Abstract
Objective
To evaluate the efficacy and safety of adalimumab (ADA) versus hyaluronic acid (HA) by intra-articular injection for moderate to severe knee osteoarthritis.
Methods
Fifty-six consecutive patients with moderate to severe knee osteoarthritis were randomly allocated to either the ADA group or HA group. On day 0, patients in the ADA group received 10 mg of ADA by intra-articular injection, while those in the HA group received 25 mg of HA. All patients received celecoxib at 200 mg/day for 4 weeks. The pain visual analog scale (VAS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Patient Global Assessment (PGA), and Physician Global Assessment (PhGA) scores were assessed.
Results
At baseline, the pain VAS, WOMAC, PGA, and PhGA scores were similar between the two groups. The decrease in the pain VAS score, WOMAC pain score, WOMAC physical function score, and WOMAC total score from baseline to week 4 were greater in the ADA than HA group. A greater decrease in the PGA and PhGA scores from baseline to week 4 was noted in the ADA than HA group. No difference in adverse events was observed between the two groups.
Conclusion
ADA by intra-articular injection was effective and tolerated for moderate to severe knee osteoarthritis.
Keywords: Efficacy, safety, adalimumab, intra-articular, knee osteoarthritis, hyaluronic acid
Introduction
Osteoarthritis (OA), a major source of pain, stiffness, and motor disorders, has a remarkable influence on the quality of life of affected patients.1As a dominant type of OA, knee OA is characterized by degeneration of the knee joint, pain, swelling, and snapping joints.2 It mainly affects people aged ≥45 years.3 Knee OA has a high incidence and risk of mortality and affects more than 250 million people worldwide,4 and it accounts for 1.0% to 2.5% of the gross domestic product.5
Inflammation has been implicated in the pathogenesis of knee OA.3 Inflammatory pain is considered the most prominent feature in patients with moderate to severe knee OA.6 However, some studies have revealed that a neuropathic mechanism that induces peripheral neuropathy with predominant sensory nerve fiber lesions7,8 may lead to neuropathic pain during the last stage of knee OA.9,10 Several studies have indicated that the concentration of tumor necrosis factor-α (TNF-α), a key proinflammatory cytokine, becomes unbalanced in the process of catabolism and anabolism of the cartilage matrix and plays a critical role in the inflammatory mechanism of knee OA.6,8,11 Furthermore, a study involving mice showed that TNF-α may accelerate the process of knee OA by stimulating nerve growth factor (NGF) expression.12 Thus, it can be hypothesized that TNF-α inhibitors may have dual actions (anti-inflammatory and anti-neurodynia effects) in the development and progression of knee OA and could serve as a potential option for knee OA treatment. Adalimumab (ADA), a fully human anti-TNF-α monoclonal antibody, is approved for the treatment of patients with various inflammatory diseases in >90 countries worldwide.13 However, few attempts have been made to improve the treatment of patients with knee OA by ADA.
Thus, the present short-term, open-label randomized controlled trial was conducted to evaluate the efficacy and safety of intra-articular (IA) ADA versus IA hyaluronic acid (HA) treatment in patients with moderate to severe knee OA.
Patients and methods
Patients
Fifty-six consecutive patients with moderate to severe knee OA were enrolled in this study from January 2015 to August 2016 at ZhongNan Hospital of Wuhan University. The inclusion criteria were as follows: diagnosis of knee OA according to the 1986 clinical and radiographic classification of OA of the knee by the American College of Rheumatology, single-sided knee OA, moderate to severe knee OA (Kellgren–Lawrence grade II to III), a pain visual analog scale (VAS) score of >4 points within the past month, and lack of efficacy of previous treatment with one nonsteroidal anti-inflammatory drug (celecoxib at 200 mg/day, meloxicam at 7.5 mg/day, diclofenac at 75 mg/day, or ibuprofen at 1.2 g/day). The exclusion criteria were as follows: previous treatment with a TNF inhibitor, previous IA treatment within 3 months, previous knee surgery, history of a tumor or severe infection, knee joint deformity, pregnancy or lactation, cognitive impairment, and poor adherence.
This study was approved by the Ethics Committee of ZhongNan Hospital of Wuhan University. Each patient provided written informed consent for participation.
Study design
This was a short-term, open-label randomized controlled pilot study. Fifty-six patients were randomly allocated to either the ADA or HA group in a 1:1 ratio. Patients in the ADA group underwent IA injection of 10 mg of ADA into the knee on day 0 followed by celecoxib at 200 mg/day for 4 weeks Patients in the HA group underwent IA injection of 25 mg of HA into the knee on day 0 followed by celecoxib at 200 mg/day for 4 weeks.
