Abstract
Refinement of treatment regimens enlisting targeted α-radiation therapy (TAT) is an ongoing effort. Among the variables to consider are the target molecule, radionuclide, dosage, and administration route. The panitumumab F(ab′)2 fragment targeting epidermal growth factor receptor tolerated modification with the TCMC chelate as well as radiolabeling with 203Pb or 212Pb. Good specific activity was attained when the immunoconjugate was labeled with 212Pb (9.6 ± 1.4 mCi/mg). Targeting of LS-174T tumor xenografts with the 203Pb-panitumumab F(ab′)2 demonstrated comparable amounts of uptake to the similarly radiolabeled panitumumab IgG. A dose escalation study was performed to determine an effective working dose for both intraperitoneal (i.p.) and intravenous (i.v.) injections of 212Pb-panitumumab F(ab′)2. Therapeutic efficacy, with modest toxicity, was observed with 30 μCi given i.p. Results for the i.v. administration were not as definitive and the experiment was repeated with a higher dose range. From this study, 20 μCi given i.v. was selected as the effective working dose. A subsequent therapy study combined gemcitabine or paclitaxel with i.v. 212Pb-panitumumab F(ab′)2, which increased the median survival (MS) of LS-174T tumor-bearing mice to 208 and 239 d, respectively. Meanwhile, the MS of mice treated with i.v. 212Pb-panitumumab F(ab′)2 alone was 61 and 11 d for the untreated group of mice. In conclusion, the panitumumab F(ab′)2 fragment whether given by i.p. or i.v. injection, is a viable candidate as a delivery vector for TAT of disseminated i.p. disease.
Keywords: : 212Pb, F(ab′)2, α-radiation, HER1, intraperitoneal, radioimmunotherapy
Introduction
This laboratory has taken a systematic approach toward the development of targeted α-radiation therapy (TAT) regimens for the treatment of disseminated intraperitoneal (i.p.) disease. α-Particles have a high linear energy transfer (60–230 keV/μm) with energy deposition over short distances in tissues (50–100 μm). Per unit of path length, α-particles deposit ≥500 times more energy than β−-particles.1,2 The therapeutic effectiveness of α-particles is not affected by the oxygenation status of the tumor. One attribute of the shorter path length is that α-particles are more suitable for the treatment and management of patients with cancers that present as single cell (hematologic) or single layers or sheets of cells in compartments, such as the peritoneum, that is, pancreatic cancer, ovarian cancer, and carcinomatosis. α-Radiation therapy is also considered appropriate for the treatment of micrometastases or residual disease following a surgical debulking. Although there are 100 known α-emitting radionuclides, those that are reasonably medically relevant and currently available for potential clinical use are 225Ac, 211At, 212Bi, 213Bi, 212Pb, 223Ra, 149Tb, and 227Th.
Matching the half-life of the biological targeting vector, that is, monoclonal antibodies (mAbs) with the physical half-life of the radionuclide is an important consideration in designing a radioimmunotherapy (RIT) regimen. Maximal therapeutic benefit, at best, would not be realized and more likely fail if days are required for optimal targeting of a tumor lesion by a mAb, whereas the cytotoxic payload, the radionuclide, has a half-life measured in minutes. The exploitation of 212Pb as an in vivo generator of 212Bi has proven a very successful approach to “matching” the half-lives of the delivery vehicle and the payload. In essence, the 10.6 h half-life of 212Pb “extends” the half-life of 212Bi (T1/2 = 1 h), allowing for the successful delivery of a lethal dose to the tumor. In fact, the preclinical investigations targeting HER2 with 212Pb-trastuzumab culminated in a phase 1 trial at the University of Alabama at Birmingham.3–5 Patients were reported to have had no adverse reactions or toxicities following i.p. injections of the radioimmunoconjugate (RIC), 212Pb-trastuzumab. The therapeutic efficacy of TAT has also been demonstrated targeting tumor xenografts expressing human epidermal growth factor receptor (hEGFR) with 212Pb-labeled cetuximab or panitumumab.6,7 The success of these preclinical studies and the clinical trial has sustained interest in 212Pb for TAT.8–11
To date, RIT studies in this laboratory have concentrated on locoregional administration of an intact mAb for the treatment of i.p. tumor xenografts. This strategy offers immediate access of the RIC to tumor lesions, avoids unnecessary exposure of normal tissues from circulating RIC when given intravenous (i.v.), and circumvents the need of the RIC to extravasate and penetrate the tumor. However, just as the intracavitary route of injection allows the RIC to target the tumor periphery, if the size of a tumor burden exceeds the maximal path length of the α-particle, then the interior of the tumor escapes therapy. A potential solution to this dilemma would be the administration of the RIT delivered by both an i.p. and an i.v. injection, thus targeting the exterior of the tumor as well as the interior through the vasculature. To lessen the normal tissue exposure that may be incurred from an i.v. injected RIC, the F(ab′)2 fragment of a mAb would be a more appropriate delivery vehicle.12,13 Due to the reduction in size, the F(ab′)2 fragment would also result in greater penetrance of tumors.14 These combined adjustments in targeting vector parameters may provide an enhanced therapy regimen.
A recent study established that HER1-positive i.p. tumor xenografts responded to therapy with a single i.p. injection of 212Pb-labeled panitumumab and that the therapeutic efficacy was enhanced with the inclusion of chemotherapeutics in the treatment regimen.6 The F(ab′)2 fragment of panitumumab has been successfully produced by peptic digest.15 When panitumumab F(ab′)2 was radiolabeled with 111In or 86Y using the CHX-A′′-DTPA ligand, in vivo tumor targeting was comparable to panitumumab IgG, by direct quantitation and two imaging modalities, γ-scintigraphy and positron emission tomography. The results indicated that the panitumumab F(ab′)2 fragment would be an appropriate vehicle for RIT applications.
The objective of the present narrative was to evaluate the panitumumab F(ab′)2 for therapeutic efficacy in preparation for future studies assessing the dual injection routes for improving TAT. The studies reported herein include (1) the confirmation of the tumor and normal tissue distribution of panitumumab F(ab′)2 labeled with 203Pb (the isotope match for 212Pb suitable for Single-photon emission computed tomography imaging); (2) establishment of an effective dose for both i.v. and i.p. injected 212Pb-panitumumab F(ab′)2 for the therapy of i.p. LS-174T tumor xenografts; and (3) an evaluation of the combination of chemotherapeutics with i.v. injected 212Pb-panitumumab F(ab′)2.
Materials and Methods
Cells
Tumor localization and therapy studies were conducted using LS-174T, a human colon carcinoma cell line, grown in Dulbecco's minimum essential medium (Lonza, Walkersville, MD). The medium was supplemented with 1 mM glutamine (Lonza), 10% FetalPlex (Gemini Bioproducts, Inc., West Sacramento, CA), and 1 mM nonessential amino acids (Lonza) as previously described.16,17
mAb conjugation
Panitumumab (Vectibix®; Amgen, Inc., Thousand Oaks, CA), was purchased through the National Institutes of Health (NIH), Division of Veterinary Resources Pharmacy. The F(ab′)2 fragment of panitumumab was generated as previously published.15 Conjugation of panitumumab F(ab′)2 with the bifunctional ligand, 1, 4, 7, 10-tetraaza-1, 4, 7, 10-tetra-(2-carbamoyl methyl)-cyclododecane (TCMC; synthesized in the laboratory), was performed at a 10-fold molar excess of ligand to panitumumab F(ab′)2 according to established methods previously described in detail.18–21 The final concentration of the panitumumab F(ab′)2 was determined by the Lowry method using a bovine serum albumin (BSA) standard.17 The number of TCMC molecules bound to panitumumab F(ab′)2 was quantitated using spectrophotometric assays based on the titration of lead-arsenazo(III) complex.16 A nonspecific control F(ab′)2 fragment for utilization in the in vivo studies was produced from HuM195, an anti-CD33 mAb provided by Dr. M. McDevitt, Memorial Sloan Kettering Cancer Center. The HuM195 F(ab′)2 was similarly conjugated with the TCMC ligand.
Radiolabeling
203Pb was obtained from the NIH Clinical Center cyclotron facility by a 203Tl(d,n)203Pb reaction and purified from the target as previously described.22 Radiolabeling with 203Pb was performed as previously described.23 The radiolabeled product was purified with a PD-10 desalting column (GE Healthcare, Piscataway, NJ) using phosphate buffered saline (PBS) as the eluent.
The 212Pb was obtained from a 224Ra/212Pb generator (Oak Ridge National Laboratories, UT-Batelle, Oak Ridge, TN). Elution of the 212Pb for radiolabeling of panitumumab and HuM195 F(ab′)2 TCMC, and subsequent purification was performed as detailed elsewhere.1,19
Radioimmunoassay
The immunoreactivity of the TCMC-panitumumab F(ab′)2 conjugate was evaluated in a competition radioimmunoassay (RIA) using purified human epidermal growth factor receptor (hEGFR; Sigma-Aldrich, E3641-500UN).24 Briefly, EGFR (50 ng/50 μL) was adsorbed onto the wells of a 96-well plate, excess EGFR was removed, and 1% bovine serum albumin in phosphate buffered saline (BSA/PBS; 100 μL) was added to each well. Following a 0.5–1 h incubation at room temperature, the solution was removed and serial dilutions of the immunoconjugate (1000–0.017 ng in 25 μL) in BSA/PBS were added to the wells in triplicate. Following the addition of 125I-panitumumab (50,000 cpm/25 μL) to each of the wells, the plates were incubated for 4 h at 37°C. The wells were washed, the radioactivity was dissociated from the wells with 0.1 M NaOH (100 μL), adsorbed to cotton filters, and counted in a γ-scintillation counter. The immunoconjugate was compared with unmodified panitumumab F(ab′)2. The percent inhibition was calculated using the buffer control and plotted. The F(ab′)2 fragment of HuM195, a mAb that reacts with human CD33, (provided by Dr. M. McDevitt, Memorial Sloan Kettering Cancer Center) served as a negative control.
