Abstract
Objective
Embedding clinical pharmacists into ambulatory care settings needs to be assessed in the context of established medical home models.
Methods
A retrospective, observational study examined the effectiveness of the Intermountain Healthcare Collaborative Pharmacist Support Services (CPSS) program from 2012–2015 among adult patients diagnosed with diabetes mellitus (DM) and/or high blood pressure (HBP). Patients who attended this program were considered the intervention (CPSS) cohort. These patients were matched using propensity scores with a reference group (no-CPSS cohort) to determine the effect of achieving disease management goals and time to achievement.
Results
A total of 17,684 patients had an in-person office visit with their provider and 359 received CPSS (the matched no-CPSS cohort included 999 patients). CPSS patients were 93% more likely to achieve a blood pressure goal < 140/90 mmHg, 57% more likely to achieve HbA1c values < 8%, and 87% more likely to achieve both disease management goals compared with the reference group. Time to goal achievement demonstrated increasing separation between the study cohorts across the entire study period (P < .001), and specifically, at 180 days post-intervention (HBP: 48% vs 27% P < .001 and DM: 39% vs 30%, P < .05).
Conclusions
CPSS participation is associated with significant improvement in achievement of disease management goals, time to achievement, and increased ambulatory encounters compared with the matched no-CPSS cohort.
Keywords: Collaborative drug therapy management, pharmacy, population health, hypertension, diabetes mellitus
Background
Currently, organizations are charged with improving the population’s health, by “improving the health outcomes of a group of individuals”, to efficiently transition towards new mechanisms of value-based care delivery and population-based payment.1 These changes have reinforced the role of primary care in meeting the growing healthcare needs of the population and coordinating access to specialty services, while controlling overall healthcare spending.2 However, optimization of care delivery modalities is still required among high-performing practices. Embedding a clinical pharmacist in an ambulatory care setting, including primary care, is a growing trend. Data have been published to support improvement in patients’ care and decreased healthcare costs when pharmacists are added to the care team.3–5
Involvement of clinical pharmacists improves clinical outcomes in many disease states, and improves patients’ satisfaction.5–8 Optimizing medication regimens to meet clinical goals and prevent adverse events is the clinical pharmacist’s specialty. Having a pharmacist included in patients’ care improves medication-related quality measures and reduces errors. These same quality and safety metrics are increasingly tied to reimbursement and population health management initiatives.4
Outcomes from involvement of a clinical pharmacist in the care of patients with high blood pressure (HBP) and/or diabetes (DM) have been highlighted in several previous clinical trials.9–14 Hirsch et al.9 found that using a pharmacist for drug therapy management for hypertension resulted in a higher rate of patients reaching a blood pressure target (88.5%) compared with usual care (63.6%). Comprehensive management of DM by clinical pharmacists within primary care practices shows improvement in numerous diabetes-related outcomes, including a greater likelihood of reaching hemoglobin A1c (HbA1c), blood pressure, and low density lipo-protein (LDL) targets compared with patients receiving care in a clinic without a pharmacist as a member of the healthcare team.14
Each of the above-mentioned studies showed positive associations when a pharmacist was integrated into the care team. However, this study analyzed the effectiveness of the Intermountain Healthcare (IH) Collaborative Pharmacist Support Services (CPSS) program, which was deployed in established primary care medical home practices. The IH CPSS program emphasizes partnership and collaboration between clinical pharmacists and primary care teams, rather than co-location of services. CPSS provides efficiency in care delivery by maximizing the role of the pharmacist. This provides a benefit to physicians who lack the ability to actively provide additional care interactions. This program uniquely incorporates collaborative drug therapy management agreements that include the following: developing and executing treatment plans; and managing medications, including selection, titration, monitoring, and medication adjustments, while supporting existing relationships between patients and their care teams.
However, whether the IH CPSS can support short-term improvement and enhance current clinical goal achievement among patients with DM and HBP are still unknown. These chronic diseases have previously been demonstrated to have excellent baseline disease management at IH.15 This study evaluated the immediate effect of the IH CPSS to determine the associated clinical and healthcare use patterns among enrolled patients compared with patients with these conditions from a neighboring geographical region who did not participate.
Methods
A retrospective, observational design was used to study the effectiveness of the IH CPSS program, which was implemented in seven pilot clinics among patients diagnosed with HBP and/or DM. Patients who were enrolled in CPSS were considered the intervention cohort. The CPSS group was matched using propensity scores (defined below) with a reference group comprising patients who were selected from an adjacent geographic region within the healthcare system (no-CPSS cohort). This matching was performed to determine the effect of achieving disease management goals and time to achievement, increasing the number of clinical encounters, and improving the overall efficiency of care teams.
