for FD-PBV studies. A red/green/blue (RGB) color scale was employed to quantify/compare FD-PBV and magnetic resonance-derived CBV (MRCBV).
Results
Contrast values of right and left common carotid arteries did not differ significantly at CM dilutions of ≥20%. RGB analysis of FD-PBV imaging (relative to MR-CVB), showed CM dilution altered the colors (by 16%), increasing red and decreasing blue ratios.
Conclusion
Diluting CM to 20% resulted in no laterality differential of FD-PBV imaging, with left/right quantitative ratios approaching 1.1 (optimal for clinical use).
Keywords: C-arm CT, cerebral blood volume, contrast medium, parenchymal blood volume, flat-panel detector, stroke
Introduction
As shown by other means1–4 (e.g., positron emission tomography, magnetic resonance imaging (MRI)), computed tomography (CT) can be used to determine cerebral blood volume (CBV) before and after therapeutic interventions for acute ischemic stroke, which may help gauge revascularization success.3,4 Recently, animal and human studies have further demonstrated in this setting the feasibility and potential benefit of measuring parenchymal blood volume (PBV) via C-arm CT equipped with a flat-panel detector (FD) system.5–8 A self-expanding stent (to retrieve thrombi) is also deployed in the interventional suite,9,10 after which it is important to assess the extent of restored cerebral blood flow. Using the same angiographic equipment for diagnostic and therapeutic purposes could eliminate the considerable time expended to transport patients between imaging modalities. Although requiring substantial volumes of contrast medium (CM), FD-PBV imaging is the approach best suited for patients with acute ischemic stroke.
CT perfusion (CTP) imaging—one of the techniques widely applied to assess perfusion abnormalities of patients with acute stroke—is expected to improve patient outcomes using thrombolysis. CTP has certain advantages over MR perfusion, although Sasaki et al.11 reported that radiation exposure during CTP may be unexpectedly high. Therefore, low-dose protocols with de-noising techniques are essential to maintain the radiation dose at <200 mGy. The quantitative characteristics of CTP data, however, are unreliable. Man et al.12 compared PCT and MRI perfusion and found that the mean transfer time and CBF values are affected by the deconvolution method used (p < 0.05), whereas CBV values remain unchanged. When applying these various thresholds, however, the predicted ischemic core and penumbra were similar with the two methods. Thus, the predicted ischemic core and penumbra are similar with these methods when using different thresholds, specific for each deconvolution method.
The aim of this study was to determine a concentration of intra-arterial injectable CM optimal for quantifying FD-PBV images, thereby reducing overall CM usage.
Materials and methods
Patients
Between February 2013 and August 2014, a total of 10 patients (9 women, 1 man) with a median age of 58.1 years (range 40–71 years) qualified for this retrospectively study as each had a single unruptured aneurysm (average diameter, 5.7 mm, range, 3–12 mm). Two of the aneurysms were atop the basilar artery (BA-Top), six involved the internal carotid–posterior communicating artery (IC-PCA), one was at the middle cerebral artery (MCA) bifurcation, and one affected the anterior communicating artery (ACA) (Table 1). Patients were scheduled for FD-PBV and MRI perfusion studies within <24 h after granting informed consent. Measuring the perfusion with MRI is a morphological evaluation that provides functional information and improves the diagnostic ability and treatment orientation. This study was compliant with the Health Insurance Portability and Accountability Act. Our institutional ethics committee approved the study prior to initiating data collection. The study was performed according to the guidelines of our facility.
Table 1.
