Abstract
Objective
To evaluate the clinical effect of different pancreaticojejunostomy techniques in the treatment of pancreaticoduodenectomy and investigate the applicability of pancreaticojejunostomy without pancreatic duct stenting.
Methods
From January 2012 to December 2015, 87 patients who underwent pancreaticoduodenectomy were randomly assigned to either Group A (duct-to-mucosa anastomosis with pancreatic duct stenting, n = 43) or Group B (pancreas–jejunum end-to-side anastomosis without stenting (n = 44). The operative duration of pancreaticojejunostomy, postoperative hospital stay, and incidence of postoperative complications were compared between the two methods.
Results
The operative duration of pancreaticojejunostomy without use of the pancreatic duct stent was significantly shorter in Group B than in Group A (t = 7.137). The postoperative hospital stay was significantly shorter in Group B than in Group A (t = 2.408). The differences in the incidence of postoperative complications such as pancreatic fistula, abdominal bleeding, abdominal infection and delayed gastric emptying were not significantly different between the two groups (χ2 = 0.181, 0.322, 0.603, and 0.001, respectively).
Conclusion
Pancreaticoduodenectomy without pancreatic duct stenting is safe and reliable and can reduce the operative time and hospital stay. No significant differences were observed in the incidence of postoperative complications.
Keywords: Pancreaticoduodenectomy, pancreatic duct stent, pancreaticojejunostomy, patient outcomes, postoperative pancreatic fistula, randomized controlled trial
Pancreaticoduodenectomy (Whipple surgery) is one of the most extensive abdominal surgeries. It is associated with high postoperative morbidity and mortality rates because of its involvement with multiple organs, complex surgical procedures, and long surgical duration.1,2 Advances in surgical techniques have led to a decrease in the mortality rate of pancreaticoduodenectomy, which has currently fallen to <5%. However, the complication rate remains as high as 21%, most commonly because of the development of pancreatic fistulas.3 The prevalence of pancreatic fistulas is influenced by patient age, preoperative nutritional status, underlying disease, pancreas texture, pathological type, and other factors. The pancreaticojejunostomy technique and experience level of the surgeon are also influential factors in the development of pancreatic fistulas. Surgeons once considered that the incidence of pancreatic fistula could be reduced by placing a pancreatic stenting tube during pancreaticoduodenectomy, thereby allowing the pancreatic juice to be directly drained from the body and reducing corrosion at the anastomotic site caused by pancreatic juice.4,5 More recently, clinical studies have suggested that placement of a tube does not reduce the incidence of postoperative pancreatic fistula and other complications. Winter et al.6 demonstrated that pancreatic duct stenting did not decrease the frequency or severity of postoperative pancreatic fistulas; in fact, a trend toward increased pancreatic fistulas was observed in patients with soft pancreatic remnants and stents (21.1%) compared with patients with soft pancreatic remnants and no stents (10.7%). A retrospective study by Suzuki et al.7 found no differences in long-term follow-up after pancreaticoduodenectomy with duct-to-mucosa pancreaticojejunostomy anastomosis performed with versus without a stenting tube. Based on the short-term and long-term complications, the authors suggested that duct-to-mucosa pancreaticojejunostomy anastomosis can be performed more safely without than with a stenting tube.7 In addition, a study by Sachs et al.8 demonstrated that the use of pancreaticojejunal stents did not decrease the incidence or severity of clinically relevant postoperative pancreatic fistulas after proximal pancreatic resection, both overall and for high-risk scenarios. In some patients, pancreaticojejunal stents may lead to short- and long-term adverse outcomes. The authors found that pancreaticojejunostomy without a stent may be associated with a decrease in the rates of abdominal infection and abscess as well as a relative decrease in the rates of major complications and wound dehiscence.8 However, their study was not a randomized controlled trial and was therefore subject to certain limitations. We designed this randomized controlled trial to demonstrate the safety and clinical value of pancreaticojejunostomy without pancreatic duct stenting.
