Abstract
Neurotransmitters can accelerate HIV-1 replication in vitro, leading us to examine whether differences in autonomic nervous system (ANS) activity might promote residual HIV-1 replication in patients treated with highly active antiretroviral therapy. Patients who showed constitutively high levels of ANS activity before highly active antiretroviral therapy experienced poorer suppression of plasma viral load and poorer CD4+ T cell recovery over 3–11 months of therapy. ANS activity was not related to demographic or behavioral characteristics that might influence pathogenesis. However, the ANS neurotransmitter norepinephrine enhanced replication of both CCR5- and CXCR4-tropic strains of HIV-1 in vitro via chemokine receptor up-regulation and enhanced viral gene expression, suggesting that neural activity may directly promote residual viral replication.
Peripheral neurons influence the activity of several viral pathogens (e.g., herpes viruses; ref. 1), but little is known about their impact on retroviruses. Associations between stress and HIV-1 disease progression suggest that neural activity might play a role in modulating lentiviral replication (2). Neurons from the sympathetic division of the autonomic nervous system (ANS) terminate in the parenchyma of all primary and secondary lymphoid organs and release micromolar concentrations of norepinephrine into T cell-rich compartments (3, 4). Norepinephrine ligation of cellular β2 adrenoreceptors modulates leukocyte activation, localization, and cytokine production via GαS protein-mediated induction of cAMP/protein kinase A (PKA) signaling (5–7). In vitro, norepinephrine and other cAMP/PKA activators can enhance HIV-1 replication (8–11). In vivo, increases in plasma viral load have been observed after pharmacologic enhancement of cAMP activity in circulating lymphocytes (12). However, the role of neurotransmitters in regulating HIV-1 replication in vivo has not been examined.
Interactions between neural activity and HIV replication could undermine clinical efforts to minimize viral replication. Highly active antiretroviral therapy (HAART) can suppress HIV-1 plasma viral load, but infection is generally not eradicated, and residual viral replication maintains an inducible reservoir of infected cells capable of reigniting viremia upon treatment failure or termination (13–16). Residual viral replication rates vary substantially during HAART (14, 17), but little is known about the physiologic factors responsible for such differences. Identification and control of such factors is critical to the success of long-term antiretroviral therapy (18). To the extent that neural activity promotes HIV replication, nervous system activity may represent a novel physiologic target for adjunctive therapies aimed at maximally suppressing residual HIV replication.
Given evidence that neurotransmitter signaling can enhance HIV replication in vitro, we examined the role of ANS activity in immunologic and virologic response to HAART therapy in a cohort of asymptomatic HIV+ males. In an effort to identify molecular processes that might mediate any observed effects, we also examined the role of the ANS neurotransmitter norepinephrine in regulating cellular expression of viral coreceptors and HIV-1 gene expression in vitro.
Methods
Patient Sample.
HIV seropositive homosexual men were recruited from the western Los Angeles metropolitan area and screened to exclude individuals with recent seroconversion, history of AIDS-defining conditions including peripheral blood CD4+ T cell level <200/mm3, and medical conditions or drug regimens that might influence ANS activity including alcoholism, heavy recreational drug use, depression, anxiety, neuropathy or other neuropathologies, respiratory disease, glaucoma, cardiovascular disease/hypertension, and use of β blockers, antihistamines, or sympathomimetics. All participants scored within the normal range on a computerized battery of neuropsychological tests identifying HIV-induced neural impairment (19). Ages ranged from 25 to 54 years, peripheral blood CD4+ T cells levels ranged from 259 to 914/mm3 (median 449), and plasma viral load ranged from 1,646 to 422,321 copies/ml (median 46,717). Primary analyses are based on all 21 cohort members who had never taken protease inhibitors and subsequently initiated HAART, less eight who failed to adhere to treatment or ceased therapy during follow-up (to ensure that differential compliance could not account for effects). Comparable results emerged from statistical analyses including nonadherent/noncompleting individuals, and those patients did not differ from the remainder of the sample on any baseline characteristic, including ANS activity, plasma viral load, and CD4+ T cell level.
ANS Activity.
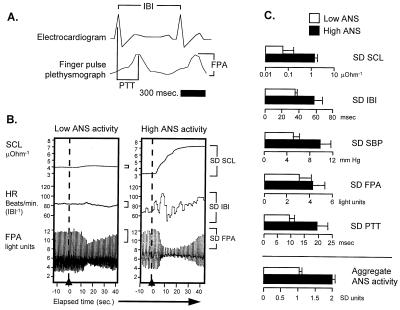
Constitutive individual differences in ANS activity were assessed on two occasions 7–13 days apart by monitoring palmar skin conductance, brachial artery systolic blood pressure (SBP), EKG interbeat interval (duration between R spikes), finger photoplethysmograph pulse peak amplitude, and peripheral pulse transit time (duration from EKG R spike to subsequent finger photoplethysmograph peak) (Fig. 1A). Each ANS indicator was monitored during the final 60 s of a 15-min resting baseline, during 90 s of metronome-paced respiration (six respiration cycles/min−1), during 12-s intervals surrounding eight auditory orienting stimuli (2-s, 80-dB, 300-Hz tone at 30-s intervals), and during the final 60 s of a 180-s verbal serial subtraction task (paced at 60 responses/min−1). ANS activity was quantified as the standard deviation of each indicator about its mean value under each assessment condition (Fig. 1B), and measures from different indicators were standardized onto a common metric by Z transformation (computed across subjects and within measurement condition and indicator). Individual ANS activity levels represent the average of individual-specific Z scores across measurement conditions and physiologic indicators. Factorial ANOVA indicated that stable differences among individuals accounted for 31% of the total variance in ANS activity (P = 0.009).
