Abstract
Objectives
We evaluate incidence and prevalence of autoimmune encephalitis and compare the epidemiology of autoimmune and infectious encephalitis.
Methods
We performed a population-based comparative study of the incidence and prevalence of autoimmune and infectious encephalitis in Olmsted County, USA. Autoimmune encephalitis diagnosis and subgroups were defined by 2016 diagnostic criteria and infectious encephalitis diagnosis required a confirmed infectious pathogen. Age- and sex-adjusted prevalence and incidence rates were calculated. Patients with encephalitis of uncertain etiology were excluded.
Results
The prevalence of autoimmune encephalitis on January 1, 2014 of 13.7/100,000 was not significantly different from that of all infectious encephalitides (11.6/100,000; p=0.63) or the viral subcategory (8.3/100,000; p=0.17). The incidence rates (1995–2015) of autoimmune and infectious encephalitis were 0.8/100,000 and 1.0/100,000 person-years respectively (p=0.58). The number of relapses or recurrent hospitalizations was higher for autoimmune than infectious encephalitis (p=0.03). The incidence of autoimmune encephalitis increased over time from 0.4/100,000 person-years (1995–2005) to 1.2/100,000 person-years (2006–2015) (p=0.02), attributable to increased recognition of autoantibody-positive cases. The incidence (2.8 vs 0.7/100,000 person-years; p=0.01) and prevalence (38.3 vs 13.7/100,000; p=0.04) of autoimmune encephalitis was higher among African-Americans than Caucasians. The prevalence of specific neural autoantibodies was: myelin-oligodendrocyte-glycoprotein (MOG) (1.9/100,000); glutamic acid decarboxylase-65 (GAD65) (1.9/100,000); unclassified neural autoantibody (1.4/100,000); leucine-rich glioma-inactivated-protein-1 (LGI1) (0.7/100,000); collapsin response-mediator protein-5 (CRMP5) (0.7/100,000); N-methyl-D-aspartate-receptor (NMDAR) (0.6/100,000); anti-neuronal nuclear antibody-2 (ANNA-2/anti-Ri) (0.6/100,000) and glial fibrillary acidic protein-α (GFAPα) (0.6/100,000).
Interpretation
This study shows that the prevalence and incidence of autoimmune encephalitis is comparable to infectious encephalitis and its detection is increasing over time.
Keywords: prevalence, incidence, encephalitis, autoimmune
Introduction
The cost of hospitalization in the United States in 2010 for encephalitis (2 billion US dollars) illustrates its severe disease burden.1 Earlier epidemiology studies have primarily focused on infectious causes.1–4 Autoimmune encephalitis is increasingly recognized as a common treatable cause of encephalitis, yet population-based studies on its incidence and prevalence are lacking. This is in part due to the only recent neural autoantibody discovery as biomarkers (e.g., N-methyl D-aspartate receptor [NMDAR] autoantibodies)5 allowing confirmation of the diagnosis and its distinction from other causes of encephalitis. In the last 15 years, the number of validated autoantibody biomarkers of encephalitis has increased dramatically. Awareness of the epidemiology of autoimmune encephalitis is essential for allocation of resources and health care planning. The diagnostic criteria for autoimmune encephalitis and its subcategories published in 2016 are utilized in this study.6 Herein we describe the incidence and prevalence of autoimmune encephalitis in Olmsted County (MN), a geographically defined region of the USA and compare the epidemiology of autoimmune encephalitis and infectious encephalitis.
Methods
Study design and participants
For this population-based study of the incidence and prevalence of autoimmune encephalitis among residents of Olmsted County, USA, we included patients of both sexes and all ages, including children and ethnic minorities. The study was approved by the Institutional Review Boards of the Mayo Clinic, and Olmsted Medical Center.
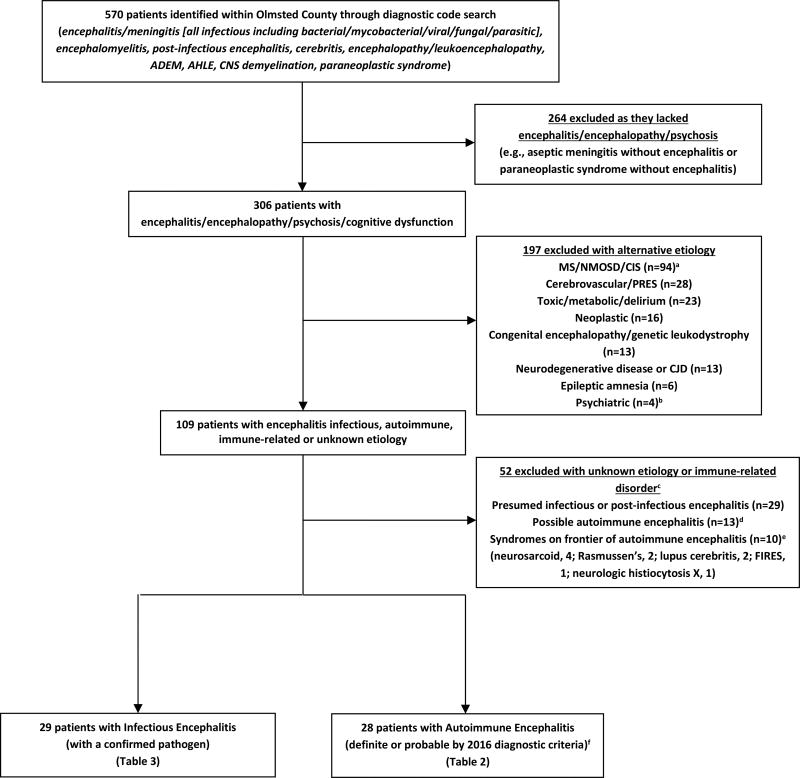
Olmsted County in southeastern Minnesota (USA) has a population of 155,285 (January 1, 2014) primarily of northern European descent and includes the city of Rochester. The medical records-linkage system of the Rochester Epidemiology Project includes all medical practitioners in Olmsted County.7 We identified all patients with encephalitis by searching the medical records from January 1, 1995 to December 31, 2015 for all potentially relevant diagnostic codes (Figure 1).
