Abstract
Experimental studies has been carried out to isolate and identify an active antifilarial compound from Vitex negundo L. plant as it has been used for treatment against filariasis in Indian traditional system of medicine. In vitro antifilarial assay has been carried out against adult filarial parasite Setaria cervi worms by both worm motility and MTT reduction assays. Levels of oxidative stress parameters MDA, carbonyl content and nitric oxide levels have been detected. The isolated compound exhibited significant antifilarial activity in dose dependent manner. The active compound has been chemically characterized and identified as 4,5-diethyl-3′-ethoxy-pyro-flavone.
Keywords: Setaria cervi, Vitex negundo L., Flavonoid, Antifilarial effect
Graphical abstract
Highlights
-
•
4,5-Diethyl-3′-ethoxy-pyro-flavone: next-generation drug for lymphatic filariasis
-
•
Vitex negundo L. has bioactivity against cattle-endosymbiont Setaria cervi.
-
•
Isolated drug has oxidative and/or nitrosative activity.
1. Introduction
The lymphatic filariasis emerged in huge majority of tropical and subtropical areas of developing countries. Filariasis is caused by the infection with parasitic filarial nematodes Wuchereria bancrofti and Brugia malayi, transmitted by mosquito vectors (Gleave et al. 2016; Chavette and Jureen, 2017). It is a major public health problem and afflicting >856 million people all over the world (WHO, 2017). Filariasis, causes disfigurement and disability in endemic areas, leading to significant economic and psychosocial effect. Around 45% of its 1 billion plus population lives in known endemic areas in India, accounting for 40% of the worldwide filariasis load and socioeconomic studies reveal that a billion U.S. dollars annual loss caused by this disease (Ramaiah et al., 1997).
WHO has recognized as major public health problem in the tropical and subtropical countries and precisely recognized this disease in its Tropical Disease Research scheme and launched a global program for elimination of lymphatic filariasis (GPELF) (www.who.int/tdr/diseases). The microfilaricidal drugs DEC (Diethylcarbamazine), Ivermectin and Albendazole (IDA) are currently used for lymphatic filariasis (Fischer et al. 2017). None of these drugs are effective in killing the adult worms, which can live in the host for several years (Sharma et al. 2016). This warrants the need for developing an effective and safe drug to kill or permanently sterilize the adult worms.
Plants are rich in resource materials for various phytomedicine utilize in traditional medicine. Tropical disease research scheme of World Health Organization recognized traditional medicine (TM) is main alternative source for novel antifilarial therapeutics. However, the major challenge in the use of traditional medicines is the lack of scientific validity. Vitex negundo L. plant has shown significant antifilarial activity and oxidative status (Sharma et al. 2010). Vitex negundo L. contains important polyphenolic compound (Prakash et al. 2017). Recent evidences show that polyphenolic compounds in plants like flavonoids behave as pro-oxidants rather than antioxidants (Tian et al. 2017). Oxidative stress is an important trigger mechanism for apoptotic signal (Song et al. 2017). With this perspective, in this present study antifilarial activity of isolated compound from Vitex negundo L. leaves methanolic extract has been conducted. Identification of active compound has been carried out and exploring their possible mechanism of action to find out the possible connection of oxidative mechanism in the antifilarial treatment.
2. Materials and methods
2.1. Procuring plant material
Leaves of Vitex negundo L. were collected from the local areas of Bhopal (M.P., India) and identified taxonomically by Department of Botany, Safia Science College, Bhopal (M.P., India) as Specimen No. 410/Bot/Safia/2012. Voucher specimen has been deposited in that department. Leaves were washed in tap water, shade dried and finally powdered.
2.2. Extraction
1.5 kg of powdered leaves of Vitex negundo L. was extracted successively with petroleum ether (60 °C–80 °C), chloroform, ethyl acetate and methanol by percolation method (ArunVijay et al. 2014).
2.3. Fractionation and isolation of active molecule
Methanolic extract had been found significant antifilarial activity and thus isolation as well as purification was carried out by fractionation in flash column chromatography. Initial separation of the active molecules present in the methanol extract was carried out by thin layer chromatography by using a mobile phase contains chloroform and methanol (8:2; v/v). Compounds were visualized at 254 nm in UV chamber and by using anisaldehyde–sulphuric acid reagent. Finally active compound was purified by flash column chromatography.