A RIA as described elsewhere was performed to assess the immunoreactivity of the radiolabeled panitumumab F(ab′)2 products.25,26 hEGFR (100 ng per well) was adsorbed to the wells of a 96-well plate, the excess hEGFR was removed, and the wells treated with BSA/PBS (100 μL). Serial dilutions of radiolabeled panitumumab (∼200,000–12,500 cpm in 50 μL of BSA/PBS) were added to the wells in triplicate and incubated for 4 h at 37°C. The wells were washed, radioactivity harvested, and counted in a γ-scintillation counter. The percentage binding was calculated for each dilution and averaged. The specificity of the radiolabeled panitumumab was confirmed by incubating one set of wells with radiolabeled panitumumab and 10 μg of unlabeled panitumumab F(ab′)2.
In vivo studies
All in vivo studies were performed using 8–12-week-old female athymic (NCr-nu/nu) mice (NCI-Frederick; Cat. No. 01B70) and were conducted according to protocols approved by the National Cancer Institute Animal Care and Use Committee.
Tumor localization
Mice (five per time point) were injected i.p. with 1 × 108 LS-174T cells in 1 mL of medium. Five days later the mice received either an i.p. or i.v. injection of ∼7.5 μCi 203Pb-panitumumab F(ab′)2 in 0.5 or 0.2 mL, respectively. To demonstrate the specificity of the tumor targeting of the 203Pb-panitumumab F(ab′)2, additional sets of mice were injected similarly with 203Pb-HuM195 F(ab′)2 The mice were euthanized by CO2 inhalation at 24, 48, 72, and 96 h. The blood, tumor, and major organs were collected, wet-weighed, and counted in a γ-scintillation counter. The %ID/g along with the standard deviations were calculated and plotted.
Radioimmunotherapy
The RIT studies detailed below were initiated at 2–3 d following i.p. injection of the mice with LS-174T as described above. 212Pb-labeled mAb F(ab′)2 was administered i.p. or i.v. to mice in 0.5 or 0.2 mL of PBS, respectively. The level of radioactivity given is specified in each of the study descriptions that follow; 212Pb-HuM195 F(ab′)2 served as a nonspecific control in each of the studies. The mice were monitored at a minimum of twice a week and the body weight was measured and recorded one to two times per week for 4–6 weeks as a measure of toxicity due to therapy. Progression of disease was observed either as an extension of the abdomen; development of ascites; or noticeable, palpable, nodules in the abdomen or, conversely, as weight loss. Mice were euthanized if found to be in distress, moribund, or cachectic or when disease progression was evident as cited above.
Studies 1 and 2 were conducted to assess the maximum effective working dose of 212Pb-panitumumab F(ab′)2 when administered i.p. or i.v., respectively. In the first study, tumor-bearing mice (groups of n = 10) were given increasing doses of 212Pb-panitumumab F(ab′)2 (10, 20, 30, 40, 50, 60, 80, or 100 μCi) by i.p. injection. The doses of HuM195 F(ab′)2 that were administered i.p. were 20, 40, 60, and 100 μCi. In the second study, tumor-bearing mice received 5, 10, 15, 30 μCi of 212Pb-panitumumab F(ab′)2 and 10, 15, and 30 μCi of HuM195 F(ab′)2 by i.v. injection. An additional set of tumor-bearing mice in each study was left untreated. All i.p. injections were administered in 0.5 mL whereas the i.v. injections were in a volume of 0.2 mL.
Study 3 was performed with two objectives. The first was to evaluate higher doses of i.v. injected 212Pb-panitumumab F(ab′)2. To this end, 20, 30, 40, and 50 μCi of 212Pb-labeled F(ab′)2 fragments of panitumumab or HuM195 were administered to tumor-bearing mice by i.v. injection. The second goal was to confirm the dose selected for i.p.-administered RIT. Therefore, extra sets of mice were given 30 μCi of either 212Pb-labeled panitumumab F(ab′)2 or HuM195 F(ab′)2 through i.p. injection.
Study 4 was designed to assess the potentiation of the therapeutic efficacy of 212Pb-RIT with 212Pb-panitumumab F(ab′)2 when combined with chemotherapeutics. The chemotherapeutics, GEM (GEMZAR; Eli Lilly and Company, Indianapolis, IN) and paclitaxel (Hospira, Inc., Lake Forest, IL) were purchased through the NIH, Division of Veterinary Resources Pharmacy. Established from previous studies, mice (groups of n = 10) bearing i.p. LS-174T tumors were injected i.p. with 1 mg of GEM or 0.6 mg of paclitaxel 24 h before i.p. administration of the 212Pb-panitumumab F(ab′)2. These treatment groups were compared with mice pretreated with GEM or paclitaxel followed by 212Pb-HuM195 F(ab′)2. Control groups included mice receiving no treatment, 212Pb-panitumumab F(ab′)2, 212Pb-HuM195 F(ab′)2, paclitaxel, or GEM only.
Statistical analyses
Kaplan–Meier survival (time to sacrifice or natural death) analysis was conducted using SigmaPlot 12.5; groups were compared using a log-rank test. A pairwise comparison was performed to test for differences between treatment groups (Holm–Sidak method). All reported p-values correspond to two-sided tests.
Results
Conjugation and radiolabeling
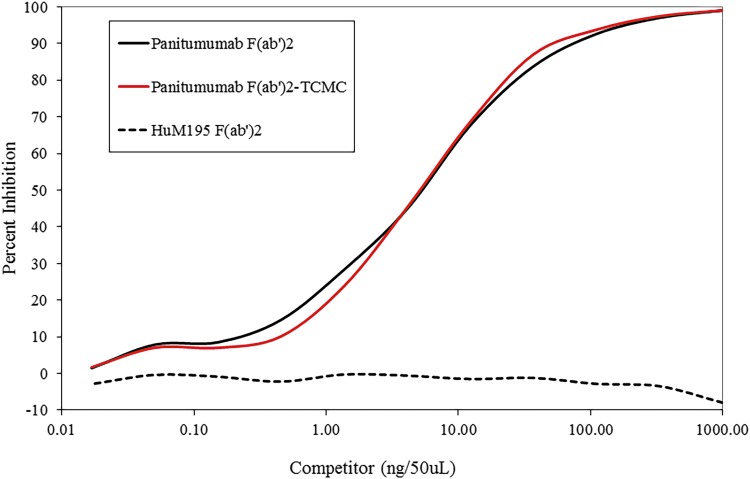
Conjugation of the TCMC ligand to the F(ab′)2 fragment of panitumumab resulted in a chelate:protein ratio of 12 ± 6. Modification with the TCMC ligand did not affect immunoreactivity of the panitumumab F(ab′)2. As shown in Figure 1, 5 ng/50 μL was required to obtain 50% inhibition of the binding of 125I-panitumumab with hEGFR by both panitumumab F(ab′)2-TCMC and the unmodified panitumumab F(ab′)2. Radiolabeling of panitumumab F(ab′)2 with 203Pb and 212Pb resulted in specific activities of 7 and 9.6 mCi/mg, respectively. Immunoreactivity of the RICs was maintained following the radiolabeling procedure with a percent bound of 75% following a 4-h incubation with hEGFR coated in the wells of a 96-well plate. Specificity of this reaction was confirmed by the addition of 10 μg of unlabeled panitumumab F(ab′)2 to a set of wells to compete with the 212Pb-panitumumab F(ab′)2. The excess unlabeled panitumumab reduced the percent bound to <1%.
FIG. 1.
Immunoreactivity of panitumumab F(ab′)2-TCMC conjugate was evaluated in a competition radioimmunoassay.