The randomization was carried out by a statistician using a block randomization method. All documents were sent to and kept at Shanghai QeeJen Bio-Tech Co., Ltd. (Shanghai, China), a medical and statistics service company. When a patient was eligible for the study, a call was made to QeeJen Bio-Tech and a unique subject identification number was provided from the randomized module.
Endpoints
The primary endpoint was a decline in the pain VAS score from baseline to week 4 in the two groups. The secondary endpoints were a reduction in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain, stiffness, physical function, and total scores from baseline to week 4; a decrease in the Patient Global Assessment (PGA) score from baseline to week 4; and a decrease in the Physician Global Assessment (PhGA) score from baseline to week 4.
Statistics
Statistical analysis was performed with SSPS 21.0 (IBM Corp., Armonk, NY, USA). Data are mainly presented as mean ± standard deviation or count and percentage. Intention-to-treat analysis was used. Differences between two groups were compared by Student’s t test or the chi-square test. Differences between each visit and baseline were compared by the paired t test. A P-value of <0.05 was considered statistically significant.
Results
Study flow and patients’ characteristics
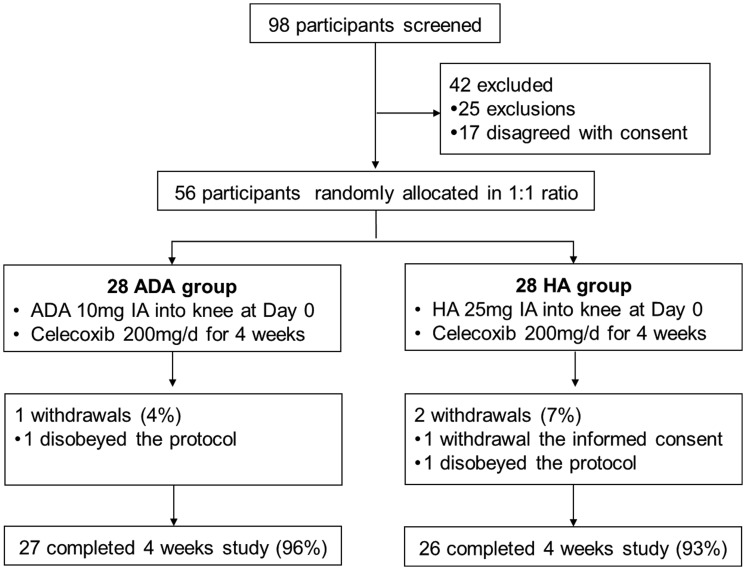
In total, 98 patients were screened for eligibility, among whom 46 were excluded (25 met the exclusion criteria and 17 did not provide informed consent). The remaining 56 patients were included in the randomization and allocated to either the ADA group (n = 28) or HA group (n = 28) as presented in Figure 1. A total of 27 (96%) patients in the ADA group completed the 4-week study, while 26 (93%) patients in the HA group completed the study.
Figure 1.
Study flow chart. ADA, adalimumab; HA, hyaluronic acid; IA, intra-articular.
The baseline demographic and clinical characteristics of the patients in both the ADA and HA groups are presented in Table 1. No significant differences were observed in age, sex, body mass index, or Kellgren–Lawrence grade between the two groups (Table 1). Likewise, the disease assessment findings and history of pharmacological therapy were similar between the two groups (Table 1).
Table 1.
Baseline characteristics in ADA and HA groups.