2.4. Spectral analysis
Compounds were finally purified by High Performance Liquid Chromatography and chromatographic separations were performed using C18-column (250 cm × 4.6 cm, 5 μm) and the column temperature had maintained at 27 ± 2 °C. The mobile phase consist acetonitrile and ammonium acetate buffer at a flow rate of 1.0 ml/min. Purified compounds were subjected to chemical characterization by UV, FT-IR and NMR analysis. FT-IR analysis was carried out on Agilent Cary 630 FT-IR spectrometer with a scan range 450 to 4000/cm with a resolution of 4/cm. 1H and 13C NMR spectra in CDCl3 were recorded on a Bruker Advance 400 (FT-NMR-400).
2.5. In vitro motility inhibition and MTT reduction assay
Adult Setaria cervi strains had been collected from the peritoneal cavity of freshly slaughtered cattle. The worms were washed repeatedly with normal saline (0.85%) to free them of any extraneous material and used for further assay.
The worms were transferred to 0.01%-streptopenicillin and 10% heat-inactivated fetal calf serum added DMEM (Dulbecco's modified eagle's medium). 100 μl diluted extract of Vitex negundo L. in DMSO (dimethyl sulphoxide) was added to 3 ml medium in a sterile disposable Petri dish (35 mm diameter and 5 ml capacity). Screening had been carried out at concentrations ranging from 0.0005 to 0.02 mg/ml. A control had been kept in 100 μl DMSO in 3 ml of the medium without the plant extracts. Two worms (one male and one female) were kept into each Petri dish. The worms were incubated at 37 °C for 24 h. in 5%-CO2 incubator and motility observed after 2 h interval.
Motility was assessed by microscopy after 24 h of the observations were recorded as the number of non-motile out of all the 100 ml taken in each well for the study and represented as percentage (%) reduction in motility (Rao and Well 2002). A 10× eyepiece was fitted in its focal plane with an opaque disk which had a whole 0.8 mm in diameter in the centre. This permitted the observation of a field 10 pm in diameter. The motility was assessed by counting the worm traversed this field in a given time. This “motility count” was routinely made over 1 min, repeating this in two another times, and taking the mean. After the experiment, the worms were collected and washed twice with fresh medium and transferred to another set of fresh Petri dish containing fresh medium to find out whether any of the immotile worm regained motility in the 2 h post-treatment period in drug free medium. If the worms did not revive, the condition was considered as irreversible and the concentration lethal (Murthy 1999).
Effect of purified compounds VN1, VN2 and VN3 on adult female Setaria cervi worms had been studied by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide)–formazan reduction assay following the method described in Strote et al. (1998).
2.6. Malondialdehyde (MDA) estimation
To 0.5 ml of culture supernatants of plant compounds and MDA standards (2.5–40 nM/ml), 2.5 ml of 20%-trichloroacetic acid (TCA) + 1.0 ml of 0.67%-thiobarbituric acid (TBA) were added and mixed by using vortex. Then the mixtures were boiled in hot water bath for 30 min. After cooling in cold-water bath, the resultant chromogen was extracted with 4 ml of n-butyl alcohol and separation of organic phase was done by centrifugation at 3000 rpm for 10 min. Absorbance of the butyl alcohol extracts of standards and samples was measured at 530 nm against butyl alcohol as blank. The standard curve had been plotted and concentrations of total MDA in samples calculated and expressed as nM MDA/ml (Zeb and Ullah 2016).
2.7. Protein carbonylation assay
Protein carbonylation estimation of the samples was carried out by treating the samples with 10%- TCA to react with 0.5 ml of 10 mM DNPH (in 2 M HCl) at room temperature for 1 h. The precipitated yield was obtained after centrifugation at 5000 rpm for 5 min and the pellet was washed thrice with ethanol-ethyl acetate (1:1). The washed pellet dissolved in 1.5 ml of protein dissolving solution (2 g SDS and 50 mg EDTA in 100 ml 80 mM/l phosphate buffer of pH 8) and kept at 37 °C for 10 min. The color intensity measured at 370 nm against 2 M HCl. Carbonyl content had been calculated by using molar extinction-coefficient (21 × 103 l·mol−1·cm−1) (Suzuki et al. 2010).