Patients' characteristics.
| Patient no. | Sex | Age (yr) | PMH | Clinical presentation (imaging method) |
|---|---|---|---|---|
| 1 | F | 71 | HL | Right cerebral aneurysm (MRI, for vertigo) |
| 2 | F | 59 | – | Left ICA aneurysm (MRI) |
| 3 | M | 50 | HT | Right MCA stenosis |
| 4 | F | 56 | – | Bilateral cerebral aneurysms |
| 5 | F | 69 | HT | Right PCA aneurysm (MRI) |
| 6 | F | 59 | – | Left cerebral aneurysm (MRI) |
| 7 | F | 61 | HT | BA aneurysm, top (MRI) |
| 8 | F | 40 | – | Left cerebral aneurysm (MRI) |
| 9 | F | 70 | HT, HL | ACA aneurysm (MRI) |
| 10 | F | 46 | – | Left cerebral aneurysm (MRI) |
Abbreviations: ACA, anterior communicating artery; BA, basilar artery; F, female; HL, hyperlipidemia; HT, hypertension; ICA, intracranial artery; M, male; MCA, middle cerebral artery; MRI, magnetic resonance imaging; PCA, posterior cerebral artery; PMH, past medical history.
Contrast value for comparison of right–left common carotid artery
FD-PBV acquisitions were obtained via a biplane FD (30 × 40 cm) angiography system (Artis zee BA Twin; Siemens AG Healthcare, Erlangen, Germany). The C-arm set gained upfront entry to the right and left common carotid arteries (CCAs) and the heart. The lateral C-arm set centered on the whole skull, with the pig-tail catheter tip placed at the aortic valve. We positioned the catheter at the ascending aorta and started the native run, after which the bolus-watching angiogram (as described in reference 10) and the injection of contrast medium were initiated simultaneously. When the veins and sinus were visible, we started the full run. The contrast values for comparing the right–left CCAs involved two-dimensional digital subtraction angiography (2D-DSA), using a power injector (Press Pro; Nemoto Co, Tokyo, Japan), and arranging the FD-PBV time delay. Injector infusion conditions included a diluted CM volume of 12 mL, a radiographic delay time of 0 sec, and a 6.0-mL/sec injection rate. The CM (Iopamidol 300; Bayer HealthCare, Osaka, Japan) of choice was diluted to 28%, 24%, 20%, 16%, and 12% with saline, meaning that there were mixtures of 3.36 CM/8.64 saline (28%), 2.88 CM/9.12 saline (24%), 2.4 CM/9.6 saline (20%), and so on.
Contrast values for the right and left CCAs were measured five times, based on a circular region of interest (ROI), and expressed as the mean ± standard deviation (SD). We determined the ratios of the right and left CCA contrast values at the various CM dilutions. In addition, we determined the duration of scan delay according to the contrast values, including the time to the transverse sinus (TS), using color-coded DSA imaging13 (iFlow software: syngo X Workplace, Siemens AG Healthcare).
FD-PBV quantitation
FD-PBV was generated from 400 radiographic images with a 5122 matrix (pixel size 0.15 mm2) obtained during rotation of the X-ray source approximately 200° within 6 sec. Data sets were transferred to a commercially available workstation (syngo X Workplace, Siemens AG Healthcare) for post-processing. The proprietary software included smoothing algorithms:14,15 binning, 4 × 4; kernel, smooth. Exposures entailed default settings (tube voltage, 70 kV; pulse width, 5 ms; radiation-exposed dose, 0.360 µGy/pulse; focus size, large) with an HU kernel type and smooth image characteristic as the conditions of acquisition. Dilute CM (90 mL) was injected at 6.0 mL/sec. CM diluted to 28%, 24%, 20%, 16%, and 12% by saline yielded volumes of 25.3 mL, 21.6 mL, 18.0 mL, 14.4 mL, and 10.8 mL, respectively.
The data were post-processed to generate FD-PBV images using prototype software (Siemens AG, Healthcare) on a dedicated research workstation (Leonardo, Siemens AG, Healthcare). For PBV image processing,16 the subtracted data were normalized with an input function automatically estimated from the histographic analysis of the vascular tree. We then performed color-coding with a standard color table to generate the PBV color-coded maps for visualization and interpretation. The PBV values are expressed in units of ml/1000 ml of cerebral tissue. PBV values for the ROI analysis were converted to more commonly used units of ml/100 g using a simple conversion factor that takes into account the cerebral tissue density (1.05 g/ml).