| Characteristics | ADA group (n = 28) | HA group (n = 28) | P-value |
|---|---|---|---|
| Age (years) | 54.3 ± 8.7 | 56.9 ± 9.1 | 0.279 |
| Sex | |||
| Female | 19 (68) | 21 (75) | 0.554 |
| Male | 9 (32) | 7 (25) | |
| Side of knee OA | |||
| Left | 12 (43) | 15 (54) | 0.422 |
| Right | 16 (57) | 13 (46) | |
| BMI (kg/m2) | 25.3 ± 3.2 | 24.7 ± 3.3 | 0.493 |
| KL grade | |||
| II | 17 (61) | 20 (71) | 0.391 |
| III | 11 (39) | 8 (29) | |
| Prior medication use | |||
| Celecoxib | 15 (54) | 14 (50) | 0.535 |
| Meloxicam | 7 (25) | 4 (14) | |
| Diclofenac | 1 (4) | 3 (11) | |
| Ibuprofen | 5 (18) | 7 (25) | |
| Pain VAS score | 7.1 ± 2.1 | 6.7 ± 1.9 | 0.458 |
| WOMAC score | |||
| Pain | 15.7 ± 4.2 | 14.8 ± 4.5 | 0.442 |
| Stiffness | 5.9 ± 2.1 | 5.3 ± 1.9 | 0.267 |
| Physical function | 47.7 ± 13.8 | 42.1 ± 15.2 | 0.155 |
| Total | 69.3 ± 18.4 | 62.2 ± 19.8 | 0.170 |
| PGA score | 7.2 ± 1.6 | 6.9 ± 1.8 | 0.513 |
| PhGA score | 7.5 ± 1.9 | 7.1 ± 1.9 | 0.434 |
Data are presented as mean ± standard deviation or n (%). Significant differences were determined by Student’s t test or the chi-square test. A P-value of <0.05 was considered statistically significant.
ADA, adalimumab; HA, hyaluronic acid; OA, osteoarthritis; BMI, body mass index; KL, Kellgren–Lawrence; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; PGA, Patient Global Assessment; PhGA, Physician Global Assessment.
Primary endpoint
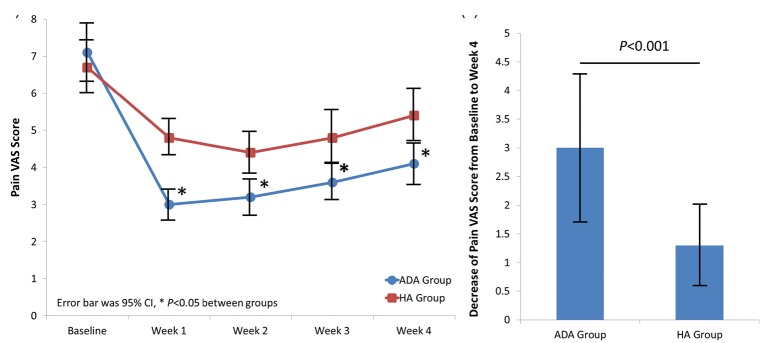
The pain VAS scores of patients in the ADA and HA groups were similar at baseline but lower in the ADA than HA group at each follow-up visit (all P < 0.05) (Figure 2(a)). The decrease in the pain VAS score of patients from baseline to week 4 was greater in the ADA than HA group (P < 0.001) (Figure 2(b)).
Figure 2.
Changes in pain VAS scores. (a) Pain VAS scores of patients in the ADA and HA groups post-treatment. (b) Decrease in pain VAS scores of patients in the ADA and HA groups from baseline to week 4. Differences between the two groups were compared by Student’s t test. A P-value of <0.05 was considered statistically significant. VAS, visual analog scale; ADA, adalimumab; HA, hyaluronic acid; CI, confidence interval.
Secondary endpoint
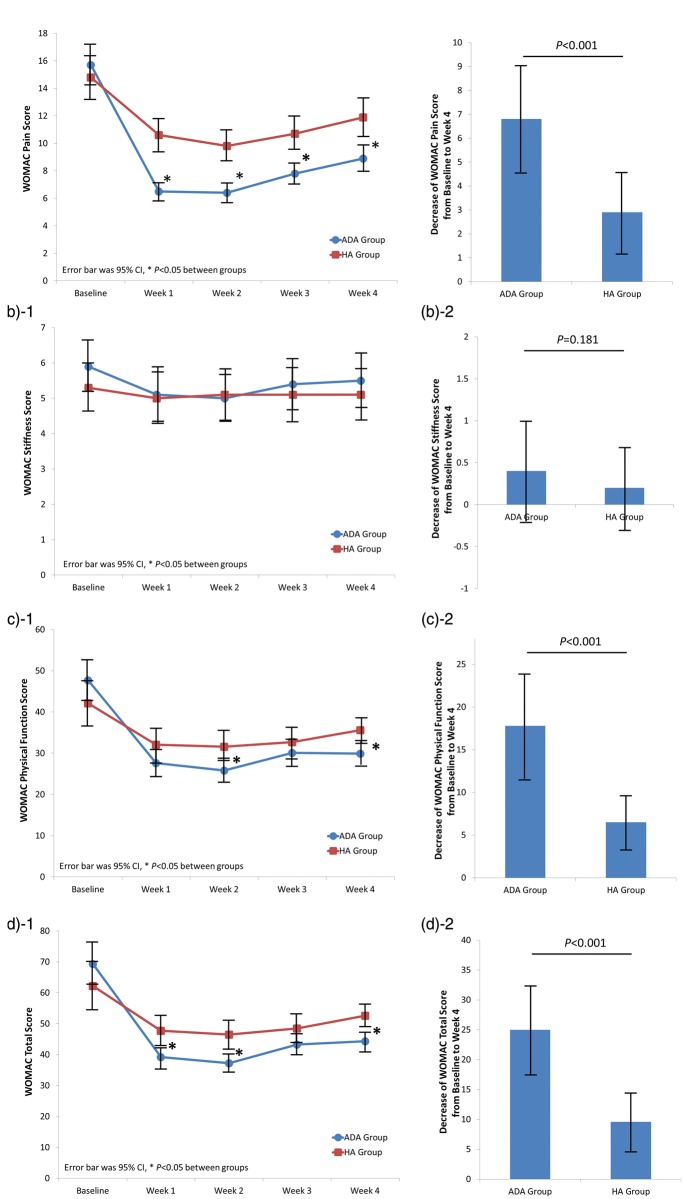