2.8. Nitric oxide assay
The adult parasite culture supernatants were checked for nitric oxide levels. The amount of nitric oxide (NO) in the 24 h. culture supernatants was estimated using Griess reagent (1%-sulfanilamide, 0.1%-napthylenediamine hydrochloride and 2.5%-phosphoric acid) (Yang et al. 2009). 100 μl of the Griess reagent was added to 100 μl of culture supernatant in 96 well microtitre plates. Absorbance was read at 542 nm after 10 min incubation. The O.D. values of culture supernatants had been plotted on standard graph of concentration 0.005 to 0.08 μM/ml, and concentration of nitric oxide in test samples were calculated. For comparison of results between test and respective controls, Student's t-test was used. P < 0.05 was considered as significant.
3. Results
3.1. In vitro motility inhibition assay
Three major spots were visualized along with minor impurities on TLC chromatograms. Isolated compound through flash-chromatography was further used for antifilarial screening against adults of the cattle filarial worm Setaria cervi. Concentrations from 0.005 to 0.02 mg/ml of VN3 caused complete immobilization of the worms at their respective incubation at 2 h. while, in control, all the worms were active (Fig.3). The results shown that, VN3 compound at lower concentrations, the inhibition in motility was found significant as compared to other two compounds (VN1 and VN2).
Fig. 3.
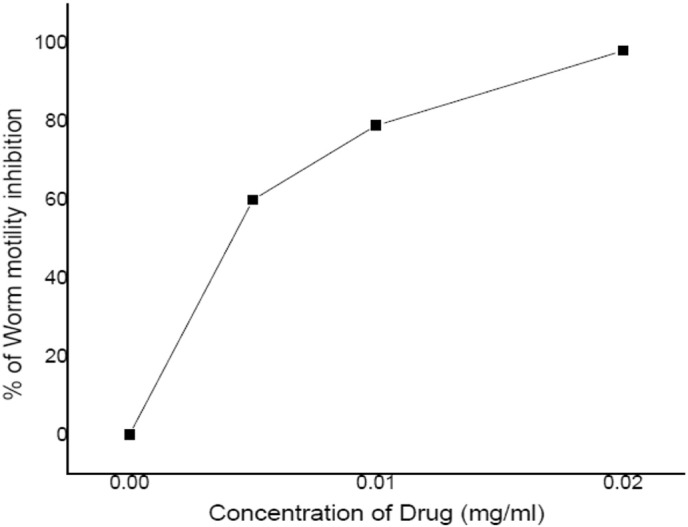
Dose-response curve of VN3 after 2 h motility inhibition.
3.2. MTT–formazan colorimetric assay
The macro-filaricidal effect of plant compound was confirmed by MTT–formazan colorimetric assay. During the assay, the formazan formed is extracted with DMSO and quantitated calorimetrically. The very low absorbance value (0.325) observed for the heat-killed worms was due to the least production of formazan in dead worms. The percentage inhibition considered (>50%) significant, was 64.1, 79.8 and 99.5% at 0.005, 0.01 and 0.02 mg/ml at 10, 6 and 2 h. incubation period indicating the significant effect of the compound (Table 1), respectively. IC50, at which 50% of the motility inhibition achieved, found to be 0.0032 mg/ml.
Table 1.
In vitro antifilarial activity of compound against adult filarial parasite in term of MTT reduction assay.
| Sample | Incubation time (in h) | Test concentrations (in mg/ml) | Absorbance at 492 nm (mean ± S.E.M.) | % reduction relative to solvent controlC, heat killedH & treated wormsT | IC50 |
|---|---|---|---|---|---|
| CControl | 24 | – | 1.007 ± 0.03 | – | |
| HHeat killed | 0.5 | – | 0.325 ± 0.028 | – | |
| TCompound | 24 | 0.0005 | 0.905 ± 0.005⁎ | 15 | |
| 20 | 0.001 | 0.816 ± 0.005⁎ | 28.1 | ||
| 14 | 0.002 | 0.709 ± 0.006⁎ | 43.7 | 0.0032 | |
| 10 | 0.005 | 0.570 ± 0.003⁎ | 64.1 | ||
| 6 | 0.01 | 0.463 ± 0.008⁎ | 79.8 | ||
| 2 | 0.02 | 0.329 ± 0.144⁎ | 99.5 |
CPositive control, Hnegative control, Ttreated worm with compound.