In vivo studies
Tumor and normal tissue distribution
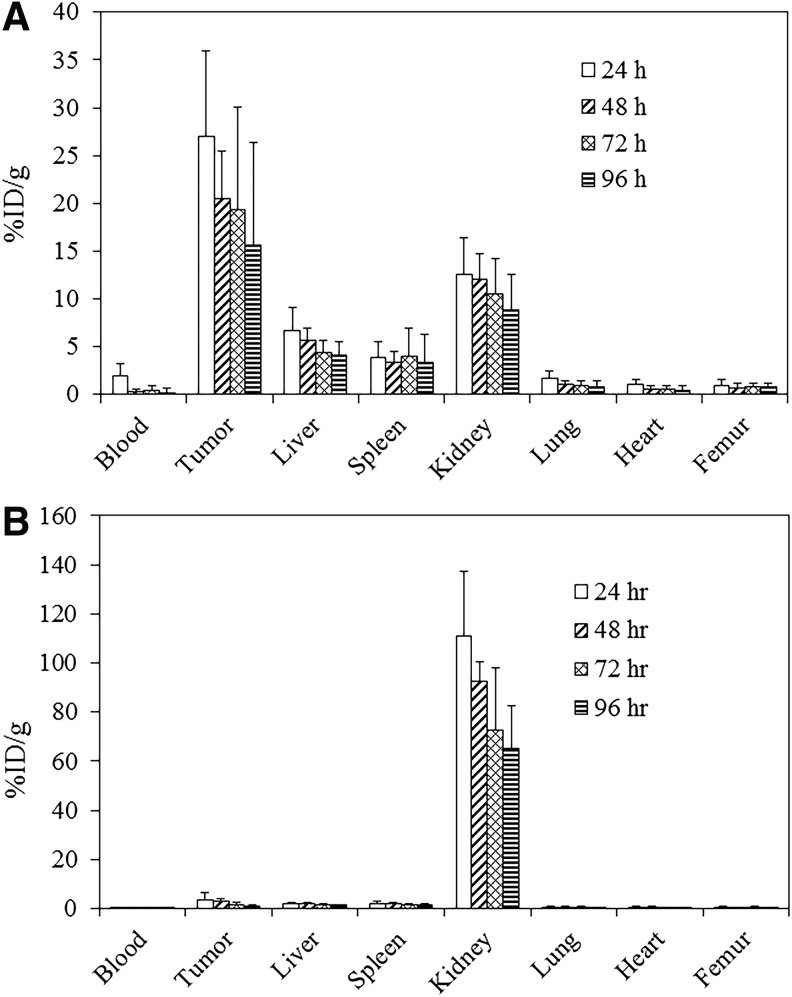
A tumor localization study was performed with 203Pb-panitumumab F(ab′)2 to evaluate the potential of the immunoconjugate for RIT applications. Mice bearing LS-174T i.p. tumor xenografts were administered 203Pb-panitumumab F(ab′)2, either i.p. or i.v., and then euthanized at 24, 48, 72, or 96 h later for analysis of the tumor and tissue distribution of the RIC. Twenty-four hours following i.p. injection, the %ID/g of the 203Pb-panitumumab F(ab′)2 in the tumor was 26.96 ± 8.96. At 48 h, the level of radioactivity was still high with a value of 20.55 ± 4.87 (Fig. 2A). A decrease was then observed in the tumor with a final %ID/g of 15.60 ± 9.03 at 96 h. Of the normal organs, the highest %ID/g was obtained in the kidneys, with a maximum value of 12.59 ± 3.83 at 24 h, which also decreased over the 4-d study, ending with a %ID/g of 8.85 ± 2.09. Of the remaining normal organs, the activity in the liver was the only other normal organ to exceed 5 %ID/g; 6.73 ± 2.33 at 24 h, which decreased to 4.18 ± 1.12 by 96 h. In contrast, the amount of 203Pb-HuM195 F(ab′)2 (Fig. 2B) that was detected in the LS-174T i.p. tumor xenografts following i.p. injection was negligible with a maximal %ID/g of 3.65 ± 2.76 at 24 h and ended with a %ID/g 1.09 ± 0.21 by 96 h. An even greater disparity between the specific and nonspecific targeted F(ab′)2 fragments was evident in the sequestration of radioactivity in the kidneys. At 24 h, there was an 8.8-fold greater amount of radioactivity in the kidneys of the mice that had received an i.p. injection of 203Pb-HuM195 F(ab′)2 than the mice administered 203Pb-panitumumab F(ab′)2.
FIG. 2.
Tumor and normal tissue distribution of 203Pb-panitumumab F(ab′)2. Athymic mice bearing 5 d LS-174T i.p. tumor xenografts were injected i.v. with ∼7.5 μCi of 203Pb-panitumumab F(ab′)2 (A) or 203Pb-HuM195 F(ab′)2 (B). The mice were euthanized (n = 5 per time point) at 24, 48, 72, and 96 h after the injection. The tumor and tissues were harvested, wet-weighed, and the radioactivity measured in a γ-counter. The %ID/g and standard deviation were calculated and plotted. i.p., intraperitoneal; i.v., intravenous.
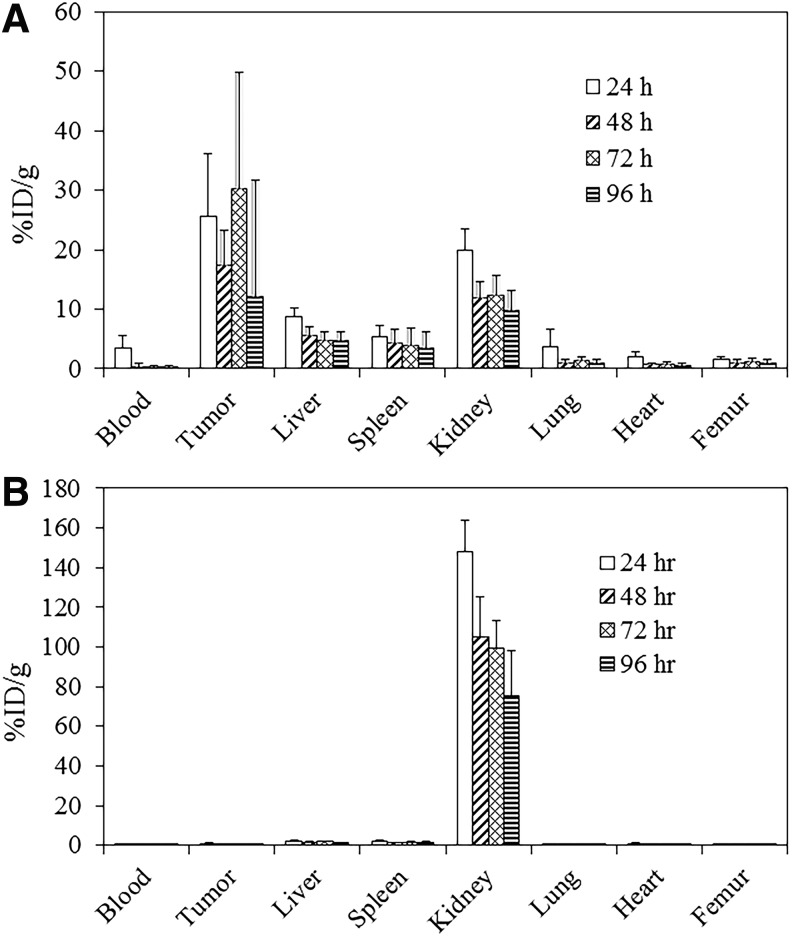
The tumor and normal tissue distribution of the i.v. injected 203Pb-panitumumab F(ab′)2 followed a similar relationship to that of the i.p. administration route (Fig. 3A). At 24 h, the tumor %ID/g was 25.56 ± 10.63 and the final value of 12.04 ± 6.73 at 96 h. At 72 h, however, there was a spike with a %ID/g of 30.26 ± 19.66. Of the normal organs, the kidneys presented with the highest %ID/g, 19.85 ± 3.74 at 24 h, which decreased to 9.70 ± 1.25 by 96 h. The liver and spleen had the next highest values. Figure 3B clearly depicts an absence of tumor targeting following the i.v. injection of 203Pb-HuM195 F(ab′)2, thus validating the specificity of tumor targeting by the 203Pb-panitumumab F(ab′)2. The calculated tumor %ID/g at 24 h was 0.91 ± 0.50, which was the greatest value attained for the 203Pb-HuM195 F(ab′)2. Meanwhile, the radioactivity measured in the kidneys after the i.v. injection of 203Pb-HuM195 F(ab′)2 was higher than what was observed with i.p. injection of the same RIC. At 24 h the %ID/g was 148.16, which was 7.5-fold higher than the i.v. administered 203Pb-panitumumab F(ab′)2.
FIG. 3.
Tumor and normal tissue distribution of 203Pb-panitumumab F(ab′)2. Athymic mice bearing 5 d LS-174T i.p. tumor xenografts were injected i.p. with ∼7.5 μCi of 203Pb-panitumumab F(ab′)2 (A) or 203Pb-HuM195 F(ab′)2 (B) The mice were euthanized (n = 5 per time point) at 24, 48, 72, and 96 h after the injection. The tumor and tissues were harvested, wet-weighed, and the radioactivity measured in a γ-counter. The %ID/g and standard deviation were calculated and plotted.