As shown in Figure 3(a)-1, the WOMAC pain score was markedly lower in patients in the ADA than HA group at all follow-up visits, and the decrease in the WOMAC pain score was also greater in the ADA than HA group (P < 0.001) (Figure 3(a)-2). Additionally, the WOMAC physical function score was notably lower in patients in the ADA than HA group at weeks 2 and 4, while no difference was found at the other follow-up visits (Figure 3(c)-1). The decline in the WOMAC physical function score in the ADA group was also larger than that in the HA group (P < 0.001) (Figure 3(c)-2). However, as presented in Figure 3(b)-1 and Figure 3(b)-2, the WOMAC stiffness score at each follow-up visit and its reduction from baseline to week 4 were similar between the two groups. The WOMAC total score was lower in the ADA than HA group at weeks 1, 2, and 4 but not at week 3 (Figure 3(d)-1, Figure 3(d)-2).
Figure 3.
Changes in WOMAC scores. (a-1) WOMAC pain score of patients in the ADA and HA groups. (a-2) Changes in WOMAC pain scores from baseline to week 4 post-treatment. (b-1) WOMAC stiffness scores of patients in the ADA and HA groups. (b-2) Changes in WOMAC stiffness scores from baseline to week 4 post-treatment. (c-1) WOMAC physical function scores of patients in the ADA and HA groups. (c-2) Changes in the WOMAC physical function scores from baseline to week 4 post-treatment. (d-1) WOMAC total scores of patients in the ADA and HA groups. (d-2) Changes in the WOMAC total scores from baseline to week 4 post-treatment. Differences between the two groups were compared by Student’s t test. A P-value of <0.05 was considered statistically significant. WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; ADA, adalimumab; HA, hyaluronic acid; CI, confidence interval.
The PGA and PhGA scores were decreased from baseline to week 4 in both the ADA and HA groups (Table 2). However, the changes in the PGA (P = 0.003) and PhGA (P = 0.002) scores from baseline to week 4 were substantially higher in the ADA than HA group.
Table 2.
Changes in PGA and PhGA scores from baseline to week 4.
| Items | ADA group (n = 28) | HA group (n = 28) | P-value |
|---|---|---|---|
| PGA score | |||
| Baseline | 7.2 ± 1.6 | 6.9 ± 1.8 | 0.513 |
| Week 4 | 4.7 ± 1.1 | 5.2 ± 1.4 | 0.143 |
| Change from baseline to week 4 | 2.5 ± 1.0 | 1.7 ± 0.9 | 0.003 |
| PhGA score | |||
| Baseline | 7.5 ± 1.9 | 7.1 ± 1.9 | 0.434 |
| Week 4 | 4.9 ± 1.3 | 5.4 ± 1.6 | 0.205 |
| Change from baseline to week 4 | 2.6 ± 1.1 | 1.7 ± 1.0 | 0.002 |
Data are presented as mean ± standard deviation. Significant differences were determined by Student’s t test. A P-value of <0.05 was considered statistically significant.
ADA, adalimumab; HA, hyaluronic acid; PGA, Patient Global Assessment; PhGA, Physician Global Assessment.