Figure 1.
(A) ANS activity levels were assessed on two occasions 7–13 days apart by monitoring palmar skin conductance (SCL), brachial artery systolic blood pressure (SBP), EKG interbeat interval (duration between R spikes; IBI), finger photoplethysmograph pulse peak amplitude (FPA), and peripheral pulse transit time (duration from EKG R spike to subsequent finger photoplethysmograph peak, PTT) during the final 60 s of a 15-min resting baseline, during 90 s of metronome-paced respiration (six respiration cycles/min−1), during 12-s intervals surrounding eight auditory orienting stimuli (2-s, 80-dB, 300-Hz tone at 30-s intervals), and during the final 60 s of a 180-s verbal serial subtraction task (paced at 60 responses/min−1). (B) Representative palmar skin conductance, interbeat interval, and finger photoplethysmograph pulse peak amplitude responses to an unexpected stimulus in individuals showing the lowest and highest overall ANS activity scores (dashed line marks inflation of blood pressure cuff on the arm contralateral to the palmar skin conductance and finger photoplethysmograph pulse peak amplitude transducers). (C) Magnitude of activity on each physiologic indicator for individuals showing aggregate ANS activity level above the sample median (filled bars) vs. below (open bars).
HIV-1 Viral Load and CD4+ T Cell Levels.
HIV-1 RNA and CD4+ T lymphocyte levels were measured over 2 weeks before initiation of HAART and again after an average 6.3 months of therapy. Plasma viral load was quantified by Amplicor assay in a clinical reference laboratory blind to patient characteristics. CD4+ T cell levels were measured by flow cytometry for CD3+/CD4+ lymphocytes. Blood specimens were obtained under resting conditions before experimental procedures to ensure that ANS activity did not artifactually alter blood parameters. To determine whether differences in post-HAART plasma viral load stemmed from mutations conferring antiretroviral resistance, viral reverse transcriptase and protease genes were sequenced (Trugene HIV-1) in all patients who maintained detectable levels of plasma HIV-1 at follow-up.
Statistical Analysis.
Constitutive ANS activity levels were normally distributed (normal scores test, r = 0.94) and thus treated as a continuous variable in primary statistical analyses. Relationships between ANS activity and HAART-induced changes in (log10-transformed) plasma viral load and CD4+ T cell level were quantified using linear regression for primary analyses and multiple linear regression for analyses controlling potential confounders. In all cases, residual distribution assumptions were verified by normal-scores analysis (r > 0.90 in all instances), and residual magnitude was verified to be independent of regressor values (all r < 0.2 magnitude). To ensure reliability, analyses were repeated using outlier-resistant statistical models (e.g., rank regression and Spearman correlation). Primary analyses quantified CD4+ T cell levels as a percentage of total lymphocytes because this variable showed the best distributional characteristics. However, comparable results emerged from analyses of (log-transformed) CD4/CD8 ratios or CD4+ T cells/mm3.
For presentational convenience, ANS activity levels were also arbitrarily classified into high and low ANS activity groups by median split. To ensure robustness of statistical results, normal-theory univariate analyses (Student's t test and ANOVA) were confirmed with nonparametric tests (Brown–Mood median test and Kruskal–Wallis rank ANOVA).
In Vitro Studies of the Effect of Norepinephrine on HIV-1 Replication.
Healthy donor peripheral blood mononuclear cells (PBMC) were infected with CXCR4-tropic (NL4–3 or SF-162) or CCR5-tropic (Ba-L or Ada-M) HIV-1 and cultured for 8 days after treatment with phytohemagglutinin (PHA)-P, IL-2, and 0, 1, or 10 μM norepinephrine (or comparable concentrations of the parasympathetic neurotransmitter acetylcholine). Multiplicities of infection ranged from 0.025 to 0.05. Viral replication was measured by ELISA of HIV-1 p24 gag protein, and differences in exponential viral growth curves were analyzed by least-squares regression.
Coreceptor Expression.
Cell-surface CXCR4 and CCR5 expression was quantified by flow cytometry in uninfected PHA-stimulated PBMC exposed to 0 or 10 μM norepinephrine for 24 h (similar effects were observed in HIV-1-infected PBMC).
