Short abstract
Objective
Low systolic blood pressure (SBP) is associated with an increased risk for cardiovascular morbidity/mortality in older patients with chronic kidney disease (CKD). The present study evaluated the association between range in blood pressure and first care-needs certification in the Long-term Care Insurance (LTCI) system or death in community-dwelling older subjects with or without CKD.
Methods
CKD was defined as an estimated glomerular filtration rate <60 ml/min/1.73 m2 or dipstick proteinuria of + or greater. Our study was conducted in 1078 older subjects aged 65–94 years. Associations were estimated using the Cox proportional hazards model.
Results
During 5 years of follow-up, 135 first certifications and 53 deaths occurred. Among patients with CKD, moderate SBP (130–159 mmHg) was associated with a significantly lower adjusted risk of subsequent total certification (hazard ratio [HR] = 0.44) and subsequent certification owing to dementia (HR = 0.17) compared with SBP < 130 mmHg. These relationships were not observed in non-CKD subjects.
Conclusion
Lower SBP of <130 mmHg may predict a higher risk for subsequent first care-needs certification in LTCI, especially for dementia, in community-dwelling patients with CKD.
Keywords: Blood pressure, chronic kidney disease, dementia, older patients, long-term care insurance, certification
Abbreviations
BMI, body mass index; BP, blood pressure; CI, confidence interval; CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LTCI, Long-term Care Insurance; SBP, systolic blood pressure, RCSC, Regional Comprehensive Support Center
Introduction
Chronic kidney disease (CKD) is an important public health problem, especially in the older population. This is because the prevalence of CKD increases with age according to an age-dependent decline in estimated glomerular filtration rate (eGFR) in the general population.1 The prevalence of CKD in the adult Japanese population was estimated to be 13% in 2009, and is expected to further increase with aging of the Japanese population.2 Blood pressure (BP) is often elevated in people with CKD, and higher BP is associated with an increased risk of cardiovascular events, especially in older patients with CKD.3 The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014)4 recommend a target BP with antihypertensive treatment for older hypertensive patients aged ≥75 years of <150/90 mmHg. These guidelines recommend attempting to lower BP to <140/90 mmHg if possible, and for hypertensive patients with CKD, a target BP of <140/90 mmHg should be attempted. These guidelines also recommend attempting to lower BP to <130/80 mmHg when accompanied by diabetes mellitus5,6 or clinical proteinuria.7 However, recent observational cohort studies have suggested that the risk of cardiovascular morbidity/mortality in patients with CKD may be increased in those with systolic BP (SBP) in the lower range. This relationship with increased cardiovascular morbidity/mortality,8 all-cause mortality,9–12 and stroke events13 in the low range of SBP, including <130 mmHg,9,10 has been shown in patients with CKD. However, this pattern has not been observed in the general population. CKD in older people is closely related to a decline in physical function14 and cognitive function.15,16 Moreover, CKD was reported to be an independent risk factor for care-needs certification in the Long-term Care Insurance (LTCI) system in older Japanese adults.17 However, the association of a range in BP with care-needs certification in LTCI has not been examined in community-dwelling older patients with CKD. Therefore, this study aimed to determine whether a lower range in BP of <130/80 mmHg is a risk factor for care-needs certification in LTCI or death among community-dwelling older subjects by comparing risk factors between those with CKD and without CKD (non-CKD).
Materials and methods
Subjects
We analysed cohort data of a town with a population of approximately 30,000. In April 2008, the local government provided public health centre-based annual health check-ups to community-dwelling uncertified older people aged ≥65 years. Among 4050 older people, complete information from the health check-up was obtained in 1091. Of these, 13 moved out of the town during 5 years, and a total of 1078 subjects were included in our study.
Baseline examinations
CKD was defined as an eGFR < 60 ml/min/1.73 m2,18 or with clinical proteinuria defined as a dipstick result of + or greater for spot urine, corresponding to a urinary protein level > 30 mg/dl.19 Major risk factors for CKD were defined based on current national guidelines. Hypertension was defined as BP ≥ 140/90 mmHg or use of antihypertensive agents. Diabetes mellitus was defined as fasting blood glucose ≥ 126 mg/dl, non-fasting glucose ≥200 mg/dl, HbA1c ≥6.5%, or use of hypoglycaemic agents or insulin. Hyperuricaemia was defined as serum uric acid levels ≥7.0 mg/dl in males and ≥6.0 mg/dl in females. Dyslipidaemia was defined as fasting serum total cholesterol levels ≥220 mg/dl, triglyceride levels ≥150 mg/dl, high-density lipoprotein cholesterol levels <40 mg/dl, or use of lipid-lowering agents. Hypoalbuminemia was defined as serum albumin levels <4 g/dl as previously reported.20 Baseline BP was measured according to JSH 20144 by trained observers.
Outcome measures
Outcome measures were first care-needs certification in LTCI by the Regional Comprehensive Support Centre (RCSC) of the local government, or death during 5 years. The services of the LTCI program are provided to frail or disabled older adults who are certified as requiring support or care, according to their care needs and the nationally standardized certification assessment system in Japan.17 We also recorded the first disorder that required certification in the primary care physician’s statement, and classified the causative disorders into four categories of arthralgia/fractures, dementia, stroke, and other diseases.