Study setting
IH is an integrated delivery system of 22 hospitals. IH is also a medical group with more than 185 ambulatory physician clinics and approximately 1100 primary and secondary care physicians. This system has an affiliated health plan that provides more than half of all healthcare services within Utah and southeastern Idaho.16 IH’s mission, “to help people live the healthiest lives possible,” is actualized through a clinical integration structure that drives clinical work processes. This is achieved through a culture of accountable leadership, continuous quality improvement, and measurement of patients’ outcomes and delivery system costs.16 IH’s Enterprise Data Warehouse collects source data from its integrated health plan and the electronic medical record (EMR). The resulting data infrastructure means that every patient who is treated at an IH inpatient or outpatient facility contributes structured data for formal learning. Therefore, IH is an example of a learning healthcare system, where routine state-of-the-art patient care also produces rapid advances in formal medical knowledge.
CPSS program
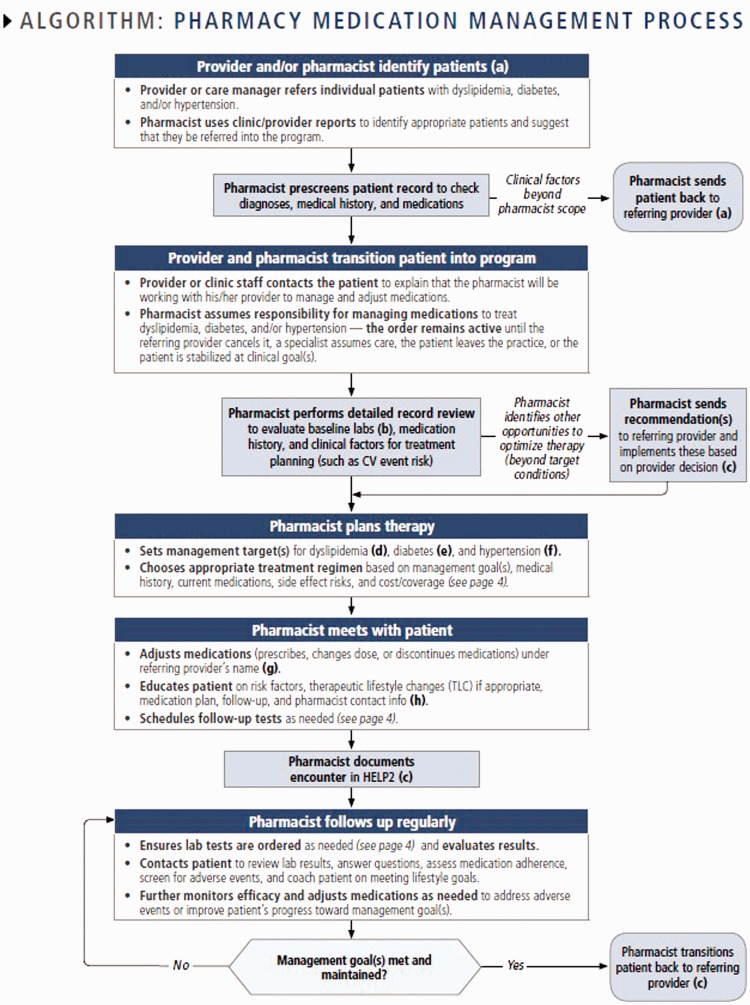
Clinical pharmacists embedded in primary care medical home practices have multiple patient care responsibilities, with co-management of DM and hypertension representing approximately 50% of the pharmacists’ responsibilities. Patients with DM, HBP, or a combination of the two, are identified for referral to the clinical pharmacist by either a primary care provider directly or review of system population health management reports (Figure 1). The majority of patients are referred to the pharmacist for management of HBP when systolic blood pressure is > 140 mmHg or diastolic blood pressure is > 90 mmHg, and for management of DM when HbA1c values are > 8%. Additionally, patients who were escalating medications or dosages were considered for referral. However, there were no strict exclusion criteria for referral.
Figure 1.
Collaborative Pharmacy Model: algorithm for the pharmacy medical management care process.
Clinical pharmacists were employed by IH and devoted 100% of their time to the intervention. Selection criteria for clinical pharmacists required board certification and an established training process (i.e., mentoring, testing and peer-review of clinical competencies) before full integration began.
Upon referral, the clinical pharmacist assumed responsibility for co-management of DM and/or hypertension under a Collaborative Drug Therapy Management (CDTM) agreement. The CDTM agreement authorizes the pharmacist to initiate, modify, and discontinue medication therapy, order labs (i.e, HbA1c, Basic Metabolic Panel, Serum Creatinine), provide education, refer patients to other healthcare professionals, and provide ongoing monitoring and follow-up. Care was individualized and there were attempts to provide avenues that best suited the needs of the enrolled patients. Three attempts at phone calls were made by the clinical pharmacist to enroll each patient who was referred for the services. When contact attempts by phone were not successful, a letter was sent to patients informing them of the services and seeking their participation. Referrals were tracked and reasons noted in the electronic medical record if a patient was not enrolled. Once enrolled, pharmacists delivered care free of charge because there was no mechanism to bill for services. Pharmacists provided follow-up every 1 to 4 weeks via face-to-face visits, telephone visits, or secure electronic messaging.
