Abstract
Objectives
To evaluate the long-term consequences of preterm birth on anthropometric parameters in women in adolescence and into adulthood.
Methods
Seventy girls born preterm (age 12.22 ± 1.52 years) and 48 born at term participated in the first stage. Eighteen years later, 13 of the same women participated in a follow-up and were compared with a control group of 27 women. We compared anthropometric results across the two examinations, and in the second stage, also assessed body composition using bioelectrical impedance analysis.
Results
No significant differences were found in anthropometric parameters or the content of individual components of the body between the preterm-born and control groups. However, the preterm-born group showed a tendency for higher average fat mass and lower fat-free and soft lean mass compared with the control group, and had a significantly higher mean waist–hip ratio.
Conclusions
Preterm birth does not adversely affect somatic development in girls during adolescence, but shows a correlation with an elevated waist–hip ratio in adulthood.
Keywords: Prematurity, somatic development, body components, obesity, bioelectrical impedance, metabolic age, longitudinal study, long-term effects
Introduction
Preterm birth, defined as birth occurring before 37 completed weeks of gestation, constitutes between 5% and 18% of all births, and its proportion is increasing worldwide.1 Premature infants are at higher risk of a wide range of well-documented early complications, including disturbances in somatic build (large head, low levels of body fat and chest deformities) and physiological function (iron deficiency anaemia, impaired breathing, low body temperature, blood circulation disorders, poor sucking reflexes, and impaired neuromuscular coordination). All of these complications are partly a consequence of reduced birth weight. Nevertheless, children who were born preterm later often show a certain “catch-up growth” phenomenon, and experience a more intense pace of development to eventually reach levels that are typical of children who were born full-term2 This process varies in intensity in different children, generally allowing them to attain somatic parameters that are typical of their peers who were born at term by the third year of life. However, this process may be delayed for various reasons and may persist into pre-school age.
Unfortunately, little is known regarding whether the achieved effects are permanent or the type of longer-term consequences of prematurity and of subsequent accelerated development that may persist into adolescence and into adulthood. Growing evidence indicates that preterm birth is one of the risk factors for metabolic syndrome in adulthood, such as hypertension, disorders of carbohydrate metabolism, and lipid disorders.3–6 Observations of premature infants have also indicated a relationship between accelerated weight gain after birth and increased risk of cardiovascular disease7 and a higher body mass index (BMI) in adulthood.8,9 Moreover, there is an association between low birth weight and body fat content in later life.10,11
However, BMI does not reflect information on the proportions of the individual components of the body (i.e., fat mass and lean tissue mass),12 or on the distribution of body fat. Therefore, BMI does not offer an ideal measure of obesity for the purposes of investigating the attendant health consequences. An increased level of abdominal fat is strongly associated with an increased risk of metabolic complications, such as insulin resistance,12 and it is an important risk factor for morbidity and mortality.13,14 Recognizing this finding, in 2008, the World Health Organization (WHO) proposed a set of recommendations for simple, inexpensive, and readily available methods of assessing the distribution of body fat to be used in evaluating potential complications associated with obesity. These methods include measurements of waist and hip circumference and the waist-hip ratio (WHR).15 Bioelectrical impedance analysis can also be used as a fast, non-invasive, and reproducible method for studying body composition16, with applications such as predicting the risk of cardiovascular and metabolic complications.17
This observational study aimed to provide evidence of potential long-term consequences (i.e., stretching into adolescence and adulthood) of prematurity on anthropometric parameters of women who were born preterm. We evaluated parameters, including BMI, WHR, and bioelectrical impedance. We performed a follow-up examination in adulthood of part of the same group of subjects who were born preterm and who had been previously examined in adolescence (each time compared with a reference group).
Material and methods
Subjects
Individuals who qualified for the study were registered at the Premature Birth Clinic of the Therapeutic Rehabilitation Department at the Institute of Mother and Child in Warsaw. These individuals had varying degrees of prematurity as children with a low birth weight (i.e., < 2500 g) or were born prematurely (i.e., before the end of the 37th week of pregnancy, but had a normal birth weight), according to their medical birth records. The mean birth weight of the preterm infants in the group was 1865.8 ±566.3 g (minimum: 1040 g, maximum: 2580 g). These children were born at 34.5 ±1.92 weeks of gestation (minimum: 32 weeks, maximum 37 weeks). The majority of premature infants (85%) were fed on infant formulas, whereas two were fed on a combination of infant formula and breast milk. Birth records did not show any bronchopulmonary dysplasia in any of the subjects.