Figure 1. Flowsheet of patient identification, inclusion and exclusion.
Key: ADEM, Acute Disseminated Encephalomyelitis; AHLE, Acute hemorrhagic leukoencephalitis; CIS, Clinically Isolated Syndrome of CNS demyelination; CJD, Creutzfeldt-Jakob Disease; CNS, central nervous system; FIRES, Febrile Infection-Related Epilepsy Syndrome; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorder; PRES, posterior reversible encephalopathy syndrome;
aPatients with MS, NMOSD (AQP4-IgG seropositive or seronegative) or CIS not meeting criteria for ADEM
bone patient had psychosis with a positive serum NMDA receptor autoantibody but negative CSF NMDA-R autoantibody and resolved with anti-psychotics alone and was excluded
cTwenty-five of these patients were tested for neural antibodies and 4 seropositive including voltage gated potassium channel complex autoantibody negative for LGI1 and CASPR2 subtyping, 2 (0.17 nmol/L and 0.48 nmol/L [normal, ≤0.02]); ganglionic acetylcholine receptor autoantibody, 1 (0.06 nmol/L [normal, ≤0.02]); and glutamic acid decarboxylase 65 autoantibody, 1 (0.06 nmol/L [normal, ≤0.02])
dby 2016 autoimmune encephalitis diagnostic criteria who did not meet criteria for definite or probable autoimmune encephalitis
eThese disorders are considered immune related disorders by the 2016 diagnostic criteria and categorized as such here
fMeeting one of the subcategories: definite autoimmune encephalitis, autoantibody-defined disease (e.g., antibodies against intracellular antigens, synaptic receptors, ion channels or other cell surface proteins that strongly associate with autoimmune encephalitis); definite autoimmune limbic encephalitis; definite acute disseminated encephalomyelitis (ADEM); autoimmune NMDA-receptor encephalitis (probable and definite); Bickerstaff’s brainstem encephalitis (probable and definite); Hashimoto encephalopathy; and “autoantibody-negative but probable” autoimmune encephalitis.6
Data Collection
Electronic and paper medical records were reviewed, for demographic details (age, sex, race/ethnicity), period of follow-up, co-existing autoimmunity, clinical data, laboratory results, radiologic findings and outcome at last follow-up using the modified Rankin score (mRS). All patients included had given consent for the passive use of their medical record for research purposes and patients that did not authorize the use of their medical records for research were excluded.
Diagnostic Criteria
Diagnoses were assigned by three neurologists (D.D., E.P.F., A.S.L.) from independent medical record review and categorized as infectious or autoimmune encephalitis.
Autoimmune encephalitis diagnosis utilized criteria from a recent position paper on a clinical approach to this diagnosis.6 The initial criteria to be met for consideration of possible autoimmune encephalitis include: 1) a compatible clinical syndrome of subacute/rapidly progressive altered mental status, memory loss or psychiatric symptoms; 2) One or more of: A) focal CNS findings; B) new seizures; C) Cerebrospinal fluid (CSF) pleocytosis; D) MRI abnormalities consistent with autoimmune encephalitis; 3) Reasonable exclusion of other etiologies.6 Patients with possible autoimmune encephalitis are then stratified via a diagnostic algorithm into specific subcategories of probable and definite autoimmune encephalitis including: definite autoimmune encephalitis, autoantibody-defined disease (e.g., antibodies against intracellular antigens, synaptic receptors, ion channels or other cell surface proteins that strongly associate with autoimmune encephalitis); definite autoimmune limbic encephalitis; definite acute disseminated encephalomyelitis (ADEM); autoimmune NMDA-receptor encephalitis (probable and definite); Bickerstaff’s brainstem encephalitis (probable and definite); Hashimoto encephalopathy (probable autoimmune); and “autoantibody-negative but probable” autoimmune encephalitis.6 Patients with a final diagnosis of possible autoimmune encephalitis not meeting criteria for a probable or definite autoimmune encephalitis category were excluded.
Comparison group of infectious encephalitis
For a comparison group, we evaluated the incidence and prevalence of infectious encephalitis including meningoencephalitis and progressive multifocal leukoencephalopathy (PML) per previously published criteria,8,9 but additionally we required conformation of an infectious pathogen (bacterial/viral/fungal/parasitic) for inclusion. Those with presumed infectious encephalitis without a confirmed pathogen or a prion disorder were excluded (Figure 1).