HIV-1 Provirus.
Vulnerability to infection was assessed by real-time PCR quantification of proviral R/U5 long-terminal repeat structures (relative to human β-globin controls) in total cellular DNA isolated 12 h after exposure to NL4–3 or Ba-L at 0.05 multiplicity of infection (similar effects observed with SF-162 and Ada-M). HIV-1 R/U5 primers (based on HIV-1 JR-CSF long-terminal repeat) used were: 5′-CAAgTAgTgTgTgCCCgTCTgT-3′ (corresponding to nucleotides 560–581 of the R region) and 5′-CTgCTAgAgATTTTCCACACTgAC-3′ (nucleotides 612–635 of U5), internal fluorescent probe 6FAM 5′-TgTgACTCTggTAACTAgAgATCCCTCAgACCC-3′ TAMRA (long-terminal repeat nucleotides 584–616). Human β-globin primers used were: 5′-CAACCTCAAACAgACACCATgg-3′ (nucleotides 846–866) and 5′-TCCACgTTCACCTTgCCC-3′ (nucleotides 911–928), and internal probe 6FAM 5′-CTCCTgAggAgAAgTCTgCCgTTACTgCC-3′ TAMRA (nucleotides 877–903).
HIV-1 Gene Expression.
Effects of norepinephrine on HIV-1 gene expression were quantified by flow cytometric detection of a virus-encoded murine heat-stable antigen (mHSA/CD24) reporter gene in PHA-stimulated PBMC treated with 0, 1, or 10 μM norepinephrine for 24 h after infection with HIV-1NL-r-HSAS (20), which contains the mHSA gene cloned into the vpr region of the HIV-1NL4–3 strain. Infections were performed at an multiplicity of infection of 0.05, and cells were subsequently cultured in 100 nM Indinavir to prevent spread of infection. To verify that differences in immunofluorescence reflected increasing reporter gene density on infected cells (rather than increasing numbers of infected cells), anti-mHSA fluorescence intensity was quantified on mHSA+ cells (gating out mHSA− cells falling below maximum isotype control fluorescence).
Results and Discussion
Baseline Characteristics.
Before the initiation of HAART, multiple physiologic indicators of ANS activity were monitored during a series of standard assessment procedures (resting baseline, auditory attentional signals, paced respiration, and mental arithmetic) (Fig. 1). ANS activity levels differed significantly across individuals (P = 0.009 by ANOVA, averaging over indicators and measurement conditions), with high ANS activity individuals showing approximately twice the absolute level of activity on each physiologic indicator as those with low ANS activity (Fig. 1). ANS activity levels were normally distributed (normal scores test r = 0.937) and thus treated as a continuous variable in primary analyses. Individual differences in ANS activity were stable over repeated measurements (intraclass r = 0.91, P < 0.0001), as were pre-HAART measures of plasma viral load (intraclass r = 0.90, P < 0.0001) and CD4+ T cell levels (intraclass r = 0.93, P < 0.0001).
After ANS assessment, 21 participants initiated HAART regimens involving one or more HIV-1 protease inhibitors in conjunction with two or more reverse transcriptase inhibitors (Table 1). Post-HAART viral load and CD4+ T cell levels were reassessed after participants had been on uninterrupted HAART for an average of 6.3 months (range 3 to 11 months), a duration sufficient to suppress plasma viremia to undetectable levels in most patients (15–18). Eight patients discontinued therapy or were nonadherent to treatment regimens during follow-up and were thus excluded from the primary results reported below. Comparable results emerged from analyses including these eight individuals (Table 2), but we sought to ensure that variable treatment compliance could not explain observed findings. Nonadherent individuals did not differ from the remainder of the sample on any variable analyzed, including ANS activity, baseline viral load, and baseline CD4+ T cell level.
Table 1.
Sample characteristics
| Patient/age | Baseline
|
Follow-up
|
||||
|---|---|---|---|---|---|---|
| Viral load* | CD4† | Prior RTI | ANS activity‡ | Duration§ | HAART | |
| 014/54 | 276,316 | 297 | AZT | −1.61 | 3.0 | SAQ, d4T, 3TC |
| 043/37 | 4,948 | 697 | −1.27 | 10.9 | NEL, d4T, 3TC | |
| 027/29 | 16,561 | 446 | AZT, 3TC | −0.88 | 10.5 | IND, NEV, d4T |
| 060/44 | 65,567 | 259 | AZT, 3TC | −0.87 | 9.1 | RIT, SAQ, d4T, ddI |
| 015/42 | 101,553 | 396 | −0.77 | 3.0 | IND, AZT, 3TC | |
| 024/42 | 13,115 | 316 | −0.18 | 11.6 | NEL, d4T, 3TC | |
| 039/35 | 1,646 | 640 | AZT, 3TC | 0.19 | 4.0 | IND, AZT, 3TC, ddC |
| 042/44 | 40,331 | 328 | AZT, ddI | 0.31 | 4.5 | SAQ, d4T, 3TC |
| 037/34 | 422,321 | 699 | 0.75 | 4.8 | SAQ, AZT, 3TC | |
| 032/43 | 16,367 | 571 | AZT, ddI | 0.93 | 3.5 | IND, d4T, 3TC |
| 058/25 | 46,717 | 449 | 0.96 | 3.0 | SAQ, d4T, 3TC | |
| 016/30 | 312,383 | 914 | AZT | 0.99 | 3.0 | SAQ, AZT, ddC |
| 062/36 | 102,632 | 650 | 1.46 | 10.9 | RIT, INV, AZT, 3TC | |
Mean plasma HIV-1 RNA copies/mm3 during 2-week baseline. IND, indinavir; NEL, nelfinavir; RIT, ritonavir; SAQ, saquinavir; AZT, zidovudine; ddI, didanosine; 3TC, lamivudine; d4T, stavudine; ddC, zalcitabine; NEV, nevirapine; DEL, delavirdine.