MR-CBV quantitation
MRI perfusion was performed in the transverse plane by gradient-echo sequences using a 1.5-T MRI scanner (Signa; GE Medical Systems, Wilmington, MA, USA) with echo planar sequence parameters as follows: TR/TE/flip angle, 1500°/40°/60°; field of view, 240 × 240 mm; slice thickness, 5 mm; matrix, 128 × 128; in-plane voxel size, 1.8 × 1.8 mm; signal bandwidth, 1470 Hz/pixel. Prior to scanning, an 18-gauge intravenous catheter was placed at the antecubital fossa for contrast administration. Imaging took place during the first pass of a standard bolus dose (0.1 mmol/kg) of gadopentetate dimeglumine (Eisai Co Ltd, Tokyo, Japan), with MRI perfusion images acquired at 1-sec intervals. Then, 13 mL was injected at 3.0 mL/sec and flushed with 30 mL of saline via an MRI-compatible power injector (SONIC SHOT50; Nemoto Kyorindo Co Ltd, Tokyo, Japan).
Post-processing for MR-CBV image calculations was performed with a Perfusion Mismatch Analyzer17 devised by the Acute Stroke Imaging Standardization Group–Japan. The arterial input function to deconvolve the dynamic tissue response curve was determined automatically. Semi-quantitative output perfusion maps of the cerebral blood volume (CBV) were obtained for clinical interpretation. Three ROIs (ACA, MCA, posterior cerebral artery (PCA) territories) were assessed by the workstation or by perfusion mismatch analysis (PMA) in each hemisphere to include all tissues and vessels. It covered the same proportion of gray/white matter for both a normal CBV range and that of the threshold of an infarct. When feasible, adjustments were made to achieve hemispheric symmetry.
Data analysis
FD-PBV and MR-CBV images were depicted as intracranial RGB displays on a black background (BG). Color-scale analysis (excluding black) was performed via ImageJ version 1.43 (National Institutes of Health, Bethesda, MD, USA). A linear two-sided t-test was performed to assess correlations between variables. Statistical significance was set as p < 0.05
Results
Contrast values for comparison of right–left CCA
Figure 1 is a DSA image of test injections at each CM dilution (BG contrast, 3220 ± 27). The right and left CCA contrast value ratios at various dilutions of CM are shown in Table 2. The mean radiographic delay time was 7.9 ± 0.7 sec (minimum, 6.5 sec; maximum, 8.5 sec).
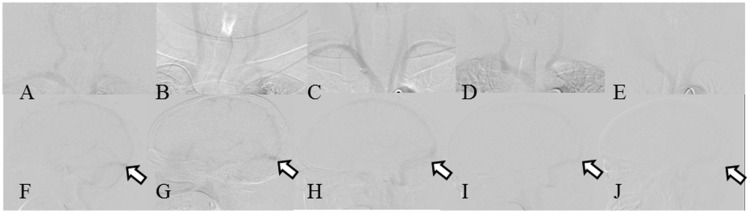
Figure 1.
Two-dimensional DSA images of the right and left CCA of five patients with test injections. (A–E) Frontal C-arm (top row). (F–J) Lateral C-arm (bottom row). (A, F) Patient 1, 28% CM. (B, G) Patient 4, 24% CM. (C, H) Patient 5, 20% CM. (D, I) Patient 7, 16% CM. (E, J) Patient 9, 12% CM (CCA, dotted line; TS, arrow). These DSA images are difficult to understand, although the images in C and H show the best contrast. This should also be the case for the images in A and F. Images of the entire series should be provided so the operator may evaluate the image quality. Abbreviations: CM, contrast medium; CCA, common carotid artery; DSA, digital subtraction angiography; TS, transverse sinus
Table 2.
Contrast values for the right CCA, left CCA, and transverse sinus, and BG values
| Patient no. | CM concentration (%) | Right CCA | Left CCA | Transverse sinus | BG value |
|---|---|---|---|---|---|
| 1 | 28 | 3083 | 3134 | 2921 | 3201 |
| 2 | 28 | 3072 | 3091 | 2973 | 3203 |
| 3 | 24 | 3145 | 3190 | 2861 | 3212 |
| 4 | 24 | 3191 | 3214 | 3187 | 3283 |
| 5 | 20 | 3079 | 3113 | 3011 | 3217 |
| 6 | 20 | 2968 | 3027 | 3000 | 3167 |
| 7 | 16 | 3153 | 3141 | 3047 | 3213 |
| 8 | 16 | 3039 | 3056 | 3121 | 3200 |
| 9 | 12 | 3077 | 3087 | – | 3184 |
| 10 | 12 | 3128 | 3162 | 3151 | 3214 |
Abbreviations: CCA, common carotid artery; CM, contrast medium; BG, background; 2D-DSA, two-dimensional digital subtraction angiography.