The somatic build of the subjects was evaluated twice: once in 1997 (puberty) and again in 2015 (adulthood). The first study involved a total of 118 individuals. The study group consisted of 70 girls who were born preterm and were age 10–14 years (12.22 ± 1.52 years). The reference group included 48 girls (12.4 ± 1.52 years), all of whom had been qualified by a physician as having been at least 6 months post-menarche. In the second stage, 18 years later, we attempted to re-establish contact with all of the individuals who participated in 1997 by sending out a request letter to their previously recorded residence twice. Only 13 of the original participants responded. Therefore, among the 70 previously surveyed subjects, 13 women who were born preterm agreed to re-participate in the study (27.6 ± 2.60 years). The reference group consisted of 27 women (28.3 ± 2.16 years). The reference groups for the first and second stages of the study comprised girls and women from the province including Warsaw and its environs (Mazowsze Voivodship), they were born at term, and were at ages corresponding to the test group. The reference group for the second stage did not consist of the same individuals as those for the first stage. Unfortunately, we had no means of contacting the original reference group participants for the follow-up study.
The basic characteristics of the two groups are shown in Table 1. All of the participants were informed about the conditions and course of the study, and gave written informed consent for participation in the research. The study received approval of the Ethics Committee at the Józef Piłsudski University of Physical Education in Warsaw.
Table 1.
Characteristics of the anthropometric groups from 1997 and 2015.
| Premature-born group, 1997 (n = 70) | Control group 1997, (n = 48) | Premature-born group, 2015 (n = 13) | Control group 2015, (n = 27) | |
|---|---|---|---|---|
| Body height (cm) | 145.32 ± 9.96 | 145.31 ± 10.13 | 162.9 ± 10.2 | 167.5 ± 5.6 |
| Body weight (kg) | 38.24 ± 11.98 | 39.00 ± 11.57 | 60.1 ± 14.77 | 61.8 ± 6.7 |
| BMI (kg/m2) | 17.8 ± 3.79 | 18.6 ± 3.30 | 22.6 ± 4.62 | 22.0 ± 1.80 |
| Chest circ. inspiration (cm) | 70.92 ± 7.33 | 71.00 ± 7.18 | 82.2 ± 9.57 | 81.6 ± 6.14 |
| Chest circ. expiration (cm) | 66.47 ± 8.52 | 66.45 ± 8.88 | 75.3 ± 9.11 | 74.1 ± 5.94 |
| Chest width (cm) | 22.43 ± 1.83 | 22.71 ± 1.93 | 23.91 ± 1.87 | 24.10 ± 1.30 |
| Chest depth (cm) | 16.72 ± 2.87 | 16.72 ± 2.34 | 17.11 ± 1.39 | 17.20 ± 1.50 |
Values are mean ± SD.
BMI, body mass index.
Study method
Two examinations were carried out at an interval of 18 years, with the first stage in 1997 and the second in 2015. All measurements were performed in the early hours of the morning at a diagnostic facility of the Central Laboratory at the University of Physical Education in Warsaw. Tests were performed at least 2 hours after the last meal. In all of the subjects, basic anthropometric measurements were recorded as follows: height and weight, circumference of the chest at the height of Xi at maximum inhalation and exhalation, depth of the chest at the narrowest point of Xi-Ths, and width of the chest at the point ThI Thl. These ratios were measured with a tape measure with a scale of 0.5 cm and a large spreading caliper. BMI was computed as weight (in kilograms) divided by the square of the height (in meters). In the second examination, waist and hip circumference were also measured and WHR values were calculated. The WHR ratio was computed as the ratio of waist circumference to hip circumference.