Determination of therapeutic dose
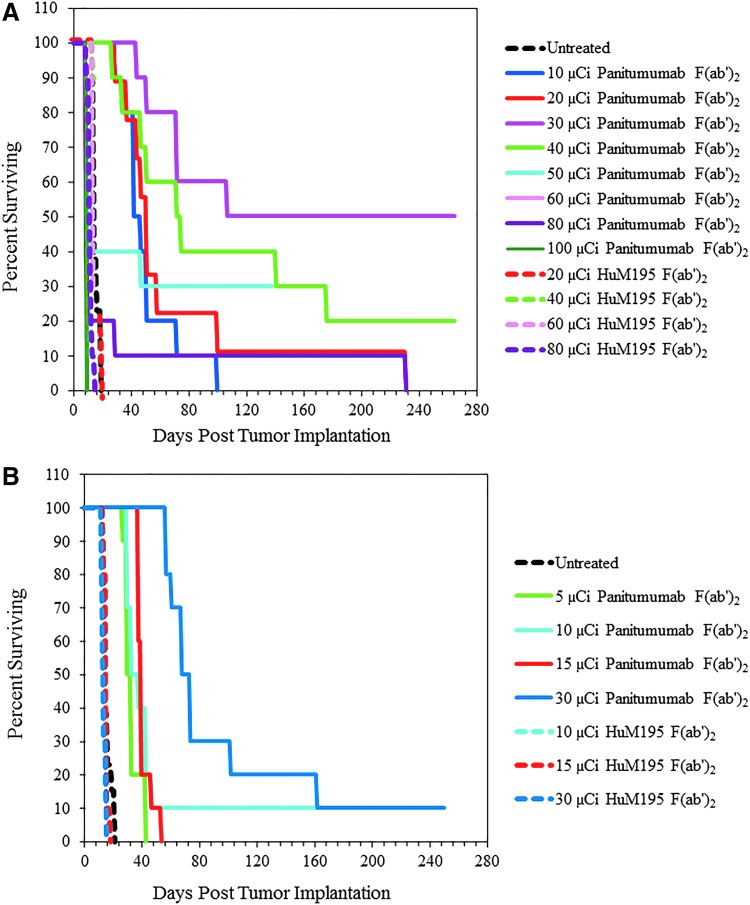
Therapy studies were then conducted to establish the effective working doses for 212Pb-panitumumab F(ab′)2 when given by i.p. and i.v. injection. The first study involved treating cohorts of tumor-bearing mice (n = 10) with 10, 20, 30, 40, 50, 60, 80, or 100 μCi of i.p. administered 212Pb-panitumumab F(ab′)2. Depicted in Figure 4A, the median survival (MS) for these treatment groups was 42, 51, 289, 72, 10, 9, 9, and 10 d, respectively. The benefit of the treatment is reflected in the therapeutic indices (TIs, MS of treatment group divided by the MS of the untreated groups), which was 3.0, 3.6, 20.6, and 5.1 for the 10, 20, 30, and 40 μCi doses and <1 for the remainder of the doses. At 265 d post-therapy with 212Pb-panitumumab F(ab′)2, 50% of the mice at the 30 μCi and 40% at the 40 μCi doses were still alive. Meanwhile, the MS for the untreated group was 14 d and 18, 19, 13, and 11 d for those groups of mice treated with 20, 40, 60, or 80 μCi of 212Pb-HuM195 F(ab′)2, the nonspecific control. Some therapeutic efficacy was observed for the 20 and 40 μCi of 212Pb-HuM195 F(ab′)2 with TI values of 1.3 and 1.4. However, the differences between the 212Pb-panitumumab F(ab′)2 and 212Pb-HuM195 F(ab′)2 groups that received the 20, 40, and 60 μCi doses were significant (p < 0.003), whereas there was no significant difference at the 80 μCi activity level (p = 1).
FIG. 4.
A dose escalation study was performed with 212Pb-panitumumab F(ab′)2 to determine an effective therapeutic dose delivered either by an i.p. or i.v. route. For the i.p. injection (A), groups of athymic mice (n = 10) bearing 3D LS-174T i.p. tumor xenografts were injected with 10, 20, 30, 40, 50, 60, 80, or 100 μCi of 212Pb-panitumumab F(ab′)2. Additional groups of mice were administered 20, 40, 60, or 80 μCi of 212Pb-HuM195 F(ab′)2, which served as a nonspecific control. The mice receiving the 212Pb-RIT by i.v. injection (B) were injected with 5, 10, 15, or 30 μCi of 212Pb-panitumumab F(ab′)2 or 10, 15, or 30 μCi of 212Pb-HuM195. An eighth cohort of mice was left untreated.
As a measure of toxicity, the weights of the mice were monitored for ∼5 weeks following administration of the 212Pb-RIT (Table 1). By the third day, weight loss was observed in all the treatment groups with the greatest losses observed in the groups that received ≥40 μCi. Recovery from the weight was apparent at 16 d after injection of the 212Pb-panitumumab F(ab′)2 in the individuals that had been given 10, 20, and 30 μCi. Based on this collective data, 30 μCi was chosen for RIT studies with i.p. administrated of 212Pb-panitumumab F(ab′)2 RIT studies using the i.p. LS-174T tumor xenograft model.
Table 1.
Effect of Increasing 212Pb-Panitumumab F(ab′)2 Doses (i.p) on the Weights of Athymic Mice Bearing i.p. LS174T Tumor Xenografts
| Days post RIT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RIT | Dose (μCi) | 0 | 3 | 6 | 11 | 16 | 19 | 24 | 31 | 34 |
| None | — | 23.5 ± 1.2 | 23.7 ± 1.7 | 23.0 ± 1.5 | 23.8 ± 1.4 | 24.8 ± 0.6 | ||||
| Panitumumab F(ab′)2 | 10 | 23.1 ± 1.4 | 21.8 ± 1.1 | 20.8 ± 1.7 | 20.8 ± 2.2 | 22.0 ± 2.3 | 22.2 ± 2.6 | 22.4 ± 1.8 | 23.0 ± 1.9 | 22.9 ± 1.5 |
| 20 | 22.1 ± 0.8 | 20.9 ± 1.1 | 20.1 ± 1.3 | 20.8 ± 1.8 | 20.8 ± 2.0 | 21.2 ± 2.0 | 21.1 ± 1.9 | 21.5 ± 1.4 | 21.7 ± 1.0 | |
| 30 | 22.3 ± 1.2 | 20.6 ± 1.2 | 19.8 ± 1.0 | 19.5 ± 1.6 | 20.8 ± 1.4 | 20.9 ± 1.8 | 20.7 ± 1.7 | 20.4 ± 1.6 | 20.5 ± 1.6 | |
| 40 | 23.8 ± 1.2 | 20.4 ± 1.0 | 19.4 ± 0.6 | 19.6 ± 0.7 | 21.1 ± 1.9 | 20.8 ± 1.8 | 20.4 ± 1.6 | 19.7 ± 1.9 | 21.3 ± 1.7 | |
| 50 | 23.0 ± 1.0 | 19.8 ± 1.1 | 17.4 ± 1.6 | 19.4 ± 0.9 | 20.1 ± 1.1 | 20.1 ± 1.3 | 20.6 ± 1.3 | 19.2 ± 0.8 | 20.6 ± 1.3 | |
| 60 | 22.4 ± 1.3 | 18.1 ± 1.4 | 15.6 ± 1.8 | |||||||
| 80 | 22.6 ± 1.7 | 18.8 ± 1.6 | 16.5 ± 2.1 | 19.5 ± 1.1 | 19.9 ± 0.5 | 19.3 ± 0.2 | 20.1 ± 0.8 | 19.6 | 20.5 | |
| 100 | 22.8 ± 1.5 | 18.2 ± 2.2 | 15.6 ± 1.5 | |||||||
| Days post RIT | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 5 | 10 | 15 | 18 | ||
| HuM195 F(ab′)2 |
20 | 23.3 ± 1.1 | 21.6 ± 1.1 | 20.2 ± 1.1 | 20.4 ± 1.2 | 21.3 ± 1.3 | 21.0 ± 1.9 |
| 40 | 23.1 ± 1.0 | 20.8 ± 0.8 | 19.4 ± 1.0 | 20.0 ± 1.1 | 20.0 ± 1.1 | ||
| 60 | 23.3 ± 0.8 | 21.5 ± 1.1 | 20.0 ± 1.2 | 19.8 ± 1.0 | 21.5 | ||
| 80 | 22.7 ± 1.0 | 19.9 ± 0.8 | 17.8 ± 1.1 | 17.8 ± 1.4 | |||
Athymic mice (n = 10) bearing 3 d i.p. LS-174T tumor xenografts were injected i.p. with 10–100 μCi 212Pb-panitumumab F(ab′)2 to establish the effective, suboptimal, therapeutic dose for subsequent studies with chemotherapeutics. Animal weights were monitored one to two times per week for 5 weeks as an indicator of toxicity. Additional groups included those that received no treatment and those that were injected with 20–80 μCi of the nonspecific control, 212Pb-HuM195 F(ab′)2. The values are the average weight (g) with the standard deviation.
i.p., intraperitoneal; RIT, radioimmunotherapy.
Therapeutic benefit was also observed in cohorts (n = 10) of i.p. tumor-bearing mice that received 5, 10, 15, or 30 μCi of 212Pb-panitumumab F(ab′)2 by i.v. injection (Fig. 4B). Correspondingly, the MS for these groups was 30, 33, 40, and 74 d compared with 14 d for the untreated mice. For the mice that were treated with 10, 15, and 30 μCi of 212Pb-HuM195 F(ab′)2, the MS was 13, 15, and 13 d. The differences between the targeted F(ab′)2 and the nontargeted F(ab′)2 were significant (p < 0.001), thus demonstrating specificity of the therapy elicited by 212Pb-panitumumab F(ab′)2. Weight loss also occurred within 2 d of the treatment reaching a nadir at 4–5 d; recovery became evident at 12 d (Table 2). Although the weights of the mice did not return to their pretherapy weights, they did appear to stabilize.
Table 2.