Data and methods
General data
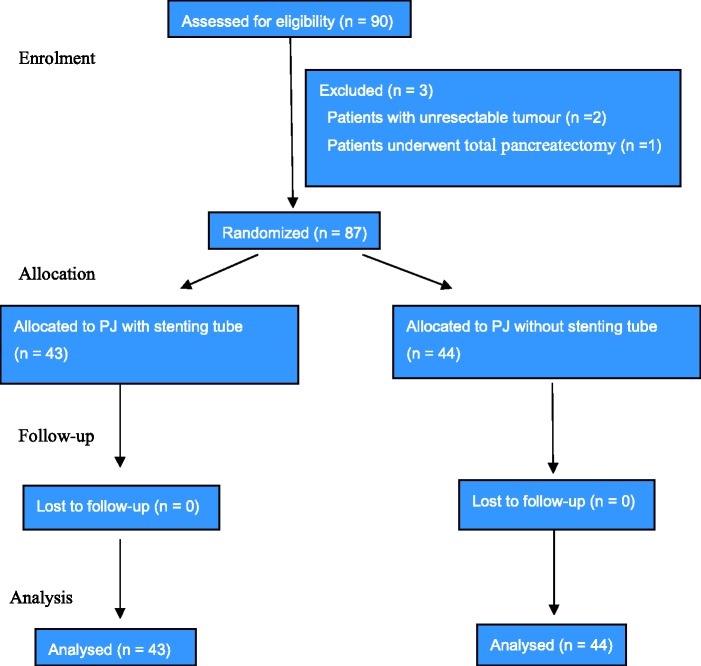
Data on 90 patients undergoing pancreaticoduodenectomy in the authors’ hospital from January 2012 to December 2015 were collected. Three patients were excluded from the study during the operation. In two cases, the tumors were unresectable because of vascular invasion of the superior mesenteric artery, and the patients therefore underwent hepaticojejunostomy. In one case, total pancreatectomy was performed when the frozen section analysis of the specimen revealed tumor involvement of the pancreatic body and tail remnant (Figure 1). The reported results include the 87 patients who completed the study (46 men and 41 women; age range, 28–78 years; mean age, 59.0 years). The reason for pancreaticoduodenectomy were pancreatic head adenocarcinoma (n = 35), ampulla of Vater adenocarcinoma (n = 13), distal common bile duct cholangiocarcinoma (n = 19), duodenal adenocarcinoma (n = 14), and chronic pancreatitis and pancreatolithiasis (n = 6). The patients were randomly divided into two groups depending on the pancreaticojejunostomy technique. Patients in Group A underwent traditional duct-to-mucosa anastomosis with pancreatic duct stenting (n = 43), while those in Group B underwent pancreas–jejunum end-to-side anastomosis without pancreatic duct stenting (n = 44). The study protocol was approved by the Ethics Committee of Yongchuan Hospital of Chongqing Medical University, and all patients provided written informed consent. There were no statistically significant differences in sex, age, preoperative laboratory parameters, or tumor site between the two groups (Table 1). There were also no statistically significant differences in pancreatic texture or pancreatic duct diameter (Table 2).
Figure 1.
Flow diagram showing progression through the phases of this randomized trial (i.e. enrollment, intervention allocation, follow-up, and data analysis).
PJ, pancreaticojejunostomy.
Table 1.
Comparison of general clinical data between the two groups of patients.