Moreover, body composition was assessed using bioelectrical impedance analysis (BIA) using a segmented body composition analyser (Jawon Medical IOI 353) with BodyPass software. We recorded the following data: actual body mass (kg), standard mass (kg), BMI (kg/m2), percentage of body fat (PBF) (%), mass of body fat (MBF) (kg), lean body mass (LBM) (kg), soft lean mass (SLM) (kg), total body water (TBW) (%), protein content (kg), and mineral content (kg). We also obtained data on the subjects’ metabolic age.
The overweight condition was defined as a BMI of 25–29.9 kg/m2 and obesity was defined as a BMI ≥ 30 kg/m2. Central fat distribution was defined as a WHR of ≥ 0.85 for women. According to WHO recommendations, the risk of metabolic complications substantially increases when the WHR is equal to or greater than 0.85.13
Statistical analysis
Statistical analysis was performed using STATISTICA (v.12). The Shapiro–Wilk test was used to assess the normality of the data. Because the studied features did not have a normal distribution, we compared variables between groups using the Mann–Whitney U test (p < 0.05). We used Spearman’s rank order correlation to analyse relationships between individual parameters. In some cases (impedance and WHR), the results were subjected to a logarithmic procedure and a normal distribution was obtained. Prematurely born individuals and those born at term were compared using post-hoc analysis of variance with Tukey’s test for various group sizes.
Results
In the first stage of the project in 1997, the results of anthropometric, body mass, and height measurements (expressed as absolute values and centiles, WHO 1995), generally placed the mean of the preterm-born group within the normal population.18 Isolated extreme cases still fell within the range of narrow standards (i.e., between the 25th and 75th percentiles). With regard to chest circumference during inhaling and exhaling, the results also did not deviate from the developmental standards for children born at term with a normal body weight. Similar observations applied to the results of anthropometric parameters of premature infants who were already adults (second stage). No significant differences in anthropometric parameters were found between preterm infants and their peers born at term. The main somatic characteristics of the preterm-born group around puberty (in 1997) and as adults (in 2015) compared with the reference groups are shown in Table 1.
In the second stage of the study in 2015, we also measured body composition using BIA (Table 2). There were no significant differences in somatic parameters (height, weight, BMI) or the content of individual components of the body between the two groups. However, the preterm-born group showed a tendency for higher average fat mass and lower fat-free and soft lean mass compared with the reference group.
Table 2.
Body components and anthropometric characteristics of the groups in 2015.
| Body components | Premature-born group, 2015 (n = 13) | Control group, 2015 (n = 27) | p value |
|---|---|---|---|
| Weight (kg) | 60.1 ± 14.77 | 61.8 ± 6.70 | 0.6097 |
| BMI (kg/m2) | 22.6 ± 4.62 | 22.0 ± 1.80 | 0.5744 |
| PBF (%) | 27.2 ± 5.59 | 26.46 ± 3.48 | 0.6485 |
| MBF (kg) | 16.92 ± 8.25 | 16.5 ± 3.55 | 0.8189 |
| LBM (kg) | 43.2 ± 7.16 | 45.3 ± 4.11 | 0.2324 |
| SLM (kg) | 39.7 ± 6.38 | 41.8 ± 3.78 | 0.2107 |
| TBW (%) | 31.1 ± 5.16 | 32.6 ± 2.96 | 0.2306 |
| TEE | 1912 ± 138.30 | 1951.4 ± 80.70 | 0.2666 |
| Impedance | 538.2 ± 63.50 | 531.8 ± 45.00 | 0.7162 |
| Mineral content (kg) | 3.4 ± 0.81 | 3.5 ± 0.37 | 0.544 |
| Protein content (kg) | 8.65 ± 1.23 | 9.14 ± 0.83 | 0.1507 |
| Metabolic age (years) | 27.7 ± 2.72 | 28.3 ± 2.31 | 0.4476 |
| Head circumference | 54.1 ± 2.49 | 54.5 ± 2.02 | 0.5826 |
| Waist circumference | 74.9 ± 10.99 | 70.2 ± 4.47 | 0.0568 |
| Hip circumference | 92.96 ± 10.49 | 91.69 ± 6.48 | 0.6378 |
BMI, body mass index; PBF, percentage of body fat; MBF, mass of body fat; LBM, lean body mass; SLM, soft lean mass; TBW, total body water; TEE, total energy expenditure. We calculated metabolic age and the waist-hip ratio (WHR).