Effect of Increasing 212Pb-Panitumumab F(ab′)2 Doses (i.v.) on the Weights of Athymic Mice Bearing i.p. LS174T Tumor Xenografts
| Days post RIT | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| RIT | Dose (μCi) | 0 | 2 | 7 | 12 | 15 | 20 | 30 | 35 |
| None | — | 23.5 ± 1.1 | 23.1 ± 0.7 | 23.6 ± 0.7 | 25.3 ± 1.3 | ||||
| Panitumumab | 5 | 23.2 ± 1.3 | 21.9 ± 1.5 | 22.3 ± 1.6 | 22.4 ± 1.5 | 22.5 ± 1.7 | 22.4 ± 1.8 | 23.1 ± 1.9 | 22.8 ± 1.6 |
| 10 | 24.1 ± 1.1 | 22.2 ± 1.2 | 21.7 ± 1.2 | 22.1 ± 1.3 | 22.4 ± 1.6 | 22.1 ± 1.8 | 23.0 ± 1.4 | 21.7 ± 2.0 | |
| 15 | 23.9 ± 1.1 | 21.6 ± 1.2 | 21.0 ± 0.9 | 21.6 ± 1.1 | 21.8 ± 1.2 | 21.3 ± 1.3 | 21.2 ± 1.3 | 21.0 ± 1.9 | |
| 30 | 23.3 ± 1.4 | 21.4 ± 1.5 | 20.4 ± 1.6 | 21.3 ± 1.7 | 21.4 ± 2.1 | 21.6 ± 1.7 | 20.9 ± 1.6 | 20.1 ± 2.0 | |
| Days post RIT | ||||||
|---|---|---|---|---|---|---|
| 0 | 2 | 5 | 10 | 15 | ||
| HuM195 | 10 | 22.9 ± 0.8 | 21.4 ± 1.0 | 20.1 ± 1.1 | 21.9 ± 1.4 | 24.4 |
| 15 | 23.0 ± 1.3 | 21.8 ± 1.3 | 20.8 ± 1.3 | 22.0 ± 1.2 | 24.2 | |
| 30 | 22.8 ± 1.0 | 21.4 ± 1.1 | 19.6 ± 1.2 | 20.0 ± 0.9 | ||
Athymic mice (n = 10) bearing 3 d i.p. LS-174T tumor xenografts were injected i.v. with 5, 10, 15, and 30 μCi of 212Pb-panitumumab F(ab′)2 to establish the effective, suboptimal, therapeutic dose for subsequent studies with chemotherapeutics. Animal weights were monitored one to two times per week for 5 weeks as an indicator of toxicity. Additional groups included those that received no treatment and those that were injected with 10, 15 and 30 μCi of the nonspecific control, 212Pb-HuM195 F(ab′)2. The values are the average weight (g) with the standard deviation.
i.v., intravenous.
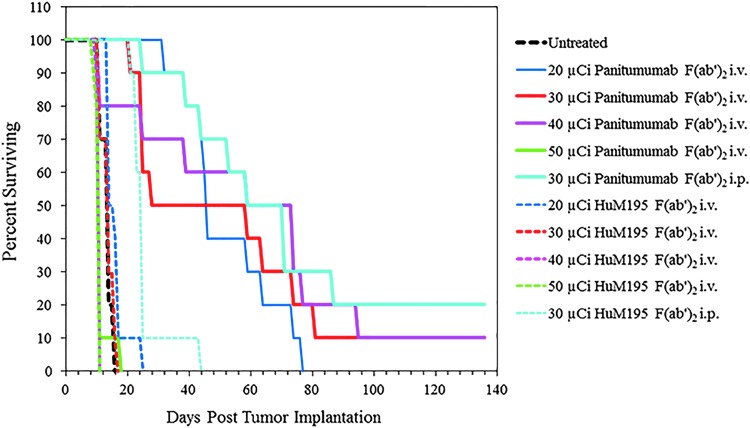
Unfortunately, the choice of a dose for the i.v. injection route was not as clear as with the i.p. injected 212Pb-panitumumab F(ab′)2; the upper dose limit was not clearly delineated. Thus, a second therapy experiment was conducted extending the doses of the i.v. injected 212Pb-panitumumab F(ab′)2. In this study, mice were injected i.v. with 20, 30, 40, or 50 μCi of 212Pb-panitumumab F(ab′)2 or 212Pb-HuM195 F(ab′)2. Additional mice were also injected i.p. with 30 μCi of specific or nonspecific F(ab′)2 fragments to validate the chosen dosage for i.p. 212Pb-RIT and one cohort of mice were left untreated. Presented in Figure 5, therapeutic benefit was provided by the 20, 30, and 40 μCi doses of 212Pb-panitumumab F(ab′)2 with MS of 46, 59, and 59 d with corresponding TIs of 3.3, 4.2, and 4.2. No therapy was observed with the 50 μCi of 212Pb-panitumumab F(ab′)2. In fact, the MS of this group of mice was lower than that of the untreated group. Likewise, there was either no difference (20 and 30 μCi) in the MS of groups treated i.v. with the 212Pb-HuM195 F(ab′)2 from the untreated control group, or, the MS was lower (40 and 50 μCi). Again, when the 20, 30, and 40 μCi dose levels of 212Pb-panitumumab F(ab′)2 and 212Pb-HuM195 F(ab′)2 were compared, the differences were found to be significant (p < 0.05).
FIG. 5.
A dose escalation study was repeated at higher doses to establish the effective, suboptimal, therapeutic dose for subsequent studies with chemotherapeutics. Athymic mice (n = 10) bearing 3 d i.p. LS-174T tumor xenografts were injected i.v. with 20, 30, 40, and 50 μCi of 212Pb-panitumumab F(ab′)2. Additional groups included those that received no treatment and those that were injected with the nonspecific control, 212Pb-HuM195 F(ab′)2 at the same dosing level as the 212Pb-panitumumab F(ab′)2. The study also included cohorts of mice that were injected i.p. with 30 μCi of the each of the 212Pb-labeled F(ab′)2 fragments to validate the i.p. dosage.
At the 20 and 30 μCi doses of 212Pb-panitumumab F(ab′)2, given i.v., the mice responded with a similar degree of weight loss as was seen in the first therapy experiment (Table 3). The greatest weight loss was noted at 11 d postdelivery of the 20, 30, and 40 μCi, whereas at the 50 μCi dose level the loss occurred at 5 d. In comparison to the other treatment groups, the weight loss realized by the 20 μCi was modest with a maximum loss of 4.3% at the 11 d time point. As with the previous therapy study, none of the mice rebound to their pretherapy weights; however, the 20 μCi treatment group experienced the least loss. Considering this lesser degree of weight loss and subsequent recovery, even though the MS was lower, 20 μCi was selected for the effective working dose for i.v. injected 212Pb-panitumumab F(ab′)2.
Table 3.
Response of Tumor-Bearing Mice to Higher Doses of i.v. Administered 212Pb-Panitumumab F(ab′)2
| Days post-RIT | ||||||||
|---|---|---|---|---|---|---|---|---|
| F(ab′)2 | Route | Dose (μCi) | −1 | 5 | 11 | 14 | 20 | 29 |
| None | — | — | 24.6 ± 2.2 | 25.7 ± 2.2 | 26.5 ± 2.4 | |||
| Panitumumab | i.v. | 20 | 25.3 ± 1.4 | 24.4 ± 1.8 | 24.3 ± 1.4 | 24.6 ± 1.4 | 24.3 ± 1.4 | 23.8 ± 1.6 |
| i.v. | 30 | 23.9 ± 1.9 | 21.8 ± 2.0 | 21.0 ± 1.9 | 21.2 ± 1.8 | 21.6 ± 1.5 | 20.8 ± 1.7 | |
| i.v. | 40 | 25.3 ± 2.0 | 23.4 ± 1.6 | 23.3 ± 1.9 | 22.9 ± 2.1 | 23.2 ± 2.1 | 21.7 ± 1.8 | |
| i.v. | 50 | 24.6 ± 2.0 | 21.7 ± 2.3 | 26.1 | 25.1 | |||
| i.p. | 30 | 23.1 ± 1.3 | 21.7 ± 1.5 | 21.5 ± 1.9 | 21.7 ± 2.0 | 21.3 ± 1.9 | 20.9 ± 1.6 | |
| HuM195 | i.v. | 20 | 25.5 ± 1.9 | 24.4 ± 2.6 | 24.2 ± 3.3 | 23.9 ± 5.0 | 22.4 | |
| i.v. | 30 | 25.6 ± 2.1 | 24.1 ± 1.7 | 22.2 ± 1.6 | 21.8 | |||
| i.v. | 40 | 24.6 ± 2.0 | 22.2 ± 1.8 | |||||
| i.v. | 50 | 24.6 ± 2.1 | 22.6 ± 1.9 | |||||
| i.p. | 30 | 23.9 ± 1.7 | 23.1 ± 1.6 | 22.6 ± 1.8 | 22.2 ± 1.9 | 21.7 ± 2.4 | 21.2 | |
A dose escalation study was repeated at higher doses to establish the effective, suboptimal, therapeutic dose for subsequent studies with chemotherapeutics. Athymic mice (n = 10) bearing 3 d i.p. LS-174T tumor xenografts were injected i.v. with 20, 30, 40, and 50 μCi of 212Pb-panitumumab F(ab′)2 and animal weights (g) were monitored one to two times per week for 4 weeks as an indicator of toxicity. Additional groups included those that received no treatment and those that were injected with the nonspecific control, 212Pb-HuM195 F(ab′)2 at the same dosing level as the 212Pb-panitumumab F(ab′)2. The study also included cohorts of mice that were injected i.p. with 30 μCi of the each of the 212Pb-labeled F(ab′)2 fragments to validate the i.p. dosage. The values are the average weight (g) with the standard deviation.