Study participants
Patients who were enrolled in CPSS between July 2012 and April 2015 were eligible for inclusion in the intervention group (CPSS). Patients were only included in the intervention group for analysis if they had an out-of-control condition (HBP and/or DM) at enrollment and a corresponding outcome measure (i.e., blood pressure measurement or HbA1c test) during the follow-up period. Independent chart review was performed by the research team (P.T. and E.H.) to ensure participation in CPSS and adjudicate results that were observed.
A reference, or control group (no-CPSS), was selected from an adult population (≥ 18 years) who was determined from IH’s network of outpatient clinics. Patients were included in the no-CPSS group if they fit the following criteria: 1) they were previously diagnosed with HBP or met the national Healthcare Effectiveness Data and Information Set (HEDIS)17 criteria for DM, and 2) had a visit to a primary care provider within a established medical home practice located in an adjacent geographic region between July 2012 and May 2015. All IH practices included in the study (i.e., intervention and control practices) were high-performing, routinized medical home practices with a similar team composition, including nurse care managers and mental health specialists. However, patients who were included in the no-CPSS group did not have access to a clinical pharmacist included as a member of the team. The earliest visit encounter date for each patient within the study period was used as the no-CPSS study enrollment date. This study was approved by the IH’s institutional review board (#1050294) and was granted a waiver of informed consent and documentation of consent for all eligible patients who were considered for analysis.
Measurements
Baseline measures
Patients were compared within the study groups to assess if differences in patients’ demographics and clinical characteristics were present prior to enrollment in the study. Baseline demographics included age, sex, race, ethnicity, and insurance status. Information on current smoking status at the time of enrollment was collected. Clinical characteristics for the study cohort included the proportion of patients with chronic conditions prior to enrollment in the study, including depression, DM, and HBP. Criteria for chronic conditions were standardized by an internal expert committee of practicing providers (Appendix Table A1). The most recent clinical biometric measures (≤ 12 months prior to enrollment) were collected and included body mass index (BMI) (BMI classes: underweight, normal, overweight, and obese), HbA1c levels, and systolic and diastolic blood pressure. Medications that were ordered prior to enrollment in the study were also included and categorized based on their class (anti-hypertensive, metformin, diabetes medications, including injectable and oral therapy other than metformin, and statins). Information on the number of patients per primary care physician (PCP) and the number of PCPs per clinic was collected at the practice level for each of the participating clinics.
Outcome measures
The primary outcome measure for the study was achievement of disease management goals as follows. 1) Control of Blood Pressure (BP) was defined by achieving < 140/90 mmHg for the general population and < 150/90 mmHg for patients aged ≥ 80 years within 7 months. 2) Control of DM was defined as HbA1c values < 8% within 12 months. The cutoffs were selected by the research team a priori to allow for a window of time for patients to return to their PCP after baseline measurement. Secondary measures included time to the disease management goal and the number of encounters with additional members of the care team that were required to achieve disease management goals. The time to goals was defined as days between study enrollment and first in-control measurement during the follow-up period. An encounter with the care team was defined as any type of ambulatory interaction with the patient. This encounter could have been a phone call or visit in person with a pharmacist, PCP, specialist, or other member of the primary care management team.
Statistical analysis
Patients were matched at a 1:4 ratio (intervention to control) based on propensity score methodology using the nearest neighbor technique.18 This methodology was operationalized by first, including potential confounders within a logit model, to predict the propensity for treatment (i.e., participation in CPSS).19 Matching characteristics included age, sex, race/ethnicity, insurance status, disease status and duration (HBP, DM, and depression), BMI, number of patients per PCP panel, and number of PCPs at the clinic. This weighting method produced an average treatment effect on the treated estimates, answering the question “Among control patients closely resembling CPSS patients, what outcomes were associated with the intervention?”18
Study group demographics were compared using chi-square analysis for categorical variables and the t-test for continuous data. Conditional logistic regression was used to test the null hypothesis that the association of achieving disease management goals was not different between participants and non-participants. Odds ratios were generated after accounting for demographic and clinical characteristic differences to assess the likelihood of achieving disease management goals. Non-adjusted Kaplan–Meier survival curves were used to visually compare achievement of clinical goals over time between the study groups.
Incidence rates (number of events per patient-years) were used to test the association of participation in CPSS and the number of encounters with the care team within the study period. An incidence rate ratio with corresponding 95% confidence intervals were computed to determine the probability of an event occurring between study groups. For all analyses, a two-sided P value ≤ .05 was considered statistically significant. All data were analyzed using Stata 12.0 (Stata Corp, College Station, TX).
Results
A total of 17,684 patients were identified within the IH delivery system as meeting the selection criteria for CPSS during the study period. A total of 489 individuals were referred to the CPSS program. However, 56 patients were excluded from analysis because they were referred for management of hyperlipidemia and 74 were referred, but never enrolled in CPSS. Patients who received CPSS (n = 359) were matched, using previously described propensity score matching, to a no-CPSS cohort (n = 999). In the CPSS group, 93 (25.9%) patients had HBP, 22 (6.1%) had DM, and 244 (68.0%) had both diagnoses.