Values are mean ± SD.
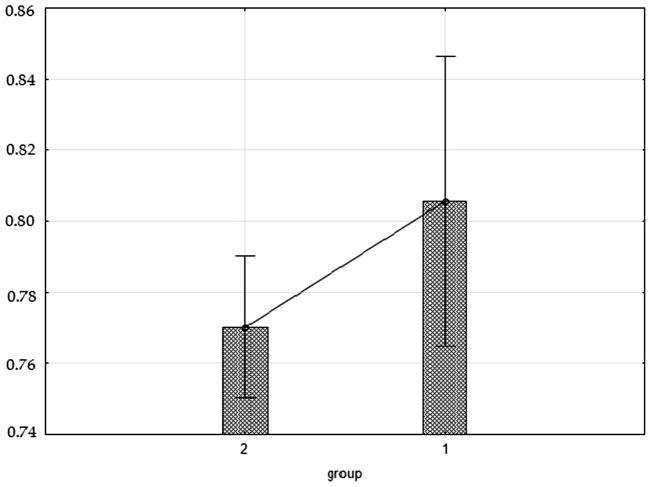
The mean WHR in the preterm-born group was significantly higher (p = 0.0462) than that in the reference group (0.81 vs. 0.77). In the preterm-born group, the WHR value of 0.85 (the lower limit for abdominal obesity for females)12 was exceeded by two women and was equal to 0.85 in a third woman. In the reference group, there were no WHRs equal to or greater than 0.85 (Figure 1).
Figure 1.
WHR in the preterm-born group (1) and the control group (2) in 2015.
Comparison of calendar age with values of metabolic age (calculated on the basis of body composition) showed no significant difference between the groups. The arithmetic mean of calendar age of the subjects was 27.6 ± 2.60 years. This was nearly identical to the mean metabolic age 27.7 ±2.72 years. Values were similar in the reference group (28.3 ± 2.16 years and 28.3 ± 2.31 years).
The best correlated anthropometric variables of body components in both groups were waist and chest circumference, which showed moderate or high correlations. Hip circumference was correlated with all of the variables shown in Table 3, except for the PBF. The relationships of body components and the WHR were noteworthy, with significant correlations of the WHR with the amount of adipose tissue. Significant correlations of the WHR with lean mass and soft tissue were not observed. Head circumference was not correlated with other body components.
Table 3.
Values of Spearman correlations between circumferences of the head and trunk and selected body components in the studied groups in 2015.
| Correlated variable | PBF (%) | MBF (kg) | LBM (kg) | SLM (kg) |
|---|---|---|---|---|
| Head circumference | −0.02 | 0.13 | 0.26 | 0.3 |
| Waist circumference | 0.54* | 0.61* | 0.46* | 0.43* |
| Hip circumference | 0.19 | 0.44* | 0.57* | 0.57* |
| WHR | 0.51* | 0.33* | 0.02 | −0.02 |
| Chest circumference at maximum inhalation | 0.45* | 0.68* | 0.66* | 0.65* |
| Chest circumference at maximum exhalation | 0.54* | 0.72* | 0.58* | 0.55* |
PBF, percentage of body fat; MBF, mass of body fat; LBM, lean body mass; SLM, soft lean mass; waist-hip ratio, WHR. *p < 0.05.
Discussion
Determining the early-life risk factors for obesity and its comorbidities is important for the global health of populations. In this observational study, we aimed to identify potential long-term effects of preterm birth on somatic development in adolescence and then again in adulthood, in a selected group of women who were born preterm. Therefore, this study extended over a period of 30 years, with part of the same study group that had been examined in adolescence undergoing a follow-up examination in adulthood.
Such long-term consequences of preterm birth are complex and notoriously problematic to study, particularly in terms of maintaining the same group of subjects until the end of the project.19,20 In our study, we ultimately succeeded in recruiting only 13 (18.6%) of the 70 original female subjects who were born preterm and had been examined twice at the age of puberty to participate in the third stage of examination in adulthood, 18 years after the first examination. This loss was largely due to the major difficulty of re-establishing contact with individuals who were previously tested after so much time had passed. The individuals who did not participate in the follow-up examination may have had a variety of reasons, including change of residence in the interim, failure to receive the letter, unwillingness or inability to participate, and a lack of interest. Regardless of this loss of follow-up, because of the relative scarcity of longitudinal data, we consider that re-examination of this sizeable of a share of the original group after such a time gap was a success.