The values shown in this table were relatively high compared with those of two-dimensional digital subtraction angiography images.
It was difficult to obtain values for the transverse sinus lesion in patient 9.
FD-PBV and MR-CBV determinations
FD-PBV and MR-CBV color-scale images are shown in Figure 2. The average RGB color distributions in the MR-CBV images in the 10 test patients are shown in Table 3. The three vascular territories of FD-PBV and MR-CBV images are shown side-by-side in Figure 3. There were no significant differences among them (ACA, p = 0.0940; MCA, p = 0.0521; PCA, p = 0.0672). Figure 4 shows the ratios for the FD-PBV/MR-CBV images in RGB color. Beyond a CM dilution of 16%, the red ratio increased, the blue ratio decreased, and the green ratio was unchanged.
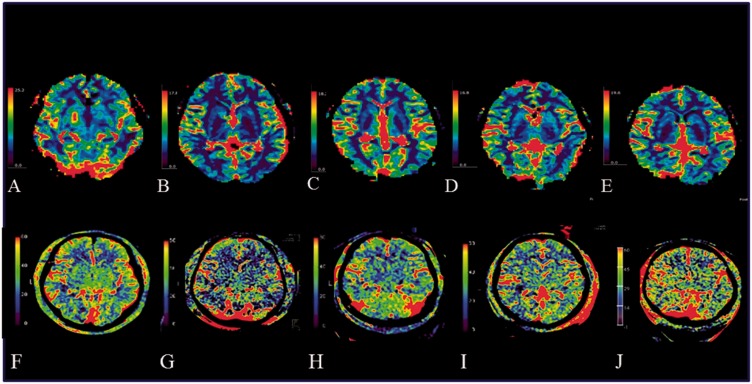
Figure 2.
Comparison of FD-PBV and MR-CBV images of five patients. (A–E) MR-CBV (top row). (F–J) FD-PBV (bottom row). (A, F) Patient 1, 28% CM. (B&G) Patient 4, 24% CM. (C, H) Patient 5, 20% CM. (D, I) Patient 7, 16% CM. (E, J) Patient 9, 12% CM. In other publications using the intravenous approach, the anatomical structures of the basal ganglia could be recognized. In our images, only in (F) (dilution 28%) could the structures of the basal ganglia can be recognized. In images (G–J), only image noise seems to be visible. Abbreviations: CBV, cerebral blood volume; FD, flat-panel detector; MR, magnetic resonance; PBV, parenchymal blood volume
Table 3.