| Group A (n = 43) | Group B (n = 44) | χ2/t | P | |
|---|---|---|---|---|
| Sex | ||||
| Men | 23 (53.5) | 23 (52.3) | 0.013 | 0.910 |
| Women | 20 (46.5) | 21 (47.7) | ||
| Age (years) | 58.60 ± 9.74 | 59.32 ± 9.43 | 0.347 | 0.729 |
| Preoperative laboratory examination | ||||
| Total bilirubin (umol/L) | 169.17 ± 56.83 | 163.48 ± 56.75 | 0.467 | 0.642 |
| Albumin (g/L) | 37.39 ± 3.15 | 36.48 ± 8.70 | 1.323 | 0.190 |
| Hemoglobin (g/L) | 116.16 ± 9.74 | 116.25 ± 8.43 | 0.048 | 0.962 |
| Type of disease | ||||
| Pancreatic head adenocarcinoma | 18 (41.9) | 17 (38.6) | 0.094 | 0.759 |
| Distal common bile duct cholangiocarcinoma | 9 (20.9) | 10 (22.7) | 0.041 | 0.839 |
| Adenocarcinoma of the ampulla of Vater | 7 (16.3) | 6 (13.6) | 0.119 | 0.730 |
| Duodenal adenocarcinoma | 6 (13.6) | 8 (18.2) | 0.288 | 0.592 |
| Pancreatolithiasis | 3 (7.0) | 3 (6.8) | 0.001 | 0.977 |
Data are presented as n (%) or mean ± standard deviation.
Table 2.
Comparison of intraoperative technique between the two groups of patients.
| Group A (n = 43) | Group B (n = 44) | χ2/t | P | |
|---|---|---|---|---|
| Pancreaticojejunostomy | ||||
| Time (min) | 21.07 ± 3.73 | 15.43 ± 3.64 | 7.137 | 0.000 |
| Blood loss (ml) | 359.30 ± 138.55 | 357.95 ± 142.21 | 0.045 | 0.964 |
| Diameter of pancreatic duct (mm) | 3.51 ± 1.18 | 3.27 ± 1.10 | 0.973 | 0.333 |
| Texture of pancreas | ||||
| Soft | 18 (41.8) | 18 (40.9) | 0.08 | 0.928 |
| Firm | 25 (58.2) | 26 (59.1) |
Data are presented as n (%) or mean ± standard deviation.
Surgical methods
Four surgeons participated in this trial, and one chief surgeon performed all the surgeries. Digestive tract reconstruction included pancreaticojejunostomy, hepaticojejunostomy, and gastrojejunostomy during the pancreaticoduodenectomy procedure. In both study groups, an end-to-side two-layer anastomosis was performed between the pancreas and jejunum. Group A underwent pancreas–jejunum end-to-side and duct-to-mucosa anastomosis with an external vinyl tube. The duct-to-mucosa pancreaticojejunostomy was created with the use of absorbable 5-0 suture. The external stenting tube traversed the anastomosis without fixation and traveled prograde down the pancreaticobiliary limb approximately 5 cm past the hepaticojejunostomy, where it was then externalized through the bowel and secured with an absorbable purse-string suture. From there, it traversed the abdominal wall, was secured to the skin with permanent sutures, and placed into a bag for gravity-induced drainage. Anastomosis of the outer layer was performed between the pancreatic parenchyma and jejunal seromuscularis using 3-0 nonabsorbable sutures. The stent was occluded 1 to 2 weeks after the operation and removed 2 months later. In contrast, the pancreaticojejunostomy method in Group B included pancreatic stump–jejunum end-to-side anastomosis without a stenting tube. The pancreaticojejunostomy procedure was performed as follows: the posterior pancreas and full layer of the jejunum were anastomosed with 4-0 Prolene interrupted sutures, and an anastomosis was created between the anterior pancreas and full layer of the jejunum. Finally, to reinforce the anastomosis, the outer layer was reformed between the pancreas serosa and the jejunal seromuscularis using 3-0 nonabsorbable sutures. The surgeons distinguished the main pancreatic duct, avoiding sutures during the operation, and performed the anterior anastomosis by interrupted sutures without knots, confirming that the main pancreatic duct was not sutured. The knots were then tied off to close the anterior anastomosis. Somatostatin was used selectively in high-risk patients who had been previously determined to have soft glands, a small pancreatic duct size (≤3 mm), high-risk pathology (anything exclusive of pancreatic adenocarcinoma or pancreatitis), and serious blood loss (>1000 mL) according to the International Study Group of Pancreatic Fistula (ISGPF) classification9. Somatostatin was started after the operation and continued for 5 to 7 days postoperatively.