Neural Autoantibody markers with high specificity for autoimmune encephalitis
As outlined in the diagnostic criteria for autoimmune encephalitis we only considered patients whose serum or CSF was positive for one or more IgG neural autoantibodies that strongly associate with and are highly specific for encephalitis as “autoantibody positive”. The following autoantibody specificities were screened for by standardized indirect immunofluorescence assay (IFA): α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor [GluA1 and GluA2 subunits], amphiphysin, anti-neuronal nuclear antibody (ANNA)-1 [anti-Hu], ANNA- 2 [anti-Ri], ANNA-3, collapsin response-mediator protein (CRMP)-5, contactin-associated protein 2 (CASPR2), dipeptidyl-peptidase-like protein 6 (DPPX), ɣ-aminobutyric acid (GABA) B receptor, glial fibrillary acidic protein (GFAP), glutamic acid decarboxylase (65 kD isoform; GAD65), leucine rich glioma-inactivated protein 1 (LGI1), Ma2 (performed through Athena diagnostics), metabotropic glutamate receptor 5 (mGluR5), NMDA-receptor [GluN1 subunit] and purkinje cell cytoplasmic autoantibody type 2/Microtubule associated protein 1B.10,11 GAD65 IgG was assessed via radioimmuno-precipitation assay and for this study GAD65 positivity was an inclusion criterion for the antibody positive subgroup only if detected in CSF or if the serum titer exceeded 20 nmol/L (normal, ≤0.02 nmol/L).12 Cell-based assays (CBAs) using human embryonic kidney (HEK) 293 cells transfected with appropriate expression plasmids were used to confirm AMPA receptor, CASPR2, DPPX, GABAB, mGluR5, LGI1 and NMDA-receptor specificities (Euroimmun, Lubeck, Germany). IFA patterns consistent with GFAPα were confirmed similarly by in-house CBA.11 Myelin oligodendrocyte (MOG) IgG were detected by live in-house (CLIA approved) flow cytometry (FACS) assay. Patients seropositive for any unclassified neural autoantibody recognized in our Neuroimmunology Laboratory’s unpublished experience to be strongly associated with autoimmune encephalitis, were included in the “autoantibody positive” group. We retested 13 specimens (11 sera, 2 CSF) for neural antibodies not tested at the time of service evaluation.
Neural autoantibodies not highly specific for autoimmune encephalitis
For this study the following autoantibodies were considered not specific for autoimmune encephalitis and seropositivity for one or more of these antibodies was insufficient to be designated antibody positive: voltage-gated Kv1 potassium channel-complex autoantibodies but negative on subtyping for CASPR2 and LGI1;13 ganglionic acetylcholine receptor autoantibodies; GAD65 positivity in serum of lower serum titer (<20 nmol/L); muscle acetylcholine receptor binding autoantibody; N or P/Q type voltage gated calcium channel autoantibodies; and striated muscle autoantibodies.12 These patients were excluded and considered antibody negative for this study unless they had a co-existing antibody strongly associated with autoimmune encephalitis or they fulfilled the much more stringent criteria for probable or definite autoimmune encephalitis subcategories that do not require antibodies.
Infectious evaluation of CSF
Infectious testing included one or more of: gram stain, aerobic and anaerobic bacterial culture, mycobacterial culture and AFB stain (selected cases), cryptococal antigen, fungal culture, IgM and IgG specific for west nile virus (WNV), LaCrosse virus and lyme disease, PCR for lyme, ehrlichia, herpes simplex virus (HSV) 1 and 2, human herpes virus (HHV) 6, varicella zoster virus (VZV), John Cunningham virus (JCV) and California encephalitis virus.
Statistical methodology
Patient and clinical characteristics were compared between autoimmune and infectious encephalitis sub-groups using Kruskal-Wallis or Fisher’s exact test, as appropriate.
Age- and sex-specific population counts were obtained from the Rochester Epidemiology Project Census of Olmsted County for January 1, 2014 and annually for 1995 through 2015. Prevalence rates were calculated as the number of patients with autoimmune or infectious encephalitis (or their specific sub-groups) divided by the population count as of January 1, 2014, and were reported per 100,000 people. The incidence rates were calculated as the number of patients with autoimmune or infectious encephalitis (or their specific sub-groups) divided by the total number of person-years at risk and were reported per 100,000 person-years. These rates were adjusted using the direct method to the sex and age distribution of the total United States population in 2010.
Prevalence and incidence rates for autoimmune and infectious encephalitis were compared using generalized logit models in which the 3 patient levels were those that were disease free, those with autoimmune encephalitis and those with infectious encephalitis. Prevalence and incidence rates for autoimmune encephalitis were separately calculated for non-Hispanic white (Caucasian) patients and for African-American patients using the corresponding race-specific Olmsted County population counts. Incidence rates were separately calculated within each encephalitis group and sub-group for 1995–2005 and for 2006–2015. Poisson regression models adjusted for age and sex were used to compare the race-specific rates as well as the incidence rates across the two-time periods. Analyses were performed using SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC).