Statistical analysis
The study population was first divided into CKD and non-CKD subjects. The population was further classified into three groups based on baseline SBP (<130, 130–159, and ≥160 mmHg) and diastolic BP (DBP; <80, 80–89, and ≥90 mmHg). The associations of CKD and range of BP with first care-needs certification in LTCI or death were analysed using Kaplan–Meier analysis and the log-rank test. Multivariate Cox proportional hazards regression analysis was used to evaluate the association between the range in BP and care-needs certification in LTCI or death. Using Cox proportional regression analysis, the adjusted hazard ratio (HR) for each baseline BP (SBP: 130–159 mmHg; DBP: 80–89 mmHg) in non-CKD subjects and the 95% confidence interval (CI) were estimated. Variables for Cox regression hazard analysis were selected according to the results of χ2 analysis (or Fisher’s exact test when needed) for categorical variables and Mann–Whitney U analysis with Bonferroni’s correction for ordinal variables in baseline clinical characteristics. A value of P < 0.20 was used as a cutoff in this analysis. Analysis was performed in all subjects, and then in subgroups of subjects stratified by age ≥75 years, male sex, receiving antihypertensive treatment, diabetes mellitus, and clinical proteinuria. We also examined models using the four classes of causative diseases for care-needs certification in LTCI as outcomes. Data were analysed using SPSS (version 22.0, Chicago, IL, USA).
Ethical considerations
This study was conducted in accordance with the guidelines of the Declaration of Helsinki, and was formally approved by the Clinical Research Ethics Committee of Kanazawa Medical University (receipt no. 48). We received baseline data and information on new care-needs certification in LTCI or death during the follow-up period. These data were anonymised from the RCSC of the local government.
Results
Study population
Among the 1078 community-dwelling older subjects, 135 were first certified as needing support/care. Fifteen subjects died after care-needs certification in LTCI and 38 died without certification. A total of 53 subjects died during the 5-year period. Subjects with first care-needs certification in LTCI or death showed a significantly higher mean age and higher female ratio compared with older residents with certification-free survival (Table 1). Age, sex, living alone, hypertension, diabetes mellitus, CKD, hyperuricaemia, hypoalbuminaemia, prior history of stroke, and prior history of heart disease were associated with the risk of first care-needs certification in LTCI, in total or by any cause, or death in univariate analyses (Table 1). Accordingly, these variables were included as confounders in the final Cox hazards regression model. Among the total subjects, 468 (43%) had CKD. Of these, 380 were diagnosed with CKD because of their eGFR (range in eGFR: 22 to <60 ml/min/1.73 m2), and 88 were diagnosed with CKD because of clinical proteinuria, despite having an eGFR ≥ 60 mL/min/1.73 m2. Patients with CKD were associated with a more advanced age, a higher BMI, a higher rate of diabetes mellitus, receiving antihypertensive treatment, hyperuricaemia, and a history of heart disease compared with non-CKD subjects (Table 2). In the CKD group, a lower range of SBP (<130 mmHg) or DBP (<80 mmHg) was related to a lower rate of receiving antihypertensive treatment and a higher rate of history of heart disease (Table 2).
Table 1.
Baseline characteristics of the total population
| Whole population |
Certification-free
survival† |
Support/care-need certification |
Death |
|||||
|---|---|---|---|---|---|---|---|---|
| Total |
Arthralgia/fractures |
Dementia |
Stroke |
Other diseases |
||||
| Characteristics | n = 1078 | n = 905 | n = 135 | n = 50 | n = 32 | n = 15 | n = 38 | n = 53 |
| Age (years) | 73.4 ± 6.1 | 72.4 ± 5.8 | 80.0 ± 5.4*** | 80.2 ± 5.1*** | 79.4 ± 4.5*** | 78.1 ± 6.3** | 80.9 ± 5.9*** | 78.0 ± 6.4*** |
| Age ≥ 75 years, n (%) | 440 (40.8) | 303 (33.5) | 114 (84.4)*** | 42 (84.0)*** | 28 (87.5)*** | 12 (80.0)*** | 32 (84.2)*** | 38 (71.7)*** |
| Male, n (%) | 424 (39.3) | 363 (40.1) | 40 (29.6)# | 7 (14.0)*** | 10 (31.3) | 8 (53.3) | 15 (39.6)# | 29 (54.7)# |
| Living alone, n (%) | 190 (17.6) | 146 (16.1) | 36 (26.7)* | 13 (26.0) | 10 (31.3) | 1 (6.7) | 12 (31.6) | 9 (17.0) |
| BMI (kg/m2) | 22.9 ± 3.2 | 22.9 ± 3.1 | 2.7 ± 3.6 | 23.4 ± 3.8 | 22.3 ± 2.5 | 23.0 ± 3.1 | 22.2 ± 4.2 | 22.4 ± 3.8 |
| Complications | ||||||||
| CKD, n (%) | 468 (43.4) | 376 (41.5) | 71 (52.6)# | 23 (46.0) | 18 (56.3) | 9 (60.0) | 21 (55.3) | 31 (58.5)# |
| eGFR < 60 ml//min/1.73 m2 | 380 (35.3) | 304 (33.6) | 59 (43.7)# | 21 (42.0) | 15 (46.9) | 9 (60.0) | 14 (36.8) | 23 (43.4) |
| Clinical proteinuria: n (%) | 158 (14.7) | 118 (13.0) | 31 (23.0)* | 6 (12.0) | 5 (15.6) | 6 (40.0)# | 14 (36.8)*** | 15 (28.3)* |
| Diabetes mellitus: n (%) | 234 (21.7) | 175 (19.3) | 51 (37.8)*** | 16 (32.0)# | 13 (40.6)* | 9 (60.0)*** | 13 (34.2)# | 17 (32.1)# |
| Hypertension: n (%) | 673 (62.4) | 557 (61.5) | 92 (68.1) | 31 (62.0) | 24 (75.0) | 14 (93.3)# | 23 (60.5) | 34 (64.2) |
| Antihypertensive treatment, n (%) | 570 (52.9) | 474 (52.4) | 74 (54.8) | 25 (50.0) | 18 (59.4) | 13 (86.7)* | 17 (44.7) | 31 (58.5) |
| Hyperuricemia, n (%) | 100 (9.3) | 81 (9.0) | 13 (9.6) | 3 (6.0) | 3 (9.4) | 6 (40.0)*** | 1 (2.6) | 9 (17.0) |
| Dyslipidaemia, n (%) | 640 (59.4) | 546 (60.3) | 70 (51.9) | 28 (56.0) | 18 (56.3) | 7 (46.7) | 17 (44.7) | 31 (58.5) |
| Hypoalbuminaemia, n (%) | 51 (4.7) | 30 (3.3) | 16 (11.9)*** | 5 (10.0)# | 2 (6.3) | 3 (20.0) | 6 (15.8)*** | 11 (20.8)*** |
| Prior history of stroke, n (%) | 55 (5.1) | 38 (4.2) | 10 (7.4) | 4 (9.5) | 0 (0) | 3 (20.0) | 3 (7.9) | 9 (17.0)** |
| Prior history of heart disease, n (%) | 203 (18.8) | 97 (10.7) | 31 (23.0)*** | 7 (14.0) | 7 (21.9) | 4 (26.7) | 13 (34.2)*** | 9 (17.0) |
Results are expressed as mean ± SD or n (%). BMI, body mass index; CKD, chronic kidney disease, eGFR, estimated glomerular filtration rate. Mann–Whitney U analysis or χ2 analysis (or Fisher’s exact test when needed) was used. #P < 0.20/6; *P < 0.05/6; **P < 0.01/6; ***P < 0.001/6 vs. †group with certification-free survival with Bonferroni’s correction. The number “6” associated with the P values represents the number of comparisons with the reference group.