P value represents the level of significance P < 0.05 when comparing the mean value of absorbance observed for the formazan formed between treated and control worms.
3.3. Chemical characterization of VN3
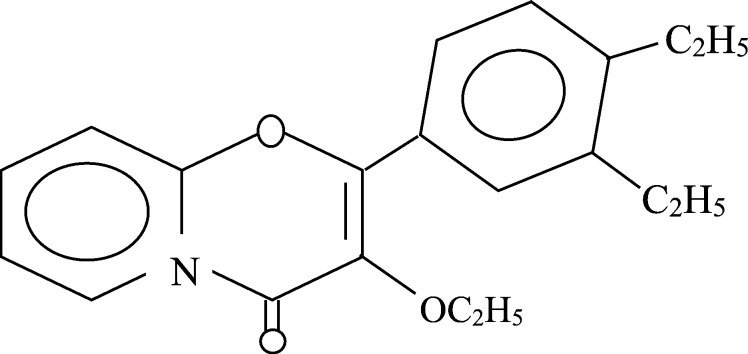
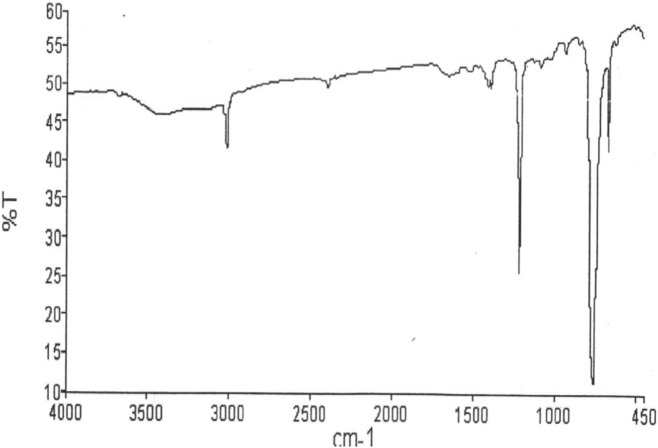
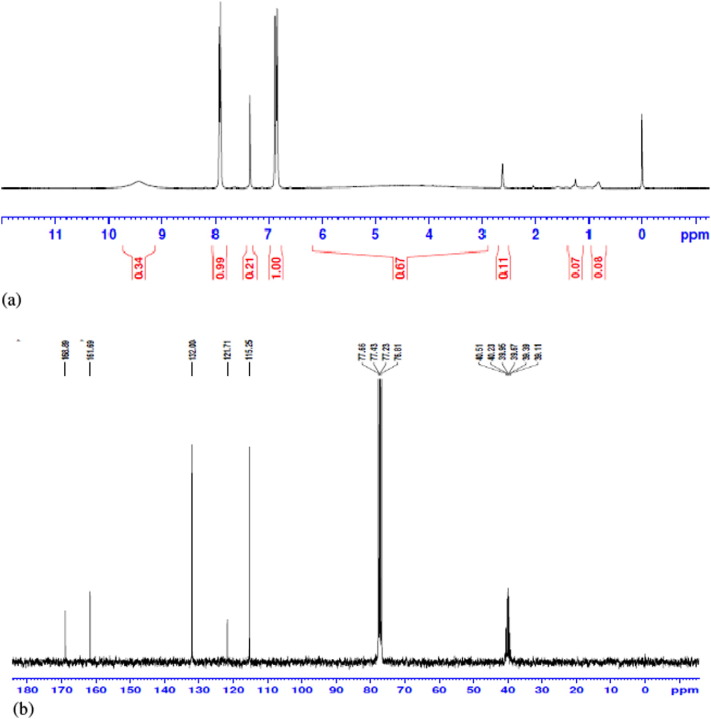
FT-IR, 1H NMR and 13C NMR (Figs. 1 and 2) assays of isolated compound were carried out successfully. Rf value of the purified VN3 on TLC chromatogram is 0.2. The 1H NMR data are in agreement with the number and type of protons present in the molecule (Fig. 2). 1H NMR revealed (300 MHz) H 1.25 (6H, s, 2× CH3), 2.61 (5H, m, COO CH2CH3), 2.60 (1H, s, CH N), 7.93 (1H, s, 2-OH), 9.43 (4H, ArH); and 13C NMR (300 MHz) 36.93, 39.20, 39.48, 39.76, 40.03, 77.74, 78.18, 78.62, 116.13, 117.4, 118.9, 131.09, 132.51, 158.60, 163.02 (Fig. 3b). The FT-IR spectrum in KBr has been found as: 3433.29 (OH), 3019.65 (N—H), 1654.03 (C O, ester), 1385.08 (C N), 1215.53 (—CONH), 1084.18 (—CH), 669.23 (Substitution) cm−1 (Fig. 1). Thus, VN3 is 4,5-diethyl-3′-ethoxy-pyro-flavone (C15H35O3N) as identified by study of the chemical data (Fig. 5).
Fig. 1.
FT-IR chromatogram of the isolated compound VN3.
Fig. 2.
(a) 1H NMR and (b) 13C NMR analysis of the isolated compound in CDCl3.
Fig. 5.
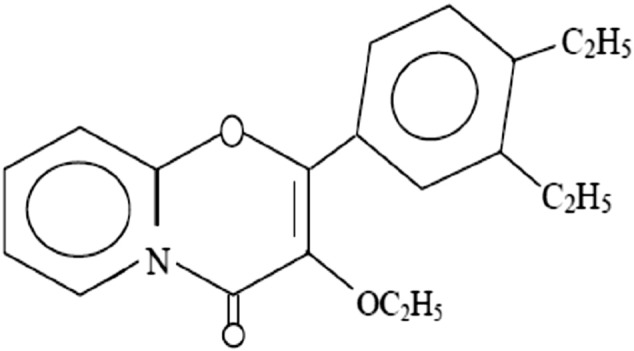
Chemical structure of VN3 [4,5-diethyl-3′-ethoxy-pyro-flavone].