Excluded patients with encephalitis of unknown etiology and immune related disorders
Fifty-two patients with encephalitis of unknown etiology and immune related disorders were excluded (Figure 1). Twenty-five of these patients were tested for neural antibodies and 4 were seropositive for antibodies not specific for autoimmune encephalitis including: voltage gated potassium channel complex autoantibody negative for LGI1 and CASPR2 subtyping, 2 (0.17 nmol/L and 0.48 nmol/L [normal, ≤0.02]); ganglionic acetylcholine receptor autoantibody, 1 (0.06 nmol/L [normal, ≤0.02]); and glutamic acid decarboxylase autoantibody, 1 (0.06 nmol/L [normal, ≤0.02]). Eight excluded patients with possible autoimmune encephalitis received immunotherapy treatment trials and six responded.
Results
Comparison of Autoimmune Encephalitis and Infectious Encephalitis
The prevalence and incidence rates for autoimmune and infectious encephalitis did not differ (respectively, 13.7 vs 11.6/100,000; p=0.63 and 0.8 vs 1.0/100,000 person-years, p=0.58; Tables 2 & 3). Autoimmune encephalitis prevalence slightly exceeded that of viral encephalitis but the difference was not significant (13.7 vs 8.3/100,000; p=0.17); incident rates were about equivalent (0.8 [autoimmune] vs 0.6 [viral] per 100,000 person-years; p=0.36). The number of relapses or recurrent hospitalizations was higher for autoimmune than infectious encephalitis (Table 1) but neither mRS at last follow-up nor the number of documented deaths differed significantly. Demographics and clinical data for the 2 patient groups are compared in Table 1. The prevalence and incidence data for autoimmune encephalitis and infectious encephalitis, and their sub-groups, are summarized in Table 2 and 3 and Figure 2 (A and B). Figure 3 (A–H) shows MRI examples of autoimmune and infectious encephalitis etiologies.
Table 2.
Age- and sex-adjusted prevalence and incidence rates for autoimmune encephalitis and its subtypes, in the Olmsted County population.
| Olmsted County total prevalent cases 1/1/2014 |
Olmsted County total incident cases (1995–2015) |
||||
|---|---|---|---|---|---|
| Olmsted population or person-years |
155,285 | 2,961,635 | |||
| N | Prevalence (per 100,000 population)a |
N | Incidence (per 100,000 person- years)a |
||
| Autoimmune encephalitis | All cases | 21 | 13.7 | 24 | 0.8 |
| Definite autoimmune encephalitis, specific disease with Ab | 10 | 6.5 | 11 | 0.4 | |
| Definite Limbic encephalitis (without Ab) | 3 | 2.0 | 5 | 0.2 | |
| Definite ADEM without Abb | 5 | 3.3 | 3 | 0.1 | |
| Probable Autoimmune encephalitis without Ab | 2 | 1.3 | 4 | 0.1 | |
| Hashimoto’s encephalitis | 1 | 0.6 | 1 | 0.03 | |
| Neural autoantibodies with high specificity for autoimmune encephalitis | MOGc,d | 3 | 1.9 | 3 | 0.1 |
| GAD65e,f | 3 | 1.9 | 3 | 0.1 | |
| NMDARd | 1 | 0.6 | 1 | 0.03 | |
| LGI1c,e | 1 | 0.7 | 0 | -- | |
| GFAPc | 1 | 0.6 | 1 | 0.03 | |
| ANNA-2 | 1 | 0.6 | 1 | 0.04 | |
| CRMP5c,e | 1 | 0.7 | 0 | -- | |
| AMPARc | 0 | -- | 1 | 0.03 | |
| Unclassified Ab | 2 | 1.4 | 2 | 0.07 | |
Key: Ab, antibody; ADEM, Acute disseminated encephalo-myelitis; AMPAR, amino-3-hydroxy-5-methyl-4-isoxazolepropionic receptor; ANNA-2, antineuronal nuclear antibody-2; CRMP5, collapsin response-mediator protein 5; GAD65, glutamic acid decarboxylase 65; GFAP, glial fibrillary acidic protein; LGI1, leucine rich glioma-inactivated protein 1; MOG, myelin oligodendrocyte glycoprotein; NMDAR, N-methyl-D-aspartate receptor.
Prevalence and incidence rates were directly adjusted to the US total population for age and sex using the 2010 census. The Olmsted County population was completely enumerated, not sampled, so confidence intervals were not reported.
One patient not evaluated for MOG antibodies.
Antibodies detected only in serum.
One patient had both MOG and NMDAR antibodies.
One patient had CRMP5, LGI1 and GAD65 antibodies. He also had other antibodies associated with myasthenia gravis and thymoma including muscle acetylcholine receptor binding (titer 18.4 nmol/L), acetylcholine receptor modulating (100%) and striational antibodies (titer 307200).
One patient with GAD65 IgG and bilateral limbic encephalitis had antibodies evaluated only in serum (titer 147.0 nmol/L).
Low titer GAD65 IgG (titer <20 nmol/L) was detected in four patients who met criteria for definite autoimmune encephalitis: two MOG-IgG seropositive (GAD65 IgG titer 0.06 nmol/L and 0.15 nmol/L), one AMPA-R Ig (GAD65 IgG titer 0.11 nmol/L) and one unclassified antibody (GAD65 IgG titer 0.03 nmol/L)
Table 3.
