Abstract
A 45-year-old man presented 4 months after ABOi renal transplantation with febrile illness and bicytopenia necessitating cessation of mycophenolate mofetil. Dengue non-structural protein 1 antigen (NS1 Ag) test was positive. Lowest total leucocyte count was 3.1×109/L and platelet count was 14×109/L. As fever subsided, patient became tachypneic with abdominal distention and hypotension. Ultrasonographic evaluation revealed ascites, gall bladder wall oedema and bilateral pleural effusion consistent with dengue capillary leak syndrome. He developed massive ascites with abrupt weight gain of 4 kg within 24 hours and worsening renal dysfunction. Patient was deteriorating rapidly in spite of adequate supportive care and we gave a trial of intravenous immunoglobulin (0.5 g/kg/day) for 5 days. Patient improved from day 2, and by day 3, he became haemodynamically stable and recovered completely. Patient was stable at discharge and is on regular follow-up.
Keywords: immunological products and vaccines, renal system, nifectious diseasest, tropical medicine (infectious disease), renal transplantation
Background
Dengue infection is a major vector-borne viral infection transmitted by Aedes mosquito, common in tropical regions. Renal allograft recipients in these areas are as susceptible to dengue like the general population. However, these patients are in a delicate immunological balance and when complications of dengue set in, they deteriorate rapidly. Apart from supportive care, no other therapeutic option has been described to be appropriate in the literature. We herein report a renal allograft recipient with dengue shock syndrome who recovered completely with intravenous immunoglobulin (IVIG) therapy. IVIG may be a suitable therapeutic option in transplant recipients with severe dengue.
Case presentation
A 45-year-old male patient with end-stage renal disease due to diabetic nephropathy underwent renal transplantation with wife as donor in July 2015 on basiliximab induction. Being an ABOi renal allograft transplantation, he had received rituximab with five sessions of Plasma exchange (PE) and five doses of IVIG in the pretransplant period as per our institute’s protocol and one session of PE with single dose IVIG in the post-transplant period. His immunosuppressive medication consisted of tacrolimus, mycophenolate mofetil (MMF) and prednisolone. Patient was on regular follow-up with stable graft function.
He presented to our emergency with complaints of fever of 2 days duration with myalgia and fatigue. On account of ongoing dengue epidemic, dengue non-structural protein 1 antigen (NS1 Ag) test was done which was positive. Patient had bicytopenia (leucopenia and thrombocytopenia) necessitating cessation of MMF, and four units of random donor platelets were transfused. Lowest total leucocyte count (TLC) was 3.1×109/L and platelet count was 14×109/L. As fever subsided, patient became tachypneic with pain and distention of abdomen, pedal oedema and fall in blood pressure. Ultrasonographic evaluation revealed ascites with gall bladder wall oedema and bilateral pleural effusion. This was consistent with the diagnosis of dengue capillary leak syndrome. The serum creatinine level increased from 1 to 1.7 mg/dL. With adequate intravenous fluid resuscitation to restore his haemodynamic status, creatinine settled to 1.4 mg/dL. He developed massive ascites with abrupt weight gain of 4 kg within 24 hours. Patient was deteriorating rapidly and we decided to give a trial of IVIG therapy; 0.5 g/kg of IVIG was administered daily for 5 days. Patient improved from day 2, and by day 3, he became haemodynamically stable and renal function settled to a baseline creatinine of 1 mg/dL. Both white cell counts and platelet counts increased thereafter and MMF was restarted. Patient was discharged with normal blood counts and serum creatinine and is on regular follow-up.
Investigations
Trend of in-hospital renal function and haematological parameters is depicted in figure 1.
Figure 1.
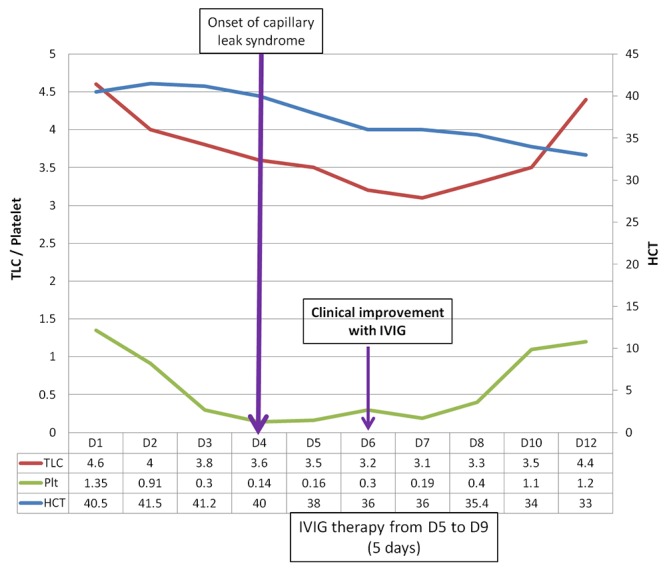
Graph depicting clinical course of the patient in relation to intravenous immunoglobulin (IVIG) therapy. The x-axis shows the days of hospitalisation. Primary y-axis (left) is for platelets (×1010/L) and total leucocyte count (×109/L) and secondary y-axis (right) for haematocrit. Patient was given IVIG after onset of capillary leak syndrome and he started improving from day 2 of IVIG therapy.