Table 2.
Baseline characteristics by each grade of SBP and DBP in older patients with CKD and without CKD.
| Total |
SBP (mmHg) |
DBP (mmHg) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-CKD subjects† |
Patients with CKD |
Non-CKD subjects |
Patients with CKD |
Non-CKD subjects |
Patients with CKD |
|||||||||
| <130 |
130–159†† |
≥160 |
< 130 |
130–159 |
≥160 |
<80 |
80–89††† |
≥90 |
<80 |
80–89 |
≥90 |
|||
| n = 610 | n = 468 | n = 246 | n = 335 | n = 29 | n = 203 | n = 240 | n = 25 | n = 330 | n = 227 | n = 53 | n = 253 | n = 156 | n = 59 | |
| Demographics | ||||||||||||||
| Age(years) | 72.8 ± 5.7 | 74.3 ± 6.5*** | 71.6 ± 5.4*** | 73.5 ± 5.7 | 74.9 ± 6.8 | 73.5 ± 6.7 | 75.0 ± 6.4** | 75.0 ± 5.7 | 72.6 ± 6.1 | 72.9 ± 5.6 | 72.7 ± 6.0 | 75.1 ± 6.9 | 74.3 ± 6.7 | 73.3 ± 5.0 |
| Age ≥75 years, n (%) | 224(36.7%) | 216(46.2%)** | 67(27.2%)*** | 143(42.7%) | 14(48.3%) | 79(38.9%) | 124(51.7%)* | 13(52.0%) | 35(35.0%) | 169(37.0%) | 20(37.7%) | 42(46.2%) | 149(46.9%) | 25(42.4%) |
| Male, n (%) | 233(38.2%) | 191(40.8%) | 100(40.7%) | 122(36.4%) | 11(37.9%) | 87(42.9%)# | 94(39.2%) | 10(40.0%) | 34(34.0%) | 183(40.0%) | 16(30.2%)# | 38(41.8%) | 127(39.9%) | 26(44.1%) |
| BMI (kg/m2) | 22.6 ± 3.0 | 23.2 ± 3.4** | 22.1 ± 3.0** | 22.9 ± 2.9 | 23.6 ± 3.6 | 22.8 ± 3.3 | 23.5 ± 3.4* | 23.7 ± 3.3 | 21.8 ± 3.1** | 22.7 ± 3.0 | 23.1 ± 2.8 | 22.3 ± 3.5** | 23.2 ± 3.2 | 24.4 ± 3.5* |
| Living alone, n (%) | 111(18.2%) | 79(16.9%) | 47(19.1%) | 57(17.0%) | 7(24.1%) | 25(12.3%)# | 53(22.1%)# | 1(4.0%)# | 24(24.0%)# | 78(17.1%) | 9(17.0%) | 11(12.1%)# | 61(19.2%) | 7(11.9%) |
| Complications | ||||||||||||||
| eGFR < 60 ml//min, n (%) | 0(0%) | 380(81.2%)*** | 0(0%) | 0(0%) | 0(0%) | 175(86.2%)*** | 189(78.9%)*** | 16(64.0%)*** | 0(0%) | 0(0%) | 0(0%) | 210(83.0%)*** | 124(79.5%)*** | 46(78.0%)*** |
| Clinical proteinuria, n (%) | 0(0%) | 158(33.8%)*** | 0(0%) | 0(0%) | 0(0%) | 55(27.1%)*** | 89(37.1%)*** | 14(56.1%)*** | 0(0%) | 0(0%) | 0(0%) | 75(29.6%)*** | 63(40.4%)*** | 20(33.9%)*** |
| Diabetes mellitus, n (%) | 109(17.9%) | 125(26.7%)*** | 40(16.3%) | 64(19.1%) | 5(17.2%) | 51(25.1%)# | 68(28.3%)** | 6(24.0%) | 69(20.9%)# | 34(15.0%) | 6(11.3%) | 71(28.1%)** | 39(25.0%)* | 15(25.4%)# |
| Hypertension, n (%) | 365(59.8%) | 308(65.8%) | 76(30.9%)*** | 260(77.6%) | 29(100.0%)** | 98(48.3%)*** | 185(77.1%) | 25(100.0%)** | 159(48.2%)*** | 153(67.4%) | 53(100.0%)*** | 143(56.5%)* | 106(67.9%) | 59(100.0%)*** |
| With antihypertensives, n (%) | 303(49.7%) | 267(57.1%)* | 75(30.5%)*** | 207(61.8%) | 21(72.4%) | 97(47.8%)** | 148(61.7%) | 22(88.0%)** | 149(45.2%)# | 117(51.5%) | 37(69.8%)* | 134(53.0%) | 92(59.0%) | 41(69.5%)* |