Age- and sex-adjusted prevalence and incidence rates for infectious encephalitis in the Olmsted County population.
| Olmsted County total prevalent cases 1/1/2014 |
Olmsted County total incident cases (1995–2015) |
|||||
|---|---|---|---|---|---|---|
| Olmsted population or person-years | 155,285 | 2,961,635 | ||||
| N | Prevalence (per 100,000 population)b |
N | Incidence (per 100,000 person- years)b |
|||
| Infectious encephalitisa |
All cases | 18 | 11.6 | 28 | 1.0 | |
| Viral Encephalitis | All cases | 13 | 8.3 | 18 | 0.6 | |
| HSV-1/HSV-2 | 4 | 2.5 | 7 | 0.2 | ||
| VZV | 1 | 0.6 | 2 | 0.07 | ||
| WNV | 2 | 1.3 | 3 | 0.1 | ||
| Lacrosse virus | 2 | 1.3 | 1 | 0.03 | ||
| HHV-6 | 1 | 0.6 | 1 | 0.03 | ||
| Enterovirus | 1 | 0.7 | 1 | 0.03 | ||
| EBV | 1 | 0.6 | 1 | 0.04 | ||
| JCV (PML) | 1 | 0.7 | 2 | 0.07 | ||
| Bacterial encephalitis/meningoencephalitis | All cases | 5 | 3.3 | 8 | 0.3 | |
| E.coli | 1 | 0.7 | 2 | 0.07 | ||
| Streptococcus Pneumoniae | 2 | 1.3 | 2 | 0.07 | ||
| Group B streptococcus | 1 | 0.6 | 1 | 0.03 | ||
| Listeria | 0 | -- | 1 | 0.04 | ||
| Mycoplasma | 1 | 0.6 | 1 | 0.03 | ||
| Ehrlichia | 0 | -- | 1 | 0.03 | ||
| Fungal (aspergillus) meningo-encephalitis | 0 | -- | 1 | 0.03 | ||
| Parasitic (toxoplasma) encephalitis | 0 | -- | 1 | 0.04 | ||
Key: EBV, Epstein–Barr virus; E. coli, Escherichia coli; HHV-6, human herpes virus-6; HSV-1, herpes simplex virus-1; HSV-2, herpes simplex virus-2; JCV = John Cunningham virus; PML, progressive multifocal leukoencephalopathy; VZV, varicella zoster virus; WNV, West Nile virus.
Four patients were tested for neural autoantibodies and all were negative.
Prevalence and incidence rates were directly adjusted to the US total population for age and sex using the 2010 census. The Olmsted County population was completely enumerated, not sampled, so confidence intervals were not reported.
Table 1.
Comparison of the characteristics of prevalent and incident autoimmune and infectious encephalitis cases.
| Autoimmune encephalitis (n=28)a |
Infectious encephalitis (n=29)a |
P-value | |
|---|---|---|---|
| Demographics | |||
| Onset age, yrs., median (range) | 43.0 (2.0–74.0) | 43.0 (0.0–91.0) | 0.64 |
| Female sex, N (%) | 10 (36) | 20 (69) | 0.01 |
| Race, African-American (%) | 4 (14) | 1 (3) | 0.19 |
| Clinical data | |||
| Follow-up, yrs., median (range) | 6.9 (0–22.8) | 7.6 (0–17.2) | 0.79 |
| Abnormal brain MRI (%)# | 26 (93) | 16/29 (55) | 0.001 |
| Inflammatory CSF (%)## | 14/24 (58) | 27/29 (93) | 0.003 |
| Malignancy (%) | 6 (21) | 1 (3) | 0.009 |
| Number of patients with relapses (%) | 9 (32) | 2 (7) | 0.01 |
| Median # of Relapses (range) | 1 (0–10) | 0 (0–3) | 0.03 |
| Median hospitalization period, months (range) | 5.5 (0–62) | 7.5 (0–45) | 0.60 |
| mRS of last follow up | 2 (0–6) | 2 (0–5) | 0.70 |
| Death, N (%) | 2 (7) | 7 (24) | 0.14 |
| Interval from diagnosis to death, median, years (range) | 6.2 (0.1–12.2) | 0.1 (0–9.8) | 0.24 |
Key: CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; mRS, modified Rankin score.
Includes patients with autoimmune or confirmed infectious encephalitis, both prevalent and incident cases.
Consistent with encephalitis.
CSF protein >50 mg/dL and/or CSF nucleated cells >5/dL.
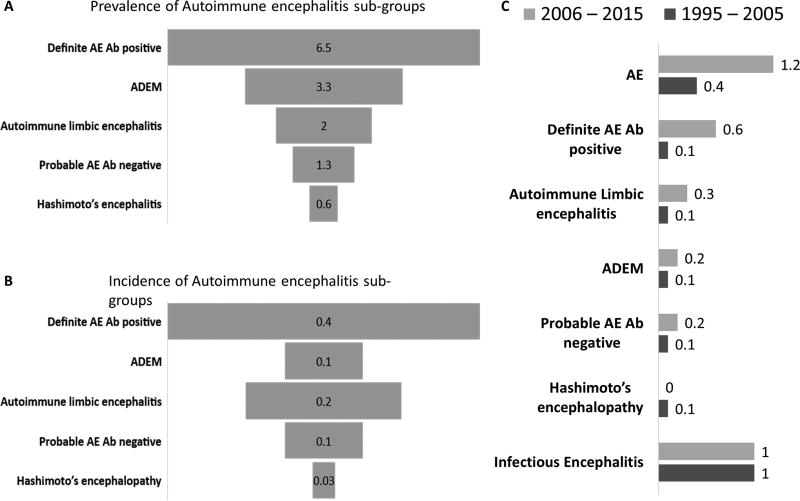
Figure 2. (A–C): Prevalence and incidence of autoimmune encephalitis sub-groups and incidence trends of encephalitis etiologies over the last two decades.