Mean peripheral blood CD3+/CD4+ lymphocytes/mm3 during 2-week baseline.
ANS activity level, averaging across indicators and measurement conditions, expressed as SD units relative to mean value (e.g, −1.0 = 1 SD below mean value).
Duration of HAART at follow-up (months).
Table 2.
Results for fully adherent patients and full sample
| Fully adherent (n = 18) | All patients (n = 21) | |
|---|---|---|
| Median baseline plasma viral load (copies/mL) | ||
| High ANS | 74,675 | 74,675 |
| Low ANS | 16,561 | 14,838 |
| Difference (high − low ANS) | P = 0.156 | P = 0.103 |
| Median follow-up plasma viral load (copies/ml) | ||
| High ANS | 44,776 | 44,776 |
| Low ANS | <400 | 488 |
| Difference (high − low ANS) | P = 0.013 | P = 0.004 |
| Correlation of change in viral load with ANS activity | r = 0.701 | r = 0.629 |
| P = 0.008 | P = 0.009 | |
| Median baseline CD4+ T cells/mm3 peripheral blood | ||
| High ANS | 602 | 611 |
| Low ANS | 396 | 421 |
| Difference (high − low ANS) | P = 0.877 | P = 0.993 |
| Median follow-up CD4+ T cells/mm3 peripheral blood | ||
| High ANS | 651 | 627 |
| Low ANS | 551 | 558 |
| Difference (high − low ANS) | P = 0.015 | P = 0.035 |
| Correlation of change in viral load with ANS activity | r = 0.701 | r = 0.629 |
| P = 0.008 | P = 0.009 |
Virologic Response.
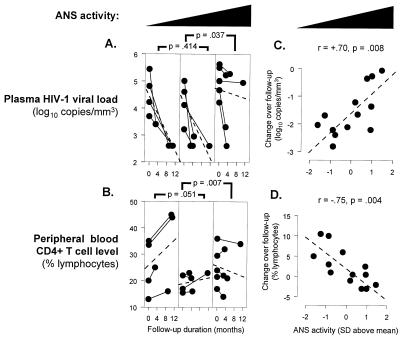
Among patients who maintained uninterrupted HAART, median plasma viral load declined from 46,717 copies/ml to <400 (P < 0.0001). However, consistent with previous observations (14, 17, 21–23), post-HAART plasma viral load varied significantly across individuals (range = <400 to 186,146 copies/ml, SD = 67,702). Posttreatment viral load was positively correlated with pretreatment levels (r = 0.77, P = 0.002), but not with pretreatment CD4+ T cell level (P = 0.981). Consistent with HAART having achieved its maximal effect on plasma viral load, posttreatment viral load was also uncorrelated with the duration of HAART at follow-up (P = 0.725). However, post-HAART steady-state plasma viral load was significantly elevated in individuals showing constitutively high (above median) levels of ANS activity before treatment (P = 0.017 by two-sample median test). Among individuals showing below-median ANS activity, plasma viral load declined from a median of 16,561 copies/ml (range 1,646 to 276,316) to <400 copies/ml after HAART. In contrast, among those showing above-median ANS activity, viral load declined from a pretreatment median of 74,675 copies/ml (range 16,367 to 422,321) to 44,776 copies/ml (<400 to 186,146) after HAART. These changes translate into a median decline in plasma viremia of more than 40-fold (−1.61 log10) for low ANS activity individuals vs. less than 10-fold (−0.86 log10) among those showing high ANS activity (P = 0.017 by two-sample median test). Similar results emerged from analyses distinguishing high, intermediate, and low ANS activity levels (Fig. 2).
Figure 2.
HAART-induced change in plasma HIV-1 viral load (A) and CD4+ T cell level (B) as a function of ANS activity levels (averaging across indicators and assessment conditions). Dashed lines represent average slope (statistical results derived from Helmert contrasts in the context of Kruskal–Wallis rank ANOVA). ANS activity was normally distributed and thus treated as a continuous linear predictor of HAART-induced changes in plasma HIV-1 viral load (C) and CD4+ T cell recovery (D).