Treatment
On account of cytopenia, MMF was withheld. Hydration was ensured with intravenous fluid administration. As the patient’s clinical status was deteriorating, 0.5 g/kg of IVIG was administered daily for 5 days. Patient improved significantly with IVIG administration.
Outcome and follow-up
Patient had complete recovery from dengue capillary leak syndrome. His renal function normalised. Patient is on regular follow-up postdischarge for over 6 months with normal renal function and no complications.
Discussion
Dengue infection usually presents as an acute febrile illness with myalgia, retro-orbital pain and fatigue. Classical dengue fever passes through three phases: febrile, critical and recovery. In most cases, patients progress directly from the febrile phase to recovery. The critical phase occurs at the time of defervescence around 4–7 days after onset of illness. Severe dengue presents as either dengue haemorrhagic fever (DHF) or dengue shock syndrome (DSS).1 2 In severe dengue, plasma leak occurs abruptly over 24–48 hours characterised by drop in blood pressure, tachycardia, sudden onset pleural effusion and ascites with varying severity of bleeding manifestations. Our patient developed features of DSS 4 days after onset of fever with significant drop in blood pressure necessitating withdrawal of all three antihypertensive agents and intravenous fluid resuscitation. Features suggestive of capillary leak and third space fluid accumulation were apparent with sudden ascites, pleural effusion and weight gain.
Though intravenous fluids and blood products are of paramount importance in the management of dengue, at times they are insufficient to stop the abrupt worsening.1 Therapies tried in refractory cases include anti-D immunoglobulin, IVIG and recombinant activated factor VII.3 These are of uncertain benefit and not recommended for routine use. Dengue capillary leak syndrome and shock occurs due to massive T cell activation, with release of cytokines and complement activation followed by plasma leakage.4 Direct interaction of dengue virus with endothelial cells with consequent increase in vascular permeability has been reported.5 Anti-inflammatory action of IVIG are postulated to be due to FcR blockade, anti-idiotypic Abs in IVIG, inhibition of complement deposition, increased regulatory T cell involvement and direct and indirect modulation of cellular immune mediators.6 7 Hence, use of IVIG is justified based on pathophysiological basis.
An initial report of clinical utility of IVIG in DHF was published as early as 2003 where five cases treated with IVIG had rapid recovery.8 However, the randomised controlled trial by Dimaano et al with 30 patients (15 received IVIG) failed to show beneficial effect of IVIG in hastening the recovery of platelet counts.9 There were no adverse drug effects. It has to be noted that this trial included patients with lesser degrees of severity (classical dengue with no e/o DHF) in which the immunological process may not be that rampant to show response to IVIG. Moreover, seriously ill patients were excluded and there were no cases of DSS where IVIG may have been of benefit. An unpublished randomised controlled trial in Philippines by Frias et al mentioned in the report by Alejandria showed mortality benefit with IVIG in children with DSS.10 The place of IVIG in therapy of DSS/DHF remains uncertain till adequately powered randomised controlled clinical trials are undertaken. The high cost of the therapy and availability issues coupled with the geographical distribution of dengue infection have limited clinical studies on IVIG therapy.
With regard to dengue infection in renal allograft recipients, the risk of cytopenias is further accentuated by use of immunosuppressive drugs in this population. This is of considerable importance in tropical countries where dengue is rampant and also for travellers from temperate zones. Four case series of dengue infection in renal allograft recipients are available in the literature with the largest series consisting of 27 patients of which one patient developed DHF and died.11 In the case series from India, three out of eight patients developed DHF and expired.12 Four patients out of 10 dengue cases developed DHF in the series from Brazil but none of them expired.13 The very first series from Singapore with six patients did not have any cases of DHF.14 The reason for reduced incidence of complications in renal transplant patients is probably due to the relative immunosuppressive state seen in post-transplant patients thereby reducing the risk of immune-mediated complications of severe dengue.14 There is no mention of use of IVIG and newer therapies for dengue in any of these series.
Idiopathic systemic capillary leak syndrome (Clarkson disease) is a rare disease of unknown aetiology characterised by recurrent episodes of systemic capillary leak. Role of vascular endothelial growth factor, angiopoietin, tumour necrosis factor-alpha and other cytokines is proposed for the vascular hyperpermeability but not conclusively proven. IVIG has been shown to be useful in this condition and is effective for prophylaxis but its efficacy in treating acute episodes is still unproven.15 The same mechanism of action may be responsible for the efficacy of IVIG in DSS also.
Data regarding the impact of immunosuppressants on severity of dengue infection is sparse. In the study by Nasim et al, it was reported that tacrolimus may be associated with more severe manifestations of dengue when compared with cyclosporine, although the sample size was too small for significance.16 Our centre is located in an area endemic for dengue and caters to over 2500 transplant recipients. We suggest that antiproliferative agents be withheld when serial counts show a trend for persistent decline (with TLC count <3.5×109/L and platelet count <10×109/L). They are restarted as soon as the counts show an increasing trend.
