Abstract
Aminoglycosides (AmAn) are widely used for their great efficiency against gram-negative bacterial infections. However, they can also induce ototoxic hearing loss, which has affected millions of people around the world. As previously reported, individuals bearing mitochondrial DNA mutations in the 12S rRNA gene, such as m.1555A>G and m.1494C>T, are more prone to AmAn-induced ototoxicity. These mutations cause human mitochondrial ribosomes to more closely resemble bacterial ribosomes and enable a stronger aminoglycoside interaction. Consequently, exposure to AmAn can induce or worsen hearing loss in these individuals. Furthermore, a wide range of severity and penetrance of hearing loss was observed among families carrying these mutations. Studies have revealed that these mitochondria mutations are the primary molecular mechanism of genetic susceptibility to AmAn ototoxicity, though nuclear modifier genes and mitochondrial haplotypes are known to modulate the phenotypic manifestation.
Keywords: Aminoglycosides ototoxicity, Genetic susceptibility, Mitochondrial DNA mutations
1. Introduction
Sensorineural hearing loss is a common human disorder affecting approximately 275 million people around the world, which occurs because of damaged or deficient cochlear hair cell function (Mathers et al., 2007). The human cochlea contains only about 5000 hair cells (HCs) and do not have the capability to self-repair. Genetic defects may cause the hair cells to be abnormal at birth. Additionally, there are many environmental factors, including noise, aging, and ototoxic drugs, which can cause damage to the HCs. Aminoglycoside (AmAn) antibiotics are common clinical drugs characterized by amino sugars with glycosidic linkages. These compounds are widely used throughout the world to treat gram-negative bacterial infections, which are not responsive to conventional antibiotics, such as penicillin. However, AmAn is difficult to metabolize and can be concentrated in the perilymph and endolymph of the inner ear (Henley and Schacht, 1988), which results in irreversible auditory and vestibular ototoxicity (Fischel-Ghodsian, 2005, Guan, 2005, Al-Malky et al., 2011, Alharazneh et al., 2011, Huth et al., 2011, Li and Steyger, 2011). The abuse of these drugs such as gentamicin, kanamycin, particularly in developing countries has caused millions of people to suffer from ototoxic side effects. The incidence of ototoxicity has been suggested to range from 2% to 45% for adults and 0–2% for infants (Matz, 1993, Fausti et al., 1999). It has been found that there is unusual susceptibility to AmAn ototoxicity related to genetic background in many individuals. In this mini-review, we briefly introduce the molecular mechanism of genetic susceptibility to AmAn ototoxicity hearing loss.
1.1. Aminoglycosides destroy bacteria without harming mammalian cells by irreversibly binding the 30S subunit of the bacterial ribosome
The ribosome is a complex molecular machine found within all living cells, which serves as the site of biological protein synthesis (translation). Ribosomal proteins and rRNAs are arranged into two distinct ribosomal pieces of different sizes, known generally as the large and small subunit of the ribosome. The ribosomal subunits of prokaryotes and eukaryotes are very similar. Prokaryotes have 70S ribosomes, each consisting of a small (30S) and a large (50S) subunit, while eukaryotes have 80S ribosomes consisting of a 40S and a 60S subunit (Palade, 1955, Czernilofsky et al., 1975, Wimberly et al., 2000). The structural differences between the bacterial 70S ribosomes and eukaryotic 80S ribosomes have been exploited to create antibiotics to combat bacterial infections without harming cells of the infected person (Gutell et al., 1994). Aminoglycosides take effect by directly binding to the base pairs C1409–G1491 at the A-site of bacterial 16S rRNA (Fig. 1A), which acts as an essential part of the decoding site, and this interaction results in protein mistranslation or premature termination of protein synthesis (Davies and Davis, 1968, Noller, 1991).
Fig. 1.
The sites of m.1555A>G and m.1494C>T mutations in the decoding region of human mitochondrial 12S rRNA. The A-site is shown at the secondary structure of E. coli 16S rRNA in A. The corresponding position of human mitochondrial 12S rRNA is shown as the wild-type version and in the version carrying the m.1555A>G and m.1494C>T mutations in B, C and D.
Ribosomes are also found in chloroplasts and mitochondria of eukaryotes, and are similar to bacterial ribosomes. According to the endosymbiotic theory, these organelles are believed to be descendants of bacteria that became incorporated into eukaryotic cells for the implementation of oxidative mechanisms (Vellai et al., 1998, Selmer et al., 2006). Mitochondria are thought to originate from α-purple bacteria. During the course of their evolution, the endosymbiont transferred many of its essential genes to the nuclear chromosomes of eukaryotes and developed into the present-day mitochondria in the eukaryotic cell. Nevertheless, mitochondria still carry hallmarks of their bacterial ancestors. For instance, mitochondria use fMet-tRNA as an initiator of protein synthesis. Mammalian mitochondrial ribosomes have small 28S and large 39S subunits, forming a 55S protein complex, which is active in mitochondria and functions to translate mitochondrial mRNAs encoded in mtDNA (Collatz et al., 1976). Even though mitochondria ribosomes are similar to bacterial ribosomes, normal cells show low toxicity to regular dosages of AmAn. This is because mitochondria are surrounded by a double membrane which does not easily admit these antibiotics into the organelle. More importantly, mammalian mitochondrial ribosomes possess an A-to-G substitution in the aminoglycoside binding site in 16S rRNA, leading to the significantly reduced toxicity of AmAn in eukaryotic cells (Hutchin et al., 1993, Gutell et al., 1994, Guan, 2005, Ruiz-Pesini and Wallace, 2006, Avent et al., 2011, Du et al., 2014, Huth et al., 2015).