The magnitude of decline in plasma viral load showed a strong linear relationship to ANS activity levels (r = 0.70, P = 0.008) (Fig. 2), and this relationship remained statistically significant in multiple regression controlling for pretreatment viral load and duration of HAART therapy (partial r = 0.57, P = 0.016). To ensure reliability, analyses were repeated by using outlier-resistant correlation coefficients (Spearman r = 0.66, P = 0.013) and omitting cases in which viral load dropped by less than 10-fold (r = 0.65, P = 0.046). Restriction of analyses to individuals with no prior antiretroviral therapy also produced comparable results (r = 0.89, P < 0.03). Viral genotyping showed no evidence of resistance to protease inhibitors or non-nucleoside reverse transcriptase inhibitors, and resistance to nucleoside reverse transcriptase inhibitors was distributed similarly among individuals with low vs. high levels of ANS activity (50% vs. 60%, respectively). Multiple regression analyses controlling for nucleoside reverse transcriptase inhibitor resistance continued to indicate a direct correlation between ANS activity and post-HAART viral load (partial r = 0.67, P = 0.007).
High ANS activity subjects showed higher plasma viral load before initiating HAART (74,674 vs. 16,561 copies/ml). However, these differences were small relative to within-group variability (SD = 169,142 and 98,794 copies/ml, respectively) and not statistically significant (P = 0.805 by two-sample median test). Duration of HAART therapy at follow-up did not differ significantly for individuals with high vs. low ANS activity levels (linear r = −0.218, P = 0.473; average 7.4 and 5.4 months for the low and high ANS activity groups, respectively, difference P = 0.410 by two-sample median test), nor did groups differ in the incidence of previous reverse transcriptase inhibitor therapy (50% vs. 43% for high and low ANS activity groups, respectively). Group differences in post-HAART viral load remained statistically significant in multiple regression analyses controlling for variance in baseline viral load, follow-up duration, and previous antiretroviral therapy (P = 0.028). Underscoring the magnitude of differential virologic response to HAART, 71% of low ANS activity individuals achieved posttreatment plasma viral loads <400 copies/ml vs. only 17% of those with high ANS activity.
CD4+ T Cell Recovery.
During HAART, peripheral blood CD4+ T cell levels increased from a pretreatment median of 449 cells/mm3 (22% of circulating lymphocytes) to 594 cells/mm3 (27%). Consistent with the variable virologic response, CD4+ T cell recovery varied substantially across individuals (range = −461 to +310 cells/mm3 or −2% to +30% lymphocytes, SD = 245 cells/mm3 or 7% lymphocytes). Changes in viral load were strongly predictive of the magnitude of CD4+ T cell recovery after HAART (r = −0.64, P = 0.018), and posttreatment CD4+ T cell levels were significantly correlated with pretreatment levels (r = 0.91, P < 0.001).
CD4+ T cell recovery was significantly reduced in individuals showing constitutively elevated ANS activity (r = −0.75, P = 0.004) (Fig. 2). Among those showing low levels of ANS activity, the median CD4+ T cell level increased from 396 cells/mm3 (23.2% of lymphocytes) to 551 (28.1%) after HAART (P = 0.023 by paired-sample t test). In contrast, CD4+ T cell levels failed to increase significantly after HAART in individuals showing high ANS activity (611 to 627 cells/mm3, or 23.9% to 23.2%, P = 0.452) (difference between groups, P = 0.018 by two-sample median test). In multiple regression analyses controlling for pretreatment CD4+ T cell level and the duration of HAART, ANS activity levels continued to show an inverse relationship to CD4+ T cell recovery (partial r = −0.57, P = 0.002). Comparable results emerged from analyses using outlier-resistant correlation coefficients (Spearman r = −0.80, P = 0.001), omitting cases in which viral load dropped by less than 10-fold (r = −0.78, P = 0.007) and restricting analysis to individuals with no prior antiretroviral therapy (r = −0.87, P < 0.03). As with viral load, CD4+ T cell response to HAART did not differ as a function of prior reverse transcriptase inhibitor therapy (P = 0.410 by two-sample median test), and relationships between ANS activity and CD4+ T cell recovery remained statistically significant in multiple regression analyses controlling for previous antiretroviral therapy (P = 0.005) and the presence of nucleoside reverse transcriptase inhibitor-resistant viral genotypes (P = 0.003).
Treatment, Demographic, and Behavioral Mediators.