Baseline demographic and clinical characteristics are shown in Table 1. No significant differences in age, female sex, race/ethnicity, type of insurance, smoking status, duration and prevalence of HBP and/or DM, prevalence of depression, baseline BMI (kg/m2), and BMI class were found between patients in the reference and intervention groups. CPSS patients were significantly more likely to already be on established evidence-based medication regimens, including anti-hypertensives, metformin, and oral and injectable diabetes medications excluding metformin compared with controls (P < .001). CPSS patients were more likely to have higher HbA1c values (P < .001), and systolic (P < .001) and diastolic (P = .05) blood pressure when averaged over the last 12 months from enrollment compared with controls.
Table 1.
Baseline characteristics of the study population stratified by participation in Collaborative Pharmacist Support Services.
| CPSS group n = 359 |
No-CPSS group n = 999 |
||||
|---|---|---|---|---|---|
| n | Mean ± SD or % | n | Mean ± SD or % | P value | |
| Characteristics | |||||
| Age, y | 359 | 61.5 ± 12.0 | 999 | 61.0 ± 12.9 | .50 |
| Female sex, % | 165 | 50.8 | 504 | 51.7 | .90 |
| Race/ethnicity, % | .78 | ||||
| White | 306 | 94.2 | 910 | 92.4 | |
| Black | 2 | 0.6 | 9 | 0.9 | |
| Asian | 5 | 1.5 | 23 | 2.3 | |
| Other/unknown | 12 | 3.7 | 43 | 4.4 | |
| Insurance product, % | .43 | ||||
| Commercial | 167 | 51.4 | 535 | 54.3 | |
| Medicare (FFS) | 79 | 24.3 | 239 | 24.3 | |
| Medicare Advantage | 61 | 18.8 | 166 | 16.9 | |
| Medicaid | 4 | 1.3 | 20 | 2.0 | |
| Self-pay/unknown | 14 | 4.3 | 25 | 2.6 | |
| Health indicators | |||||
| Smoking status, % | .13 | ||||
| Current | 25 | 6.6 | 96 | 9.6 | |
| Not current | 334 | 93.4 | 903 | 90.4 | |
| Clinical characteristics | |||||
| High blood pressure | |||||
| Prevalence, % | 337 | 93.9 | 911 | 91.2 | .78 |
| Duration, y | 337 | 8.2 ± 5.7 | 911 | 7.6 ± 5.2 | .11 |
| Diabetes mellitus | |||||
| Prevalence, % | 266 | 74.1 | 754 | 75.5 | .99 |
| Duration, y | 266 | 6.9 ± 5.1 | 754 | 6.1 ± 4.6 | .15 |
| Depression, % | 151 | 46.5 | 439 | 44.6 | .55 |
| Medication class, % | |||||
| Anti-hypertensive | 334 | 93.0 | 658 | 65.9 | < .001 |
| Metformin | 222 | 61.8 | 365 | 36.5 | < .001 |
| Other DM medications* | 248 | 69.1 | 342 | 34.2 | < .001 |
| Statins | 251 | 77.2 | 774 | 78.6 | .61 |
| Body mass index, kg/m2 | 325 | 34.6 ± 7.6 | 985 | 34.5 ± 7.8 | .90 |
| Body mass index classes, % | |||||
| Underweight | 1 | 0.3 | 3 | 0.3 | .99 |
| Normal | 25 | 7.7 | 71 | 7.2 | |
| Overweight | 69 | 21.2 | 213 | 21.6 | |
| Obese | 230 | 70.8 | 698 | 70.9 | |
| Mean hemoglobin A1c values within 12 months of enrollment, % | 266 | 8.7 ± 2.1 | 754 | 7.4 ± 1.8 | < .001 |
| Mean blood pressure within 12 months of enrollment | |||||
| Systolic, mm Hg | 337 | 138.3 ± 18.4 | 911 | 129.2 ± 15.3 | < .001 |
| Diastolic, mm Hg | 337 | 78.1 ± 12.0 | 911 | 76.6 ± 10.3 | .05 |
Other diabetes medications included injectable and oral medications excluding metformin.