Monitoring indicators
Factors compared between Groups A and B included intraoperative bleeding; the surgical duration of pancreaticojejunostomy; postoperative complications such as pancreatic fistula, abdominal bleeding, abdominal infections, and delayed gastric emptying; and the mean postoperative hospital stay. The ISGPF defines a postoperative pancreatic fistula as a drain output of any measurable volume of fluid on or after postoperative day 3 with an amylase content greater than three times the serum amylase activity.10 The pancreatic fistulas in the present study were grade B and C. The definition of bleeding established by the International Study Group of Pancreatic Surgery (ISGPS) was used,11 and grade B and C bleeding was included in the present study. The definition of delayed gastric emptying established by the ISGPS was used,12 and grade B and C delayed gastric emptying was included in the present study.
Statistical analysis
Statistical software (SPSS v.24.0; IBM Corp., Armonk, NY, USA) was used for data analysis. Measurement data were analyzed using the t test, while count data were analyzed using the χ2 test. The difference was statistically significant at P < 0.05.
Results
Comparison of intraoperative situation
The surgical duration of pancreaticojejunostomy in Group B (pancreas–jejunum end-to-side anastomosis without pancreatic duct stenting) was significantly shorter than that in Group A (duct-to-mucosa anastomosis with pancreatic duct stenting and outer drainage) (t = 7.192, P < 0.01) (Table 2).
Postoperative complications
The postoperative complications included pancreatic fistula, abdominal bleeding, abdominal infection, and delayed gastric emptying. According to the Clavien–Dindo classification, in Group A, four patients had grade I complications, nine patients had grade II complications, and one patient had grade IIIb complications. In Group B, four patients had grade I complications, six patients had grade II complications, one patient had a grade IIIa complication, and one patient had a grade IIIb complication. There were no statistically significant differences in these postoperative complications between the two groups (χ2 = 0.181, 0.332, 0.603, and 0.001, respectively). The patients with a pancreatic fistula in both Group A and Group B showed improvement after treatment with drainage. Patients in Group B experienced a significantly shorter hospital stay than those in Group A (t = 2.408, P < 0.05) (Table 3). The patients in both groups were followed up for 3 months. The patients in Group A with pancreatic duct stenting developed stent occlusion 1 to 2 weeks after the operation, and the pancreatic stent was removed 2 months later. One patient developed a complication involving the stent falling out, but no serious outcomes occurred. The remaining patients did not experience complications after removal of the pancreatic duct stent. No patients in the two groups developed complications such as pancreatic duct stenosis or pancreatitis after a 3-month follow-up.
Table 3.
Comparison of postoperative complications between the two groups of patients.
| Group A (n = 43) | Group B (n = 44) | χ2/t | P | |
|---|---|---|---|---|
| Pancreatic fistula | 4 (9.3) | 3 (6.8) | 0.181 | 0.670 |
| Abdominal bleeding | 1 (2.3) | 2 (4.5) | 0.322 | 0.570 |
| Abdominal infection | 5 (11.6) | 3 (6.9) | 0.603 | 0.438 |
| Delayed gastric emptying | 4 (9.3) | 4 (9.1) | 0.001 | 0.973 |
| Postoperative hospital stay (days) | 13.19 ± 2.85 | 11.70 ± 2.89 | 2.408 | 0.018 |
| Mortality | 0 (0.0) | 0 (0.0) |
Data are presented as n (%) or mean ± standard deviation.
