Abstract
A 22-year-old woman presented with symptoms and signs consistent with acute severe asthma. After significant doses of beta-agonist, she developed a significant lactic acidosis. Significant issues arose in this patient’s history with regards to purchase of medications, compliance and follow-up with respiratory service. Beta-adrenergic receptors when stimulated have been hypothesised to increase lipolysis, producing free fatty acids, which inhibit the conversion of pyruvate to coenzyme A within the Krebs cycle. Additional pyruvate is generated through stimulation of glycolysis and glycogenolysis through simultaneous catecholamine surge. This increased pyruvate load is shunted through anaerobic glycolysis, producing increased lactate. Steroid use during an asthma attack enhances the beta-2 receptor sensitivity, further potentiating lactate production. The hyperadrenergic state in this young asthmatic likely resulted in pyruvate and therefore lactate rise and thus metabolic acidosis as mentioned before. This piece highlights a physiological phenomenon that may occur in the context of iatrogenic hyperadrenergism.
Keywords: Respiratory System, Asthma
Background
Asthma continues to place a significant burden of disease within Ireland, affecting 12% of the population.1 The Asthma Insights and Reality in Europe (AIRI) study demonstrated 50% of Irish patients with asthma suffer from nocturnal symptoms and nearly 75% are limited in their activities of daily living due to asthma.2
Inhaled corticosteroids have revolutionised asthma care and are a cornerstone of GINA guidelines for stepwise treatment of asthma. They have been found to prevent the majority of asthma hospitalisations and deaths.3 Beta-agonist therapy is a mainstay of reliever therapy recommended from Steps 1 to 5 in the GINA guidelines.
Sparse case reports exist within the literature detailing lactic acidosis in the context of high-dose inhaled beta-2-agonist therapy in acute severe asthma.4–7 This case details a patient with a severe asthma exacerbation treated with beta-agonist therapy subsequently developing significant lactic acidosis. This lactic acidosis in the context of an acute exacerbation of asthma was felt secondary to a hyperadrenergic state.
Case presentation
A 22-year-old woman was admitted with progressive dyspnoea, wheeze and cough. Her background comprised poorly controlled asthma diagnosed at the age of 7 and smoked approximately 10 cigarettes a day over the last 8 years.
She had three prior admissions for asthma exacerbations, with a prior intensive care unit (ICU) admission. Maintenance medications included budesonide/formoterol and terbutaline sulfate inhalers with poor compliance.
Her inhalers were bought abroad as they were considerable cost savings made. The patient was also on an unknown imported nebulised therapy and used her nebulizers as required approximately once or twice every few days.
Initial physical examination at the emergency department demonstrated respiratory distress, tachypnoea with a respiratory rate of 30–35 breaths/min and a tachycardia of 110 beats per minute. Initial oxygen saturations were 90% on room air. On respiratory examination, she had widespread polyphonic wheeze bilaterally and marked difficulty in completing sentences.
Investigations
Initial blood tests demonstrated normal inflammatory markers with a normal full blood count, liver function tests and renal indices. Her chest X-ray demonstrated no infiltrate. Her initial arterial blood gas demonstrated hypoxia with a pO2 of 9.88 kPa and a lactate of 2.4 mmol/L. Her peak flow was recorded at 35% personal best.
Differential diagnosis
Beta-agonist therapy.
Tissue hypoxaemia.
Ethanol toxicity.
Acute liver injury.
Diabetic ketoacidosis.
Drug toxicity—includes metformin, linezolid and isoniazid.
Treatment
She was commenced on 100% O2 via non-rebreather mask and received 200 mg intravenous hydrocortisone on arrival as well as multiple atrovent/salbutamol doses (15 mg total salbutamol over 24 hours) via nebuliser. Intravenous magnesium sulfate 2 g followed shortly as she failed to improve as well as commencement on intravenous beta-lactam and macrolide therapy.
After initial improvement, she deteriorated with worsening dyspnoea and wheeze, and desaturated to SpO288% on 6 L O2. She went on to have multiple further high doses of nebulised beta-agonist-based bronchodilator (20 mg additional) and intravenous steroid therapy, commencement on montelukast before transfer to ICU, where she was commenced on an aminophylline infusion with multiple further doses of beta-agonist bronchodilator therapy.