ANS activity levels did not differ as a function of socioeconomic status (income, education), recreational drug use, alcohol consumption, age, ethnicity, anxiety, depression, or high-risk sexual activity. Moreover, statistical analyses controlling for these variables continued to indicate poorer immunologic and virologic outcomes in HAART-treated patients showing high levels of ANS activity (all P < 0.039 in multiple regressions of change in plasma viral load or CD4+ T cell level on ANS activity, baseline viral load or CD4, and indicated control variables). ANS activity levels were uncorrelated with characteristics of antiretroviral treatment, including prior therapy with reverse transcriptase inhibitors alone (22, 23), characteristics of the HAART regimen (number of nucleoside vs. non-nucleoside reverse transcriptase inhibitors, first- vs. second-generation protease inhibitors), and treatment adherence. Multiple regression analyses controlling for these treatment-related variables continued to show significantly poorer suppression of plasma viral load and CD4+ T cell recovery among individuals with high levels of ANS activity (partial regression of change in plasma viral load or CD4+ T cell level on ANS activity, P < 0.015 in each analysis). Thus, differences in demographic, behavioral, or treatment characteristics do not appear to account for the impaired response to HAART among individuals with high ANS activity.
Effect of Norepinephrine on HIV-1 Replication in Vitro.
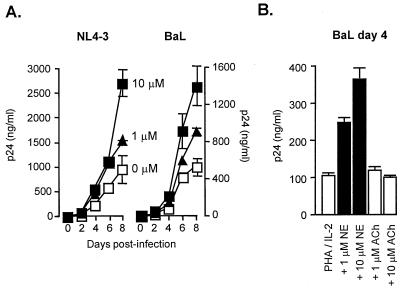
To determine whether ANS activity might directly influence HIV-1 replication, we examined the effects of the ANS sympathetic neurotransmitter norepinephrine on viral replication in in vitro-infected PBMC from randomly selected healthy donors. Confirming previous observations (9), norepinephrine significantly accelerated the replication of CXCR4-tropic strains of HIV-1 (Fig. 3). Similar results emerged from studies of CCR5-tropic strains of HIV-1 (Fig. 3), which are likely to be more representative of the viral species present in vivo during asymptomatic infection. Norepinephrine-induced enhancement of HIV-1 replication was abrogated by β-adrenergic antagonists (but not α-adrenergic antagonists) and by inhibitors of PKA activity (but not protein kinase C activity) (9) (data not shown), suggesting that norepinephrine regulates viral replication via the adrenoreceptor/GαS protein/adenylyl cyclase/cAMP/PKA signaling pathway. In contrast to the effects of the sympathetic ANS neurotransmitter, the parasympathetic neurotransmitter acetylcholine failed to significantly influence HIV-1 replication in vitro (Fig. 3B).
Figure 3.
(A) HIV-1 replication in healthy donor PBMC infected with CXCR4-tropic (NL4–3 shown, similar results with SF-162) or CCR5-tropic HIV-1 (Ba-L shown, similar results with Ada-M) and stimulated with PHA, IL-2, and 0, 1, or 10 μM norepinephrine. Viral replication was measured by ELISA of HIV-1 p24 gag protein, and differences in exponential viral growth curves were analyzed by least-squares regression (both P < 0.0001). (B) Effects of norepinephrine and the parasympathetic neurotransmitter, acetylcholine, on HIV-1 gag levels (day 4 postinfection). Norepinephrine significantly enhanced viral replication (P < 0.0001), whereas acetylcholine-treated cultures failed to differ from stimulated controls (P = 0.543). Comparable results were observed in experiments with CXCR4-tropic NL4–3 (data not shown).
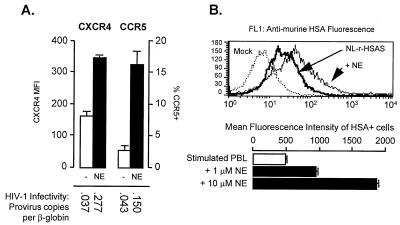
Norepinephrine up-regulated cellular expression of both CXCR4 and CCR5, enhanced cellular vulnerability to infection, and increased viral gene expression in previously infected cells (Fig. 4). These findings are consistent with previous results showing that cAMP/PKA signaling can enhance cellular vulnerability to HIV-1 infection (8) and increase transcription of genes under control of the HIV-1 long-terminal repeat (24).
Figure 4.
(A) Cell-surface CXCR4 and CCR5 expression by uninfected PHA-stimulated PBMC exposed to 0 or 10 μM norepinephrine for 24 h (similar effects were observed in HIV-1-infected PBMC). Vulnerability to infection was assessed by real-time PCR quantification of proviral R/U5 long-terminal repeat structures (relative to human β-globin controls) in total cellular DNA isolated 12 h. after exposure to NL4–3 or Ba-L at 0.05 multiplicity of infection (similar effects observed with SF-162 and Ada-M). (B) Effects of norepinephrine on HIV-1 gene expression as quantified by flow cytometric detection of a virus-encoded murine heat-stable antigen (mHSA/CD24) reporter gene in PHA-stimulated PBMC treated with 0, 1, or 10 μM norepinephrine for 24 h after infection with HIV-1NL-r-HSAS (20). To verify that differences in immunofluorescence reflected increasing reporter gene density on infected cells (rather than increasing numbers of infected cells), anti-mHSA fluorescence intensity was quantified on mHSA+ cells (gating out mHSA− cells falling below maximum isotype control fluorescence).