3.4. Antifilarial activity of VN3
The culture of parasite was checked for lipid peroxidation by estimating the levels of malondialdehyde (MDA) in each of the concentration. This was carried out by following (TBA) thiobarbituric acid method, modified by Yoon et al. (2009). The standard graph has been plotted from MDA concentrations ranging from 2.50 nM/ml, 5.00 nM/ml, 10.00 nM/ml, 20.00 nM/ml to 40.00 nM/ml against the O.D. values of culture supernatants (Table 2). The MDA levels in culture supernatant as antifilarial activity dependent manner are 0.5, 0.9, 1.6, 1.8, 2.3 and 3.5 for control 0.3 nM/ml (Fig. 4a).
Table 2.
Levels of MDA, carbonyl content and nitric oxide levels in culture supernatants (nM/ml) of compound: results shown are mean ± S.E.M.
| Concentration of compound (mg/ml) | MDA levels (nM/ml) | Carbonyl content (nM/mg) | Nitric oxide levels (μM/ml) |
|---|---|---|---|
| Control | 0.3 ± 0.057 | 0.02 ± 0.008 | 0.004 ± 0.001 |
| 0.0005 | 0.5 ± 0.057⁎ | 0.06 ± 0.05 | 0.007 ± 0.01⁎ |
| 0.001 | 0.9 ± 0.009⁎ | 0.12 ± 0.02⁎ | 0.011 ± 0.06⁎ |
| 0.002 | 1.6 ± 0.152⁎ | 0.29 ± 0.009⁎ | 0.023 ± 0.001⁎ |
| 0.005 | 1.8 ± 0.057 | 0.54 ± 0.009⁎ | 0.049 ± 0.01⁎ |
| 0.01 | 2.3 ± 0.152⁎ | 0.73 ± 0.025⁎ | 0.076 ± 0.001⁎ |
| 0.02 | 3.5 ± 0.152 | 0.96 ± 0.011⁎ | 0.095 ± 0.001⁎ |
Significant at the level of P < 0.05 when compared with respective control level.