| Hyperuricaemia, n (%) | 27(4.4%) | 73(15.6%)*** | 8(3.3%) | 16(4.8%) | 3(10.3%)# | 31(15.3%)*** | 36(15.0%)*** | 6(24.0%)** | 14(4.2%) | 10(4.4%) | 3(5.7%) | 35(13.8%)*** | 28(17.9%)*** | 10(16.9%)** |
| Dyslipidaemia, n (%) | 357(58.5%) | 283(60.5%) | 143(58.1%) | 195(58.2%) | 19(65.5%) | 121(59.6%) | 145(60.4%) | 17(68.0%) | 190(57.6%) | 135(59.5%) | 32(60.4%) | 154(60.9%) | 96(61.5%) | 33(55.9%) |
| Hypoalbuminaemia: n (%) | 26(4.3%) | 25(5.3%) | 10(4.1%) | 14(4.2%) | 2(7.1%) | 11(5.4%) | 13(5.4%) | 1(4.0%) | 14(4.2%) | 11(4.9%) | 1(1.9%) | 18(7.1%) | 5(3.2%) | 2(3.4%) |
| History of stroke, n (%) | 28(4.6%) | 27(6.0%) | 7(2.8%)# | 18(5.4%) | 3(10.3%)# | 15(7.4%) | 12(5.0%) | 0(0%) | 9(2.7%)* | 15(6.6%) | 4(7.5%) | 13(5.1%) | 11(7.1%) | 3(5.1%) |
| History of heart disease, n (%) | 66(10.8%) | 69(14.8%)# | 24(9.8%) | 41(12.2%) | 1(3.4%) | 33(16.3%)# | 34(14.2%) | 2(8.0%) | 36(10.9%) | 24(10.6%) | 6(11.3%) | 41(16.2%)# | 20(12.8%) | 8(13.6%) |
| Outcomes | ||||||||||||||
| Death, n (%) | 22(3.6%) | 31(6.6%)* | 8(3.3%) | 13(3.9%) | 1(3.4%) | 10(4.9%) | 17(7.1%)# | 4(16.0%)* | 12(3.6%) | 9(4.0%) | 1(1.9%) | 15(5.9%) | 12(7.7%)# | 4(6.8%) |
| Care-needs certification, n (%) | 64(10.5%) | 71(15.2%)* | 18(7.3%)# | 41(12.2%) | 5(17.2%)# | 38(18.7%)* | 29(12.1%) | 4(16.0%) | 37(11.2%) | 18(7.9%) | 9(17.0%)* | 40(15.8%)** | 26(16.7%)** | 5(8.5%) |
| Certification for arthralgia/fractures, n (%) | 27(4.4%) | 23(4.9%) | 7(2.8%)# | 17(5.1%) | 3(10.3%)# | 9(4.4%) | 11(4.6%) | 3(12.0%)# | 13(3.9%) | 9(4.0%) | 5(9.4%)# | 12(4.7%) | 8(5.1%) | 3(5.1%) |
| Certification for dementia, n (%) | 14(2.3%) | 18(3.8%)# | 3(1.2%) | 10(3.0%) | 1(3.4%) | 13(6.4%)# | 4(1.7%) | 1(4.0%) | 8(2.4%) | 4(1.8%) | 2(3.8%) | 11(4.3%)# | 7(4.5%)# | 0(0%)# |
| Certification for stroke, n (%) | 6(1.0%) | 9(1.9%)# | 1(0.4%) | 5(1.5%) | 0(0%) | 3(1.5%) | 6(2.5%)# | 0(0%) | 3(0.9%) | 1(0.4%) | 2(3.8%)# | 4(1.6%) | 4(2.6%)# | 1(1.7%) |
| Certification for other diseases, n (%) | 17(2.8%) | 21(4.5%)# | 7(2.8%) | 9(2.7%) | 1(3.4%) | 13(6.4%)* | 8(3.3%) | 0(0%) | 13(3.9%) | 4(1.8%) | 0(0%) | 13(5.1%)# | 7(4.5%)# | 1(1.7%) |
Results are expressed as mean ± S.D or n (%). CKD, chronic kidney disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; eGFR, estimated glomerular filtration rate. Mann–Whitney U analysis or χ2 analysis (or Fisher’s exact test when needed) was used. #P < 0.20; *P < 0.05; **P < 0.01; ***P < 0.001 compared with control groups of †total non-CKD subjects, ††non-CKD subjects with SBP of 130–159 mmHg, and †††non-CKD subjects with DBP of 80–89 mmHg.