1.2. Mitochondrial DNA mutations m.1555A>G and m.1494C>T are associated with aminoglycoside ototoxicity
Mitochondria have their own genome with a modified genetic code. The mammalian mitochondrial genome is transmitted exclusively through the female germ line. Indeed, pedigree analysis has shown that the aminoglycoside susceptibility exhibits a maternally inherited transmission. The most well studied mutations are m.1555A>G and m.1494C>T in the 12S rRNA gene of the 39S subunit (Moazed and Noller, 1987, Purohit and Stern, 1994, Fourmy et al., 1998). Prezant et al. (1993) first suggested the molecular basis of ototoxic hearing loss by analyzing the mitochondrial genome of three Chinese pedigrees and a large Arab-Israeli pedigree with maternally inherited non-syndromic hearing loss. The m.1555A>G in the 12S rRNA gene was identified in these matrilineal subjects, but not found in 278 control subjects (Prezant et al., 1993). As shown in Fig. 1, the A nucleotide at the 1555 position in the 12S rRNA gene in human mitochondria is equivalent to position 1491 in the 16S rRNA gene in wild-type Escherichia coli (Bottger, 2010). When the 1555 A is mutated to G, the secondary structure of 12S rRNA more closely resembles the corresponding region of 16S rRNA in E. coli (Fig. 1C). Thus, it was speculated that this newly generated G-C pair creates a binding site for aminoglycoside. Indeed, Guan et al. reported that the m.1555A>G mutation alters the binding properties of aminoglycosides at the A-site of rRNA and leads to conformational changes in 12S rRNA. Families with inherited patterns of deafness carrying the m.1555A>G mutation have subsequently been reported worldwide, including in Asian, Caucasian, and African populations, as well as in many sporadic individuals (Matthijs et al., 1996, Pandya et al., 1997, Estivill et al., 1998, del Castillo et al., 2003, Li et al., 2004, Jacobs et al., 2005, Young et al., 2005, Leveque et al., 2007, Tang et al., 2007, Ballana et al., 2008, Chen et al., 2008, Wang et al., 2008, Lu et al., 2010a, Hakli et al., 2015, Jiang et al., 2015). Zhao et al. identified a homoplastic mutation at position 1494 (m.1494C>T) in the mitochondria genome in a large Chinese pedigree with ototoxic deafness (Zhao et al., 2004a). It is reasonable to conclude that this alteration also contributes to the hypersensitivity to AmAn, since m.1494 is the corresponding position of m.1555 at the highly conserved A-site of 12S rRNA gene (Fig. 1D). In addition, the researchers found both the severity of deafness and the age of onset vary among subjects in the absence of AmAn exposure, and that the administration of AmAn can induce or aggravate hearing loss in matrilineal relatives. This finding was subsequently validated by additional studies in both Chinese (Wang et al., 2006, Chen et al., 2007, Han et al., 2007, Yuan et al., 2007, Zhu et al., 2009) and Spanish pedigrees (Rodriguez-Ballesteros et al., 2006).
Although the m.1555A>G and m.1494C>T mutations are located at corresponding positions and both mutations were reported in hearing loss subjects among various ethnic backgrounds, the m.1555A>G mutation is likely to be more frequent than m.1494C>T. Moreover, there is a distinctly ethnic and geographical pattern of the m.1555A>G mutation in hearing loss. Considering only drug-induced deafness, the incidence of the m.1555A>G mutation was approximately 33% in two Japanese ethnic groups (Usami, 2000, Noguchi et al., 2004), 13%, 12.29%, 10.4%, and 5% in four Chinese ethnic groups (Hutchin et al., 1993, Li et al., 2005, Lu et al., 2010a, Li et al., 2014), and 17% in two white ethnic groups from the United States and Spain (Fischel-Ghodsian et al., 1993, Estivill et al., 1998). However, the frequency of the m.1494C>T mutation is much lower. In 1642 Chinese children subjects who suffered from hearing loss, the frequency of the m.1555A>G mutation was 3.96% while the frequency of the m.1494C>T mutation was only 0.18% (Lu et al., 2010a). Additionally, in a study of sporadic Spanish hearing-impaired subjects, only three cases were found to have the m.1494C>T mutation among 1340 people (Rodriguez-Ballesteros et al., 2006).
1.3. Other mitochondrial DNA mutations related to aminoglycoside ototoxicity
Based on sequence analysis of the complete mitochondrial genome, several additional mitochondrial DNA mutations were found to be associated with ototoxic hearing loss. Zhao et al. reported that the m.1095T>C mutation correlated with ototoxicity and non-syndromic hearing loss in three Chinese families with ototoxic hearing loss (Zhao et al., 2004b). This T-to-C mutation, located in the 12S rRNA gene which disrupts the conserved stem loop of helix 25 (Neefs et al., 1991), was found in affected individuals, but not in 364 Chinese control individuals. The alternation may affect the initiation of mitochondrial protein synthesis and, consequently, result in mitochondrial dysfunction (Thyagarajan et al., 2000). Moreover, insertion or deletion at the 961 position was reported to be associated with ototoxicity deafness in several genetically unrelated families (Bacino et al., 1995, Casano et al., 1999, Tang et al., 2002, Konings et al., 2008). This position is located at the C-cluster between loops 21 and 22 of 12S rRNA (Neefs et al., 1991). However, this region is not evolutionarily conserved and its functions remain unclear, especially its interaction with aminoglycoside in bacterial homologs. Furthermore, after screening 12S rRNA gene sequences collected from Chinese children diagnosed with hearing loss, mitochondrial DNA mutations including m.745A>G, m.792C>T, m.801A>G, m.839A>G, m.856A>G, m.1027A>G, m.1192C>T, m.1192C>A, m.1310C>T, m.1331A>G, m.A374A>G, m.1452T>C and m.1537C>T were suggested to be related to AmAn ototoxicity or non-syndromic hearing loss (Li et al., 2004, Leveque et al., 2007, Konings et al., 2008, Lu et al., 2010a). All of these mutations are in highly conserved nucleotides in the 12S rRNA and were not found in the 449 control samples. However, the frequencies of these variants were very low. For instance, the incidence of the mutation 1095C>T was 0.61% and an insertion or deletion at the 961 position was 1.8% in a large Chinese pediatric population (Lu et al., 2010a). The frequencies of the other mutations were even lower (Leveque et al., 2007, Konings et al., 2008, Lu et al., 2010a, Wu et al., 2015).