Safety
Only one patient in the ADA group developed a pulmonary infection within 3 months after injection; no infections occurred in the HA group. No other adverse events were observed in the two groups for 3 months after the injection. The pulmonary infection in the ADA group occurred 10 weeks after injection of ADA and was categorized as a possibly drug-related adverse event. After symptom onset, a sputum sample was obtained and two positive cultures for methicillin-resistant Staphylococcus aureus were obtained. Teicoplanin was administered at 6 mg/kg every 12 hours for three doses followed by 6 mg/kg per day for 14 days, which cured the pulmonary infection.
Discussion
In this study, patients with knee OA treated with IA injection of ADA into the knee showed better clinical outcomes than patients treated with IA injection of HA into the knee. These better outcomes were evidenced by improvements in the pain VAS score, WOMAC pain score, WOMAC physical functional score, WOMAC total score, PGA score, and PhGA score. Additionally, no difference in adverse events was observed between the two groups.
Knee OA, which is characterized by irreversible cartilage loss, bone destruction, and hyperostosis, is a type of proliferative arthritis driven by many proinflammatory mediators.1,14 Several studies have shown that proinflammatory cytokines including TNF-α and interleukin (IL)-1 are considered to be the major factors involved in the pathophysiological mechanism of OA.6,8,11,15,16 In patients with knee OA, TNF-α expression is increased in the synovial fluid, synovial membrane, and subchondral bone and cartilage. It increases sharply in the acute phase of knee OA and is responsible for driving the development and severity of inflammation.11 Several in vitro and in vivo studies have revealed that TNF-α also acts as a mediator for the activity of NGF expression, which causes neuropathic pain in patients with OA.12,17 Additionally, a recent study involving mice showed that suppressing the expression of TNF-α contributes to the inhibition of OA progression.18
ADA is the most frequently used TNF inhibitor worldwide and is indicated for several inflammatory diseases.13,19 ADA downregulates the expression of proinflammatory cytokines such as TNF-α, IL-1, IL-6, and IL-819 and expresses adhesion molecules by blocking endothelial cells, which attracts leukocytes into the affected joints. Moreover, ADA decreases the rate of synthesis of metalloproteinases by synovial macrophages, fibroblasts, osteoclasts, and chondrocytes.20 Furthermore, research involving a mouse model of OA showed that ADA treatment curbed cartilage degradation by suppressing matrix metalloproteinase-13 expression, which is related to the modulation of cartilage metabolism, and improved the subchondral trabecular bone alterations in the knee joints compared with normal saline treatment.21 These results suggest that ADA may be beneficial for patients with OA by inhibiting inflammation, protecting articular cartilage, and improving the function of the subchondral bone structure. However, few studies have been performed to assess the efficacy and safety of ADA by IA injection for patients with moderate to severe knee OA.
A previous single-armed study of 20 patients with knee OA treated by subcutaneous injections of ADA biweekly for 12 weeks showed that ADA alleviated pain and improved knee function.22 Additionally, a pilot study showed obvious pain relief in patients treated with direct injection of etanercept, another TNF-α inhibitor, into the knee joints as determined by the pain VAS and WOMAC scores at 4 weeks compared with HA treatment.6 A proof-of-concept study including 450 patients with knee OA revealed that the TNF-α inhibitor tanezumab had robust analgesic effects on joint pain and physical function by neutralization of NGF in patients with moderate to severe knee OA.8 Generally consistent with previous studies, we found that the pain VAS score and WOMAC score decreased after a single IA injection of ADA compared with HA. This difference might be associated with the anti-inflammatory role of ADA in OA as well as the neuropathic pain relief that occurs through inhibition of NGF expression by ADA.12,17,19
The PGA and PhGA scores also decreased by IA injection of ADA, which might be mainly due to the pain relief and improvement of physical function. This finding is consistent with the results of ADA application in other inflammatory diseases, such as rheumatoid arthritis, ankylosing spondylitis, and juvenile idiopathic arthritis.23–25
With the exception of one patient who developed a pulmonary infection in the ADA group, no differences in adverse events were found between the ADA and HA groups in this study. This suggests that IA injection of ADA in patients with knee OA is well tolerated.
This study had some limitations. First, the relatively small sample size (56 patients) might have reduced the statistical power, and some differences might not have been discovered. Second, ADA and HA were injected only once at baseline, preventing assessment of the long-term efficacy and safety. Finally, the influence of the ADA treatment dose was not evaluated, and a 10-mg injection was an estimated dose considering the pharmacokinetics and safety profile of ADA.
In conclusion, this study illustrated that IA injection of ADA is effective and well tolerated in patients with moderate to severe knee OA.
Acknowledgements
None.