Care-needs certification in LTCI and death
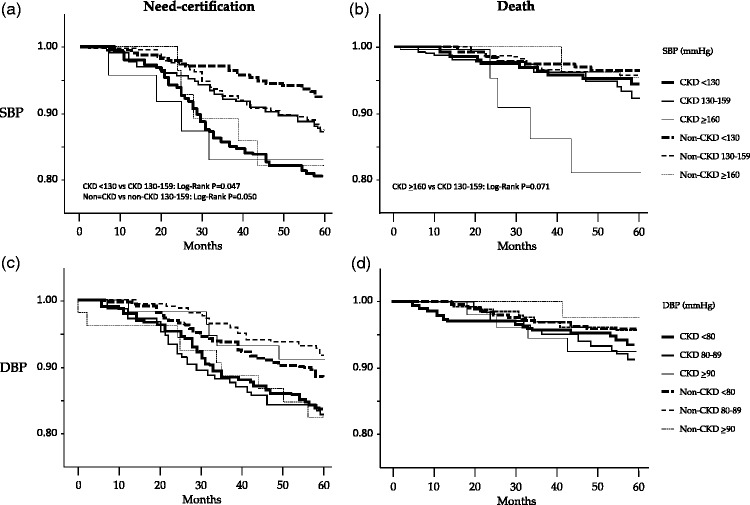
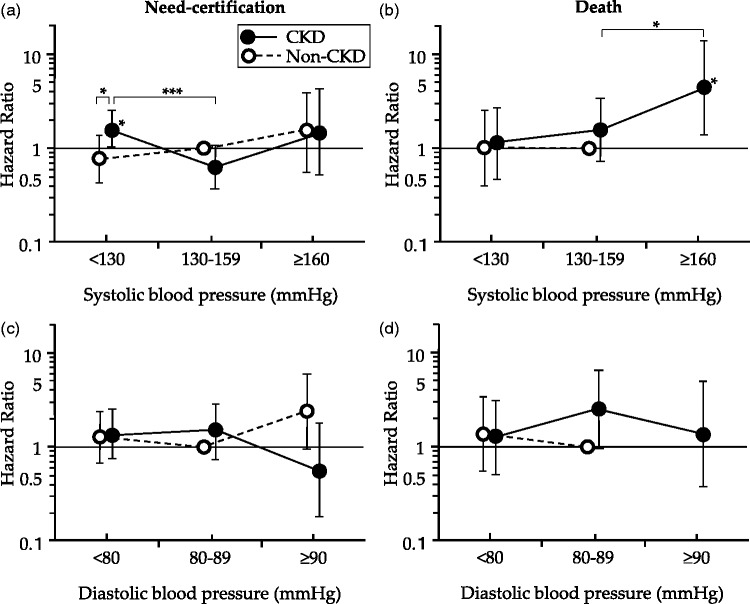
Kaplan–Meier analysis showed that the cumulative incidence of care-needs certification in LTCI in subjects with SBP <130 mmHg tended to be lower compared with those with SBP of 130–159 mmHg in the non-CKD group (P = 0.050, log-rank test), but this incidence was higher in the CKD group (P = 0.047, log-rank test) (Figure 1(a)). The adjusted HR for first care-needs certification in LTCI for 5 years was significantly higher in patients with CKD and a baseline SBP of <130 mmHg compared with patients with CKD and a baseline SBP of 130–159 mmHg (HR = 2.24, 95% CI = 1.48–4.12, P < 0.001) with non-CKD subjects with a baseline SBP of 130–159 mmHg (HR = 1.58, 95% CI = 1.01–2.52, P = 0.049), and with non-CKD subjects with a baseline SBP of <130 mmHg (HR = 2.25, 95% CI = 1.22–4.13, P < 0.001). However, there was no difference in HRs among each of the baseline SBP classes in non-CKD subjects (Figure 2(a)). The adjusted HR for death was significantly higher in patients with CKD and SBP of ≥160 mmHg compared with either patients with CKD and a baseline SBP of 130–159 mmHg (HR = 4.50, 95% CI = 1.38–14.7, P = 0.013) or non-CKD subjects with a baseline SBP of 130–159 mmHg (HR = 4.03, 95% CI = 1.09–15.2, P = 0.026) (Figure 2(b)). There was no significant difference in HR among each of the baseline DBP classes in CKD and non-CKD subjects (Figure 1(c) and 1(d)).
Figure 1.
Kaplan–Meier curves for support/care-need certification (a, c) and death (b, d) in subjects in each group with a range in systolic blood pressure of <130, 30–159, and ≥160 mmHg (a, b) and range in diastolic blood pressure of <80, 80–89, and ≥90 mmHg (c, d)
Figure 2.
Relation between baseline blood pressure and hazard ratio for first support/care-need certification (a, c) and death (b, d). Data are shown with adjusted relative risks and 95% confidence intervals with reference to subjects without chronic kidney disease with baseline systolic blood pressure of 130–159 mmHg (a, b) and those with baseline diastolic blood pressure of 80–89 mmHg (c, d). *P < 0.05; ***P < 0.001.
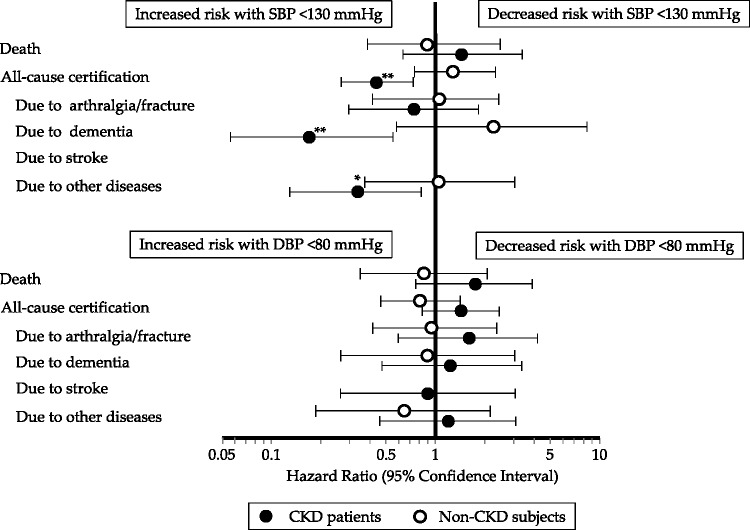
Figure 3 shows HRs for first care-needs certification in LTCI or death in each group of lower and moderate SBP (<130 vs. 130–159 mmHg) and DBP (<80 vs. 80–89 mmHg) in CKD and non-CKD subjects. Patients with CKD and a moderate baseline SBP of 130–159 mmHg had a significantly lower adjusted HR for first care-needs certification in LTCI for 5 years compared with those with a lower baseline SBP of <130 mmHg (HR = 0.44, 95% CI = 0.26–0.72, P = 0.001), although non-CKD subjects with moderate SBP did not (Figure 3). Among the 135 subjects with care-needs certification in LTCI, 50 were certified because of arthralgia/fractures, 32 for dementia, 15 for stroke, and 38 for other diseases during the study period (Table 1). Patients with CKD and a moderate baseline SBP of 130–159 mmHg also had significantly lower adjusted HRs for first care-needs certification in LTCI because of dementia (HR = 0.17, 95% CI = 0.05 –0.55, P = 0.003) and for other diseases (HR = 0.33, 95% CI = 0.13–0.82, P = 0.017) for 5 years, although non-CKD subjects with moderate SBP did not (Figure 3). There were no significant differences in the HRs for first care-needs certification in LTCI between the baseline DBP classes, or in the HRs for death between each of the baseline SBP and DBP classes, in the CKD and non-CKD groups (Figure 3).