RGB analysis of PBV and MRI images
| PBV |
MRI |
PBV/MRI |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. | CM concentration (%) | Red | Green | Blue | Red | Green | Blue | Red | Green | Blue |
| 1 | 28 | 26.2 | 31.2 | 17.6 | 22.8 | 25.8 | 20.7 | 1.1 | 1.2 | 0.9 |
| 2 | 28 | 24.4 | 25.6 | 24.9 | 21.1 | 27.7 | 26.1 | 1.2 | 0.9 | 1.0 |
| 3 | 24 | 26.1 | 24.1 | 26.1 | 21.3 | 23.3 | 30.4 | 1.2 | 1.0 | 0.9 |
| 4 | 24 | 25.0 | 23.7 | 26.3 | 19.8 | 27.6 | 27.5 | 1.3 | 0.9 | 1.0 |
| 5 | 20 | 25.2 | 24.5 | 25.3 | 23.7 | 27.4 | 23.8 | 1.1 | 0.9 | 1.1 |
| 6 | 20 | 25.6 | 29.4 | 20.0 | 22.2 | 28.2 | 24.6 | 1.2 | 1.0 | 0.8 |
| 7 | 16 | 27.0 | 28.1 | 19.9 | 22.1 | 27.8 | 25.1 | 1.2 | 1.0 | 0.8 |
| 8 | 16 | 28.2 | 27.9 | 18.9 | 21.9 | 26.1 | 27.0 | 1.3 | 1.1 | 0.7 |
| 9 | 12 | 26.9 | 27.8 | 20.4 | 20.1 | 28.0 | 26.9 | 1.3 | 1.0 | 0.8 |
| 10 | 12 | 31.3 | 27.8 | 15.9 | 22.5 | 26.0 | 26.5 | 1.4 | 1.1 | 0.6 |
| Average | 26.58 | 27.01 | 21.54 | 21.77 | 26.78 | 25.87 | 1.22 | 1.01 | 0.83 | |
| SD | 1.98 | 2.44 | 3.78 | 1.20 | 1.51 | 2.57 | 0.10 | 0.10 | 0.13 | |
Abbreviations: PBV, parenchymal blood volume; MRI, magnetic resonance imaging; RGB, red green blue; SD, standard deviation.
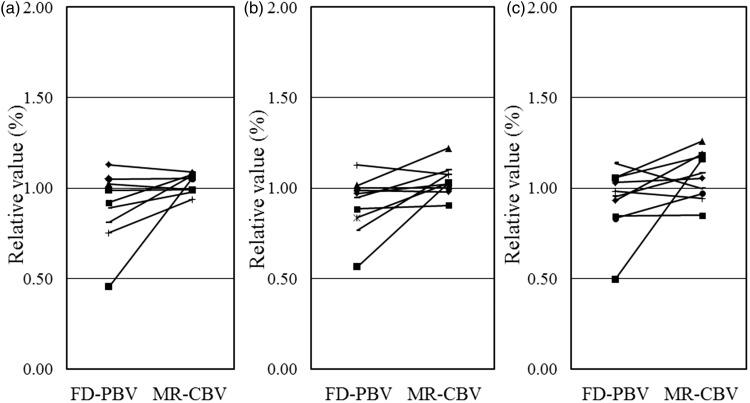
Figure 3.
Relative values of FD-PBV and MR-CBV. (a) Relative values between FD-PBV and MR-CBV in an ACA lesion. (b) Relative values of FD-PBV and MR-CBV in an MCA lesion. (c) Relative values of FD-PBV and MR-CBV in a PCA lesion. Abbreviations: ACA, anterior cerebral artery; CBV, cerebral blood volume; FD, flat-panel detector; MCA, middle cerebral artery; MRI, magnetic resonance imaging; PBV, parenchymal blood volume; PCA, posterior cerebral artery
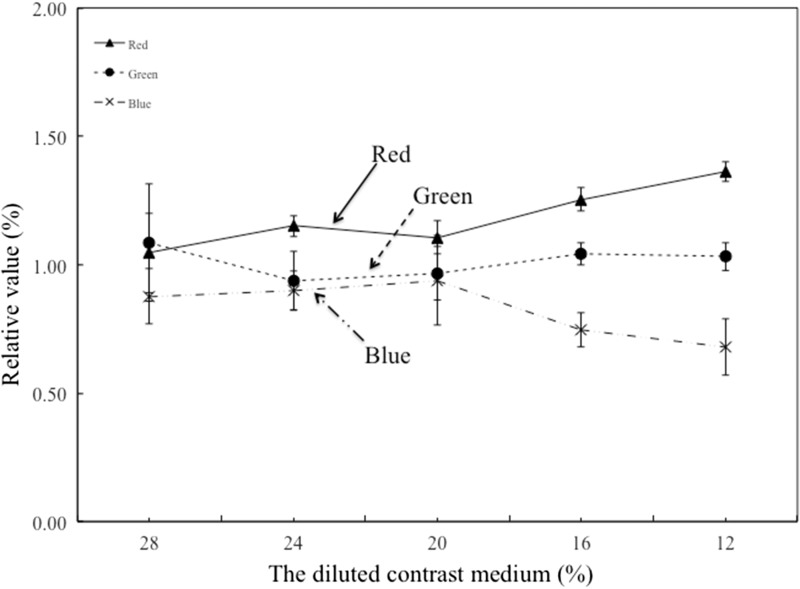
Figure 4.