1.4. Potential pathophysiological mechanisms of aminoglycoside ototoxicity
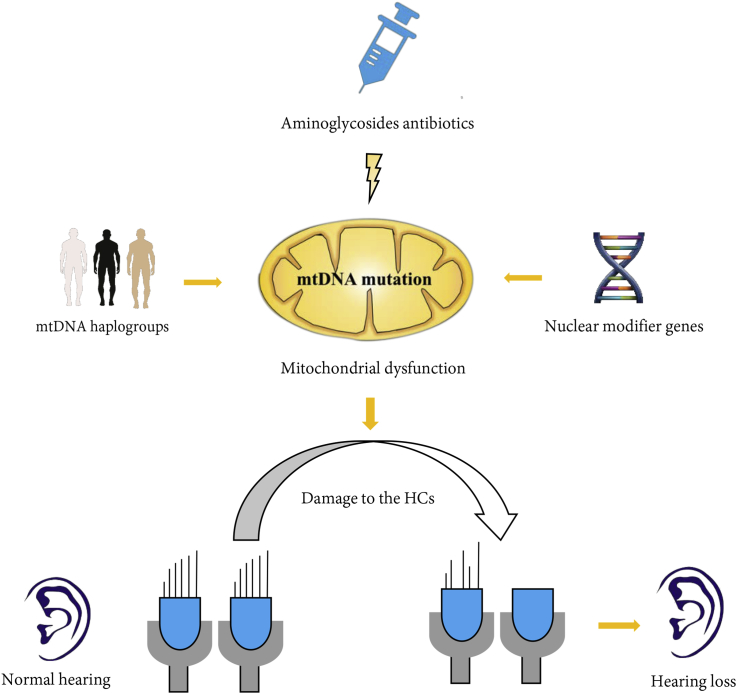
A lot of research has been performed to substantiate the theory of maternally transmitted ototoxic hearing loss (Fig. 2). The mutations m.1555A>G and m.1494C>T cause a local conformational change at the A-site of 12S rRNA (Qian and Guan, 2009), which impacts the accuracy and efficiency of codon interaction and subsequently disrupts the synthesis of mitochondrial protein (Hobbie et al., 2008). As reported, the mutations m.1555A>G and m.1494C>T induce the mistranslation of mitochondrial proteins in patient-derived lymphocyte cell lines, reducing the rate of mitochondrial protein labeling 30–40% (Guan et al., 1996, Guan et al., 2001). In addition, these cells exhibited a decline in ATP generation and an increase in production of reactive oxygen species (ROS) (Wrzesniok et al., 2013).
Fig. 2.
Schematic representation of pathways leading to hearing loss associated with mitochondrial 12S rRNA mutations. AmAn is the primary factor inducing the loss of HCs, mtDNA haplogroups and the nuclear modifier genes play important roles in the phenotype manifestation of hearing loss.
The mild biochemical defects caused by the m.1555A>G and m.1494C>T mutations are the primary factors in the development of hearing impairment, but are not themselves sufficient to produce a clinical phenotype. An RNA-directed chemical modification experiment showed that the m.1555A>G mutation increased the binding of six aminoglycosides to the 12S rRNA and changed the patterns of chemical modifications by dimethyl sulfate (DMS) in the presence of aminoglycosides. Moreover, the accumulation of AmAn in the cochlea and vestibular system worsens defects in mitochondrial protein synthesis. As previously reported, aminoglycosides selectively concentrate in the cochlea and vestibular system and are taken up into lysosomes and mitochondria in HCs (Qian and Guan, 2009). AmAn may impair mitochondrial translation in cochlear cells in susceptible subjects carrying the m.1555A>G or m.1494C>T mutations. Guan et al. found that the growth of cytoplasmic hybrid (cybrid) cells carrying the m.1555A>G or m.1494C>T mutation were significantly inhibited by paromomycin. Aminoglycosides caused an additional decrease (∼30%) in the rate of protein synthesis in cells carrying m.1555A>G or m.1494C>T mutations, leading to the overall mitochondrial translation rate being reduced below the minimal level that required for normal cell function (Guan et al., 1996, Guan et al., 2000, Zhao et al., 2004a). The decrease in protein synthesis subsequently induces the apoptosis of HCs. As reported by Ding et al., the damage begins at the base of the cochlea and progresses toward the apex after systemic administration of aminoglycoside antibiotics, meaning that outer hair cell loss precedes inner hair cell loss. Moreover, aminoglycosides can also induce acute physiological effects on the auditory nerve, thus inducing the deafness phenotype (Ding et al., 1991, Ding et al., 1995, Ding et al., 1997, Ding et al., 2010, Ding and Salvi, 2005, Ding and Salvi, 2007, Zhao et al., 2005).
However, in the absence of aminoglycosides, subjects bearing the m.1555A>G mutation can exhibit considerable phenotypic variation (Prezant et al., 1993, Matthijs et al., 1996, Estivill et al., 1998, Li et al., 2004, Young et al., 2005, Tang et al., 2007, Chen et al., 2008, Al-Malky et al., 2014). Some Chinese pedigrees carrying the m.1555A>G mutation exhibit very low penetrance of hearing loss (Young et al., 2005, Dai et al., 2006, Tang et al., 2007, Chen et al., 2008), while a large Arab-Israeli pedigree carrying the m.1555A>G mutation showed high penetrance of hearing loss (Bykhovskaya et al., 1998). After analyzing the haplogroups of 69 Chinese pedigrees carrying the m.1555A>G mutation, Lu et al. found that all of these Chinese pedigrees belonged to ten different haplogroups, including A, B, C, D, F, G, M, N, R, and Y (Lu et al., 2010b). Other studies reported that some Spanish pedigrees carrying the m.1555A>G mutation belonged to various European haplogroups such as H, I, J, K, T, U, V, and L (Torroni et al., 1999, del Castillo et al., 2003). The main haplogroups among the Chinese and Spanish pedigrees carrying the m.1555A>G mutation were found to be D and H, while the haplogroups A, C, G, R and Y were found to be rare in these families. Among the 69 Chinese pedigrees, 11 of them were haplogroup B, which displayed higher penetrance than other families. These results indicate that mitochondrial haplogroups play important roles in the phenotypic manifestation of the m.1555A>G and m.1494C>T mutations.
Further, Qian et al. showed that the rate of mitochondrial protein synthesis was reduced by approximately 28% and 50% on average in lymphocyte cell lines isolated from nine asymptomatic and ten symptomatic subjects, respectively, from an Arab-Israeli family carrying the m.1555A>G mutation (Guan et al., 1996, Guan et al., 2001). Notably, no significant difference in the average protein reduction was found in cybrid cell lines between the two groups under a constant nuclear background (35% vs 37% reduced). These data indicate that nuclear-encoded modifier genes also affect the biochemical phenotype of cells carrying the mutations. Extensive genome-wide link-age studies in Arab-Israeli and Spanish-Italian families has shown that mutations in nuclear-encoded modifier genes such as MTO1, GTPBP3 and TRMU may induce mitochondrial tRNA metabolic disorders in subjects carrying the m.1555A>G or m.1494C>T mutations (Li and Guan, 2002, Li et al., 2002, Bykhovskaya et al., 2004, Yan et al., 2005, Chen et al., 2011, Chen et al., 2015).