Conclusions
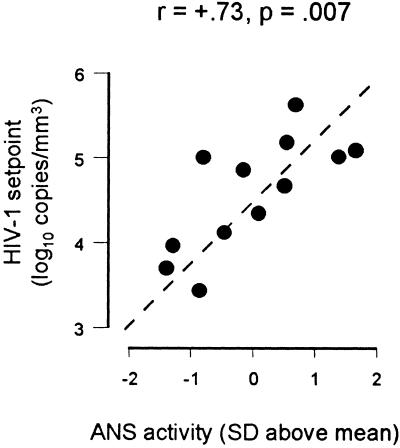
The present data document an unexpectedly strong linear relationship between constitutive individual differences in ANS activity and residual viral replication in HIV-infected patients treated with HAART. These relationships were sufficiently pronounced to reach statistical significance despite the limited power available in this sample and despite statistical control for potentially confounding demographic, treatment, and behavioral characteristics. None of the treatment variables believed to influence HAART outcomes (e.g., drug regimen, adherence, prior antiretroviral monotherapy) differed as a function of ANS activity, and there is no a priori reason to expect such a correlation in a population of normal individuals. The smooth linear relationship between ANS activity and virologic and immunologic responses to HAART (Fig. 2) suggests that differential incidence of total treatment failure is not likely to be responsible for the observed results. Such results are more consistent with a quantitative effect of ANS activity on residual viral replication, which also would explain the mild (statistically nonsignificant) elevation in baseline viral load among studied individuals with high ANS activity (r = 0.412, P = 0.161). Baseline elevations create the potential for greater HAART-induced declines among high ANS individuals, but the opposite effect was empirically observed in our analyses of posttreatment viral setpoint, change in viral load, and in both analyses controlling for baseline viral load. Elevated baseline viral load in individuals with high ANS activity is also consistent with other analyses showing a linear relationship between ANS activity levels and viral load setpoint in this cohort's antiretroviral naive patients (r = 0.73, P = 0.007; Fig. 5).
Figure 5.
Plasma HIV-1 viral load was measured on two occasions 7–14 days apart in all patients from the current cohort who had never received antiretroviral therapy. ANS activity was quantified as described in the legend for Fig. 1, and relationships between ANS activity and viral load were analyzed by Spearman rank correlation coefficient.
The consistency of these results among early-stage HIV-infected patients with normal neurological status (verified in comparison to an HIV-1 seronegative control group recruited in parallel) and varying antiretroviral treatment histories suggests that relationships between ANS activity and HIV-1 pathogenesis are of general significance and not restricted to cases of neurologic abnormality or suboptimal treatment. Peripheral autonomic neurons deliver norepinephrine directly to lymphoid and myeloid cells in the lymph node paracortex, splenic periarteriolar lymphoid sheath, and other lymphoid structures involved in HIV pathogenesis (3, 25–27). The present results show that norepinephrine can enhance cellular expression of HIV-1 coreceptors and HIV-1 viral gene expression in vitro. Further study will be required to determine whether the in vivo results observed here reflect direct effects on viral replication, indirect effects on antiretroviral pharmacokinetics, or possible effects on antiviral immune responses (norepinephrine activation of cAMP/PKA signaling can inhibit the production and lytic activity of cytotoxic T lymphocytes) (28, 29). Regardless of the mechanism involved, the present data suggest that ANS activity may significantly contribute to the biological processes supporting residual HIV replication in vivo and could thus represent a novel target for viral-suppressive therapies. Such results also suggest one physiologic mechanism by which stress can influence HIV disease progression.
Acknowledgments
This work was supported by National Institute of Allergy and Infectious Diseases Grants NIAID AI 33259, AI 36554, and AI 49135, Univ. of California, Los Angeles, AIDS Institute, Center for AIDS Research, Clinical Immunology Research Laboratories, and Univ. of California Universitywide AIDS Research Program K99-LA-030.