This is the first study on somatic development in a group of prematurely born female adolescents and young adults in Poland. Only two partially comparable long-term follow-up studies have been performed. In the first of these studies,21 the subjects were very low birthweight (VLBW) men who were examined at 19 years old. However, in the second study,20 the study group consisted of VLBW men and women who were examined at 20 years of age. In contrast to our study, body composition was not assessed in either of these studies.
Because previous studies only considered subjects who were born preterm in their first or second decade of life, there remains an important lack of data on somatic build of this group in later years. Changes in morphological and functional parameters in children with low birth weight in the period of dynamic development (puberty) require observation and should constitute an important indication for prophylactic medical care and rehabilitation. However, monitoring and testing of adults who were born prematurely is also important for identifying the longer-term effect of prematurity on health, especially in the context of work and quality of life.
In the first phase of our project in 1997 (adolescents), we found that anthropometric measurements in the study group of individuals who were born preterm were not different from the results obtained in the reference group. By the mean age of 12.22 years, these female children had evened out their deficits and showed no disparities in anthropometric features compared with the reference group. This finding suggests that these children had undergone catch-up growth during childhood. Fewtrell et al.23 studied a group of 200 children born prematurely (mean age: 11.2 years, 51% boys) and found that they were lighter at mid-childhood than a control group of children born at term. This finding is in contrast to our study. Similar findings have also been reported by some other studies as follows. Peralta-Carcelen et al.24 studied a group of 53 adolescents who were born with extremely low birth weight (mean age: 14.85 years, 41.5% male). These authors reported that they were significantly shorter and lighter than adolescents born with normal birth weight. These differences between studies may be related to the fact that the majority of subjects in our study were girls with low birthweight (below 2.499 g), not VLBW. The probability of achieving expected body weight increases with increasing birth body weight.24 Moreover, there are significant differences between the sexes in catch-up growth, with poorer catch-up growth in VLBW males because of a greater susceptibility to neonatal complications.25
In the second phase of our project, part of the same preterm-born group was subjected to re-examination nearly 2 decades later. We still found no differences in weight, height, and BMI between the premature-born and reference groups. This finding is compatible with the results of Hack et al.22, who found that VLBW females had catch-up in growth by 20 years old, unlike VLBW males who remained shorter and weighed less than normal birth weight controls. We also did not find differences in the content of individual components of the body between the two groups. However, we found a significant difference in the WHR value between groups. The average WHR of the two groups did not exceed 0.85, but it was higher in the preterm-born group than in the reference group. Additionally, the WHR was positively correlated with adipose tissue. The PBF was higher in the preterm-born group than in the reference group (not significant). In three women born prematurely, we found android obesity based on WHO recommendations (WHR ≥ 0.85) with a normal BMI, but did not observe any similar cases among women born at term. This finding suggests that premature birth may be an important independent risk factor for android obesity and numerous related metabolic complications in young women. This possibility is consistent with the mounting evidence that rapid growth during childhood may increase the risk of obesity and metabolic disturbances in adulthood.22, 28
The WHR is a good indicator in clinical practice for assessing distribution of fat.29 Central obesity is particularly dangerous because it significantly increases the risk of metabolic syndrome and diabetes, contributes to an increased cardiovascular risk, and stimulates secretion of pro-inflammatory cytokines.30–34 Our results are consistent with the results of a recent (2015) study by Sipola-Leppanen et al.36. They found that young adults born preterm (n = 376, mean age of 23.1 years) had significantly higher levels of cardiometabolic risk factors, including an increased WHR, than did their peers born at full term.