According to the accumulated genetic and biochemical evidence, we propose a mechanism for maternally transmitted aminoglycoside ototoxicity (Fig. 2). We hypothesize that mutations in mitochondrial 12S rRNA, especially the mutations m.1555A>G or m.1494C>T, induce defects in mitochondrial protein synthesis. Aminoglycosides are selectively concentrated in the cochlea and vestibular system cell mitochondria. These drugs worsen defects the mitochondrial protein synthesis caused by the 12S rRNA m.1555A>G or m.1494C>T mutations. The translational defects result in the apoptosis of HCs in the cochlea and vestibular system, which consequently induces the deafness phenotype. Additionally, mutations in nuclear modifier genes and mitochondrial haplogroups can aggravate the phenotype of hearing impairment.
1.5. Prevention of aminoglycoside ototoxicity
Mutations in the 12S rRNA gene are the molecular mechanism of aminoglycoside ototoxicity-induced hearing loss. Approximately 17% of ototoxicity subjects were found to have mutations in the 12S rRNA gene. In order to mitigate the negative effect of aminoglycosides, we can predict the ototoxicity risk of individuals by assessing their pedigree or by screening their 12S rRNA gene prior to the administration of AmAn. Medical professionals should ask the patients whether they have a family history of AmAn-induced deafness and screen for mutations in the 12S rRNA gene of the patients or their family members. Carriers of mutations in this gene should avoid exposure to AmAn and use alternative medications for antibiotic treatment to avoid the possibility of inducing ototoxic hearing loss. This will reduce the risk of ototoxicity and improve aminoglycoside antibiotic therapy.
However, aminoglycosides cannot be completely replaced by equally effective or less ototoxic drugs at present and effective alternatives are not always available. Thus, ototoxicity is still a risk to people. Efforts have been undertaken to protect the inner ear from ototoxicity. As ROS play a critical role in mediating aminoglycoside ototoxicity, administration of antioxidants is considered to be the most practical method. A large number of such compounds, some of which are classified as food supplements, can be safely administered in order to relieve stressed cells. Another approach to reduce drug-induced ototoxicity is to inhibit the transporter that selectively uptakes the ototoxic drug into the inner ear. However, it will be crucial to identify at risk patients through careful monitoring in clinical environments. The most promising therapy may be precision medicine, which would diagnose and/or cure the patients selectively at the molecular level.
Further, studies of nuclear-encoded modifying genes have provided insights into the mechanisms of genetic interaction and greatly enriched the understanding of mitochondria related disorders. We believe that the rapidly advancing field of understanding how modifying genes affect phenotypes promises many more advances to come, which can provide further understanding of mitochondrial mutation-related aminoglycoside ototoxicity, as well as identify potential therapeutic targets.
Conflict of interest
The authors declare no competing financial interests.
Acknowledgements
The authors were supported by the project from National Basic Research Priorities Program of China (2014CB541702), and National Natural Science Foundation of China (31671305).
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Al-Malky G., Suri R., Dawson S.J., Sirimanna T., Kemp D. Aminoglycoside antibiotics cochleotoxicity in paediatric cystic fibrosis (CF) patients: a study using extended high-frequency audiometry and distortion product otoacoustic emissions. Int. J. Audiol. 2011;50(2):112–122. doi: 10.3109/14992027.2010.524253. [DOI] [PubMed] [Google Scholar]
- Al-Malky G., Suri R., Sirimanna T., Dawson S.J. Normal hearing in a child with the m.1555A > G mutation despite repeated exposure to aminoglycosides. Has the penetrance of this pharmacogenetic interaction been overestimated? Int. J. Pediatr. Otorhinolaryngol. 2014;78(6):969–973. doi: 10.1016/j.ijporl.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Alharazneh A., Luk L., Huth M., Monfared A., Steyger P.S., Cheng A.G., Ricci A.J. Functional hair cell mechanotransducer channels are required for aminoglycoside ototoxicity. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0022347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avent M.L., Rogers B.A., Cheng A.C., Paterson D.L. Current use of aminoglycosides: indications, pharmacokinetics and monitoring for toxicity. Intern. Med. J. 2011;41(6):441–449. doi: 10.1111/j.1445-5994.2011.02452.x. [DOI] [PubMed] [Google Scholar]
- Bacino C., Prezant T.R., Bu X., Fournier P., Fischel-Ghodsian N. Susceptibility mutations in the mitochondrial small ribosomal RNA gene in aminoglycoside induced deafness. Pharmacogenetics. 1995;5(3):165–172. doi: 10.1097/00008571-199506000-00005. [DOI] [PubMed] [Google Scholar]
- Ballana E., Govea N., de Cid R., Garcia C., Arribas C., Rosell J., Estivill X. Detection of unrecognized low-level mtDNA heteroplasmy may explain the variable phenotypic expressivity of apparently homoplasmic mtDNA mutations. Hum. Mutat. 2008;29(2):248–257. doi: 10.1002/humu.20639. [DOI] [PubMed] [Google Scholar]
