Abstract
Non-bacterial thrombotic endocarditis (NBTE) is a well-described phenomenon associated with malignancies due to hypercoaguable state. In the setting of pancreatic cancer, NBTE is more commonly diagnosed postmortem. We describe a case of a man who was diagnosed with pancreatic carcinoma after incidental finding of NBTE. Imaging incidentally revealed multiple strokes, bilateral renal and splenic infarcts, while subsequent workup for cardioembolic source demonstrated a 1.1×0.7 cm mitral valve vegetation. As multiple blood cultures were sterile and patient lacked clinical signs of infection, an underlying malignancy was suspected. CT abdomen demonstrated a dilated pancreatic duct, MRI showed a 2.8×2.2 cm pancreatic head mass. Endoscopic biopsy of the mass revealed pancreatic adenocarcinoma. Other than NBTE, there were no other clinical or laboratory findings to clearly suggest pancreatic cancer. Thus, incidental discovery of this mitral valve vegetation led to the diagnosis of pancreatic malignancy.
Keywords: cancer - see oncology, valvar diseases, pancreas and biliary tract
Background
Non-bacterial thrombotic endocarditis (NBTE), also known as marantic endocarditis, is well documented in the literature phenomenon. This condition arises secondary to hypercoagulable state and has been associated with various malignancies, most commonly, gynaecologic.1 Pancreatic cancer is the third leading cause of cancer-related deaths in the USA with a 5-year survival rate less than 10%.2 Whereas globally, pancreatic cancer is responsible for 331 000 deaths annually and is the seventh most common cause of cancer-related deaths.3 The morbidity and mortality from pancreatic cancer is due to hypercoagulable state resulting in increased incidence of embolic events.1 Here, we report a case of NBTE as the presentation of underlying pancreatic malignancy. What makes our case unique is that diagnosis of marantic endocarditis in the setting of pancreatic cancer is made postmortem in 1.2% of patients during autopsy.4 The diagnostic challenge of marantic endocarditis in living patients is due to the small size of these vegetations, which are often not large enough to be diagnosed on echocardiography; thus, it is not surprising that the first ever antemortem case was reported in 2008.4 5 Healthcare professionals need to be aware of the association between marantic endocarditis and pancreatic malignancy, as it may lead to diagnosis and treatment of underlying neoplastic process.
Case presentation
A 66-year-old cachectic male with history of heavy alcohol use, but no known medical history presented to the emergency department after a mechanical fall while attempting to climb into his truck. He denied presyncopal symptoms preceding the fall. Imaging showed a left inferior pubic ramus fracture which orthopaedic surgery concluded was non-operative. Although the patient was alert and oriented, he was noted to have slow responses to questions and scanning speech. MRI of the brain revealed multiple subacute to acute strokes of differing ages: posterior right periventricular white matter infarcts and right posterior cerebellar infarction (figure 1). Patient was evaluated by neurology, started on atorvastatin, aspirin and clopidogrel. Transthoracic echocardiography (TTE) was negative for cardioembolic source. His clinical course was complicated by increased oxygen requirement due to aspiration pneumonia, which resolved after a course of antibiotics. Patient was discharged home in stable condition.
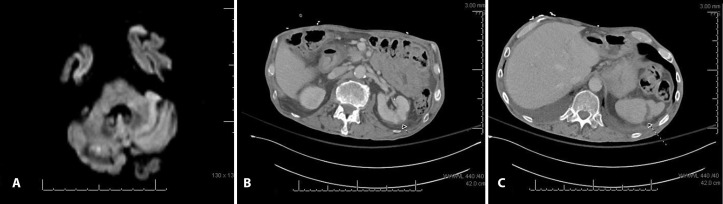
Figure 1.
(A) Brain MRI showing a focus of restricted diffusion in right cerebellum. (B) CT of abdomen demonstrating wedge-shaped area of renal cortical hypoattenuation. (C) CT of abdomen demonstrating age-indeterminate splenic infarct.
Three days later, the patient returned to the emergency department with nausea, vomiting and abdominal pain. On presentation, he was noted to have melena.
Patient was afebrile, with normal vital signs. He was cachectic, but awake, alert and oriented. Cardiac examination revealed regular rhythm with no murmur auscultated, lungs were clear to auscultation bilaterally, abdomen was non-tender with no palpable masses, and skin examination showed no jaundice or rashes. Other than anaemia, all laboratory studies including hepatic function panel were normal.
CT of abdomen and pelvis showed a duodenal ulcer with contained perforation. He had a non-surgical abdomen and was taken to interventional radiology for mesenteric angiogram which showed no source of bleeding, but the gastroduodenal artery was prophylactically embolised. Incidentally, CT imaging also revealed bilateral renal and splenic infarcts (figure 1). Due to the previously noted strokes and new multiorgan infarctions, concern for a cardiac source of emboli was again raised.
Investigations
Repeat TTE was again negative for valvular vegetation or intracardiac shunt.