Potentiation of therapeutic efficacy
Having determined the effective dose for each of the injection routes for 212Pb-panitumumab F(ab′)2, a study was designed to assess the potentiation of its therapeutic effectiveness by chemotherapeutics. The i.v. route of administration was chosen in anticipation of potentially combining the administration of 212Pb-RIT through concurrent i.v. and i.p injections. Tumor-bearing mice were given either 1 mg of GEM or 0.6 mg of paclitaxel by i.p. injection ∼24 h before being given 20 μCi (i.v.) of 212Pb-panitumumab F(ab′)2. Other treatment groups (controls) included the two chemotherapeutics alone, 212Pb-panitumumab F(ab′)2, 212Pb-HuM195 F(ab′)2, or 212Pb-HuM195 F(ab′)2 with GEM or paclitaxel along with a group that was left untreated. The 212Pb-panitumumab F(ab′)2 alone resulted in a MS of 61 d, an outcome that was consistent with the previous therapy experiment (Table 4). When GEM or paclitaxel was added to the regimen, the MS increased to 208 and 239 d, respectively. Compared with the 11 d MS of the untreated group, the GEM and paclitaxel increased the 5.5 TI of 212Pb-panitumumab F(ab′)2 to 19.0 and 21.7, respectively. GEM and paclitaxel alone had a modest effect on the LS-174T i.p. tumor xenografts with corresponding MS of 18 and 24 d, respectively. Meanwhile, 212Pb-HuM195 F(ab′)2 alone resulted in a MS of 11 d and in combination with GEM or paclitaxel increased to only 14 d (1.3 TI) and 12 d (1.1 TI), respectively. The effect of the GEM and paclitaxel on the therapeutic efficacy 212Pb-panitumumab F(ab′)2 was statistically significant (p < 0.01) and was specific when compared with the 212Pb-HuM195 F(ab′)2 treatment groups (p < 0.01).
Table 4.
Potentiation of the Therapeutic Efficacy of i.v. 212Pb-Radioimmunotherapy with Panitumumab F(ab′)2
| Chemotherapeutic | ||||
|---|---|---|---|---|
| F(ab′)2 | None | Gemcitabine | Paclitaxel | |
| None | MSa | 11 | 18 | 24 |
| TIb | 1.0 | 1.6 | 2.2 | |
| Panitumumab | MS | 61 | 208 | 239 |
| TI | 5.5 | 19.0 | 21.7 | |
| HuM195 | MS | 11 | 14 | 12 |
| TI | 1.0 | 1.3 | 1.1 | |
Median survival (MS; days) of athymic mice bearing LS-174T i.p. tumor xenografts following pretreatment with chemotherapeutics and a single injection of 212Pb-panitumumab F(ab′)2. Paclitaxel (0.6 mg) and gemcitabine (1 mg) were administered i.p. to tumor-bearing mice 24 h before RIT. Additional groups of mice included those that were treated with each of the chemotherapeutics alone, 212Pb-HuM195 F(ab′)2 alone, 212Pb-HuM195 F(ab′)2 in combination with each of the chemotherapeutics, as well as a group of mice that were left untreated.
The therapeutic index is the MS of the treatment group divided by the MS of the untreated group.
TI, therapeutic indices.
Weight loss (Table 5) was observed in all of the treatment groups on the fifth day, with the exception of the untreated group, the severest loss occurring in the mice that had received the paclitaxel before the 212Pb-panitumumab F(ab′)2. At 8 d following the 212Pb-RIT, mice were found to be rebounding from their weight loss. Yet, as was observed in the previous studies, none returned to their pretherapy weights, except for the GEM-212Pb-HuM195 F(ab′)2 treatment group, which succumbed to disease progression by 14 d.
Table 5.
Effect of i.v. 212Pb-Radioimmunotherapy in Combination with Chemotherapeutics on the Weights of Athymic Mice Bearing LS-174T i.p. Tumor Xenografts
| Days post 212Pb-RIT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F(ab′)2 | Chemotherapeutic | −1 | 5 | 8 | 12 | 15 | 19 | 26 | 29 | 33 |
| None | None | 22.3 ± 1.0 | 22.6 ± 1.2 | 23.9 ± 1.2 | ||||||
| None | GEM | 22.6 ± 1.3 | 21.7 ± 1.7 | 22.4 ± 1.8 | 23.1 ± 2.1 | 21.5 | ||||
| None | Paclitaxel | 22.9 ± 2.0 | 21.9 ± 2.4 | 23.0 ± 2.5 | 23.1 ± 2.2 | 23.0 ± 2.2 | 25.1 ± 0.2 | 24.0 | 24.5 | 24.2 |
| Panitumumab | None | 25.1 ± 2.0 | 21.9 ± 1.9 | 24.0 ± 1.9 | 21.8 ± 2.3 | 21.8 ± 2.3 | 22.8 ± 1.8 | 22.0 ± 2.0 | 21.7 ± 1.9 | 21.1 ± 1.7 |
| Panitumumab | GEM | 24.3 ± 1.2 | 20.0 ± 1.9 | 21.6 ± 2.2 | 21.3 ± 2.0 | 21.7 ± 2.0 | 22.7 ± 2.1 | 22.7 ± 1.7 | 22.5 ± 1.8 | 22.5 ± 2.2 |
| Panitumumab | Paclitaxel | 23.7 ± 2.4 | 18.8 ± 2.5 | 20.5 ± 2.8 | 21.1 ± 2.0 | 22.8 ± 2.7 | 24.1 ± 2.3 | 23.3 ± 1.7 | 23.5 ± 1.8 | 23.4 ± 2.5 |
| HuM195 | None | 21.9 ± 1.7 | 19.3 ± 1.9 | 19.2 ± 2.9 | 23.7 ± 2.2 | |||||
| HuM195 | GEM | 22.7 ± 0.9 | 20.3 ± 1.5 | 19.2 ± 1.5 | 19.1 ± 1.4 | |||||
| HuM195 | Paclitaxel | 23.0 ± 2.7 | 21.6 ± 3.0 | 20.3 ± 2.5 | 21.7 ± 3.2 | |||||
Paclitaxel (0.6 mg) and gemcitabine (1 mg) were administered i.p. to mice bearing LS-174T i.p. tumor 24 h before RIT. The 212Pb-panitumumab F(ab′)2 (20 μCi) was administered by i.v. injection. Animal weights were then monitored one to two times per week for 5 weeks as an indicator of toxicity. Additional groups included those that received no treatment, GEM or paclitaxel only, 212Pb-panitumumab F(ab′)2, 212Pb-HuM195 F(ab′)2, and 212Pb-HuM195 F(ab′)2 in combination with GEM or paclitaxel.
Discussion
As with any regimen designed for treating and managing cancer patients, a primary goal of RIT is to deliver a cytotoxic dose to a patient's tumor while minimizing damage to normal tissues. Toward this end, TAT regimens are continually being refined with numerous strategies taken to realize this goal. Among the variables to consider are: the target molecule, the targeting agent, the choice of radionuclide, and administration route.
Regarding the targeting agent, the size of the molecule, that is, a mAb, may limit extravasation as well as penetration of tumor tissue resulting in a prolonged residence time in the blood.13,27 mAbs, even with a moderate affinity, delivered through i.v. injection do not penetrate far into tumors, but instead concentrate in the region of, or immediately adjacent to blood vessels. Higher affinities might exacerbate the ability of a mAb to migrate into and penetrate tumor tissue. Furthermore, maximal concentration is not achieved until 72–96 h after injection.14,28 Penetration of tumor has been accomplished with multiple doses and at quantities of mAb much greater than what is typically injected when the mAb is a radiopharmaceutical.1,19,27 The residence time of an mAb in the blood compartment is also an issue. The whole body clearance (T1/2β) of a chimerized or humanized mAb in tumor-bearing mice is in the range of 2–3 d.6,20,29–31
Intracavitary injection of the RIC is one strategy for circumventing the challenges presented by these intrinsic properties of a mAb in RIT applications.9,32–37 The hypothesis being that locoregional administration increases the absorbed dose by providing greater direct access to the tumor while reducing toxicity to normal tissues, especially myeloid cells. The clearance rate of RICs from a cavity, such as the peritoneum, is delayed and prolonged, and the level of radioactivity does not reach the same level as that of an i.v. injection of the same dose.6,20,30,31 In fact, Meredith et al. reported minimal redistribution of 212Pb-trastuzumab out of the peritoneal cavity of patients as well as no significant uptake in major organs.3
Reduction in the size of the mAb, achieved through enzymatic digestion or genetic engineering, is another path taken by investigators in dealing with the obstacles inherent in the use of an intact immunoglobulin as a radiopharmaceutical vector.38 Fragments, such as an F(ab′)2, can be readily obtained using pepsin without loss of immunoreactivity.13,15,39 F(ab′)2 fragments have been shown to have a faster extravasation rate and localization to tumor, greater penetration of the tumor, and have an overall lower residence time of unbound RIC in the body.13–15,40 With these advantages, the F(ab′)2 fragment would be an appropriatetargeting vehicle for i.v. administration of targeted α-radiation. Disadvantages, however, may reside in an increased whole body clearance rate thereby arguing for their use with radionuclides with relatively shorter half-lives than might be used with an intact IgG. While there may be some clear advantages to the use of fragments in this specific case and model system that conclusion can only be extended further to other models through speculation, and real empirical determinations will prove or disprove what choice(s) will be most efficacious in any given disease setting and model.