- Bottger E.C. Mutant A1555G mitochondrial 12S rRNA and aminoglycoside susceptibility. Antimicrob. Agents Chemother. 2010;54(7):3073–3074. doi: 10.1128/AAC.01819-09. author reply 3074–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykhovskaya Y., Mengesha E., Wang D., Yang H., Estivill X., Shohat M., Fischel-Ghodsian N. Phenotype of non-syndromic deafness associated with the mitochondrial A1555G mutation is modulated by mitochondrial RNA modifying enzymes MTO1 and GTPBP3. Mol. Genet. Metab. 2004;83(3):199–206. doi: 10.1016/j.ymgme.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Bykhovskaya Y., Shohat M., Ehrenman K., Johnson D., Hamon M., Cantor R.M., Aouizerat B., Bu X., Rotter J.I., Jaber L., Fischel-Ghodsian N. Evidence for complex nuclear inheritance in a pedigree with nonsyndromic deafness due to a homoplasmic mitochondrial mutation. Am. J. Med. Genet. 1998;77(5):421–426. doi: 10.1002/(sici)1096-8628(19980605)77:5<421::aid-ajmg13>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Casano R.A., Johnson D.F., Bykhovskaya Y., Torricelli F., Bigozzi M., Fischel-Ghodsian N. Inherited susceptibility to aminoglycoside ototoxicity: genetic heterogeneity and clinical implications. Am. J. Otolaryngol. 1999;20(3):151–156. doi: 10.1016/s0196-0709(99)90062-5. [DOI] [PubMed] [Google Scholar]
- Chen B., Sun D., Yang L., Zhang C., Yang A., Zhu Y., Zhao J., Chen Y., Guan M., Wang X., Li R., Tang X., Wang J., Tao Z., Lu J., Guan M.X. Mitochondrial ND5 T12338C, tRNA(Cys) T5802C, and tRNA(Thr) G15927A variants may have a modifying role in the phenotypic manifestation of deafness-associated 12S rRNA A1555G mutation in three Han Chinese pedigrees. Am. J. Med. Genet. A. 2008;146A(10):1248–1258. doi: 10.1002/ajmg.a.32285. [DOI] [PubMed] [Google Scholar]
- Chen C., Chen Y., Guan M.X. A peep into mitochondrial disorder: multifaceted from mitochondrial DNA mutations to nuclear gene modulation. Protein Cell. 2015;6(12):862–870. doi: 10.1007/s13238-015-0175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., He F., Fu S., Dong J. GJB2 and mitochondrial DNA 1555A>G mutations in students with hearing loss in the Hubei Province of China. Int. J. Pediatr. Otorhinolaryngol. 2011;75(9):1156–1159. doi: 10.1016/j.ijporl.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Chen J., Yang L., Yang A., Zhu Y., Zhao J., Sun D., Tao Z., Tang X., Wang J., Wang X., Tsushima A., Lan J., Li W., Wu F., Yuan Q., Ji J., Feng J., Wu C., Liao Z., Li Z., Greinwald J.H., Lu J., Guan M.X. Maternally inherited aminoglycoside-induced and nonsyndromic hearing loss is associated with the 12S rRNA C1494T mutation in three Han Chinese pedigrees. Gene. 2007;401(1–2):4–11. doi: 10.1016/j.gene.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collatz E., Kuchler E., Stoffler G., Czernilofsky A.P. The site of reaction on ribosomal protein L27 with an affinity label derivative of tRNA Met f. FEBS Lett. 1976;63(2):283–286. doi: 10.1016/0014-5793(76)80112-3. [DOI] [PubMed] [Google Scholar]
- Czernilofsky A.P., Kurland C.G., Stoffler G. 30S ribosomal proteins associated with the 3'-terminus of 16S RNA. FEBS Lett. 1975;58(1):281–284. doi: 10.1016/0014-5793(75)80279-1. [DOI] [PubMed] [Google Scholar]
- Dai P., Liu X., Han D., Qian Y., Huang D., Yuan H., Li W., Yu F., Zhang R., Lin H., He Y., Yu Y., Sun Q., Qin H., Li R., Zhang X., Kang D., Cao J., Young W.Y., Guan M.X. Extremely low penetrance of deafness associated with the mitochondrial 12S rRNA mutation in 16 Chinese families: implication for early detection and prevention of deafness. Biochem. Biophys. Res. Commun. 2006;340(1):194–199. doi: 10.1016/j.bbrc.2005.11.156. [DOI] [PubMed] [Google Scholar]
- Davies J., Davis B.D. Misreading of ribonucleic acid code words induced by aminoglycoside antibiotics. The effect of drug concentration. J. Biol. Chem. 1968;243(12):3312–3316. [PubMed] [Google Scholar]
- del Castillo F.J., Rodriguez-Ballesteros M., Martin Y., Arellano B., Gallo-Teran J., Morales-Angulo C., Ramirez-Camacho R., Cruz Tapia M., Solanellas J., Martinez-Conde A., Villamar M., Moreno-Pelayo M.A., Moreno F., del Castillo I. Heteroplasmy for the 1555A>G mutation in the mitochondrial 12S rRNA gene in six Spanish families with non-syndromic hearing loss. J. Med. Genet. 2003;40(8):632–636. doi: 10.1136/jmg.40.8.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D., Jiang H., Salvi R.J. Mechanisms of rapid sensory hair-cell death following co-administration of gentamicin and ethacrynic acid. Hear Res. 2010;259(1–2):16–23. doi: 10.1016/j.heares.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D., Jin X., Zhao J. Accumulation sites of kanamycin in cochlear basal membrane cells. Chin. J. Otorhinolaryngol. 1995;30(6):323–325. [PubMed] [Google Scholar]
- Ding D., Jin X., Zhao J. Accumulation sites of kanamycin in the organ of Corti by microautoradiography. Chin. J. Otorhinolaryngol. 1997;32(6):348–349. [PubMed] [Google Scholar]
- Ding D., Luo D., Guo Y., Huangfu M. Probe into the ototoxic mechanism of aminoglycoside antibiotic. Chin. J. Otorhinolaryngol. 1991;26:154–155. [Google Scholar]
- Ding D., Salvi R. Review of cellular changes in the cochlea due to aminoglycoside antibiotics. Volta Rev. 2005;105(3):407. [Google Scholar]
- Ding D., Salvi R. Experiences in ototoxici investigations in aminoglycoside antibiotics. Chin. J. Otol. 2007;5(2) 125–125. [Google Scholar]
- Du W., Cheng J., Ding H., Jiang Z.W., Guo Y.F., Yuan H.J. A rapid method for simultaneous multi-gene mutation screening in children with nonsyndromic hearing loss. Genomics. 2014;104(4):264–270. doi: 10.1016/j.ygeno.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Estivill X., Govea N., Barcelo A., Perello E., Badenas C., Romero E., Moral L., Scozzari R., D'Urbano L., Zeviani M., Torroni A. Familial progressive sensorineural deafness is mainly due to the mtDNA A1555G mutation and is enhanced by treatment with aminoglycosides. Am. J. Hum. Genet. 1998;62(1):27–35. doi: 10.1086/301676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausti S.A., Henry J.A., Helt W.J., Phillips D.S., Frey R.H., Noffsinger D., Larson V.D., Fowler C.G. An individualized, sensitive frequency range for early detection of ototoxicity. Ear Hear. 1999;20(6):497–505. doi: 10.1097/00003446-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Fischel-Ghodsian N. Genetic factors in aminoglycoside toxicity. Pharmacogenomics. 2005;6(1):27–36. doi: 10.1517/14622416.6.1.27. [DOI] [PubMed] [Google Scholar]