Prevalence (per 100,000 population) of autoimmune encephalitis sub-groups (A). Incidence (per 100,000 person-years) of autoimmune encephalitis sub-groups (B). Trends in incident rates per 100,000 person-years) of autoimmune encephalitis, definite autoimmune encephalitis (with CNS specific antibodies), autoimmune limbic encephalitis, ADEM, probable autoimmune encephalitis, Hashimoto’s encephalitis and infectious encephalitis (1995–2005 and 2006–2015) (C).
Key: Ab, antibody; AE: autoimmune encephalitis; ADEM, Acute disseminated encephalo-myelitis
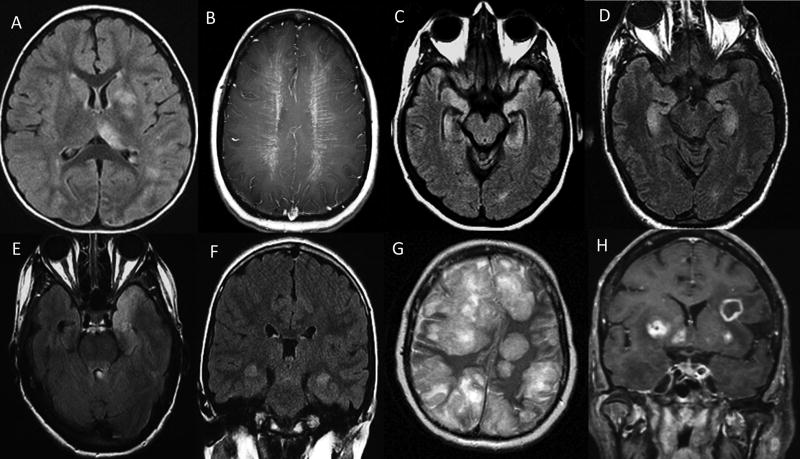
Figure 3. Depicting of MRI brain of autoimmune (A–D) and infectious (E–H) encephalitis cases.
Poorly demarcated diffuse FLAIR hyperintensities involving left putamen, thalamus, white matter and juxta-cortical regions in acute disseminated encephalomyelitis (A); peri-ventricular radial enhancement consistent with glial fibrillary acidic protein IgG associated encephalitis (B); bilateral mesial temporal lobe FLAIR hyperintensities in an antibody negative patient (C); and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor encephalitis patient (D); bilateral (left>right) anterior temporal lobe FLAIR hyperintensity in a case of herpes simplex virus-1 encephalitis (E); bilateral medial temporal lobe FLAIR hyperintensity in a case of human herpes-6 virus encephalitis (F); multifocal FLAIR hyperintensities with mass effect and right-ward midline shift in a case of disseminated aspergillosis (G); and multifocal ring-like enhancement on T1- post-gadolinium sequence in a case of toxoplasmosis (H).
Disproportionate African-American ethnicity representation in autoimmune encephalitis
The prevalence of autoimmune encephalitis was higher among African-American population (38.3/100,000) than Caucasians (13.7/100,000; p=0.04), particularly for autoimmune GAD65 encephalitis (2) and ADEM (2 [1 patient MOG-IgG positive]). The incidence of autoimmune encephalitis also was higher among African-Americans (2.8/100,000 person-years) than Caucasians (0.7/100,00 person-years, p=0.01). The ethnic proportionality did not differ among incident/prevalent infectious encephalitis.
Time trends in autoimmune encephalitis and infectious encephalitis
The incidence of autoimmune encephalitis increased from the 1995–2005 interval to the 2006–2016 interval (0.4 [1995–2005] to 1.2 [2006–2015], p=0.02) (Figure 2C). An increase in neural-specific IgG-associated encephalitis was the major contributor to this trend (0.1 [1995–2005] to 0.6 [2006–2015], p=0.03). Incident rates of infectious encephalitis remained unchanged over the two decades (0.9 [1995–2005] to 1.0 [2006–2015], p=0.87) (Figure 2C).