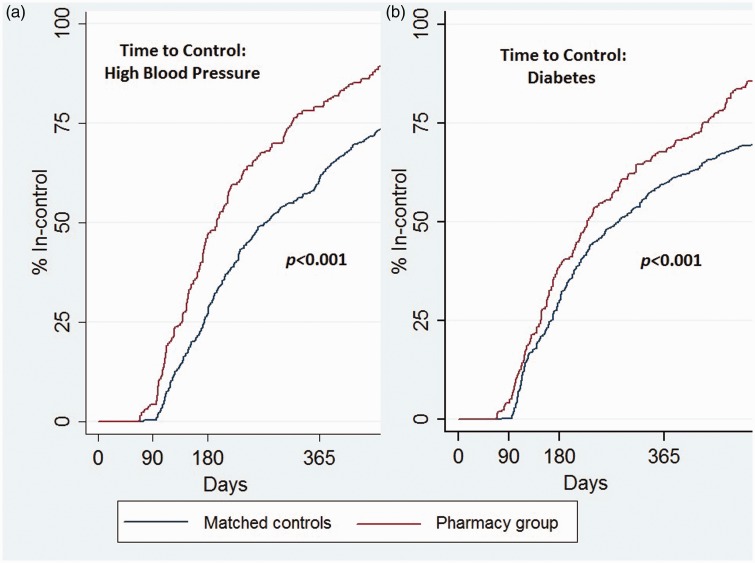
After a priori adjustment for confounders (see Table 2), patients in the CPSS group were 93% more likely to achieve a BP goal of < 140/90 mmHg (Odds Ratio (OR) = 1.93; 95% Confidence Interval (CI) = 1.40, 2.65; P < .001), 57% more likely to achieve HbA1c values of < 8% (OR = 1.57; 95% CI = 1.06, 2.34; P < .026), and 87% more likely to achieve both disease management goals (OR = 1.87; 95% CI = 1.41, 2.50; P < .001) compared with the reference group. In Kaplan–Meier survival analysis, the time to achievement of goals showed increasing separation between the study cohorts across the entire study period (P < .001), especially at 180 days post-intervention (HBP: 48% vs 27% P < .001 and type 2 DM: 39% vs. 30%, P < .05) (Figure 2).
Table 2.
Conditional logistic regression modeling for achievement of HbA1c values < 8% within 12 months and blood pressure < 140/90 mmHg in 7 months.
| BP goal < 140/90 mmHg |
HbA1c goal < 8% |
BP and HbA1c goals |
|||||
|---|---|---|---|---|---|---|---|
| #pts | #met goal | OR (95% CI) | #met goal | OR (95% CI) | #met goal | OR (95% CI) | |
| Study cohortǂ | |||||||
| No-CPSS | 999 | 283 | — | 196 | — | 145 | — |
| CPSS | 359 | 159 | 1.93 (1.40, 2.65)* | 113 | 1.57 (1.06, 2.34)** | 139 | 1.86 (1.28, 2.69)* |
CPSS: Collaborative Pharmacist Support Services; BP: blood pressure; HbA1c: hemoglobin A1c.
P < .001; **P < .05; ǂgroups were adjusted using the characteristics of age, sex, race/ethnicity, insurance status, disease status and duration (high blood pressure, diabetes, and depression), baseline systolic and diastolic BP, baseline HbA1c (%), body mass index, number of patients per PCP panel, and number of PCPs at the clinic.
Figure 2.
Time to achieve disease management goals: (a) Blood pressure was < 140/90 mmHg for patients with high blood pressure. (b) HbA1c values were <8% for patients with DM.
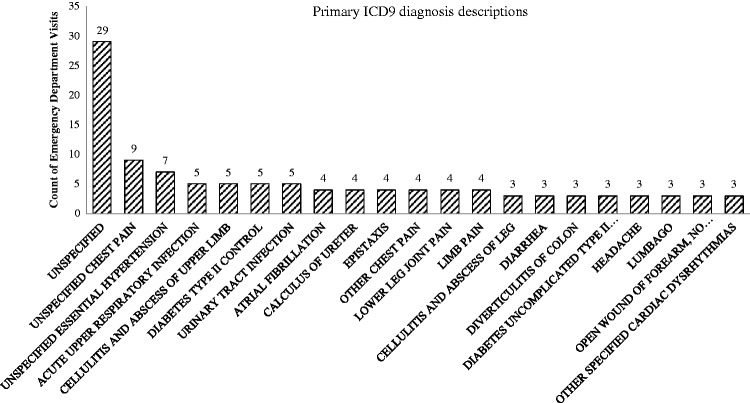
Within the CPSS cohort, there were significantly more ambulatory clinical visits compared with the no-CPSS cohort (8.4 vs. 5.1 visits per patient-year, P < .001) (Table 3). In the CPSS group, there were 1742 encounters with the clinical pharmacist, averaging 3.07 visits per patient-year (median = 3 visits) during enrollment in the study. The number of visits to primary care, specialty care, and care managers (registered nurses) were all significantly higher in the CPSS group compared with the no-CPSS group (P < .001). While there was no difference in hospital admissions, there was a significantly higher number of emergency department visits in the CPSS group compared with the no-CPSS group (0.27 vs. 0.21 visits per patient-year, P = .007). Additional post-hoc analyses were completed to identify the most common primary diagnoses that were associated with aberrant visits to the emergency department (Figure 3). Of the 133 different primary diagnoses, 27 were determined to be linked with CPSS comorbidities when reviewed by the study team.
Table 3.