- Fischel-Ghodsian N., Prezant T.R., Bu X., Oztas S. Mitochondrial ribosomal RNA gene mutation in a patient with sporadic aminoglycoside ototoxicity. Am. J. Otolaryngol. 1993;14(6):399–403. doi: 10.1016/0196-0709(93)90113-l. [DOI] [PubMed] [Google Scholar]
- Fourmy D., Recht M.I., Puglisi J.D. Binding of neomycin-class aminoglycoside antibiotics to the A-site of 16 S rRNA. J. Mol. Biol. 1998;277(2):347–362. doi: 10.1006/jmbi.1997.1552. [DOI] [PubMed] [Google Scholar]
- Guan M.X. Prevalence of mitochondrial 12S rRNA mutation associated with aminoglycoside ototoxicity. Volta. Rev. 2005;105(3):211–227. [Google Scholar]
- Guan M.X., Fischel-Ghodsian N., Attardi G. Biochemical evidence for nuclear gene involvement in phenotype of non-syndromic deafness associated with mitochondrial 12S rRNA mutation. Hum. Mol. Genet. 1996;5(7):963–971. doi: 10.1093/hmg/5.7.963. [DOI] [PubMed] [Google Scholar]
- Guan M.X., Fischel-Ghodsian N., Attardi G. A biochemical basis for the inherited susceptibility to aminoglycoside ototoxicity. Hum. Mol. Genet. 2000;9(12):1787–1793. doi: 10.1093/hmg/9.12.1787. [DOI] [PubMed] [Google Scholar]
- Guan M.X., Fischel-Ghodsian N., Attardi G. Nuclear background determines biochemical phenotype in the deafness-associated mitochondrial 12S rRNA mutation. Hum. Mol. Genet. 2001;10(6):573–580. doi: 10.1093/hmg/10.6.573. [DOI] [PubMed] [Google Scholar]
- Gutell R.R., Larsen N., Woese C.R. Lessons from an evolving rRNA: 16S and 23S rRNA structures from a comparative perspective. Microbiol. Rev. 1994;58(1):10–26. doi: 10.1128/mr.58.1.10-26.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakli S., Luotonen M., Sorri M., Majamaa K. Mutations in the two ribosomal RNA genes in mitochondrial DNA among Finnish children with hearing impairment. BMC Med. Genet. 2015;16 doi: 10.1186/s12881-015-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D., Dai P., Zhu Q., Liu X., Huang D., Yuan Y., Yuan H., Wang X., Qian Y., Young W.Y., Guan M.X. The mitochondrial tRNA(Ala) T5628C variant may have a modifying role in the phenotypic manifestation of the 12S rRNA C1494T mutation in a large Chinese family with hearing loss. Biochem. Biophys. Res. Commun. 2007;357(2):554–560. doi: 10.1016/j.bbrc.2007.03.199. [DOI] [PubMed] [Google Scholar]
- Henley C.M., Schacht J. Pharmacokinetics of aminoglycoside antibiotics in blood, inner-ear fluids and tissues and their relationship to ototoxicity. Audiology. 1988;27(3):137–146. doi: 10.3109/00206098809081584. [DOI] [PubMed] [Google Scholar]
- Hobbie S.N., Akshay S., Kalapala S.K., Bruell C.M., Shcherbakov D., Bottger E.C. Genetic analysis of interactions with eukaryotic rRNA identify the mitoribosome as target in aminoglycoside ototoxicity. Proc. Natl. Acad. Sci. U. S. A. 2008;105(52):20888–20893. doi: 10.1073/pnas.0811258106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchin T., Haworth I., Higashi K., Fischel-Ghodsian N., Stoneking M., Saha N., Arnos C., Cortopassi G. A molecular basis for human hypersensitivity to aminoglycoside antibiotics. Nucleic Acids Res. 1993;21(18):4174–4179. doi: 10.1093/nar/21.18.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huth M.E., Han K.H., Sotoudeh K., Hsieh Y.J., Effertz T., Vu A.A., Verhoeven S., Hsieh M.H., Greenhouse R., Cheng A.G., Ricci A.J. Designer aminoglycosides prevent cochlear hair cell loss and hearing loss. J. Clin. Invest. 2015;125(2):583–592. doi: 10.1172/JCI77424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huth M.E., Ricci A.J., Cheng A.G. Mechanisms of aminoglycoside ototoxicity and targets of hair cell protection. Int. J. Otolaryngol. 2011;2011:937861. doi: 10.1155/2011/937861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs H.T., Hutchin T.P., Kappi T., Gillies G., Minkkinen K., Walker J., Thompson K., Rovio A.T., Carella M., Melchionda S., Zelante L., Gasparini P., Pyykko I., Shah Z.H., Zeviani M., Mueller R.F. Mitochondrial DNA mutations in patients with postlingual, nonsyndromic hearing impairment. Eur. J. Hum. Genet. 2005;13(1):26–33. doi: 10.1038/sj.ejhg.5201250. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Huang S.S., Deng T., Wu L.H., Chen J., Kang D.Y., Xu X.F., Li R.Y., Han D.Y., Dai P. Mutation spectrum of common deafness-causing genes in patients with non-syndromic deafness in the Xiamen Area, China. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0135088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings A., Van Camp G., Goethals A., Van Eyken E., Vandevelde A., Ben Azza J., Peeters N., Wuyts W., Smeets H., Van Laer L. Mutation analysis of mitochondrial DNA 12SrRNA and tRNASer(UCN) genes in non-syndromic hearing loss patients. Mitochondrion. 2008;8(5–6):377–382. doi: 10.1016/j.mito.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Leveque M., Marlin S., Jonard L., Procaccio V., Reynier P., Amati-Bonneau P., Baulande S., Pierron D., Lacombe D., Duriez F., Francannet C., Mom T., Journel H., Catros H., Drouin-Garraud V., Obstoy M.F., Dollfus H., Eliot M.M., Faivre L., Duvillard C., Couderc R., Garabedian E.N., Petit C., Feldmann D., Denoyelle F. Whole mitochondrial genome screening in maternally inherited non-syndromic hearing impairment using a microarray resequencing mitochondrial DNA chip. Eur. J. Hum. Genet. 2007;15(11):1145–1155. doi: 10.1038/sj.ejhg.5201891. [DOI] [PubMed] [Google Scholar]
- Li H.Z., Steyger P.S. Systemic aminoglycosides are trafficked via endolymph into cochlear hair cells. Sci. Rep. 2011;1 doi: 10.1038/srep00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Ji Y.B., Han B., Zong L., Lan L., Zhao Y.L., Wang H.Y., Wang D.Y., Wang Q.J. Comparative study of mutation spectrums of MT-RNR1 m.1555A > G, GJB2, and SLC26A4 between familial and sporadic patients with nonsyndromic sensorineural hearing loss in Chinese Han. Chin. Med. J. 2014;127(18):3233–3237. [PubMed] [Google Scholar]