Categories of encephalitis for all incident and prevalent autoimmune encephalitis cases
Definite autoimmune encephalitis, autoantigen designated
One or more neural-specific IgGs were detected in 13 autoimmune encephalitis cases: all four patients who were MOG-IgG positive in serum met criteria for ADEM; two of them had coexisting serum GAD65 autoantibodies (0.06 nmol/L, 0.15 nmol/L [normal, ≤0.02]). One, a boy aged 2 years at onset who was dual seropositive for NMDA-receptor-IgG and MOG-IgG, had recurrent encephalitis, seizures, optic neuritis and multifocal MRI abnormalities (Figure 3A). NMDA-receptor-IgG was detected in CSF by cell-based assay alone and MOG-IgG was detected in serum by FACS (titer 100 [normal<2.5]). Clinically, diagnostic criteria were met for autoimmune NMDA-receptor encephalitis and ADEM; no tumor was found. Three patients had isolated GAD65-IgG (patient 1: serum 697 nmol/L, CSF 13.3 nmol/L; patient 2: serum 147.0 nmol/L; patient 3: CSF 4.9 nmol/L [normal, ≤0.02]). Their clinical findings included altered mental status, brain stem dysfunction, neuropsychiatric manifestations, seizures and refractory status epilepticus. In one patient a multifocal neurologic disorder developed including autoimmune encephalitis accompanied by focal epilepsy, dysautonomia and myasthenia gravis leading to the diagnosis of thymoma. His serum was positive for LGI1-IgG by cell-based assay, CRMP5-IgG (titer 960 [normal, <240] confirmed by western blot), GAD65-IgG (23.1 nmol/L [normal, ≤0.02]), acetylcholine receptor binding autoantibodies (6.08 nmol/L [normal, ≤0.02]) and striated muscle autoantibodies (titer 30720 [normal, <120]). One patient with serum GFAP-IgG positivity on tissue immunofluorescence confirmed by cell-based assay using GFAPα isoform had the hallmark clinical syndrome of meningoencephalitis accompanied by bilateral optic disc edema, tremors, radial enhancement on head MRI (Figure 3B)14 and brain biopsy showed perivascular chronic inflammatory infiltrates, comprised mainly of mature T-lymphocytes, microglial activation and focal microglial nodules. One patient with limbic encephalitis accompanied by bilateral mesial temporal T2-hyperintensity with subsequent atrophy on MRI (Figure 3C) was seropositive for AMPAR-IgG in serum by immunofluorescence (no titer available) and cell-based assay leading to the detection of breast carcinoma; serum GAD65 autoantibody coexisted (0.11 nmol/L; normal, ≤0.02]) in this patient. Another patient in whom breast carcinoma was found after the onset of neurologic symptoms presented with jaw dystonia, laryngospasm and neuropsychiatric manifestations and was ANNA-2-IgG seropositive in both serum (titer=7680 [normal, <240]) and CSF (titer=16 [normal, <2] and was confirmed by western blot. Two patients with CSF unclassified neural-specific antibodies (serum titers 1920, 15360 [normal, <240]) and CSF titers 128, 1024 [normal, <4]) one with coexisting serum GAD65 autoantibody (0.03 nmol/L [normal, ≤0.02]), had subacute cognitive decline and brainstem symptoms and signs. The serological findings prompted a search for cancer leading to diagnosis of seminoma in both cases.
Definite autoimmune limbic encephalitis
Five patients without detectable neural autoantibodies met criteria for diagnosis of definite autoimmune limbic encephalitis (clinical criteria, 5; bilateral MRI mesial temporal signal changes, 5 [Figure 3D]; CSF pleocytosis, 2, or EEG criteria, 3) and alternative etiologies were excluded.
Definite ADEM
Five patients without a detected neural autoantibody fulfilled diagnostic criteria for ADEM.
Bickerstaff encephalitis
No patients met diagnostic criteria for probable or definite Bickerstaff encephalitis.
Hashimoto encephalitis
One patient met criteria for Hashimoto encephalitis with encephalopathy, myoclonus, thyroid disease, thyroid peroxidase antibody seropositivity, normal MRI brain, no neural autoantibody detected in serum or CSF and response to immunotherapy.
Seronegative probable autoimmune encephalitis
Four patients with a compatible clinical syndrome met at least two of the three criteria for this diagnosis: Head MRI findings consistent with encephalitis (right medial temporal lobe T2/FLAIR hyperintensity [n=1], multifocal cortical T2/FLAIR hyperintensities [n=1], multifocal subcortical T2/FLAIR hyperintensities [n=2]); CSF inflammation (pleocytosis, 1; supernumerary oligoclonal bands/elevated IgG index, 1); brain biopsy showing inflammatory infiltrates and excluding other disorders (n=3). One of the four patients had ganglionic (α3) acetylcholine receptor (titer 0.11 nmol/L [normal, ≤0.02]) and muscle acetylcholine receptor antibodies (titer 0.07 nmol/L [normal, ≤0.02]) both considered less specific for autoimmune encephalopathy and thus was not included in the antibody positive section.
Cancer associations of autoimmune encephalitis
Six patients had paraneoplastic autoimmune encephalitis with the following neoplasms identified (3 after a predictive neural autoantibody profile was detected): breast carcinoma (2), seminoma (2; both were seronegative for Ma1 and Ma2 IgGs), thymoma (1), lymphoma (1).
Treatments and response in autoimmune encephalitis
Immunotherapy was administered to 26 of 28 patients (93%). High-dose corticosteroids (23), IVIG (2) and plasmapheresis (1) were the initial immunomodulatory therapies. Twenty-one (81%) improved clinically following immunotherapy, either as sole initial treatment (86%) or in combination with cancer therapy (14%). Thirteen patients (46%) received second line or maintenance treatment (mycophenolate mofetil [4], cyclophosphamide [3], extended IVIG [>6 months, 3], rituximab [2], extended prednisone course [>6 months, 1]).
Discussion
This study shows that the incidence and prevalence of autoimmune encephalitis approximates that of infectious encephalitis at a population level. Furthermore, the detection of autoimmune encephalitis is increasing over time. Although both infectious and autoimmune etiologies are associated with considerable morbidity, the tendency to relapse was greater in patients with autoimmune encephalitis, thus increasing the disease burden.