Abbreviations
- ANS
autonomic nervous system
- PKA
protein kinase A
- HAART
highly active antiretroviral therapy
- PBMC
peripheral blood mononuclear cells
- PHA
phytohemagglutinin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Steiner I, Kennedy G E. Mol Neurobiol. 1993;7:137–159. doi: 10.1007/BF02935640. [DOI] [PubMed] [Google Scholar]
- 2.Cole S W, Kemeny M E. Crit Rev Neurobiol. 1997;11:289–321. doi: 10.1615/critrevneurobiol.v11.i4.30. [DOI] [PubMed] [Google Scholar]
- 3.Felten D L, Felten S Y, Bellinger D L, Carlson S L, Ackerman K D, Madden K S, Olschowka J A, Livnat S. Immunol Rev. 1987;100:225. doi: 10.1111/j.1600-065x.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 4.Downing J E, Miyan J A. Immunol Today. 2000;21:281–289. doi: 10.1016/s0167-5699(00)01635-2. [DOI] [PubMed] [Google Scholar]
- 5.Landmann R. Eur J Clin Invest. 1992;22:30–36. [PubMed] [Google Scholar]
- 6.Ottaway C A, Husband A J. Immunol Today. 1994;15:511. doi: 10.1016/0167-5699(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 7.Kammer G M. Immunol Today. 1988;9:222–229. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- 8.Cole S W, Jamieson B D, Zack J A. J Immunol. 1999;162:1392–1400. [PubMed] [Google Scholar]
- 9.Cole S W, Korin Y D, Fahey J L, Zack J A. J Immunol. 1998;161:610–616. [PubMed] [Google Scholar]
- 10.Nokta M A, Pollard R B. AIDS Res Hum Retroviruses. 1992;8:1255–1261. doi: 10.1089/aid.1992.8.1255. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhury M I, Koyanagi Y, Horiuchi S, Hazeki O, Ui M, Kitano K, Golde D W, Takada K, Yamamoto N. Virology. 1993;194:345–349. doi: 10.1006/viro.1993.1265. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann B, Afzelius P, Iverson J, Kronborg G, Aabech P, Benfield T, Dybkjaer E, Nielsen J O. AIDS. 1996;10:1339–1347. doi: 10.1097/00002030-199610000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Chun T W, Davey R T J, Ostrowski M, Shawn Justement J, Engel D, Mullins J I, Fauci A S. Nat Med. 2000;6:757–761. doi: 10.1038/77481. [DOI] [PubMed] [Google Scholar]
- 14.Sharkey M E, Teo I, Greenough T, Sharova N, Luzuriaga K, Sullivan J L, Bucy R P, Kostrikis L G, Hasse A, Veryard C, et al. Nat Med. 2000;6:76–81. doi: 10.1038/71569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davey R T, Bhat N, Yoder C, Chun T W, Metcalf J A, Dewar R, Natarajan V, Lempicki R A, Adelsberger J W, Miller K D, et al. Proc Natl Acad Sci USA. 1999;96:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, et al. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 17.Ramratnam B, Mittler J E, Zhang L, Boden D, Hurley A, Fang F, Macken C A, Perelson A S, Markowitz M, Ho D D. Nat Med. 2000;6:82–85. doi: 10.1038/71577. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Rammatnam B, Tenner-Racz K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson A S, Korber B T, et al. N Engl J Med. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 19.Miller E N, Satz P, Visscher B. Neurology. 1991;41:1608–1616. doi: 10.1212/wnl.41.10.1608. [DOI] [PubMed] [Google Scholar]
- 20.Jamieson B D, Zack J A. J Virol. 1998;72:6520–6526. doi: 10.1128/jvi.72.8.6520-6526.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewin S R, Vesanen M, Kostrikis L, Hurley A, Duran M, Zhang L, Ho D D, Markowitz M. J Virol. 1999;73:6099–6103. doi: 10.1128/jvi.73.7.6099-6103.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gulick R M, Mellors J W, Havlir D, Eron J J, Gonzalez C, McMahon D, Richman D D, Valentine F T, Jonas L, Meibohm A, et al. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 23.Ledergerber B, Egger M, Opravil M, Telenti A, Hirschel B, Battegay M, Vernazza P, Sudre P, Fleep M, Furrer H, et al. Lancet. 1999;353:863–868. doi: 10.1016/s0140-6736(99)01122-8. [DOI] [PubMed] [Google Scholar]
- 24.Rabbi M F, Saifuddin M, Gu D S, Kagnoff M F, Roebuck K A. Virology. 1998;245:257–269. doi: 10.1006/viro.1998.9158. [DOI] [PubMed] [Google Scholar]
- 25.Embertson J, Zupancic M, Ribas J L, Burke A, Racz P, Tenner-Racz K, Hasse A T. Nature (London) 1993;362:359. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 26.Pantaleo G, Graziosi C, Demarest J F, Butini L, Montroni M, Fox C H, Orenstein J M, Kotler D P, Fauci A S. Nature (London) 1993;362:355. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 27.Hasse A T, Henry K, Zupancic M, Sedgewick G, Faust R A, Melroe H, Cavert W, Gebhard K, Staskus K, Zhang Z, et al. Science. 1996;274:985. doi: 10.1126/science.274.5289.985. [DOI] [PubMed] [Google Scholar]
- 28.Valitutti S, Dessing M, Lanzavecchia A. Eur J Immunol. 1993;23:790–795. doi: 10.1002/eji.1830230403. [DOI] [PubMed] [Google Scholar]
- 29.Kalinichenko V V, Mokyr M B, Graf L H J, Cohen R L, Chambers D A. J Immunol. 1999;163:2492–2499. [PubMed] [Google Scholar]