- Li R.H., Xing G.Q., Yan M., Cao X., Liu X.Z., Bu X.K., Guan M.X. Cosegregation of C-insertion at position 961 with the A1555G mutation of the mitochondrial 12S rRNA gene in a large chinese family with maternally inherited hearing loss. Am. J. Med. Genet. Part A. 2004;124a(2):113–117. doi: 10.1002/ajmg.a.20305. [DOI] [PubMed] [Google Scholar]
- Li X., Guan M.X. A human mitochondrial GTP binding protein related to tRNA modification may modulate phenotypic expression of the deafness-associated mitochondrial 12S rRNA mutation. Mol. Cell. Biol. 2002;22(21):7701–7711. doi: 10.1128/MCB.22.21.7701-7711.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Li R., Lin X., Guan M.X. Isolation and characterization of the putative nuclear modifier gene MTO1 involved in the pathogenesis of deafness-associated mitochondrial 12 S rRNA A1555G mutation. J. Biol. Chem. 2002;277(30):27256–27264. doi: 10.1074/jbc.M203267200. [DOI] [PubMed] [Google Scholar]
- Li Z., Li R., Chen J., Liao Z., Zhu Y., Qian Y., Xiong S., Heman-Ackah S., Wu J., Choo D.I., Guan M.X. Mutational analysis of the mitochondrial 12S rRNA gene in Chinese pediatric subjects with aminoglycoside-induced and non-syndromic hearing loss. Hum. Genet. 2005;117(1):9–15. doi: 10.1007/s00439-005-1276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Li Z., Zhu Y., Yang A., Li R., Zheng J., Cai Q., Peng G., Zheng W., Tang X., Chen B., Chen J., Liao Z., Yang L., Li Y., You J., Ding Y., Yu H., Wang J., Sun D., Zhao J., Xue L., Wang J., Guan M.X. Mitochondrial 12S rRNA variants in 1642 Han Chinese pediatric subjects with aminoglycoside-induced and nonsyndromic hearing loss. Mitochondrion. 2010;10(4):380–390. doi: 10.1016/j.mito.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Qian Y., Li Z., Yang A., Zhu Y., Li R., Yang L., Tang X., Chen B., Ding Y., Li Y., You J., Zheng J., Tao Z., Zhao F., Wang J., Sun D., Zhao J., Meng Y., Guan M.X. Mitochondrial haplotypes may modulate the phenotypic manifestation of the deafness-associated 12S rRNA 1555A>G mutation. Mitochondrion. 2010;10(1):69–81. doi: 10.1016/j.mito.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers C.D., Ezzati M., Lopez A.D. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Negl. Trop. Dis. 2007;1(2):e114. doi: 10.1371/journal.pntd.0000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijs G., Claes S., Longo-Mbenza B., Cassiman J.J. Non-syndromic deafness associated with a mutation and a polymorphism in the mitochondrial 12S ribosomal RNA gene in a large Zairean pedigree. Eur. J. Hum. Genet. 1996;4(1):46–51. doi: 10.1159/000472169. [DOI] [PubMed] [Google Scholar]
- Matz G.J. Aminoglycoside cochlear ototoxicity. Otolaryngol. Clin. North Am. 1993;26(5):705–712. [PubMed] [Google Scholar]
- Moazed D., Noller H.F. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987;327(6121):389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- Neefs J.M., Van de Peer Y., De Rijk P., Goris A., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1991;19(Suppl):1987–2015. doi: 10.1093/nar/19.suppl.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi Y., Yashima T., Ito T., Sumi T., Tsuzuku T., Kitamura K. Audiovestibular findings in patients with mitochondrial A1555G mutation. Laryngoscope. 2004;114(2):344–348. doi: 10.1097/00005537-200402000-00031. [DOI] [PubMed] [Google Scholar]
- Noller H.F. Ribosomal RNA and translation. Annu. Rev. Biochem. 1991;60:191–227. doi: 10.1146/annurev.bi.60.070191.001203. [DOI] [PubMed] [Google Scholar]
- Palade G.E. A small particulate component of the cytoplasm. J. Biophys. Biochem. Cytol. 1955;1(1):59. doi: 10.1083/jcb.1.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya A., Xia X., Radnaabazar J., Batsuuri J., Dangaansuren B., Fischel-Ghodsian N., Nance W.E. Mutation in the mitochondrial 12S rRNA gene in two families from Mongolia with matrilineal aminoglycoside ototoxicity. J. Med. Genet. 1997;34(2):169–172. doi: 10.1136/jmg.34.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezant T.R., Agapian J.V., Bohlman M.C., Bu X.D., Oztas S., Qiu W.Q., Arnos K.S., Cortopassi G.A., Jaber L., Rotter J.I., Shohat M., Fischelghodsian N. Mitochondrial ribosomal-RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat. Genet. 1993;4(3):289–294. doi: 10.1038/ng0793-289. [DOI] [PubMed] [Google Scholar]
- Purohit P., Stern S. Interactions of a small RNA with antibiotic and RNA ligands of the 30S subunit. Nature. 1994;370(6491):659–662. doi: 10.1038/370659a0. [DOI] [PubMed] [Google Scholar]
- Qian Y., Guan M.X. Interaction of aminoglycosides with human mitochondrial 12S rRNA carrying the deafness-associated mutation. Antimicrob. Agents Chemother. 2009;53(11):4612–4618. doi: 10.1128/AAC.00965-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Ballesteros M., Olarte M., Aguirre L.A., Galan F., Galan R., Vallejo L.A., Navas C., Villamar M., Moreno-Pelayo M.A., Moreno F., del Castillo I. Molecular and clinical characterisation of three Spanish families with maternally inherited non-syndromic hearing loss caused by the 1494C->T mutation in the mitochondrial 12S rRNA gene. J. Med. Genet. 2006;43(11):e54. doi: 10.1136/jmg.2006.042440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Pesini E., Wallace D.C. Evidence for adaptive selection acting on the tRNA and rRNA genes of human mitochondrial DNA. Hum. Mutat. 2006;27(11):1072–1081. doi: 10.1002/humu.20378. [DOI] [PubMed] [Google Scholar]
- Selmer M., Dunham C.M., Murphy F.V., Weixlbaumer A., Petry S., Kelley A.C., Weir J.R., Ramakrishnan V. Structure of the 70S ribosome complexed with mRNA and tRNA. Science. 2006;313(5795):1935–1942. doi: 10.1126/science.1131127. [DOI] [PubMed] [Google Scholar]