Smaller alternatives to F(ab′)2 fragments include molecules, such as nanobodies, affibodies, and peptidomimetics.41–44 The potential of a peptidomimetic having therapeutic benefit for two late-stage prostate cancer patients has recently been related in a communication, the authors acknowledge that a larger cohort of patients will need to be studied.44 In general, the smaller molecules result in lower tumor %ID/g and higher renal uptake of radioactivity.
In pre-clinical studies, panitumumab has proven to be an excellent candidate for 212Pb-RIT of HER1-positive disseminated i.p. disease.6,20 However, the LS-174T tumor model that has been used by this laboratory for RIT studies is aggressive to the point that the effectiveness of TAT was found to be dramatically decreased when mice bearing a 5 d tumor burden were treated.45 Furthermore, macroscopic inspection of the disease burden after only 3 d revealed that tumor masses were already attaching to the peritoneum walls and to organ surfaces as well as developing vasculature (unpublished observations). Unlike the controlled setting of the laboratory, patients do not present with a 3 d tumor burden. Managing patients with aggressive disease and/or greater tumor burdens might require more than an intracavitary therapy treatment regimen. The delivery of 212Pb-RIT using a mAb fragment was considered a worthwhile pursuit and the F(ab′)2 fragment of panitumumab was generated and evaluated as a delivery vector for α-particle radiation therapy.
The panitumumab F(ab′)2 was conjugated with the TCMC ligand and assessed in vitro and in vivo for retention of immunoreactivity and the ability to target tumor. The data from the in vitro analysis of the panitumumab F(ab′)2 was consistent not only with what has been published for the parental panitumumab IgG, demonstrating that the F(ab′)2 fragment has retained the qualities of the intact IgG, but also with other mAb F(ab′)2 fragments that have been evaluated for similar applications.13,15,20,32,40,46,47
The initial in vivo study provided information on the tumor targeting and normal distribution of the panitumumab F(ab′)2 when modified with the TCMC chelate and labeled with a Pb(II) isotope. The 203Pb-panitumumab F(ab′)2 was evaluated in the LS-174T i.p. tumor model, injected either i.p. or i.v. The i.p. injection did achieve the higher tumor %ID/g, however, the i.v. injected 203Pb-panitumumab F(ab′)2 resulted in a tumor %ID/g that was comparable at 24 h to what was observed in s.c. tumors after the i.v. injection of 111In-panitumumab F(ab′)2.15 Furthermore, peak tumor targeting of the panitumumab F(ab′)2 was attained at 24 h (the earliest time point of the study) in contrast to the 48–72 h required by panitumumab IgG.20 These data are consistent with what has been reported by others comparing radiolabeled forms of a mAb in not only animal models, but also in human clinical trials.13,46,47 In a RIT clinical trial, patients with metastatic colorectal cancer received either the IgG and the F(ab′)2 forms of the anticarcinoembryonic antigen mAb, A5B7 labeled with 131I. The study found no statistical difference between the uptake of 131I-A5B7 IgG and 131I-A5B7 F(ab′)2 in patient tumors.47 Examples also exist in the literature of an F(ab′)2 fragment having either a greater level of tumor targeting than the parental mAb immunoglobulin, or, conversely, a decreased level.48,49 Such examples simply highlight the necessity of performing these experiments empirically to validate each RIC. The lack of tumor targeting by the 203Pb-HuM195 F(ab′)2 fragment, injected by either the i.p. or i.v. route, attests to the specificity of the 203Pb-panitumumab F(ab′)2 fragment. Furthermore, in the absence of antigen, or antigen sink, a F(ab′)2 fragment is eliminated through renal excretion.
The therapy studies that followed with 212Pb-panitumumab F(ab′)2 clearly indicated that the therapeutic potential of this mAb form would be worthwhile to further investigate. The MS of tumor-bearing mice receiving an i.p. injection of the 212Pb-panitumumab F(ab′)2 (30 μCi) was similar to what was reported for 212Pb-panitumumab.6 Even more encouraging was the therapeutic benefit that was observed with the i.v. injected 212Pb-panitumumab F(ab′)2. Although the MS of the tumor-bearing mice at the final selected dose of 20 μCi was not as great as the F(ab′)2 i.p.-delivered RIT, the therapeutic benefit was still realized. Furthermore, the therapeutic efficacy was specific with the differences between the targeted 212Pb-panitumumab F(ab′)2 and the nonspecific HuM195 F(ab′)2 being significant (p < 0.001). The therapeutic efficacy of the i.v. administered 212Pb-panitumumab F(ab′)2 could also be enhanced by the chemotherapeutics, gemcitabine or paclitaxel, which were administered i.p.
Studies are conflicted as to whether or not dual administration of RIT provides any additional benefit of either administration route alone. For example, in an early study with colorectal cancer patients with i.p. disease, 131I-B72.3 was administered by i.v. and i.p. injection.50 The authors concluded that the i.p. route was more efficient for peritoneal tumor masses, whereas the i.v. route had greater advantage for local recurrences of tumor as well as lymph node metastases. There was an advantage noted for the concomitant i.v. and i.p. RIT. In contrast, no advantage was found when ovarian cancer patients were treated with the chimerized antifolate receptor mAb, MOv18.51 Both i.v. administration and i.p. administration of 212Pb-panitumumab F(ab′)2 provided therapeutic benefit. The i.v. administration of 212Pb-panitumumab F(ab′)2 in combination with i.p. administered 212Pb-RIT may provide a means of attaining more effective therapy, especially in well-vascularized tumor burdens through the additional targeting of the tumor interior. The dual route of RIT administration may also prove to have greater efficacy in the treatment of larger tumor burdens.
Conclusions
The panitumumab F(ab′)2 fragment is a viable candidate as a delivery vector for TAT. Future studies will include evaluating the penetration of i.p. disseminated tumors by the panitumumab F(ab′)2 fragment following i.v. and i.p. injections and combination thereof. Studies will also proceed toward optimization of TAT implementing the dual injection route alone and in combination with chemotherapeutics.
Acknowledgment
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Disclosure Statement
No competing financial interests exist.
References
- 1.Baidoo KE, Milenic DE, Brechbiel MW. Methodology for labeling proteins and peptides with lead-212 (212Pb). Nucl Med Biol 2013;40:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brechbiel MW. Targeted alpha-therapy: Past, present, future? Dalton Trans 2007;4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meredith RF, Torgue J, Azure MT, et al. . Pharmacokinetics and imaging of 212Pb-TCMC-trastuzumab after intraperitoneal administration in ovarian cancer patients. Cancer Biother Radiopharm 2014;29:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meredith RF, Torgue JJ, Rozgaja TA, et al. . Safety and outcome measures of first-in-human intraperitoneal alpha radioimmunotherapy with 212Pb-TCMC-trastuzumab. Am J Clin Oncol 2016; [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meredith R, Torgue J, Shen S, et al. . Dose escalation and dosimetry of first-in-human alpha radioimmunotherapy with 212Pb-TCMC-trastuzumab. J Nucl Med 2014;55:1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milenic DE, Baidoo KE, Kim YS, et al. . Targeted alpha-particle radiation therapy of HER1-positive disseminated intraperitoneal disease: An investigation of the human anti-EGFR monoclonal antibody, panitumumab. Transl Oncol 2017;10:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milenic DE, Baidoo KE, Kim YS, et al. . Evaluation of cetuximab as a candidate for targeted alpha-particle radiation therapy of HER1-positive disseminated intraperitoneal disease. MAbs 2015;7:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boudousq V, Bobyk L, Busson M, et al. . Comparison between internalizing anti-HER2 mAbs and non-internalizing anti-CEA mAbs in alpha-radioimmunotherapy of small volume peritoneal carcinomatosis using 212Pb. PLoS One 2013;8:e69613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasten BB, Arend RC, Katre AA, et al. . B7-H3-targeted 212Pb radioimmunotherapy of ovarian cancer in preclinical models. Nucl Med Biol 2017;47:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su FM, Beaumier P, Axworthy D, et al. . Pretargeted radioimmunotherapy in tumored mice using an in vivo 212Pb/212Bi generator. Nucl Med Biol 2005;32:741. [DOI] [PubMed] [Google Scholar]
- 11.Tan Z, Chen P, Schneider N, et al. . Significant systemic therapeutic effects of high-LET immunoradiation by 212Pb-trastuzumab against prostatic tumors of androgen-independent human prostate cancer in mice. Int J Oncol 2012;40:1881. [DOI] [PubMed] [Google Scholar]
- 12.Covell DG, Barbet J, Holton OD, et al. . Pharmacokinetics of monoclonal immunoglobulin G1, F(ab′)2, and Fab′ in mice. Cancer Res 1986;46:3969. [PubMed] [Google Scholar]