FD-PBV/MR-CBV ratio in the RGB scale. MR-CBV image represents the amount of contrast medium inflowing and outflowing. Thus, a laterality differential of the CBV was not seen for either cerebral hemisphere, as shown in Figure 1. According to contrast values for comparison of the right–left CCA, we confirmed that no laterality differential was seen between CCA and VA during aortic artery flow. FD-PBV is a color-coded image that represents the quantity of the contrast medium. relative blood flow. Red = high relative blood flow. Blue = low relative blood flow. To compare FD-PBV with MR-CBV could enable a relatively fixed-quantity evaluation. Abbreviations: CBV, cerebral blood volume; FD, flat-panel detector; PBV, parenchymal blood volume; RGB, red/green/blue
Discussion
This study is the first to report quantitative assessment of FD-PBV imaging using various CM dilutions in Asian subjects. A CM dilution to 20% appears optimal for clinical use of FD-PBV quantitation.
Contrast value for comparison right–left CCA
MRI-CBV might not show a laterality of the contrast value for the right–left brain because the average CM concentration was unreliable due to mixing by cardiac activity.18 In this study, however, we were able to confirm laterality of the contrast value for the right–left CCA. When laterality was not present, the CM volume was circulated equivalently in the right–left brain. The symmetrical appearance of the CM is shown in Figure 1.
Royalty et al.6 described use of a 60% CM dilution injected intravenously at 3.5 mL/sec, although an 8-sec radiographic delay proved excessive. There was already significant enhancement of the basilar artery at the time of the initial image acquisition in this canine study. In a clinical study, Lin et al.10 also used a 60% CM dilution given via an arterial injection at 1.5 mL/sec. The time to maximum opacification of the superior sagittal sinus in 34 patients was 6.0 ± 2.1 sec. In our cohort, intra-arterial injection of diluted CM at 6 mL/sec required 7.9 ± 0.7 sec, on average, for maximum opacification of the superior sagittal sinus, and the related standard deviation was less than that cited by Lin et al.10. The time required for the superior sagittal sinus to reach full opacity might vary according to the total amount used, the dilution of the CM administered, and the positioning of the angiographic catheters. Thus, we anticipate that a 9-sec radiographic delay would allow sufficient whole-brain dissemination of CM (Figure 1).
Regarding the injection rate, Rumberger et al.19 investigated varying intravenous injections to determine whether faster injection was preferable for a set volume of CM and whether diluted CM, injected at an equivalent volume, conferred similar results. Ishida et al.18 likewise studied a human cohort, finding no significant improvement by increasing the intravenous injection rate from 4 mL/sec to 5 mL/sec. In a study of swine, Ganguly7 determined that a higher intra-arterial injection rate produced better results, presumably due to better flow and improved mixing of CM in the carotid arteries and less loss of iodinated contrast in subclavian arteries at the higher flow rates, which at most were 4-6 mL/sec. The contrast values of the right and left CCAs, shown in Figure 1, did not differ significantly at 20% CM (p = 0.0746). With no laterality differential, equivalent PBV imaging is therefore expected.
FD-PBV and MR-CBV comparison
In each of the 10 patients studied, the catheters were consistently positioned at the aortic valve, near the frontal FD, and no acute ischemia stroke or stenotic vessel was encountered. The similar vascular territories of FD-PBV and MR-CBV determinations (ACA, p = 0.0940; MCA, p = 0.0521; PCA, p = 0.0672) are shown in Figure 4 Still, the relations are marginal. Greater clarity may be achieved by enlisting a larger patient population (e.g., ≥30 subjects). Lin et al.13 reported that the ability to measure CBV in the angiography suite facilitated the following situations: (1) when there is a significant delay between the diagnosis and the beginning of the endovascular procedure; (2) if a procedure has been too long or unsuccessful, the operator can assess the evolution of the infarct core; (3) being able to identify the prognostic factors and rule out hemorrhage immediately after finishing the procedure. If we want to use this new imaging modality as a first-line tool, we should use a pig-tail catheter (which is potentially dangerous) instead of an intravenous approach, which is minimally invasive. Measurement of the CBV and cerebral flow in an angiography room might be a useful tool for emergency cases, such as a patient with acute stroke.