- Tang H.Y., Hutcheson E., Neill S., Drummond-Borg M., Speer M., Alford R.L. Genetic susceptibility to aminoglycoside ototoxicity: how many are at risk? Genet. Med. 2002;4(5):336–345. doi: 10.1097/00125817-200209000-00004. [DOI] [PubMed] [Google Scholar]
- Tang X., Yang L., Zhu Y., Liao Z., Wang J., Qian Y., Tao Z., Hu L., Wu G., Lan J., Wang X., Ji J., Wu J., Ji Y., Feng J., Chen J., Li Z., Zhang X., Lu J., Guan M.X. Very low penetrance of hearing loss in seven Han Chinese pedigrees carrying the deafness-associated 12S rRNA A1555G mutation. Gene. 2007;393(1–2):11–19. doi: 10.1016/j.gene.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Thyagarajan D., Bressman S., Bruno C., Przedborski S., Shanske S., Lynch T., Fahn S., DiMauro S. A novel mitochondrial 12SrRNA point mutation in parkinsonism, deafness, and neuropathy. Ann. Neurol. 2000;48(5):730–736. [PubMed] [Google Scholar]
- Torroni A., Cruciani F., Rengo C., Sellitto D., Lopez-Bigas N., Rabionet R., Govea N., Lopez De Munain A., Sarduy M., Romero L., Villamar M., del Castillo I., Moreno F., Estivill X., Scozzari R. The A1555G mutation in the 12S rRNA gene of human mtDNA: recurrent origins and founder events in families affected by sensorineural deafness. Am. J. Hum. Genet. 1999;65(5):1349–1358. doi: 10.1086/302642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami S., Abe S., Akita J., Namba A., Shinkawa H., Ishii M., Iwasaki S., Hoshino T., Ito J., Doi K., Kubo T., Nakagawa T., Komiyama S., Tono T., Komune S. Prevalence of mitochondrial gene mutations among hearing impaired patients. J. Med. Genet. 2000;37(1):38–40. doi: 10.1136/jmg.37.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellai T., Takacs K., Vida G. A new aspect to the origin and evolution of eukaryotes. J. Mol. Evol. 1998;46(5):499–507. doi: 10.1007/pl00006331. [DOI] [PubMed] [Google Scholar]
- Wang Q., Li Q.Z., Han D., Zhao Y., Zhao L., Qian Y., Yuan H., Li R., Zhai S., Young W.Y., Guan M.X. Clinical and molecular analysis of a four-generation Chinese family with aminoglycoside-induced and nonsyndromic hearing loss associated with the mitochondrial 12S rRNA C1494T mutation. Biochem. Biophys. Res. Commun. 2006;340(2):583–588. doi: 10.1016/j.bbrc.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Wang X., Lu J., Zhu Y., Yang A., Yang L., Li R., Chen B., Qian Y., Tang X., Wang J., Zhang X., Guan M.X. Mitochondrial tRNAThr G15927A mutation may modulate the phenotypic manifestation of ototoxic 12S rRNA A1555G mutation in four Chinese families. Pharmacogenet Genomics. 2008;18(12):1059–1070. doi: 10.1097/FPC.0b013e3283131661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimberly B.T., Brodersen D.E., Clemons W.M., Jr., Morgan-Warren R.J., Carter A.P., Vonrhein C., Hartsch T., Ramakrishnan V. Structure of the 30S ribosomal subunit. Nature. 2000;407(6802):327–339. doi: 10.1038/35030006. [DOI] [PubMed] [Google Scholar]
- Wrzesniok D., Beberok A., Otreba M., Buszman E. Modulation of melanogenesis and antioxidant defense system in melanocytes by amikacin. Toxicol. In Vitro. 2013;27(3):1102–1108. doi: 10.1016/j.tiv.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Wu J., Hao Z.J., Fu D.G., Hei N., Zhang B., Zhou A.F., Hu X.J., Yao C., Dong Y.P., Ring H.J.Z., Ring B.Z. Mitochondrial mutations associated with aminoglycoside ototoxicity and hearing loss susceptibility identified by meta-analysis. J. Med. Genet. 2015;52(2):95–103. doi: 10.1136/jmedgenet-2014-102753. [DOI] [PubMed] [Google Scholar]
- Yan Q., Li X., Faye G., Guan M.X. Mutations in MTO2 related to tRNA modification impair mitochondrial gene expression and protein synthesis in the presence of a paromomycin resistance mutation in mitochondrial 15 S rRNA. J. Biol. Chem. 2005;280(32):29151–29157. doi: 10.1074/jbc.M504247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W.Y., Zhao L., Qian Y., Wang Q., Li N., Greinwald J.H., Jr., Guan M.X. Extremely low penetrance of hearing loss in four Chinese families with the mitochondrial 12S rRNA A1555G mutation. Biochem. Biophys. Res. Commun. 2005;328(4):1244–1251. doi: 10.1016/j.bbrc.2005.01.085. [DOI] [PubMed] [Google Scholar]
- Yuan H., Chen J., Liu X., Cheng J., Wang X., Yang L., Yang S., Cao J., Kang D., Dai P., Zhai S., Han D., Young W.Y., Guan M.X. Coexistence of mitochondrial 12S rRNA C1494T and CO1/tRNA(Ser(UCN)) G7444A mutations in two Han Chinese pedigrees with aminoglycoside-induced and non-syndromic hearing loss. Biochem. Biophys. Res. Commun. 2007;362(1):94–100. doi: 10.1016/j.bbrc.2007.07.161. [DOI] [PubMed] [Google Scholar]
- Zhao H., Li R., Wang Q., Yan Q., Deng J.H., Han D., Bai Y., Young W.Y., Guan M.X. Maternally inherited aminoglycoside-induced and nonsyndromic deafness is associated with the novel C1494T mutation in the mitochondrial 12S rRNA gene in a large Chinese family. Am. J. Hum. Genet. 2004;74(1):139–152. doi: 10.1086/381133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Young W.Y., Yan Q., Li R., Cao J., Wang Q., Li X., Peters J.L., Han D., Guan M.X. Functional characterization of the mitochondrial 12S rRNA C1494T mutation associated with aminoglycoside-induced and non-syndromic hearing loss. Nucleic Acids Res. 2005;33(3):1132–1139. doi: 10.1093/nar/gki262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Young W.Y., Li R., Wang Q., Qian Y., Guan M.X. Clinical evaluation and sequence analysis of the complete mitochondrial genome of three Chinese patients with hearing impairment associated with the 12S rRNA T1095C mutation. Biochem. Biophys. Res. Commun. 2004;325(4):1503–1508. doi: 10.1016/j.bbrc.2004.10.199. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Li Q., Chen Z., Kun Y., Liu L., Liu X., Yuan H., Zhai S., Han D., Dai P. Mitochondrial haplotype and phenotype of 13 Chinese families may suggest multi-original evolution of mitochondrial C1494T mutation. Mitochondrion. 2009;9(6):418–428. doi: 10.1016/j.mito.2009.07.006. [DOI] [PubMed] [Google Scholar]