Figure 3.
Sensitivity analysis of the association of lower baseline systolic blood pressure (<130 mmHg) and moderate baseline systolic blood pressure (130–159 mmHg), or lower baseline diastolic blood pressure (<80 mmHg) and moderate baseline diastolic blood pressure (80–89 mmHg) with hazard ratio for first support/care-need certification, stratified by the presence or absence of chronic kidney disease, in community-dwelling older subjects aged ≥65 years
Data are presented with adjusted relative risks and 95% confidence intervals. *P < 0.05; **P < 0.01.
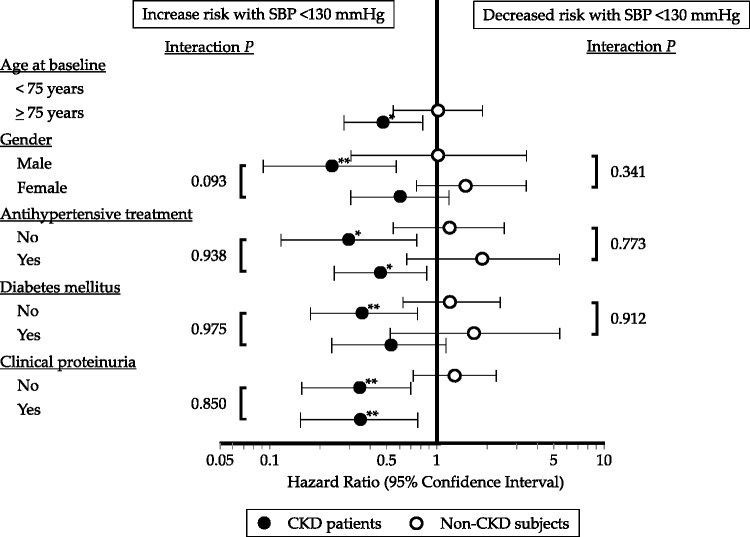
A J-shaped relationship with a beneficial effect of SBP of 130–159 mmHg on total care-needs certification in LTCI compared with SBP of <130 mmHg was maintained in the subgroup of patients with CKD aged ≥75 years (HR = 0.47, 95% CI = 0.28–0.81, P = 0.007). This relationship tended to be more evident in male patients with CKD than in female patients with CKD (HR = 0.23, 95% CI = 0.09–0.58, P = 0.002), with a P value for interaction of 0.093 (Figure 4). A beneficial effect of SBP of 130–159 mmHg on total care-needs certification in LTCI compared with SBP of <130 mmHg was observed in patients with CKD with and without antihypertensive treatment, in those without diabetes mellitus, and in those with and without clinical proteinuria. However, the interaction P values of these confounders were not significant (Figure 4).
Figure 4.
Sensitivity analysis of the association of lower baseline systolic blood pressure (<130 mmHg) and moderate baseline systolic blood pressure (130–159 mmHg) with adjusted hazard ratio for first total support/care-need certification, stratified by the presence or absence of chronic kidney disease in various subgroups of confounding factors for cardiovascular diseases.
*P < 0.05; **P < 0.01.
Discussion
The present study showed that 43% of community-dwelling older Japanese subjects had either an eGFR <60 ml/min/1.73 m2 or clinical proteinuria. This finding indicates that CKD is common in this population. Several previous studies have demonstrated an association of lower SBP with increased cardiovascular morbidity/mortality in patients with CKD.8–13 The present study adds evidence that comorbidity of CKD is one of the main factors associated with an increase in HR at a lower SBP of <130 mmHg for subsequent care-needs certification in LTCI in Japanese older subjects.