- 13.Milenic DE, Yokota T, Filpula DR, et al. . Construction, binding properties, metabolism, and tumor targeting of a single-chain Fv derived from the pancarcinoma monoclonal antibody CC49. Cancer Res 1991;51:6363. [PubMed] [Google Scholar]
- 14.Yokota T, Milenic DE, Whitlow M, et al. . Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res 1992;52:3402. [PubMed] [Google Scholar]
- 15.Wong KJ, Baidoo KE, Nayak TK, et al. . In vitro and in vivo pre-clinical analysis of a F(ab′)2 fragment of panitumumab for molecular imaging and therapy of HER1 positive cancers. EJNMMI Res 2011;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dadachova E, Chappell LL, Brechbiel MW. Spectrophotometric method for determination of bifunctional macrocyclic ligands in macrocyclic ligand-protein conjugates. Nucl Med Biol 1999;26:977. [DOI] [PubMed] [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, et al. . Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265. [PubMed] [Google Scholar]
- 18.Chappell LL, Dadachova E, Milenic DE, et al. . Synthesis, characterization, and evaluation of a novel bifunctional chelating agent for the lead isotopes 203Pb and 212Pb. Nucl Med Biol 2000;27:93. [DOI] [PubMed] [Google Scholar]
- 19.Milenic DE, Garmestani K, Brady ED, et al. . Alpha-particle radioimmunotherapy of disseminated peritoneal disease using a (212)Pb-labeled radioimmunoconjugate targeting HER2. Cancer Biother Radiopharm 2005;20:557. [DOI] [PubMed] [Google Scholar]
- 20.Ray GL, Baidoo KE, Wong KJ, et al. . Preclinical evaluation of a monoclonal antibody targeting the epidermal growth factor receptor as a radioimmunodiagnostic and radioimmunotherapeutic agent. Br J Pharmacol 2009;157:1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C, Gansow OA, Brechbiel MW. Evaluation of methods for large scale preparation of antibody ligand conjugates. Nucl Med Biol 1999;26:339. [DOI] [PubMed] [Google Scholar]
- 22.Garmestani K, Milenic DE, Brady ED, et al. . Purification of cyclotron-produced 203Pb for labeling Herceptin. Nucl Med Biol 2005;32:301. [DOI] [PubMed] [Google Scholar]
- 23.Milenic DE, Baidoo KE, Brechbiel MW. Bench to bedside: Stability studies of GMP produced trastuzumab-TCMC in support of a clinical trial. Pharmaceuticals (Basel) 2015;8:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nayak TK, Garmestani K, Milenic DE, et al. . HER1-targeted 86Y-panitumumab possesses superior targeting characteristics than 86Y-cetuximab for PET imaging of human malignant mesothelioma tumors xenografts. PLoS One 2011;6:e18198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milenic DE, Wong KJ, Baidoo KE, et al. . Cetuximab: Preclinical evaluation of a monoclonal antibody targeting EGFR for radioimmunodiagnostic and radioimmunotherapeutic applications. Cancer Biother Radiopharm 2008;23:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H, Baidoo K, Gunn AJ, et al. . Design, synthesis, and characterization of a dual modality positron emission tomography and fluorescence imaging agent for monoclonal antibody tumor-targeted imaging. J Med Chem 2007;50:4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freeman DJ, McDorman K, Ogbagabriel S, et al. . Tumor penetration and epidermal growth factor receptor saturation by panitumumab correlate with antitumor activity in a preclinical model of human cancer. Mol Cancer 2012;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinstein JN, Holton OD, 3rd, Black CD, et al. . Regional delivery of monoclonal antitumor antibodies: Detection and possible treatment of lymph node metastases. Prog Clin Biol Res 1986;212:169. [PubMed] [Google Scholar]
- 29.Colcher D, Milenic D, Roselli M, et al. . Characterization and biodistribution of recombinant and recombinant/chimeric constructs of monoclonal antibody B72.3. Cancer Res 1989;49:1738. [PubMed] [Google Scholar]
- 30.Milenic DE, Wong KJ, Baidoo KE, et al. . Targeting HER2: A report on the in vitro and in vivo pre-clinical data supporting trastuzumab as a radioimmunoconjugate for clinical trials. MAbs 2010;2:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers BE, Roberson PL, Shen S, et al. . Intraperitoneal radioimmunotherapy with a humanized anti-TAG-72 (CC49) antibody with a deleted CH2 region. Cancer Biother Radiopharm 2005;20:502. [DOI] [PubMed] [Google Scholar]
- 32.Boskovitz A, Akabani GH, Pegram CN, et al. . Human/murine chimeric 81C6 F(ab′)(2) fragment: Preclinical evaluation of a potential construct for the targeted radiotherapy of malignant glioma. Nucl Med Biol 2004;31:345. [DOI] [PubMed] [Google Scholar]
- 33.Brown MT, Coleman RE, Friedman AH, et al. . Intrathecal 131I-labeled antitenascin monoclonal antibody 81C6 treatment of patients with leptomeningeal neoplasms or primary brain tumor resection cavities with subarachnoid communication: Phase I trial results. Clin Cancer Res 1996;2:963. [PubMed] [Google Scholar]
- 34.Elgqvist J, Andersson H, Back T, et al. . Fractionated radioimmunotherapy of intraperitoneally growing ovarian cancer in nude mice with 211At-MX35 F(ab′)2: Therapeutic efficacy and myelotoxicity. Nucl Med Biol 2006;33:1065. [DOI] [PubMed] [Google Scholar]
- 35.Grossi PM, Ochiai H, Archer GE, et al. . Efficacy of intracerebral microinfusion of trastuzumab in an athymic rat model of intracerebral metastatic breast cancer. Clin Cancer Res 2003;9:5514. [PubMed] [Google Scholar]
- 36.Huber R, Seidl C, Schmid E, et al. . Locoregional alpha-radioimmunotherapy of intraperitoneal tumor cell dissemination using a tumor-specific monoclonal antibody. Clin Cancer Res 2003;9:3922S. [PubMed] [Google Scholar]
- 37.Schlom J, Hand PH, Greiner JW, et al. . Innovations that influence the pharmacology of monoclonal antibody guided tumor targeting. Cancer Res 1990;50:820s. [PubMed] [Google Scholar]
- 38.Milenic DE. Antibody engineering—Optimizing the delivery vehicle. In: Reilly RM. (ed.), Monoclonal Antibody and Peptide targeted Radiotherapy of Malignancies. Hoboken, NJ: John Wiley and Sons, 2010;1 [Google Scholar]
- 39.Milenic DE, Esteban JM, Colcher D. Comparison of methods for the generation of immunoreactive fragments of a monoclonal antibody (B72.3) reactive with human carcinomas. J Immunol Methods 1989;120:71. [DOI] [PubMed] [Google Scholar]
- 40.Dumolyn C, Schoonooghe S, Moerman L, et al. . Generation and in vivo characterization of a chimeric αvβ5-targeting antibody 14C5 and its derivatives. EJNMMI Res 2013;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benesova M, Schafer M, Bauder-Wust U, et al. . Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med 2015;56:914. [DOI] [PubMed] [Google Scholar]
- 42.Choi J, Vaidyanathan G, Koumarianou E, et al. . Astatine-211 labeled anti-HER2 5F7 single domain antibody fragment conjugates: Radiolabeling and preliminary evaluation. Nucl Med Biol 2018;56:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dekempeneer Y, Keyaerts M, Krasniqi A, et al. . Targeted alpha therapy using short-lived alpha-particles and the promise of nanobodies as targeting vehicle. Expert Opin Biol Ther 2016;16:1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kratochwil C, Bruchertseifer F, Rathke H, et al. . Targeted Alpha Therapy of mCRPC with (225)Actinium-PSMA-617: Swimmer-Plot analysis suggests efficacy regarding duration of tumor-control. J Nucl Med 2018;59:795. [DOI] [PubMed] [Google Scholar]
- 45.Milenic DE, Garmestani K, Brady ED, et al. . Targeting of HER2 antigen for the treatment of disseminated peritoneal disease. Clin Cancer Res 2004;10:7834. [DOI] [PubMed] [Google Scholar]
- 46.Boyle AJ, Cao PJ, Hedley DW, et al. . MicroPET/CT imaging of patient-derived pancreatic cancer xenografts implanted subcutaneously or orthotopically in NOD-scid mice using (64)Cu-NOTA-panitumumab F(ab′)2 fragments. Nucl Med Biol 2015;42:71. [DOI] [PubMed] [Google Scholar]
- 47.Lane DM, Eagle KF, Begent RH, et al. . Radioimmunotherapy of metastatic colorectal tumours with iodine-131-labelled antibody to carcinoembryonic antigen: Phase I/II study with comparative biodistribution of intact and F(ab′)2 antibodies. Br J Cancer 1994;70:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buchegger F, Pelegrin A, Hardman N, et al. . Different behaviour of mouse-human chimeric antibody F(ab′)2 fragments of IgG1, IgG2 and IgG4 sub-class in vivo. Int J Cancer 1992;50:416. [DOI] [PubMed] [Google Scholar]
- 49.Spiridon CI, Guinn S, Vitetta ES. A comparison of the in vitro and in vivo activities of IgG and F(ab′)2 fragments of a mixture of three monoclonal anti-Her-2 antibodies. Clin Cancer Res 2004;10:3542. [DOI] [PubMed] [Google Scholar]
- 50.Colcher D, Esteban J, Carrasquillo JA, et al. . Complementation of intracavitary and intravenous administration of a monoclonal antibody (B72.3) in patients with carcinoma. Cancer Res 1987;47:4218. [PubMed] [Google Scholar]
- 51.van Zanten-Przybysz I, Molthoff CF, Roos JC, et al. . Influence of the route of administration on targeting of ovarian cancer with the chimeric monoclonal antibody MOv18: i.v. vs. i.p. Int J Cancer 2001;92:106. [PubMed] [Google Scholar]