Renal allograft recipients are a special group of patients where even a small insult tilts the balance causing fatal complications. The time available for remedial actions is limited and hence all therapeutic measures have to be tried as in our case. Our case is the first renal allograft recipient in whom IVIG has been tried in DSS and has been successful. A randomised controlled trial in allograft recipients would not be feasible for all practical reasons. Hence, IVIG may be thought of as a therapeutic option in renal allograft recipients with manifestations of severe dengue.
Learning points.
Dengue shock syndrome is an uncommon but serious complication of dengue infection.
Renal transplant recipients are susceptible equally to complications of dengue.
Careful titration of immunosuppressant doses is needed to maintain renal function in the event of dengue induced cytopenia.
Adequate hydration and supportive care needs to be ensured in dengue patients.
Intravenous immunoglobulin can be tried for dengue shock syndrome even in renal allograft recipients.
Footnotes
Contributors: All authors were involved in the clinical management of the patient and approved the final manuscript. AS, SM and SKA were involved in the conception of work; AKS and RKY were involved in data acquisition; AS, SM and RKY were involved in drafting the work; SM and SKA revised it critically.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.WHO Guidelines Approved by the Guidelines Review Committee. Dengue: guidelines for diagnosis, treatment, prevention and control. New Edition Geneva: World Health Organization, 2009. http://www.ncbi.nlm.nih.gov/books/NBK143157/ [PubMed] [Google Scholar]
- 2.Srikiatkhachorn A, Rothman AL, Gibbons RV, et al. . Dengue--how best to classify it. Clin Infect Dis 2011;53:563–7. 10.1093/cid/cir451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alejandria MM. Dengue haemorrhagic fever or dengue shock syndrome in children. BMJ Clin Evid 2009;2009. [PMC free article] [PubMed] [Google Scholar]
- 4.Green S, Rothman A. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr Opin Infect Dis 2006;19:429–36. 10.1097/01.qco.0000244047.31135.fa [DOI] [PubMed] [Google Scholar]
- 5.Halstead SB. Dengue. The Lancet 2007;370:1644–52. 10.1016/S0140-6736(07)61687-0 [DOI] [PubMed] [Google Scholar]
- 6.Sibéril S, Elluru S, Negi VS, et al. . Intravenous immunoglobulin in autoimmune and inflammatory diseases: more than mere transfer of antibodies. Transfus Apher Sci 2007;37:103–7. 10.1016/j.transci.2007.01.012 [DOI] [PubMed] [Google Scholar]
- 7.Rajapakse S. Intravenous immunoglobulins in the treatment of dengue illness. Trans R Soc Trop Med Hyg 2009;103:867–70. 10.1016/j.trstmh.2008.12.011 [DOI] [PubMed] [Google Scholar]
- 8.Ostronoff M, Ostronoff F, Florêncio R, et al. . Serious thrombocytopenia due to dengue hemorrhagic fever treated with high dosages of immunoglobulin. Clin Infect Dis 2003;36:1623–4. 10.1086/374870 [DOI] [PubMed] [Google Scholar]
- 9.Dimaano EM, Saito M, Honda S, et al. . Lack of efficacy of high-dose intravenous immunoglobulin treatment of severe thrombocytopenia in patients with secondary dengue virus infection. Am J Trop Med Hyg 2007;77:1135–8. [PubMed] [Google Scholar]
- 10.Alejandria M. Dengue fever. Clin Evid 2005;13:887–95. [PubMed] [Google Scholar]
- 11.Azevedo LS, Carvalho DB, Matuck T, et al. . Dengue in renal transplant patients: a retrospective analysis. Transplantation 2007;84:792–4. 10.1097/01.tp.0000280547.91617.25 [DOI] [PubMed] [Google Scholar]
- 12.Prasad N, Bhadauria D, Sharma RK, et al. . Dengue virus infection in renal allograft recipients: a case series during 2010 outbreak. Transpl Infect Dis 2012;14:163–8. 10.1111/j.1399-3062.2011.00699.x [DOI] [PubMed] [Google Scholar]
- 13.Costa SD, da Silva GB, Jacinto CN, et al. . Dengue fever among renal transplant recipients: a series of 10 cases in a tropical country. Am J Trop Med Hyg 2015;93:394–6. 10.4269/ajtmh.15-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renaud CJ, Manjit K, Pary S. Dengue has a benign presentation in renal transplant patients: a case series. Nephrology 2007;12:305–7. 10.1111/j.1440-1797.2007.00785.x [DOI] [PubMed] [Google Scholar]
- 15.Druey KM, Parikh SM. Idiopathic systemic capillary leak syndrome (Clarkson disease). J Allergy Clin Immunol 2017;140:663–70. 10.1016/j.jaci.2016.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nasim A, Anis S, Baqi S, et al. . Clinical presentation and outcome of dengue viral infection in live-related renal transplant recipients in Karachi, Pakistan. Transpl Infect Dis 2013;15:516–25. 10.1111/tid.12114 [DOI] [PubMed] [Google Scholar]