Previous studies have shown that a lower range in SBP is associated with an increased risk of mortality, which varies with a more advanced mean age in patients with CKD. Indeed, the risk of cardiovascular mortality was increased in patients with CKD and SBP <110 mmHg in relatively younger CKD populations in the Gonryo study, with a mean age of 60.0 years.8 All-cause mortality was increased in patients (mean age, 68.1 years) with CKD and SBP <133 mmHg in the Salem Veterans Affairs Medical Centre study, 9 in those (mean age, 68.4 years) with SBP <130 mmHg in the Indianapolis Veterans Affairs Hospital study,10 and in those (mean age, 73.8 years) with SBP <130 mmHg in US veterans.11 Moreover, Weiss et al.12 showed a J-shaped relation for a higher risk of mortality with a stepwise increase in the lower range of SBP according to more advanced age in a cohort of older patients with CKD. They found a higher risk of mortality with SBP <131 mmHg in those aged 65–70 years, with <141 mmHg in those aged 71–80 years, and with <151 mmHg in those aged > 80 years. The mean age of patients with CKD in the present study was 74.3 years. Therefore, the range in SBP of <130 mmHg, which was associated with an increased risk for first care-needs certification in LTCI, appears to be partially compatible with these previous studies.9–11 The Systolic Blood Pressure Intervention Trial (SPRINT) was a randomized, controlled trial in subjects aged ≥50 years that excluded those with diabetes mellitus or a history of stroke.21 This trial recently demonstrated more beneficial effects of lowering SBP to a target of <120 mmHg (intensive treatment) on cardiovascular morbidity/mortality compared with those with a target of <140 mm Hg (standard treatment). One of the reasons for the discrepancy between the present study results and SPRINT results could be a difference in outcome measures. The support/care-need certification is assessed based on a decline in physical and mental functions of individual older people. Therefore, not only new onset of causative disorders, but also occurrence of harmful adverse events by hypertension treatment would affect the outcome. In fact, rates of serious adverse events, including hypotension, syncope, electrolyte abnormalities, and acute kidney injury or failure, were higher in the intensive-treatment group than in the standard-treatment group in the SPRINT.21 Findings in these previous studies and in our study suggest the importance of avoiding BP that is too low because a lower BP may relate to unexpected appearance of a J-shaped relationship in high-risk older populations, including older patients with CKD.22
The present study showed that the disadvantageous effect of a low range in SBP of <130 mmHg on subsequent care-needs certification in LTCI in patients with CKD was maintained in those aged ≥75 years, and it was more prominent in men (Figure 4). Moreover, stratified analysis showed that a low range in SBP was associated with an increased risk of subsequent certification because of dementia (Figure 3). The mechanism(s) that might contribute to the association of the low range of SBP and increased risk of dementia in older patients with CKD remains unclear. Okumiya et al.23 reported a relationship with a significant decline in cognitive function during 3-year follow-up when SBP was <125 mmHg in community-dwelling older Japanese subjects. CKD is an independent potential risk for small vessel disease.24 Therefore, a possible explanation for this association may be that hypoperfusion caused by low BP could increase the risk of cerebral small vessel disease with consequent worsening of cognitive impairment.25 Indeed, patients with vascular cognitive impairment25 or vascular dementia26 are older and more frequently male. Another explanation for this association may be that older patients with CKD and baseline SBP <130 mmHg might be originally at high risk of Alzheimer dementia. This is because SBP has been reported to decline in the years before the onset of Alzheimer dementia, but not vascular dementia, in community-dwelling older subjects.27 Recent evidence has also showed an association of mild CKD with an increased risk of Alzheimer dementia, as well as an association of moderate to severe CKD with vascular dementia.28 The precise mechanism(s) responsible for onset of dementia in the low range of SBP remains to be determined.
Previous studies have shown an advantageous effect of a low range in SBP of <130 mmHg in patients with hypertension and diabetes mellitus for a reduction in risk of stroke based on a meta-analysis of 31 intervention trials29 and for renoprotective outcomes.5,6 However, the ONTARGET study of 25,584 patients with antihypertensive treatment showed a J-shaped relationship for the risk of cardiovascular morbidity and mortality, except for stroke.30 This study showed nadirs in SBP at the J-shaped relationship of 129 and 130 mmHg in non-diabetic and diabetic hypertensive patients, respectively. Our findings of a J-shaped association of SBP of <130 mmHg for an increased risk for subsequent care-needs certification in LTCI in non-diabetic CKD subjects and in patients CKD with and without antihypertensive treatment (Figure 4) may be partly compatible with the findings of the ONTARGET study.30 Kovesdy et al.9 found a J-shaped association between SBP and all-cause mortality, and the outcome was associated with older age and lower proteinuria after multivariate adjustments in veterans with CKD. Agarwal10 also demonstrated that the J-shaped association between SBP and all-cause mortality was especially pronounced in patients with CKD and an older age or the absence of clinical proteinuria. In the present study, a J-shaped association between the lowest range in SBP and an increase in risk of subsequent certification was observed in patients with CKD with and without clinical proteinuria (Figure 4). The reason(s) for this difference between studies should be evaluated in the future. Nevertheless, our findings suggest that introduction of antihypertensive drugs and treatment goals should be individually evaluated in older fragile patients with CKD, especially in those aged ≥75 years.
This study has several limitations. First, we conducted a single community study. Second, we could not consider CKD data in the majority of community-dwelling older people who did not undergo an annual health check-up, and who might have a higher prevalence and different subclass patterns of CKD and BP because of less concern about their health. Third, our study had relatively small numbers of patients with CKD and non-CKD subjects, especially those with a higher SBP of ≥160 mmHg. Finally, the analyses performed in our study do not imply causation between antihypertensive drug overtreatment and an increased risk of care-needs certification in LTCI. This is because a J-shaped relationship was observed in patients with CKD with and without antihypertensive treatment (Figure 4). The risk of an excess reduction in BP on frailty or dementia in older hypertensive patients with CKD should be examined in future, randomized studies.
In conclusion, in older patients with CKD, the lower range in SBP of <130 mmHg may be a useful prognostic marker for subsequent care-needs certification in LTCI, especially for dementia.
Contributors
All authors have reviewed the final version of this manuscript and approved its submission for publication.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was partly supported by Research Funding for Longevity Sciences (20 A-02) from the National Center for Geriatrics and Gerontology (NCGG) Japan.