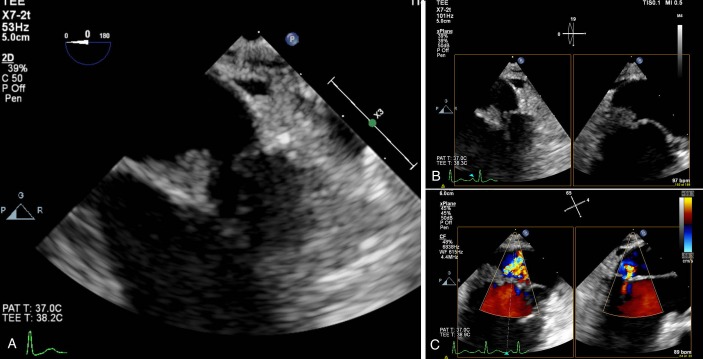
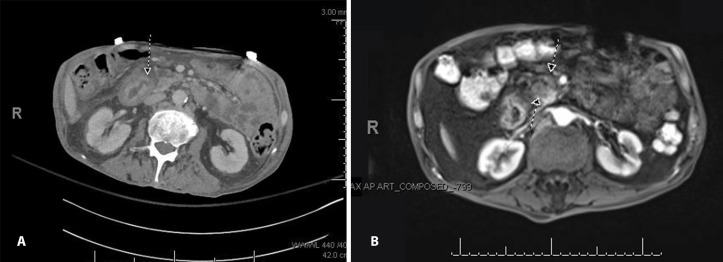
As four sets of serial blood cultures taken over the course of a month remained negative and lack of evidence to explain multiorgan embolic events, a transoesophageal echocardiography (TEE) was ordered. Follow-up (TEE) revealed a 1.1×0.7 cm vegetation on the posterior leaflet of the mitral valve (figure 2). CT abdomen on initial presentation showed a 5 mm dilation of the main pancreatic duct with no definite evidence of obstructing lesion (figure 3). Outpatient non-emergent MRI was recommended; however, abdominal MRI was ordered inpatient due to suspected malignancy in the setting of NBTE. MRI revealed a 2.8×2.2 cm soft tissue mass in the pancreatic head that resulted in pancreatic duct stricture (figure 3). Endoscopic ultrasound demonstrated an irregular 14 mm hypoechoic mass with poorly defined endosonographic borders, invading into the portal vein. The parenchyma of the pancreas appeared to have honeycombing and lobularity. The pancreatic duct was dilated to 6 mm with a mural nodule seen within pancreatic duct at the genu. Needle aspiration was performed and cytology revealed pancreatic adenocarcinoma.
Figure 2.
(A and B) Transoesophageal echocardiography showing a 1.1×0.7 cm vegetation on the posterior leaflet of the mitral valve. (C) The presence of mitral valve regurgitation.
Figure 3.
(A) Dilation of main pancreatic duct up to 5 mm shown on abdominal CT. (B) Abdominal MRI demonstrating an ill-defined soft tissue in the pancreatic neck/head measuring 2.8×2.2 cm, obstructing the pancreatic duct.
Differential diagnosis
Culture-negative infective endocarditis: patient had no evidence of infective endocarditis on clinical examination and no signs or symptoms of underlying infection.
NBTE secondary to malignancy: patient’s age, cachectic appearance and negative infectious workup made malignancy high on our differential.
Antiphospholipid syndrome: antinuclear antibody was negative, and given the patient’s age and lack of clinical symptoms, systemic lupus erythematosus and secondary antiphospholipid syndrome were less likely diagnoses. However, primary antiphospholipid syndrome can truly be ruled out by negative laboratory workup with lupus anticoagulant, anticardiolipin antibody or anti- β2 glycoprotein-I antibody at least 12 weeks apart.6
Treatment
In the setting of mitral valve NBTE complicated by cerebral, renal and splenic infarcts, the patient was started on systemic anticoagulation for prevention of recurrent thromboemboli. Treatment of his underlying malignancy is also crucial. On hospital discharge, patient followed up with surgical, medical and radiation oncology.
Outcome and follow-up
Given his poor performance status and borderline resectable tumour with the involvement of portal vein, he was deemed not a surgical candidate at this time. He elected to pursue neoadjuvant therapy with chemo and radiation and may still consider surgery in the future if his performance status improves and tumour responds.