Declaration of conflicting interest
The author declares that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003; 81: 646–656. [PMC free article] [PubMed] [Google Scholar]
- 2.Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med 2015; 162: 46–54. [DOI] [PubMed] [Google Scholar]
- 3.Hochberg MC. Osteoarthritis: The Rheumatologist’s Perspective. HSS J 2012; 8: 35–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2197–2223. [DOI] [PubMed] [Google Scholar]
- 5.Hiligsmann M, Cooper C, Arden N, et al. Health economics in the field of osteoarthritis: an expert’s consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum 2013; 43: 303–313. [DOI] [PubMed] [Google Scholar]
- 6.Ohtori S, Orita S, Yamauchi K, et al. Efficacy of Direct Injection of Etanercept into Knee Joints for Pain in Moderate and Severe Knee Osteoarthritis. Yonsei Medical Journal 2015; 56: 1379–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finan PH, Buenaver LF, Bounds SC, et al. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum 2013; 65: 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lane NE, Schnitzer TJ, Birbara CA, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med 2010; 363: 1521–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutolo M, Berenbaum F, Hochberg M, et al. Commentary on recent therapeutic guidelines for osteoarthritis. Semin Arthritis Rheum 2015; 44: 611–617. [DOI] [PubMed] [Google Scholar]
- 10.Hochman JR, Gagliese L, Davis AM, et al. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthritis Cartilage 2011; 19: 647–654. [DOI] [PubMed] [Google Scholar]
- 11.Dias CN, Vasilceac FA, Durigan JL, et al. Analysis of local and systemic TNF-alpha and IL1-alpha expression in the acute phase of knee osteoarthritis of rats. Cytokine 2014; 66: 164–165. [DOI] [PubMed] [Google Scholar]
- 12.Takano S, Uchida K, Miyagi M, et al. Nerve Growth Factor Regulation by TNF-alpha and IL-1beta in Synovial Macrophages and Fibroblasts in Osteoarthritic Mice. J Immunol Res 2016; 2016: 5706359–5706359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bain B, Brazil M, Bain B, et al. Fresh from the Pipeline: Adalimumab. Dressnature Reviews Drug Discovery 2003; 2: 693–694. [DOI] [PubMed] [Google Scholar]
- 14.da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet 2016; 387: 2093–2105. [DOI] [PubMed] [Google Scholar]
- 15.Loeser RF, Goldring SR, Scanzello CR, et al. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis & Rheumatism 2012; 64: 1697–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 2011; 7: 33–42. [DOI] [PubMed] [Google Scholar]
- 17.Manni L, Lundeberg T, Fiorito S, et al. Nerve growth factor release by human synovial fibroblasts prior to and following exposure to tumor necrosis factor-alpha, interleukin-1 beta and cholecystokinin-8: the possible role of NGF in the inflammatory response. Clin Exp Rheumatol 2003; 21: 617–624. [PubMed] [Google Scholar]
- 18.Zhao YP, Liu B, Tian QY, et al. Progranulin protects against osteoarthritis through interacting with TNF-α and β-Catenin signalling. Ann Rheum Dis 2015; 74: 2244–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bain B, Brazil M. Adalimumab. Nat Rev Drug Discov 2003; 2: 693–694. [DOI] [PubMed] [Google Scholar]
- 20.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 2001; 344: 907–916. [DOI] [PubMed] [Google Scholar]
- 21.Ma CH, Lv Q, Yu YX, et al. Protective effects of tumor necrosis factor-alpha blockade by adalimumab on articular cartilage and subchondral bone in a rat model of osteoarthritis. Braz J Med Biol Res 2015; 48: 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maksymowych WP, Russell AS, Chiu P, et al. Targeting tumour necrosis factor alleviates signs and symptoms of inflammatory osteoarthritis of the knee. Arthritis Res Ther 2012; 14: R206–R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansen JP, Buckley F, Dejonckheere F, et al. Comparative efficacy of biologics as monotherapy and in combination with methotrexate on patient reported outcomes (PROs) in rheumatoid arthritis patients with an inadequate response to conventional DMARDs – a systematic review and network meta-analysis. Health Qual Life Outcomes 2014; 12: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turina MC, Ramiro S, Baeten DL, et al. A psychometric analysis of outcome measures in peripheral spondyloarthritis. Ann Rheum Dis 2016; 75: 1302–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walters HM, Pan N, Lehman TJ, et al. The impact of disease activity and tumour necrosis factor-alpha inhibitor therapy on cytokine levels in juvenile idiopathic arthritis. Clin Exp Immunol 2016; 184: 308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]