Fig. 4.
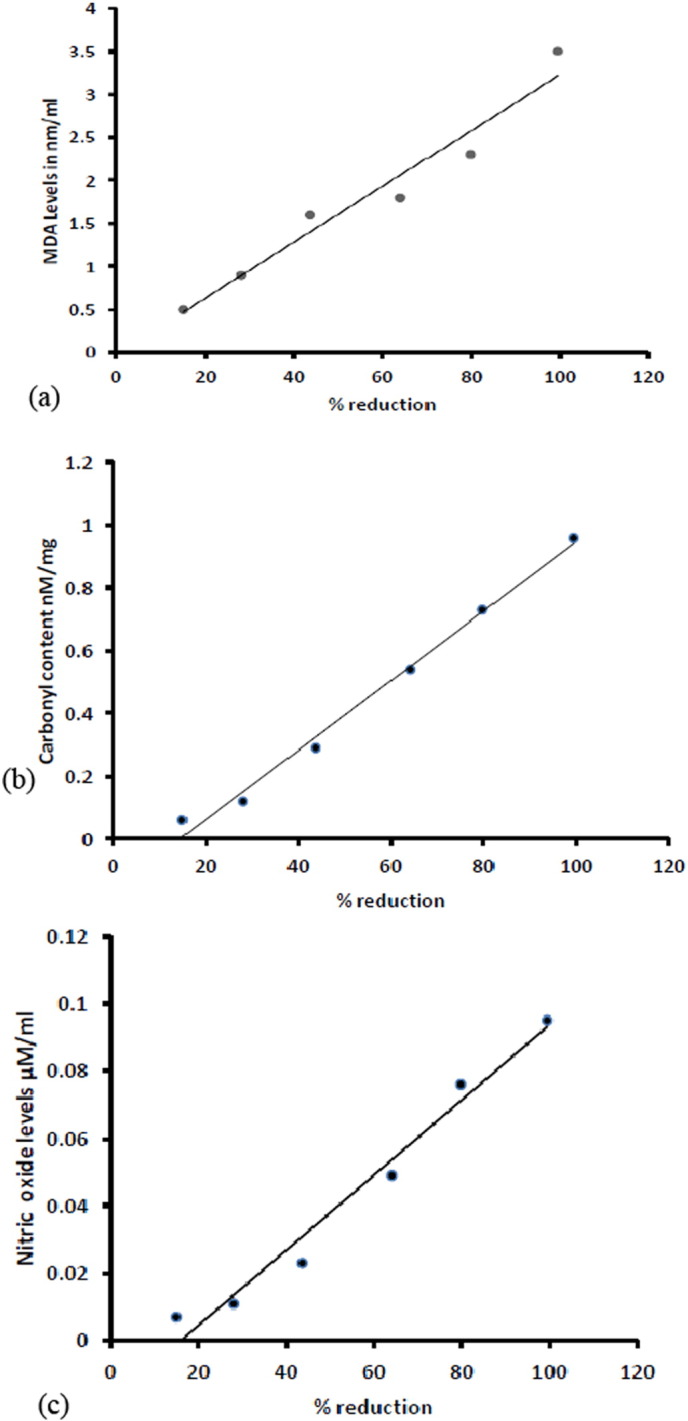
Levels of (a) MDA, (b) carbonyl content and (c) nitric oxide after 24 h incubation in the in vitro experiments for the effect of the compound on parasite motility.
The culture of parasite was checked for protein carbonylation in each of the concentration. The levels of protein carbonylation in culture supernatants after 24 h. are expressed in nM/mg unit. Carbonyl content are found as 0.06, 0.12, 0.29, 0.54, 0.73 and 0.96 nM/mg in culture supernatants as antifilarial activity achieved and for control 0.02 nM/mg (Table 2) has been calculated (Fig. 4b).