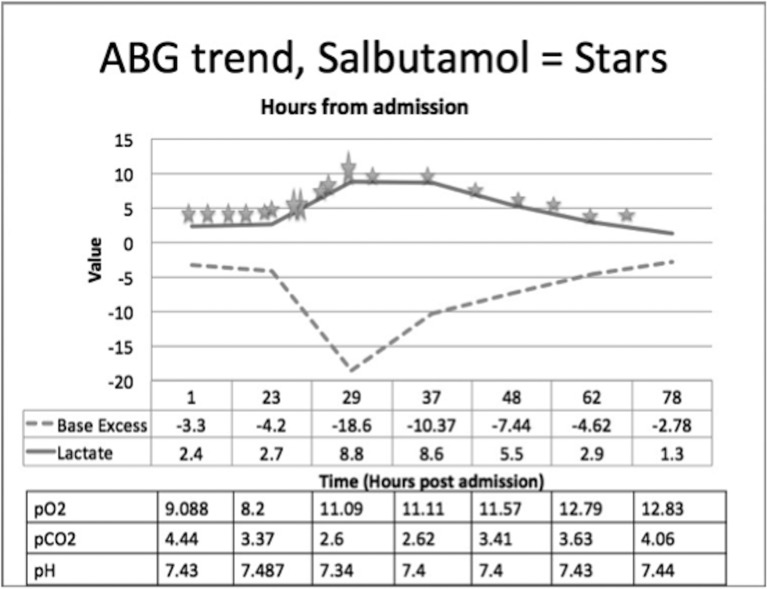
Serial arterial blood gases (ABGs) demonstrated a rising lactate and deteriorating acidosis as seen in figure 1. She began to improve with peak flow rising to 60% personal best and her beta-agonist therapy was spaced gradually to 4 hourly. A more ipratropium-based therapy was initiated from mid-day day 3 admission.
Figure 1.
Arterial blood gas (ABG) trend. X-axis, time in hours from admission; Y-axis, value. Small stars represent 2.5 mg salbutamol, larger stars represent 5 mg equivalent. Table underneath represents other values on ABG over the same time.
Outcome and follow-up
She was discharged after 5 days on budesonide/formoterol 200 µg/6 µg one inhalation twice a day, salbutamol two inhalations 100 µg 4 to 6 hourly as needed and oral montelukast 10 mg once a day; tapering steroid therapy with outpatient follow-up.
Discussion
This case demonstrates a severe asthma attack and highlights potential difficulties in management. Despite age-standardised death rates from asthma falling by about one-third between 1990 and 2010, from 250 per million to 170 per million among men, and from 130 per million to 90 per million among women,8 mortality rates remain quite high. In 2013, Ireland had the sixth highest adult mortality in Europe.9
This case illustrates many challenges faced in dealing with asthma in Ireland. The AIRI study revealed only 8% of patients with asthma in Ireland are well controlled.2 The results of the study highlight that management of asthma fail to achieve the goals outlined in national management guidelines. Further evidence from the International Study of Asthma and Allergies in Childhood Study (Ireland) identified 37% of Irish teenagers with severe symptoms of asthma had neither been diagnosed nor treated for asthma.10
The patient described had noted marked difficulty with compliance to treatment. Fifty per cent of patients are partially adherent and 20% non-adherent to the prescribed dose.11 Maintenance therapy is essential to the care of these patients. Low doses of inhaled corticosteroid therapy prevents the major burden of asthma-associated morbidity and mortality.3
This case points towards disparities in medication cost within the European Union. Twenty-five per cent of diagnosed asthmatics in Ireland travel abroad to purchase their prescribed medications at considerable discount2; 40% do not take all their prescribed medications due to pricing. The current cost of therapy ranges from €100 to €144 per month in Ireland.2 The patient obtained her inhalers from Spain, as she could not afford them domestically. This is seen across multiple prescribed long-term medication classes and adds an extra level of complexity in the management of patients with chronic diseases that require life-long expensive medications.
This case highlights a unique metabolic complication in asthma management. The common metabolic disturbance in acute asthma is respiratory alkalosis followed by respiratory acidosis as fatigue sets in.12 In this case, a rapid metabolic acidosis was the primary acid–base disturbance witnessed. There are a number of physiological mechanisms that may have developed.
The commonly held theory is that the metabolic acidosis is secondary to a hyperadrenergic state6 7 driven by culpable exogenous agents, such as salbutamol, and associated anxiety.
Beta-adrenergic receptors when stimulated have been hypothesised to increase lipolysis, producing free fatty acids, which inhibit the conversion of pyruvate to coenzyme A within the Krebs cycle. Additional pyruvate is generated through stimulation of glycolysis and glycogenolysis through simultaneous catecholamine surge. This increased pyruvate load is shunted through anaerobic glycolysis, producing increased lactate.13 Steroid use during an asthma attack enhances the beta-2 receptor sensitivity, further potentiating lactate production.14
This hyperadrenergic state in this young asthmatic likely resulted in pyruvate and therefore lactate rise and thus metabolic acidosis as mentioned before. The absence of sepsis criteria, hepatic impairment and other possible iatrogenic agents would lead against alternative hypothesis.