Incidence rate of healthcare use encounters stratified by participation in the Collaborative Pharmacist Support Services program.
| Healthcare use | CPSS group (n = 359) |
No-CPSS group** (n = 999) |
Rate ratio | 95% CI | P value | ||
|---|---|---|---|---|---|---|---|
| #events | #events/ patient-year | #events | #events/ patient-year | ||||
| Number of ambulatory encounters† | 6590 | 8.37 | 11,856 | 5.09 | 1.64 | 1.60, 1.70 | < .001 |
| PCP | 2511 | 3.20 | 6701 | 2.88 | 1.11 | 1.06, 1.17 | < .001 |
| Specialist* | 2007 | 2.56 | 4831 | 2.07 | 1.24 | 1.17, 1.30 | < .001 |
| Pharmacist | 1742 | 3.07 | 0 | 0 | — | — | — |
| Care manager (RN) | 330 | 0.42 | 324 | 0.14 | 3.03 | 2.60, 3.54 | < .001 |
| Number of ED visits | 243 | 0.27 | 512 | 0.22 | 1.23 | 1.06, 1.45 | .007 |
| Number of hospital admissions | 115 | 0.13 | 264 | 0.11 | 1.14 | 0.90, 1.42 | .25 |
PCP: primary care physician; CPSS: Collaborative Pharmacist Support Services; CI: confidence interval; ED: emergency department.
Number of visits to the provider without primary care specialty designation; †number of ambulatory encounters combining PCP, specialty, care management, and pharmacy services; **the control group was propensity matched using the characteristics of age, sex, race/ethnicity, insurance status, disease status and duration (high blood pressure, diabetes, and depression), body mass index, baseline HbA1c (%), baseline systolic and diastolic BP, number of patients per PCP panel, and number of PCPs at the clinic.
Figure 3.
Twenty most common diagnoses associated with emergency department visits in the intervention group
CPSS: Collaborative Pharmacist Support Services; ICD-9: International Classification of Diseases, Ninth Revision; ED: emergency department.
Discussion
Patients in the CPSS program showed significant improvement in disease management goals and time to achievement compared with a patient group who did not enroll in this program. Patients in the CPSS program experienced a significant increase in the rate of all ambulatory encounters (i.e., visits with primary and specialty care providers, nurse care managers, and pharmacists), which indicated greater overall effectiveness with better coordinated care delivery. Additionally, patients in the CPSS program showed a significantly higher rate of visits to the emergency department compared with those who did not enroll in this program. However, on further review, the emergency services that were rendered did not appear attributable to the program and the clinical relevance is unclear. Nonetheless, these results originated from a population of patients who had established medical home practices. This population has previously demonstrated exceptional baseline medical therapy and achievement of disease management targets.15
A large body of evidence supports intensification of treatment and adherence to the treatment regimen as significantly leading to improved control of DM and HBP.20 A lack of these factors is defined as a failure to either increase a drug dose or change a drug class. Findings in our study support previous clinical trials that showed an improvement of approximately 20% in achievement of clinical targets and a $1600 reduction in healthcare use costs.3 While these studies validate the results in our study, the IH CPSS program went beyond what has been previously reported. This program uniquely authorized the pharmacist to take accountability for a patient’s disease management experience within a medical home practice, while efficiently coordinating care among the whole clinical team.
However, even with success of the IH CPSS program, operational resources to deliver non-traditional models of ambulatory care require substantial investment and a pay-for-value strategy. In this program, clinical pharmacists were unable to bill for the collaborative services that were rendered. The pharmacists’ salary was paid for by institution to test the effectiveness of this modality for improving adherence to clinical management goals. Ultimately, implementing CPSS will be an ongoing investment where outcomes that provide value to the health system may need to be realized over time. If this program is supported by value-based or capitated reimbursement structures, it could represent decreased healthcare expenses.
With growing patient panels for physicians (average for an IH physician is > 2000 patients), three additional encounters were needed to further refine clinical disease targets, as demonstrated in this study to achieve superior results compared with a matched control group. Using ancillary clinical team members to perform CDTM becomes an operational necessity to provide access and efficiency in delivering these services. As healthcare delivery systems move towards capitated reimbursement models, investing in collaborative pharmacy services may reduce the professional salary cost by as much as half to deliver chronic disease management. This could result in major savings to accountable care organizations and/or capitated systems.21 Further rigorous economic analyses studying the financial effects of this program in relation to other integration improvement activities were not part of the scope of this study. However, these analyses are required to prioritize and support the spread of innovations that provide the most value to customers, with the patient and their families foremost.