The levels of nitric oxide in culture supernatant after 24 h. are expressed in μM/ml unit (Table 2). Nitric oxide levels are 0.007, 0.011, 0.023, 0.049 and 0.076 found in culture supernatant as antifilarial activity dependent manner and 0.095 for control 0.004 μM/ml (Fig. 4c).
4. Discussion
Looking on the enormous socio-economic burden of filarial disease on the developing countries, in accordance to WHO mandate, development of novel antifilarial therapeutic drug is essential. In this experimental study compound has been isolated from the methanolic extract of Vitex negundo L. leaves and identified as a flavonoid compound, 4,5-diethyl-3′-ethoxy-pyro-flavone, shows significant antifilarial activity at their respective concentration.
Vitex negundo L. contains various polyphenolic compounds as active ingredients (Tripathi et al., 2017). Polyphenolic compounds from plant origin are usually active antioxidant in nature (Nile et al. 2017) and this may due to the reversible nature of redox reaction (Lee et al. 2017). On other hand, polyphenolic agents like flavonoids in Vitex negundo L. roots may be responsible for such higher level of protein oxidation (Balasooriya et al. 2017).
In this study, we have found direct effect of 4,5-diethyl-3′-ethoxy-pyro-flavone on adult parasite Setaria cervi. The oxidative markers MDA, carbonyl content and nitric oxide levels in parasitic culture has been found to be increasing in a dose dependent manner parallel to the reduction in adult motility. Similarity in the results are obtained for stress parameters specify a close involvement of oxidative and nitrosative rationale in antifilarial effect. Significant correlation between each of the parameter and the reduction in worm motility over the dose range specify a fundamental cause of such oxidative damage in the antifilarial effect of isolated compound. Lower concentration reveals significant association with oxidative parameters in a dose dependent manner for filarial parasite. These experimental results shows that direct antifilarial effect might be as a function of oxidative and/or nitrosative role.
In summary, compound isolated from methanolic extract of Vitex negundo L. has exhibited promising macrofilaricidal activity against adult filarial worm Setaria cervi, possible mechanism has been identified as oxidative and the active principle responsible for this is 4,5-diethyl-3′-ethoxy-pyro-flavone.
Conflict of interest
The authors declare no conflict of interest.
References
- ArunVijay M., Natheer Hassan Y., Srinivasa Kumar K.P. Evaluation of phytoconstituents and antibacterial activity of Vitex negundo var. Negundos. Int. J. Adv. Interdis. Res. 2014;1:1–4. http://ijaidr.co.in/files/arun1-4.pdf [Google Scholar]
- Balasooriya E.R., Jayasinghe C.D., Jayawardena U.A., Ruwanthika R.W.D., de Silva R.M., Udagama P.V. Honey mediated green synthesis of nanoparticles: new era of safe nanotechnology. J. Nanomat. 2017 doi: 10.1155/2017/5919836. [DOI] [Google Scholar]
- Chavette J.-M., Jureen R. Imported asymptomatic bancroftian filariasis discovered from a Plasmodium vivax infected patient: a case report from Singapore. Case Rep. Infect. Dis. 2017 doi: 10.1155/2017/1972587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer P.U., King C.L., Jacobson J.A., Weil G.J. Potential value of triple drug therapy with Ivermectin, Diethylcarbamazine, and Albendazole (IDA) to accelerate elimination of lymphatic filariasis and onchocerciasis in Africa. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleave K., Cook D., Taylor M.J., Reimer L.J. Filarial infection influences mosquito behavior and fecundity. Sci. Rep. 2016;6:36319. doi: 10.1038/srep36319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.T., Lin W.C., Yu B., Lee T.T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals — a review. Asian Australas. J. Anim. Sci. 2017;30:299–308. doi: 10.5713/ajas.16.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy P.K. Evaluation of two in vitro systems employing Brugia malayi parasite for prescreening of potential antifilarials. Curr. Sci. 1999;77(8):1084–1089. [Google Scholar]