Reducing the amount of CM in both diagnostic and interventional procedures is an important issue, especially for elderly patients. FD-PBV affords easy laterality of imaging,5–8 depending on the position of the catheter and acquisition timing, among other factors. The contrast values of the right–left CCA may be symmetrical using CM at low concentrations and a high injection rate. At our facility, we always have CM (100 mL) ready when DSA for cerebral endovascular treatment is scheduled. Should the patient suffer a cerebral event or need an FD-PBV study, CM may be added. FD-PBV imaging enables evaluation of both the physiological and cerebral perfusion states of patients in the angiography suite before, during, and after therapeutic interventions.
All color maps17 delineating FD-PBV and MRI-CBV were based on default ranges—whether software- or PMA-generated. In Figure 3, FD-PBV images at 16% CM dilution showed an increase in the red ratios and a decrease in blue ratios, with the green ratio unchanged. Compared with its 20% counterpart, the 16% CM dilution was inadequate for color-scale depiction of FD-PBV. In Figure 2, if we think in images “G”, “H”, “I”, and “J”, only image noise is visible—nothing else. Thus, the color analyzed showed a different malignancy at ≥20% CM concentration. In the future, we believe that this application will be developed so that MR-perfusion-CBV and CT-perfusion-CBV are equivalent.
Royalty et al.6 obtained qualitative and quantitative measurements of CBF and CBV via C-arm CT acquisition, injecting 28 mL of CM at 3.5 mL/sec. The radiographic delay time was detailed, and CM was simply flushed by the saline. Cigarroa et al.,20 however, determined an upper limit for CM volume based on the following formula,21 to preclude a high incidence of nephropathy:
Thus, we must reduce the CM volume during cerebral endovascular treatments.
Limitations
Limitations of this study include a small patient group, so the outcomes are useful only as a reference for initial comparisons. In addition, although this study concluded that a CM dilution to 20% resulted in no laterality differential of FD-PBV imaging, with left–right quantitative ratios approaching values of 1:1, we did not compare the asymmetry in the PBV images—only the CM ratio at the CCA. Therefore, it is difficult to reach conclusions. We also used MR perfusion imaging as a control, which varies among individuals and is affected by several factors. Higher correlation with the MR-CBV image does not mean it is better. Finally, it has become increasingly evident that color mapping is of limited value as a research tool.
Conclusions
We investigated serial reductions in CM concentrations for use in FD-PBV imaging. The most important goal is not to create an asymmetrical image. At 20% CM dilution, no imaging laterality differential was observed, with the left–right quantitative ratio approaching 1:1. Therefore, this study may support a reduction of CM if the results can clearly show that the doses are optimal.