Finally, this program had operational challenges. Further evaluation is ongoing to study facilitators and barriers to implementation and patients’ reports of their experience. Similar to the literature, engagement of patients and activation were major impediments observed by clinical pharmacists for obstructing the services that they rendered.22 Additionally, the cost of treatment (i.e., medications, monitoring tools, and devices) needed to optimize the disease management process for patients contributes to stagnant intensification or poor adherence. This could potentially lead to avoidable incident disease and complications over time.20 Uncertainty still exists regarding how to finance new mechanisms of value-based care delivery. Additionally, determining who is responsible within the care delivery system to cover the expenses of the CPSS program still continues to affect expansion and dissemination. Despite these issues, the CPSS program transformed the practice culture among the intervention clinics. Physicians and care management nurses worked in concert with pharmacists to provide improved and timely care. This study showed that integrating clinical pharmacists into established medical home practices provided additional levels of success in managing chronic disease within a learning healthcare system. The CPSS program may be used nationally as demonstration of a promising practice for improving adherence to clinical goals.
Limitations
The control group was carefully selected from an adjacent geographical region that was not participating in this program to account for possible selection bias. However, patients who were enrolled in the CPSS program may have been more ready to change than those who did not participate. Subsequently, there remains a possibility that PCPs only referred patients that they felt would participate in CPSS. Unfortunately, data were not available to measure this possibility (i.e., through documentation in the EMR or through measurement of health literacy, patient activation, or engagement levels) and should be an area of future study. Additionally, patients who were enrolled in CPSS were prescribed proportionally more medications, presenting a possible selection bias. However, biometrics (i.e., HbA1c and blood pressure) were also elevated in this group of patients, potentially indicating a more complicated clinical course.
The IH Diabetes Registry does not distinguish between type 1 or type 2 DM. Therefore, the study groups may still include both disease phenotypes within the patient population. In this study, we identified > 40,000 patients who were diagnosed with DM and/or HBP. However, only a small proportion of this group was enrolled because of the pragmatic nature of this evaluation related to resource and clinical constraints within primary care teams. We acknowledge that the study population was largely derived from patients who had visited a primary care provider within a large integrated healthcare system and may not be generalizable to populations outside of IH. Data that include social determinants of health (income and education level, number of family members or dependents, and the contextual elements of the neighborhood or geographical location where the patient lives) are predictive of outcomes in control of disease management; however, these data were not available for this study. The level of satisfaction with the program, patient activation and/or engagement were not measured and secondary data collection was not available for analysis. Additionally, the exact mode of the pharmacist’s interaction was not differentiated in the medical record (i.e., interaction via face-to-face visits, telephone visits, or secure electronic messaging) and thus this information was not available for study. Iteration on data collection processes is planned to allow for future understanding on the best method of delivering the intervention.
Conclusions
Integrating clinical pharmacists to function in collaboration with established care teams for direct management of chronic diseases using CDTM is proposed. This could optimize operational utility, promote evidence-based change within a learning healthcare system, and demonstrate improved adherence to clinical goals. Furthermore, this study highlights the promise of improving the efficiency of care teams, while reducing the burden of disease management, which is traditionally exclusively attributed to physicians.
Acknowledgements
This research was supported by the combined efforts of IH’s Medical Group, Pharmacy Services, the Primary Care Clinical Program, and the Institute for Healthcare Delivery Research.
Appendix
Table A1.
Definitions of chronic conditions.
| Chronic condition | Diagnoses (ICD-9-CM)* | Encounters (CPT)* | Exclusions |
|---|---|---|---|
| High blood pressure | 360.42, 362.11, 401, 401.0, 401.1, 401.9, 402, 402.0, 402.00, 402.01, 402.1, 402.10, 402.11, 402.9, 402.90, 402.91, 403, 403.0, 403.00, 403.1, 403.10, 403.9, 403.90, 404, 404.0, 404.00, 404.01, 404.1, 404.10, 404.11, 404.90, 404.9, 404.91, 405, 405.0, 405.01, 405.09, 405.1, 405.11, 405.19, 405.9, 405.91, 405.99, 437.2 | Outpatient visit for the following: 99201-05, 99211-15, 99241-45, 99341-50, 99381-87, 99391-97, 99401-04, 99411-12, 99420, 99429, 99455-56 | No documentation of renal transplant |
| Depression | 296.2, 296.20, 296.21, 296.22, 296.23, 296.24, 296.25, 296.26, 296.3, 296.30, 296.31, 296.32, 296.33, 296.34, 296.35, 296.36, 296.82, 296.90, 298, 298.0, 300.4, 309.1, 309.28, 311 | Hospital admission, emergency department visit, or outpatient visit for the following: 99201-05, 99211-15, 99241-45, 99341-50, 99381-87, 99391-97, 99401-04, 99411-12, 99420, 99429, 99455-56 | None |
| Diabetes mellitus | 250.x | Hospital admission, emergency department visit, or two outpatient visits for the following: 99201-05, 99211-15, 99241-45, 99341-50, 99381-87, 99391-97, 99401-04, 99411-12, 99420, 99429, 99455-56 Dispensed insulin or hypoglycemic/anti-hyperglycemics on an ambulatory basis | None |
ICD-9-CM: International Classification of Diseases, Ninth Revision, Clinical Modification; CPT: current procedural terminology.