Discussion
In 1888, Zeigler used the term thromboendocarditis to describe thrombi depositing on heart valves. Later, the term evolved into cachectic, or marantic, endocarditis; while finally in 1936, Gross and Friedberg coined ‘non-bacterial thrombotic endocarditis’.4 7 8 NBTE most commonly affects patients between the fourth and eight decade of life, without a specific gender predilection.4 NBTE results from deposition of thrombi composed of platelets and sterile fibrin on heart valves, with associated morbidity and mortality stemming from embolisation of these into the central nervous system.9 Mitral and aortic valves are the most common sites of vegetation.10 Although the pathogenesis of this process still remains unclear, underlying malignancy or high-inflammatory states result in elevated levels of cytokines (tumour necrosis factor and interleukin-1), which in turn cause local tissue damage with activation of coagulation cascade resulting in vegetation formation.9 The first ever antemortem case of marantic endocarditis as the presentation of underlying pancreatic malignancy was published in 2008 by Smeglin et al.1 5 In pancreatic cancer, these vegetations are often too small and friable, leaving remnants that are not large enough to be identified by echocardiography, making it difficult to diagnose NBTE in a living patient.4 Yet, these lesions have significant morbidity with the overall reported incidence of embolic events being 42%, most commonly affecting spleen, kidney, brain and heart, respectively.4 11
In addition to the treatment of underlying malignancy, the most important guidelines when it comes to treatment of NBTE focus on the use of anticoagulation to prevent recurrent thromboembolic events.
Unfortunately, these stem from general anticoagulation guidelines in malignancy, as there is scarcity of studies specifically in NBTE. Therefore, some suggest the use of low-molecular-weight heparin as the preferred antithrombotic agent, whereas others argue that unfractionated heparin is the therapy of choice.9 12 13 Meanwhile, the role of direct oral anticoagulants in cancer-related thrombotic events, and in NBTE in particular, needs to be addressed in further studies.13
Learning points.
Pancreatic cancer is the third leading cause of the cancer-related deaths in the USA with a 5-year survival rate less than 10%.2 Whereas globally, pancreatic cancer is responsible for 331 000 deaths annually and is the seventh most common cause of cancer-related deaths.3
Non-bacterial thrombotic endocarditis (NBTE) is a rare but known presenting feature of pancreatic cancer.
Healthcare professionals must suspect NBTE in the setting of multiorgan infarcts and lack of infectious signs or symptoms. It is prudent to obtain a transoesophageal echocardiography in the setting of negative transthoracic echocardiography.
Treatment of NBTE consists of systemic anticoagulation and treating the underlying malignancy.
Footnotes
Contributors: ES, EAR and EB contributed to clinical management. SS: attending physician managing the patient. All authors contributed to the writing and editing the manuscript and completing the submission process.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Jameson GS, Ramanathan RK, Borad MJ, et al. Marantic endocarditis associated with pancreatic cancer: a case series. Case Rep Gastroenterol 2009;3:67–71. 10.1159/000207195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rahib L, Fleshman JM, Matrisian LM, et al. Evaluation of pancreatic cancer clinical trials and benchmarks for clinically meaningful future trials: a systematic review. JAMA Oncol 2016;2:1209 10.1001/jamaoncol.2016.0585 [DOI] [PubMed] [Google Scholar]
- 3. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E386. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 4. Lopez JA, Ross RS, Fishbein MC, et al. Nonbacterial thrombotic endocarditis: a review. Am Heart J 1987;113:773–84. 10.1016/0002-8703(87)90719-8 [DOI] [PubMed] [Google Scholar]
- 5. Smeglin A, Ansari M, Skali H, et al. Marantic endocarditis and disseminated intravascular coagulation with systemic emboli in presentation of pancreatic cancer. J Clin Oncol 2008;26:1383–5. 10.1200/JCO.2007.12.9148 [DOI] [PubMed] [Google Scholar]
- 6. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. 10.1111/j.1538-7836.2006.01753.x [DOI] [PubMed] [Google Scholar]
- 7. Zeigler R. Uber den bau die entstehung endocaritischer efflorescenzen. Werh Dtsch Kong Intern Med 1888;7:399. [Google Scholar]
- 8. Gross L, Friedberg C. Nonbacterial thrombotic endocarditis. Classification and general description. Arch Intern Med 1936;936:620–40. [Google Scholar]
- 9. Piovanelli B, Rovetta R, Bonadei I, et al. Pancreatic cancer endocardite trombotica non batterica in associazione con una neoplasia pancreatica, 2013:189–92. [Google Scholar]
- 10. Deppisch LM, Olusegun Fayemi A. Non-bacterial thrombotic endocarditis. Am Heart J 1976;92:723–9. 10.1016/S0002-8703(76)80008-7 [DOI] [PubMed] [Google Scholar]
- 11. Rosen P, Armstrong D. Nonbacterial thrombotic endocarditis in patients with malignant neoplastic diseases. Am J Med 1973;54:23–9. 10.1016/0002-9343(73)90079-X [DOI] [PubMed] [Google Scholar]
- 12. Mantovani F, Navazio A, Barbieri A, et al. A first described case of cancer-associated non-bacterial thrombotic endocarditis in the era of direct oral anticoagulants. Thromb Res 2017;149:45–7. 10.1016/j.thromres.2016.11.016 [DOI] [PubMed] [Google Scholar]
- 13. Liu J, Frishman WH. Nonbacterial thrombotic endocarditis: pathogenesis, diagnosis, and management. Cardiol Rev 2016;24:244–7. 10.1097/CRD.0000000000000106 [DOI] [PubMed] [Google Scholar]