References
- 1.Castro AF, Coresh J. CKD surveillance using laboratory data from the population-based National Health and Nutrition Examination Survey (NHANES). Am J Kidney Dis 2009; 53: S46–S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imai E, Horio M, Watanabe T, et al. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol 2009; 13: 621–630. [DOI] [PubMed] [Google Scholar]
- 3.Iyer AS, Ahmed MI, Filippatos GS, et al. Uncontrolled hypertension and increased risk for incident heart failure in older adults with hypertension: findings from a propensity-matched prospective population study. J Am Soc Hypertens 2010; 4: 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimamoto K, Ando K, Fujita T, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res 2014; 37: 253–390. [DOI] [PubMed] [Google Scholar]
- 5.de Galan BE, Perkovic V, Ninomiya T, et al. Lowering blood pressure reduces renal events in type 2 diabetes. J Am Soc Nephrol 2009; 20: 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uzu T, Kida Y, Yamauchi A, et al. The effects of blood pressure control levels on the renoprotection of type 2 diabetic patients without overt proteinuria. J Am Soc Hypertens 2012; 6: 124–131. [DOI] [PubMed] [Google Scholar]
- 7.Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ 2013; 185: 949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto T, Nakayama M, Miyazaki M, et al. Relationship between low blood pressure and renal/cardiovascular outcomes in Japanese patients with chronic kidney disease under nephrologist care: the Gonryo study. Clin Exp Nephrol 2015; 19: 878–886. [DOI] [PubMed] [Google Scholar]
- 9.Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, et al. Association of low blood pressure with increased mortality in patients with moderate to severe chronic kidney disease. Nephrol Dial Transplant 2006; 21: 1257–1262. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R. Blood pressure components and the risk for end-stage renal disease and death in chronic kidney disease. Clin J Am Soc Nephrol 2009; 4: 830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovesdy CP, Bleyer AJ, Molnar MZ, et al. Blood pressure and mortality in US veterans with chronic kidney disease. Ann Intern Med 2013; 159: 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss JW, Peters D, Yang X, et al. Systolic BP and mortality in older adults with CKD. Clin J Am Soc Nephrol 2015; 10: 1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiner DE, Tighiouart H, Levey AS, et al. Lowest systolic blood pressure is associated with stroke in stages 3 to 4 chronic kidney disease. J Am Soc Nephrol 2007; 18: 960–966. [DOI] [PubMed] [Google Scholar]
- 14.Brogan DJ, Haber M, Kutner NG. Functional decline among older adults: Comparing a chronic disease cohort and controls when mortality rates are markedly different. J Clin Epidemiol 2000; 53: 847–851. [DOI] [PubMed] [Google Scholar]
- 15.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate renal impairment and risk of dementia among older adults: The Cardiovascular Health Cognition Study. J Am Soc Nephrol 2004; 15: 1904–1911. [DOI] [PubMed] [Google Scholar]
- 16.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: The health, aging, and body composition study. J Am Soc Nephrol 2005; 16: 2127–2133. [DOI] [PubMed] [Google Scholar]
- 17.Yamada M, Arai H, Nishiguchi S, et al. Chronic kidney disease (CKD) is an independent risk factor for long-term care insurance (LTCI) need certification among older Japanese adults: a two-year prospective cohort study. Arch Gerontol Geriatr 2013; 57: 328–332. [DOI] [PubMed] [Google Scholar]
- 18.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 2012; 307: 1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakayama M, Metoki H, Terawaki H, et al. Kidney dysfunction as a risk factor for first symptomatic stroke events in a general Japanese population–the Ohasama study. Nephrol Dial Transplant 2007; 22: 1910–1915. [DOI] [PubMed] [Google Scholar]
- 20.Iritani O, Koizumi Y, Hamazaki Y, et al. Association between blood pressure and disability-free survival among community-dwelling elderly patients receiving antihypertensive treatment. Hypertens Res 2014; 37: 772–778. [DOI] [PubMed] [Google Scholar]
- 21.SPRINT Research Group, Wright JT, Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373: 2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banach M, Aronow WS. Blood pressure J-curve: current concepts. Curr Hypertens Rep 2012; 14: 556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okumiya K, Matsubayashi K, Wada T, et al. J-curve relation between blood pressure and decline in cognitive function in older people living in community, Japan. J Am Geriatr Soc 1997; 45: 1032–1033. [DOI] [PubMed] [Google Scholar]
- 24.Wada M, Nagasawa H, Iseki C, et al. Cerebral small vessel disease and chronic kidney disease (CKD): results of a cross-sectional study in community-based Japanese elderly. J Neurol Sci 2008; 272: 36–42. [DOI] [PubMed] [Google Scholar]
- 25.Pavlovic AM, Pekmezovic T, Tomic G, et al. Baseline predictors of cognitive decline in patients with cerebral small vessel disease. J Alzheimers Dis 2014; 42: S37–S43. [DOI] [PubMed] [Google Scholar]
- 26.Fujishima M, Kiyohara Y. Incidence and risk factors of dementia in a defined elderly Japanese population: the Hisayama study. Ann N Y Acad Sci 2002; 977: 1–8. [DOI] [PubMed] [Google Scholar]
- 27.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet 1996; 347: 1141–1145. [DOI] [PubMed] [Google Scholar]
- 28.Miwa K, Tanaka M, Okazaki S, et al. Chronic kidney disease is associated with dementia independent of cerebral small-vessel disease. Neurology 2014; 82: 1051–1057. [DOI] [PubMed] [Google Scholar]
- 29.Reboldi G, Gentile G, Angeli F, et al. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: A meta-analysis in 73,913 patients. J Hypertens 2011; 29: 1253–1269. [DOI] [PubMed] [Google Scholar]
- 30.Sleighta P, Redonb J, Verdecchia P. et al. Prognostic value of blood pressure in patients with high vascular risk in the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial study. J Hypertens 2009; 27: 1360–1369. [DOI] [PubMed] [Google Scholar]