To be identified with a chronic condition. Specifications require at least one CPT and ICD-9-CM code to be paired on the same day.
Declaration of conflicting interest
The Authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Kindig D and Stoddart G. What is population health? Am J Public Health 2003; 93: 380–383. [DOI] [PMC free article] [PubMed]
- 2.Reiss-Brennan B, Brunisholz KD, Dredge C, et al. Association of integrated team-based care with health care quality, utilization, and cost. JAMA 2016; 316: 826–834. [DOI] [PubMed] [Google Scholar]
- 3.Smith M, Giuliano MR, Starkowski MP. In Connecticut: improving patient medication management in primary care. Health Aff (Millwood) 2011; 30: 646–654. [DOI] [PubMed] [Google Scholar]
- 4.Houle SK, Chuck AW, McAlister FA, et al. Effect of a pharmacist-managed hypertension program on health system costs: an evaluation of the study of cardiovascular risk intervention by pharmacists-hypertension (SCRIP-HTN). Pharmacotherapy 2012; 32: 527–537. [DOI] [PubMed] [Google Scholar]
- 5.Bunting BA, Smith BH, Sutherland SE. The Asheville Project: clinical and economic outcomes of a community-based long-term medication therapy management program for hypertension and dyslipidemia. J Am Pharm Assoc (2003) 2008; 48: 23–31. [DOI] [PubMed] [Google Scholar]
- 6.American College of Clinical Pharmacy McBane SE, Dopp AL, et al. Collaborative drug therapy management and comprehensive medication management-2015. Pharmacotherapy 2015; 35: e39–e50. [DOI] [PubMed] [Google Scholar]
- 7.American Pharmacists Association Foundation and American Pharmacists Association. Consortium recommendations for advancing pharmacists' patient care services and collaborative practice agreements. J Am Pharm Assoc (2003) 2013; 53: e132–e141. [DOI] [PubMed] [Google Scholar]
- 8.Services USDoHaH. Special Report to Senate Appropriations Committee: Advancing Clinical Pharmacy Services in Programs Funded by the Health Resources and Services Administration and its Safety-Net Providers. 2010; https://docs.340bpvp.com/documents/public/resourcecenter/HRSA_ACPS_report.pdf.
- 9.Hirsch JD, Steers N, Adler DS, et al. Primary care-based, pharmacist-physician collaborative medication-therapy management of hypertension: a randomized, pragmatic trial. Clin Ther 2014; 36: 1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McConnell KJ, Zadvorny EB, Hardy AM, et al. Coronary artery disease and hypertension: outcomes of a pharmacist-managed blood pressure program. Pharmacotherapy 2006; 26: 1333–1341. [DOI] [PubMed] [Google Scholar]
- 11.Parker CP, Cunningham CL, Carter BL, et al. A mixed-method approach to evaluate a pharmacist intervention for veterans with hypertension. J Clin Hypertens (Greenwich) 2014; 16: 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAdam-Marx C, Dahal A, Jennings B, et al. The effect of a diabetes collaborative care management program on clinical and economic outcomes in patients with type 2 diabetes. J Manag Care Spec Pharm 2015; 21: 452–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taveira TH, Dooley AG, Cohen LB, et al. Pharmacist-led group medical appointments for the management of type 2 diabetes with comorbid depression in older adults. Ann Pharmacother 2011; 45: 1346–1355. [DOI] [PubMed] [Google Scholar]
- 14.Johnson KA, Chen S, Cheng IN, et al. The impact of clinical pharmacy services integrated into medical homes on diabetes-related clinical outcomes. Ann Pharmacother 2010; 44: 1877–1886. [DOI] [PubMed] [Google Scholar]
- 15.Muhlestein JB, Lappe DL, Lima JA, et al. Effect of screening for coronary artery disease using CT angiography on mortality and cardiac events in high-risk patients with diabetes: the FACTOR-64 randomized clinical trial. JAMA 2014; 312: 2234–2243. [DOI] [PubMed] [Google Scholar]
- 16.James BC, Savitz LA. How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood) 2011; 30: 1185–1191. [DOI] [PubMed] [Google Scholar]
- 17./2012 HEDIS Quality Assurance Reporting Requirements. New York2011.
- 18.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika 1983; 70: 41–55. [Google Scholar]
- 19.Rose S, Laan MJ. Why match? Investigating matched case-control study designs with causal effect estimation. Int J Biostat 2009; 5: Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Professional practice committee for the standards of medical care in diabetes-2016. Diabetes Care 2016; 39(Suppl 1): S107–S108. [DOI] [PubMed] [Google Scholar]
- 21.Occupational Outlook Handbook. 2016; http://www.bls.gov/ooh/home.htm. Accessed November 15, 2016.
- 22.Smith M, Bates DW, Bodenheimer T, et al. Why pharmacists belong in the medical home. Health Aff (Millwood) 2010; 29: 906–913. [DOI] [PubMed] [Google Scholar]