Both clinical manifestations of lactic acidosis (non-specific but can include tachypnoea, confusion, headache and palpitations) and/or a deteriorating metabolic acidosis should alert the clinician that a ceiling of therapy with escalating doses of inhaled salbutamol has been reached, and temporary cessation, followed by a reduction in dosing frequency guided by ABGs, should be warranted. This strategy can be used in combination with ipratropium. Clinical assessment alone can be challenging as tachypnoea can be confused with deteriorating asthma and care can be compounded by further beta-agonist therapy. Peak flow can aid in distinguishing between a lactic acidosis due to beta-agonists (which may present as a worsening of dyspnoea, but in the face of improving peak flow) or lactic acidosis because of the onset of tissue hypoxaemia in life-threatening asthma (when the peak flow rate will decline or become un-recordable).
Approximately 2%–20% of admissions to ICUs are attributed to severe asthma, with intubation and ventilation necessary in up to one-third of asthmatic patients. In extreme cases, extracorporeal membrane oxygenation can be used as rescue therapy to bridge the patient until respiratory mechanics improve.
This was an interesting case demonstrating the challenges facing care of a severe asthmatic patient and a potential physiological phenomenon that may occur in the context of iatrogenic hyperadrenergism.
Learning points.
Iatrogenic hyperadrenergism and resultant lactic acidosis can arise following beta-adrenergic therapy in the treatment of the asthmatic patient.
Where a ceiling of beta-agonist therapy has been reached, temporary cessation, followed by a reduction in dosing frequency guided by arterial blood gases, should be warranted. This strategy can be used in combination with ipratropium.
Several challenges exist in the care of the asthmatic patient within Ireland.
Acknowledgments
The authors would like to acknowledge the ICU staff at Our Lady’s Hospital who were actively involved in the critical care of this patient.
Footnotes
Contributors: ZS: involved primarily in case. Primarily responsible for writing up case. MA-A: consultant overseeing case. Responsible for key therapeutic decisions in case.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Asthma Society of Ireland. Annual Report 2007.
- 2.Rabe KF, Vermeire PA, Soriano JB, et al. Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) study. Eur Respir J 2000;16:802–7. 10.1183/09031936.00.16580200 [DOI] [PubMed] [Google Scholar]
- 3.Suissa S, Ernst P. Inhaled corticosteroids: impact on asthma morbidity and mortality. J Allergy Clin Immunol 2001;107:937–44. 10.1067/mai.2001.115653 [DOI] [PubMed] [Google Scholar]
- 4.Claret PG, Bobbia X, Boutin C, et al. Lactic acidosis as a complication of β-adrenergic aerosols. Am J Emerg Med 2012;30:1319 10.1016/j.ajem.2011.05.011 [DOI] [PubMed] [Google Scholar]
- 5.McGonigle R, Woods RA. Take my breath away: a case of lactic acidosis in an asthma exacerbation. CJEM 2013;2011:284–8. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigo GJ, Rodrigo C. Elevated plasma lactate level associated with high dose inhaled albuterol therapy in acute severe asthma. Emerg Med J 2005;22:404–8. 10.1136/emj.2003.012039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauritsen L, Sahl C, Thorsen S. [Nebulized salbutamol as a possible cause of lactate acidosis in a patient with acute asthma]. Ugeskr Laeger 2013;175:111–2. [PubMed] [Google Scholar]
- 8.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224–60. 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson GJ, Loddenkemper R, Lundbäck B, et al. Respiratory health and disease in Europe: the new European Lung White Book. Eur Respir J 2013;42:559–63. 10.1183/09031936.00105513 [DOI] [PubMed] [Google Scholar]
- 10.Yarnell JW, Stevenson MR, MacMahon J, et al. Smoking, atopy and certain furry pets are major determinants of respiratory symptoms in children: the International Study of Asthma and Allergies in Childhood Study (Ireland). Clin Exp Allergy 2003;33:96–100. 10.1046/j.1365-2222.2003.01572.x [DOI] [PubMed] [Google Scholar]
- 11.Yeung M, O’Connor SA, Parry DT, et al. Compliance with prescribed drug therapy in asthma. Respir Med 1994;88:31–5. 10.1016/0954-6111(94)90171-6 [DOI] [PubMed] [Google Scholar]
- 12.Mountain RD, Heffner JE, Brackett NC, et al. Acid–base disturbances in acute asthma. Chest 1990;98:651–5. 10.1378/chest.98.3.651 [DOI] [PubMed] [Google Scholar]
- 13.Haffner CA, Kendall MJ. Metabolic effects of beta 2-agonists. J Clin Pharm Ther 1992;17:155–64. 10.1111/j.1365-2710.1992.tb01285.x [DOI] [PubMed] [Google Scholar]
- 14.Manthous CA. Lactic acidosis in status asthmaticus : three cases and review of the literature. Chest 2001;119:1599–602. [DOI] [PubMed] [Google Scholar]