Discussion
Pancreaticoduodenectomy is a complex operation involving substantial bodily injury because the pancreas is located in the retroperitoneum and surrounded by many important anatomical structures and organs. In addition, the operation includes complex procedures and requires a long surgical time. Multiple postoperative complications associated with this procedure have led researchers and surgeons to seek new ways to simplify the technique, reduce the operation time, and reduce the risk of postoperative complications. Pancreatic fistula is the most common and dangerous complication of pancreaticoduodenectomy.13 Effective prevention of pancreatic leakage is the key to successful pancreaticoduodenectomy, and choosing the proper pancreaticojejunostomy technique is regarded as the most critical step of the whole operation. A significantly higher incidence and clinical severity of pancreatic fistula are associated with pancreaticojejunostomy.14 More than 30 methods have been used to reduce the incidence of pancreatic fistula; however, an effective method with which to completely avoid the occurrence of pancreatic fistula has not been established. The main factors involved in pancreatic fistula formation include the pancreatic texture, thickness of the pancreatic duct, experience of the surgeon, high pressure placed on the bowel, delayed healing of the pancreaticojejunostomy, presence of residual pancreas tissue, and digestion and destruction of the pancreaticojejunostomy site by pancreatic enzymes. The traditional view is that placement of a pancreatic duct stenting tube during the pancreaticojejunostomy allows for direct drainage of the pancreatic juice from the body, which can reduce the incidence of pancreatic fistula because of the decrease in corrosion of the pancreaticojejunostomy site by the pancreatic juice. However, recent studies performed worldwide have revealed that the absence of a pancreatic duct stent is safe and reliable and has no effect on the development of pancreatic fistula.15–17 One randomized controlled study showed that the incidence of postoperative pancreatic fistula did not increase and that no statistically significant difference was present when pancreaticojejunostomy without a pancreatic stenting tube was performed compared with a control group. Other complications such as delayed gastric emptying, abdominal infection, bleeding, and perioperative mortality were not significantly different. The operative procedures were simplified in the experimental group, which did not undergo pancreatic stenting, and the operative duration of pancreaticojejunostomy and postoperative hospital stay were significantly shortened. The patients did not have an external pancreatic duct stent, which can result in an improved quality of life because the tube does not need to be utilized for a long period of time, thereby reducing the patients’ experience of trauma and pain. In addition, treating patients becomes more convenient because they no longer need to use the tube after they leave the hospital nor do they need to return to the hospital for removal of the drainage tube.
Notably, the performance of duct-to-mucosa anastomosis with a pancreatic stenting tube is not applicable to every patient because it is very difficult to place a pancreatic duct stent during the operation in patients with a soft pancreatic texture, small pancreatic duct diameter, or thin pancreatic duct wall. The external pancreatic duct stent may become blocked or fall off of the drainage tube, which may lead to further complications including pancreatic fistula.18,19 It can also lead to migration of the pancreaticojejunostomy stent into the bile duct, potentially resulting in small bowel perforation.20,21 The method of pancreaticojejunostomy without a pancreatic stent that the authors use in their clinic can simplify the operation procedure, reduce the duration of pancreaticojejunostomy, and decrease the postoperative hospitalization time. Furthermore, the absence of an external drainage tube of the pancreatic duct facilitates postoperative nursing care of patients and improves their quality of life. The method used in this study was safe and reliable, and no significant difference was found in postoperative complications compared with pancreaticojejunostomy involving placement of a pancreatic stenting tube.