With regard to body fat, the same trend that we observed in our study was shown by Breukhoven et al.37 in young adults born prematurely (n = 167; age: 20.7 years). They reported a significantly higher PBF in young adults born prematurely compared with the control group. In a study by Mathai et al.38, in which body composition was also measured by dual-energy x-ray absorptiometry, adults who were born preterm (n = 52; 54% female, aged 35.7 years) had a significantly higher PBF and greater amount of abdominal adiposity compared with those born at term. A systematic review and meta-analysis by Parkinson et al.5 reported no significant differences in PBF, BMI, WHR, and biochemical markers of metabolic syndrome, such as the lipid profile
Conclusions
Preterm birth does not adversely affect somatic development in girls during adolescence. However, in early adulthood, women born prematurely have a significantly elevated WHR, which is a one of the indicators of abdominal obesity — a major component of metabolic syndrome. Such findings are particularly important because the number of adults who are born prematurely is constantly increasing yearly. Notably, most of the risk factors for cardiovascular disease are modifiable. Overall, paediatricians should draw special attention to premature infants. Premature infants should be provided appropriate conditions for optimal growth in early infancy and later in childhood. Proper nutrition and other elements of a healthy lifestyle, such as regular physical activity, can lead to a significant reduction in the risk of cardiovascular disease in adulthood. Individuals born preterm should be subject to comprehensive health monitoring to enable prevention, diagnosis, and treatment of long-term health consequences in the early stage. Further studies following this group into middle adulthood should be performed to enable better understanding of the long-term health consequences of prematurity and to determine whether women who are born prematurely continue to have accelerated abdominal fat accumulation.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This study was supported by the Polish National Science Centre (Project number: 2012/07/D/NZ7/03265).
References
- 1.World Health Organizatin http://www.who.int/mediacentre/factsheets/fs363/en/ 2015.
- 2.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008; 371: 261–269. [DOI] [PubMed] [Google Scholar]
- 3.Keijzer-Veen MG, Dulger A, Dekker FW, et al. Very preterm birth is a risk factor for increased systolic blood pressure at a young adult age. Pediatr Nephrol 2010; 25: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Jong F, MOnuteaux MC, van Elburg RM, et al. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension 2012; 59: 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parkinson JR, Hyde MJ, Gale C, et al. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta-analysis. Pediatrics 2013; 131: e1240–e1263. [DOI] [PubMed] [Google Scholar]
- 6.Hovi P, Andersson S, Eriksson JG, et al. Glucose regulation in young adults with very low birth weight. N Engl J Med 2007; 356: 2053–2063. [DOI] [PubMed] [Google Scholar]
- 7.Singhal A, Cole TJ, Fewtrell M, et al. Is slower early growth beneficial for long-term cardiovascular health? Circulation 2004; 109: 1108–1113. [DOI] [PubMed] [Google Scholar]
- 8.Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr 1994; 59: 955–959. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson J, Forsén T, Osmond C, et al. Obesity from cradle to grave. Int J Obes Relat Metab Disord 2003; 27: 722–727. [DOI] [PubMed] [Google Scholar]
- 10.Irving RJ, Belton NR, Elton RA, et al. Adult cardiovascular risk factors in premature babies. Lancet 2000; 355: 2135–2136. [DOI] [PubMed] [Google Scholar]
- 11.Leunissen RW, Kerkhof GF, Stijnen T, et al. Fat mass and apolipoprotein E genotype influence serum lipoprotein levels in early adulthood, whereas birth size does not. J Clin Endocrinol Metab 2008; 93: 4307–4314. doi: 10.1210/jc.2008-0621. [DOI] [PubMed] [Google Scholar]
- 12.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000; 894: i–xii, 1–253. [PubMed] [Google Scholar]
- 13.Bigaard J, Frederiksen K, Tjonneland A, et al. Waist circumference and body composition in relation to all-cause mortality in middle-aged men and women. Int J Obes (Lond) 2005; 29: 778–784. [DOI] [PubMed] [Google Scholar]
- 14.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med 2008; 359: 2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Waist circumference and waist–hip ratio: report of a WHO expert consultation, Geneva, 8–11 December 2008. 2011. World Health Organization.