- Nile S.H., Nile A.S., Keum Y.-S. Total phenolics, antioxidant, antitumor, and enzyme inhibitory activity of Indian medicinal and aromatic plants extracted with different extraction methods. 3 Biotech. 2017;7 doi: 10.1007/s13205-017-0706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash V., Rana S., Sagar A. Studies on analysis of antioxidant and enzyme inhibitory activity of Vitex negundo Linn. Int. J. Pharmacognosy Pythochem. Res. 2017;9:833–839. doi: 10.25258/phyto.v9i6.8187. [DOI] [Google Scholar]
- Ramaiah K.D., Vijay Kumar K.N., Ramu K., Das P.K. Functional impairment caused by lymphatic filariasis in rural areas of South India. Trop. Med. Int. Health. 1997;2:832–838. doi: 10.1046/j.1365-3156.1997.d01-406.x. [DOI] [PubMed] [Google Scholar]
- Rao R., Well G.J. In vitro effect of antibiotics on Brugia malayi worm survival and reproduction. J. Parasitol. 2002;83:605–611. doi: 10.1645/0022-3395(2002)088[0605:IVEOAO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Sharma R.D., Veerpathran A.R., Dakshinamoorthy G., Sahare K.N., Goswami K., Reddy M.V.R. Possible implication of oxidative stress in antifilarial effect of certain traditionally used medicinal plants in vitro against Brugia malayi microfilariae. Pharm. Res. 2010;2:350–354. doi: 10.4103/0974-8490.75453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Jayoussi G.A., Tyrer H.E., Gamble J., Hayward L., Guimaraes A.F., Davies J., Waterhouse D., Cook D.A.N., Myhill L.J., Clare R.H., Cassidy A., Steven A., Johnston K.L., Ford L., Turner J.D., Ward S.A., Taylor M.J. Minocycline as a re-purposed anti-Wolbachia macrofilaricide: superiority compared with doxycycline regimens in a murine infection model of human lymphatic filariasis. Sci. Rep. 2016;6:23458. doi: 10.1038/srep23458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Fu B., Zhang J., Zhao J., Yuan M., Peng W., Zhang Y., Wu H. Sodium fluoride induces nephrotoxicity via oxidative stress-regulated mitochondrial SIRT3 signaling pathway. Sci. Rep. 2017;7:672. doi: 10.1038/s41598-017-00796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strote G., Bonow I., Kromer M., Rubio de Kromer T., Attah S., Opoku N. Chemotherapy for onchocerciasis: results of in vitro experiments with promising new compounds. Trop. Med. Int. Health. 1998;3:397–407. [PubMed] [Google Scholar]
- Suzuki Y.J., Carini M., Butterfield D.A. Protein carbonylation. Antioxid. Redox Signal. 2010;12:323–325. doi: 10.1089/ars.2009.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T., Wang Z., Zhang J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid. Med. Cell. Longev. 2017 doi: 10.1155/2017/4535194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi I.P., Tripathi R., Tiwari A. Investigation of biologicaly active phytoconstituents present in selected plants material of Verbenaceae, Lamiaceae and Fabaceae family. Int. J. Multidiscip. Curr. Res. 2017;5:31–37. http://ijmcr.com/wp-content/uploads/2017/01/Paper531-37.pdf [Google Scholar]
- WHO Report Lymphatic Filariasis. 2017. http://www.who.int/mediacentre/factsheets/fs102/en/
- Yang E.-J., Yim E.-Y., Song G., Hyun C.-G. Inhibition of nitric oxide production in lipopolysaccharide-activated RAW 264.7 macrophages by Jeju plant extracts. Interdiscip. Toxicol. 2009;2:245–249. doi: 10.2478/v10102-009-0022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon W.J., Kim S.S., oh T.H., Lee N.H., Hyun C.G. Abies koreana essential oil inhibits drug-resistant skin pathogen growth and LPS-induced inflammatory effects of murine macrophage. Lipids. 2009;44:471–476. doi: 10.1007/s11745-009-3297-3. [DOI] [PubMed] [Google Scholar]
- Zeb A., Ullah F. A simple spectrophotometric method for the determination of thiobarbituric acid reactive substances in fried fast foods. J. Anal. Methods Chem. 2016 doi: 10.1155/2016/9412767. [DOI] [PMC free article] [PubMed] [Google Scholar]