Disclaimers
This study received no external funding.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Notation of prior abstract publication/presentation
An abstract of this study was presented at the 31st Japanese Society for Neuro-endovascular Therapy, Okayama, Japan, November 19-21, 2015
References
- 1.Vagal AS, Leach JL, Fernandez-Ulloa M, et al. The acetazolamide challenge: techniques and applications in the evaluation of chronic cerebral ischemia. AJNR Am J Neuroradiol 2009; 30: 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eastwooda JD, Lev MH, Wintermark M, et al. Correlation of early dynamic CT perfusion imaging with whole-brain MR diffusion and perfusion imaging in acute hemispheric stroke. AJNR Am J Neuroradiol 2003; 24: 1869–1875. [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich JO, Hsu D, Wang G, et al. Magnetic resonance imaging results can affect therapy decisions in hyperacute stroke care. Acta Radiol 2008; 49: 550–557. [DOI] [PubMed] [Google Scholar]
- 4.Kosior JC, Ryder RC, Andersen LB, et al. MRI of ischemic stroke in canines: applications for monitoring intraarterial thrombolysis. J Magn Reson Imaging 2007; 26: 1421–1428. [DOI] [PubMed] [Google Scholar]
- 5.Struffert T, Deuerling-Zheng Y, Kloska S, et al. Flat Detector CT in the evaluation of brain Parenchyma, intracranial vasculature, and cerebral blood volume: a pilot study in patients with acute symptoms of cerebral ischemia. AJNR Am J Neuroradiol 2010; 31: 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Royalty K, Manhart M, Pulfer K, et al. C-arm CT measurement of cerebral blood volume and cerebral blood flow using a novel high-speed acquisition and a single intravenous contrast injection. AJNR Am J Neuroradiol 2013; 34: 2131–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganguly A, Fieselmann A, Marks M, et al. Cerebral CT perfusion using an interventional C-arm imaging system: cerebral blood flow measurements. AJNR Am J Neuroradiol 2011; 32: 1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiorella D, Turk A, Chaudry I, et al. A prospective, multicenter pilot study investigating the utility of flat detector derived parenchymal blood volume maps to estimate cerebral blood volume in stroke patients. J NeuroIntervent Surg 2014; 6: 451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saver JL, Goyal M, Bonafe A, et al. Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 10.Lin CJ, Yu M, Hung SC, et al. In-room assessment of cerebral blood volume for guidance during intra-arterial thrombolytic therapy. Interventional Neuroradiology 2012; 18: 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasaki M, Kudo K, Oikawa H. CT perfusion for acute stroke: current concepts on technical aspects and clinical applicatios. Int Congr Ser 2006; 1290: 30–36. [Google Scholar]
- 12.Man F, Patrie JT, Xin W, et al. Delay-sensitive and delay-insensitive deconvolution perfusion-CT: similar ischemic core and penumbra volumes if appropriate threshold selected for each. Neuroradiology 2015; 57: 573–581. doi: 10.1007/s00234-015-1507-7. [DOI] [PubMed] [Google Scholar]
- 13.Lin CJ, Hung SC, Guo WY, et al. Monitoring Peri-therapeutic cerebral circulation time: a feasibility study using color-coded quantitative DSA in patients with steno occlusive arterial disease. AJNR Am J Neuroradiol 2012; 33: 1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manduca A, Yu L, Trzasko JD, et al. Projection space denoising with bilateral filtering and CT noise modeling for dose reduction in CT. Med Phys 2009; 36: 911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Supanich M, Tao Y, Nett B, et al. Radiation dose reduction in timeresolved CT angiography using highly constrained back projection reconstruction. Phys Med Biol 2009; 54: 4575–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zellerhoff M, Deuerling-Zheng Y, Strother CM, et al. Measurement of cerebral blood volume using angiographic C-arm systems. In: Proceedings of SPIE. Lake Buena Vista, FL, USA: 2009, 72620H-72620H-8.
- 17.Kudo K, Sasaki M, Yamada K, et al. Differences in CT perfusion maps generated by different commercial software: quantitative analysis by using identical source data of acute stroke patients. Radiology 2010; 254: 200–209. [DOI] [PubMed] [Google Scholar]
- 18.Ishida M, Sakuma H, Murashima S, et al. Absolute blood contrast concentration and blood signal saturation on myocardial perfusion MRI: estimation from CT data. J Magn Reson Imaging 2009; 29: 205–210. [DOI] [PubMed] [Google Scholar]
- 19.Rumberger JA, Bell MR. Measurement of myocardial perfusion and cardiac output using intravenous injection methods by ultrafast (cine) computed tomography. Invest Radiol 1992; 27: S40–S46. [DOI] [PubMed] [Google Scholar]
- 20.Cigarroa RG, Lange RA, Williams RH, et al. Dosing of contrast material to contrast nephyropathy with renal desease. Am J Med 1989; 86: 649–652. [DOI] [PubMed] [Google Scholar]
- 21.Cigarroa RG, Lange RA, Williams RH, et al. Dosing of contrast material to prevent contrast nephropathy in patients with renal disease. AJM 1989; 86: 649–652. [DOI] [PubMed] [Google Scholar]