Declaration of conflicting interests
The material has not been published previously and will not be submitted for publication elsewhere, in whole or in part. All authors listed have approved the manuscript and have no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Kawai M, Yamaue H. Analysis of clinical trials evaluating complications after pancreaticoduodenectomy: a new era of pancreatic surgery. Surg Today 2010; 40: 1011–1107. [DOI] [PubMed] [Google Scholar]
- 2.Wellner UF, Sick O, Olschewski M, et al. Randomized controlled single-center trial comparing pancreatogastrostomy versus pancreaticojejunostomy after partial pancreatoduodenectomy. J Gastrointest Surg 2012; 16: 1686–1695. [DOI] [PubMed] [Google Scholar]
- 3.Reid-Lombardo KM, Fanell MB, Crippa S, et al. Pancreatic anastomotic leakage after pancreaticoduodenectomy in 1,507 patients: a report from the pancreatic anastomotic leak study group. J Gastrointest Surg 2007; 11: 1451–1458. [DOI] [PubMed] [Google Scholar]
- 4.Motoi F, Egawa S, Rikiyama T, et al. Randomized clinical trial of external stent drainage of the pancreatic duct to reduce postoperative pancreatic fistula after pancreaticojejunostomy. Br J Surg 2012; 99: 524–531. [DOI] [PubMed] [Google Scholar]
- 5.Patel K, Teta A, Sukharamwala P, et al. External pancreatic duct stent reduces pancreatic fistula: a meta-analysis and systematic review. Int J Surg 2014; 12: 827–832. [DOI] [PubMed] [Google Scholar]
- 6.Winter JM, Cameron JL, Campbell KA, et al. Does pancreatic duct stenting decrease the rate of pancreatic fistula following pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg 2006; 10: 1280–1290. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki S, Kaji S, Koike N, et al. Pancreaticoduodenectomies with a duct-to-mucosa pancreaticojejunostomy anastomosis with and without a stenting tube showed no differences in long-term follow-up. J Hepatobiliary Pancreat Sci 2011; 18: 258–262. [DOI] [PubMed] [Google Scholar]
- 8.Sachs TE, Pratt WB, Kent TS, et al. The pancreaticojejunal anastomotic stent: friend or foe? Surgery 2013; 153: 651–662. [DOI] [PubMed] [Google Scholar]
- 9.Pratt WB, Callery MP, Vollmer CM., Jr Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg 2008; 32: 419–428. [DOI] [PubMed] [Google Scholar]
- 10.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005; 138: 8–13. [DOI] [PubMed] [Google Scholar]
- 11.Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an international study group of pancreatic surgery (ISGPS) definition. Surgery 2007; 142: 20–25. [DOI] [PubMed] [Google Scholar]
- 12.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the international study group of pancreatic surgery (ISGPS). Surgery 2007; 142: 761–768. [DOI] [PubMed] [Google Scholar]
- 13.Shrikhande SV, D’Souza MA. Pancreatic fistula after pancreatectomy: evolving definitions, preventive strategies and modern management. World J Gastroenterol 2008; 14: 5789–5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Addeo P, Delpero JR, Paye F, et al. Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: a multicentre study of the French Surgical Association. HPB (Oxford) 2014; 16: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong JJ, Altaf K, Mukherjee R, et al. Systematic review and meta-analysis of outcomes after intraoperative pancreatic duct stent placement during pancreaticoduodenectomy. Br J Surg 2012; 99: 1050–1061. [DOI] [PubMed] [Google Scholar]
- 16.Usuba T, Misawa T, Ito R, et al. Safety of Non-stented Pancreaticojejunostomy in Pancreaticoduodenectomy for Patients with Soft Pancreas. Anticancer Res 2016; 36: 6619–6623. [DOI] [PubMed]
- 17.Watanobe I, Omori S, Miyano S, et al. Results of pancreaticojejunal end-to-side anastomosis using the invagination method without a pancreatic stenting tube. Hepatogastroenterology 2015; 62: 447–450. [PubMed] [Google Scholar]
- 18.Suzuki S, Kaji S, Koike N, et al. Pancreaticojejunostomy of duct to mucosa anastomosis can be performed more safely without than with a stenting tube. Am J Surg 2009; 198: 51–54. [DOI] [PubMed] [Google Scholar]
- 19.Smyrniotis V, Arkadopoulos N, Kyriazi MA, et al. Does internal stenting of the pancreaticojejunostomy improve outcomes after pancreatoduodenectomy? A prospective study. Langenbecks Arch Surg 2010; 395: 195–200. [DOI] [PubMed] [Google Scholar]
- 20.Mari G, Costanzi A, Monzio N, et al. Small bowel perforation caused by pancreaticojejunal anastomotic stent migration after pancreaticoduodenectomy for periampullary carcinoma. JOP 2015; 16: 185–188. [DOI] [PubMed] [Google Scholar]
- 21.Park SH, Kim JH, Noh SY, et al. Migration of internal pancreaticojejunostomy stents into the bile ducts in patients undergoing pancreatoduodenectomy. J Gastrointest Surg 2015; 19: 1995–2002. [DOI] [PubMed] [Google Scholar]