- 16.Wiszomirska I, Krynicki B, Kaczmarczyk K, Gajewski J. The impact of functional training on postural stability and body composition in women over 60. Journal of Sports Medicine and Physical Fitness 2015; 55(6): 654–62. [PubMed] [Google Scholar]
- 17.Krachler B, Volgyi E, Saconen K, et al. BMI and an athropometry-based estimate of fat mass percentage are both valid discriminators of cardiometabolic risk: a comparison with DXA and bioimpedance. J Obes 2013; 2013: 862514–862514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Physical status:the use and interpretation of anthropometry: report of a WHO expert committee, Geneva, 1995. [PubMed]
- 19.Narang I, Rosenthal M, Cremonesini D, et al. Longitudinal evaluation of airway function 21 years after preterm birth. Am J Respir Crit Care Med 2008; 178: 74–80. [DOI] [PubMed] [Google Scholar]
- 20.Vollsæter M, Røksund OD, Eide GE, et al. Lung function after preterm birth: development from mid-childhood to adulthood. Thorax 2013; 68: 767–776. [DOI] [PubMed] [Google Scholar]
- 21.Ericson A, Kallen B. Very low birthweight boys at the age of 19. Arch Dis Child Fetal Neonatal Ed 1998; 78: F171–F174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hack M, Schluchter M, Cartar L, et al. Growth of very low birth weight infants to age 20 years. Pediatrics 2003; 112: e30–e38. [DOI] [PubMed] [Google Scholar]
- 23.Fewtrell MS, Lucas A, Cole TJ, et al. Prematurity and reduced body fatness at 8-12 y of age. Am J Clin Nutr 2004; 80: 436–440. [DOI] [PubMed] [Google Scholar]
- 24.Peralta-Carcelen M, Jackson DS, Goran MI, et al. Growth of adolescents who were born at extremely low birth weight without major disability. J Pediatr 2000; 136: 633–640. [DOI] [PubMed] [Google Scholar]
- 25.Kosińska M, Stoińska B, Gadzinowski J. Catch-up growth among low birth weight infants: Estimation of the time of occurrence of compensatory events. Anthropological Review 2004; 67: 87–95. [Google Scholar]
- 26.Stevenson DK, Verter J, Fanaroff AA, et al. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Arch Dis Child Fetal Neonatal Ed 2000; 83: F182–F185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cianfarani S, Germani D, Branca F. Low birthweight and adult insulin resistance: the “catch-up growth” hypothesis. Arch Dis Child Fetal Neonatal Ed 1999; 81: F71–F73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Law C. Adult obesity and growth in childhood. BMJ 2001; 323: 1320–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eriksson JG, Forsén T, Tuomilehto J, et al. Early growth and coronary heart disease in later life: longitudinal study. BMJ 2001; 322: 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemieux S, Prud’homme D, Tremblay A, et al. Anthropometric correlates to changes in visceral adipose tissue over 7 years in women. Int J Obes Relat Metab Disord 1996; 20: 618–624. [PubMed] [Google Scholar]
- 31.Bergman RN, Van Citters GW, Mittelman SD, et al. Central role of the adipocyte in the metabolic syndrome. J Investig Med 2001; 49: 119–126. [DOI] [PubMed] [Google Scholar]
- 32.Mathieu P, Lemieux I, Després JP. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther 2010; 87: 407–416. [DOI] [PubMed] [Google Scholar]
- 33.Sharma AM. Adipose tissue: a mediator of cardiovascular risk. Int J Obes Relat Metab Disord 2002; 26(Suppl 4): S5–S7. [DOI] [PubMed] [Google Scholar]
- 34.El-Wakkad A, Hassan Nel-M, Sibaii H, et al. Proinflammatory, anti-inflammatory cytokines and adiponkines in students with central obesity. Cytokine 2013; 61: 682–687. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt FM, Weschenfelder J, Sander C, et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS One 2015; 10: e0121971–e0121971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sipola-Leppanen M, Vääräsmäki M, Tikanmäki M, et al. Cardiometabolic risk factor in young adults who were born preterm. Am. J. Epidemiol 2015; 181: 861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breukhoven PE, Kerkhof GF, Willemsen RH, et al. Fat mass and lipid profile in young adults born preterm. J Clin Endocrinol Meta 2012; 97: 1294–1302. [DOI] [PubMed] [Google Scholar]
- 38.Mathai S, Derraik JG, Cutfield WS, et al. Increased adiposity in adults born preterm and their children. PLoS One 2013; 8: e81840–e81840. [DOI] [PMC free article] [PubMed] [Google Scholar]