To our knowledge, this is the first study to determine the epidemiology of autoimmune encephalitis at a population level; showing an incidence of 0.8/100,000 person-years and prevalence of 13.7/100,000. Antibody-positive definite autoimmune encephalitis was the most prevalent category; next was ADEM. The most frequently identified neural autoantibody specificities were MOG and GAD65 (in CSF or in serum at high titer), with a single case of NMDA-receptor encephalitis identified with coexisting MOG-IgG. Prior epidemiology studies of encephalitis have mostly focused on infectious etiologies, with “immune-mediated” syndromes occasionally analyzed as a subgroup. Studies have either utilized administrative data2,4, hospital evaluation data, or sample referral from selected hospitals to a centralized organization for epidemiological evaluation.15 A prospective study of encephalitis in the United Kingdom that enrolled patients from 24 hospitals over a period of 2 years found infectious etiology to be twice as common as autoimmune (42% vs 21% [the remainder were of uncertain etiology]); ADEM and NMDA-receptor encephalitis were the most common subcategories.16 A direct comparison to our study is limited, as that study was hospital-based rather than truly population-based, the repertoire of antibodies available has expanded, and diagnostic criteria that we used were not available at that time. Additionally, among young patients tested at a referral laboratory for infectious encephalitis agents in California (the California encephalitis project), the frequency of NMDA-receptor encephalitis was found to equate to viral causes in young individuals.17 The incident rate of encephalitis (combined autoimmune and infectious) was lower than an Olmsted County study from 1950–1981 (1.8 vs 7.4/100,0000 person-years) reflecting our exclusion of encephalitis of unknown etiology and aseptic meningitis, the increased availability of vaccines in recent years and access to MRI during our time period which is very helpful in refining diagnosis.18
Autoimmune encephalitis is a broad term that encompasses many different neurological diseases depending on the definition used; we used the 2016 diagnostic criteria for autoimmune encephalitis.6 In utilizing these criteria, we took numerous steps to avoid an overestimate of autoimmune encephalitis frequency. Firstly, only patients with probable or definite autoimmune encephalitis per the 2016 criteria were included (Figure 1). Secondly, diseases with more chronicity (e.g., Morvan syndrome), clinical presentations different to classic limbic encephalitis (e.g., progressive encephalomyelitis rigidity and myoclonus [PERM]) or immune related disorders (e.g., Rasmussen encephalitis [Figure 1]) were excluded per the 2016 diagnostic criteria. Thirdly, only antibodies highly specific for autoimmune encephalitis (often detected by cell-based assay), were included in the antibody positive category and these antibodies are only rarely found in disease or healthy controls (0.2%).19 Many patients designated antibody positive had CSF antibody detection which is preferred for some antibodies (e.g., NMDA-R). However, antibody detection in serum alone can also be highly specific and may be required if CSF testing is not available (e.g., MOG-IgG) or preferred if sensitivity is optimal in serum without loss of specificity (e.g., serum LGI1 autoantibodies); all patients in this study with serum antibody positivity alone had syndromes compatible with autoimmune encephalitis.20–22 With the exception of GAD 65 (serum >20 nmol/L or detectable in CSF), patients with neural autoantibodies less specific for autoimmune encephalitis detected by older generation techniques (e.g., immunoprecipitation assay) which can be found in controls (up to 6%)19 were designated antibody negative for the purposes of this study. Such patients were excluded unless they had a coexisting highly specific antibody or they met more stringent criteria for antibody negative autoimmune encephalitis. Care is needed in those with neural antibodies not strongly associated with autoimmune encephalitis (e.g., voltage-gated potassium-channel autoantibodies negative for LGI1 and CASPR2) as a positive result can lead to premature diagnostic closure, an alternative diagnosis being overlooked and iatrogenic morbidity from inappropriate immunosuppression.6,13,23 A positive antibody test result should never replace clinical judgement.
The 2016 diagnostic criteria focus on the limbic encephalitis phenotype. Patients with paraneoplastic/autoimmune encephalitis involving the brainstem alone (e.g., accompanying Ma2 autoantibodies) may not meet criteria. Additionally, patients with faciobrachial dystonic seizures and LGI1 autoantibodies would not meet diagnostic criteria until cognitive impairment occurred, yet rapid diagnosis and early treatment may prevent cognitive impairment.24 Future criteria need to consider how to best capture such cases, particularly if such criteria are to be used for enrollment in clinical trials. Treatment response was not a component of the 2016 criteria which aimed at diagnosis prior to treatment and sought to distinguish autoimmune encephalitis from other steroid-responsive-disorders (e.g., lymphoma). However, this could result in under-representation of some autoimmune encephalitis cases as 6 excluded patients met criteria for possible autoimmune encephalitis and responded to immunotherapy but lacked the classic features of limbic encephalitis required for inclusion into a subcategory of autoimmune encephalitis.
The detection of autoimmune encephalitis is likely to increase over time and the prevalence and incidence in our study is likely an underestimate. This is evident by the tripling of its incidence from 1995–2005 to 2006–2015, markedly in antibody-positive cases (Figure 2B). Other contributors to under-representation may include the lack of widespread recognition of what we now recognize as classic syndromes (e.g., neuropsychiatric syndrome of anti-NMDA-receptor encephalitis; faciobrachial dystonic seizures in LGI1-antibody encephalitis) over the duration of our study (1995–2015),25,26 lack of availability of samples in those with encephalitis of unknown etiology and the likelihood of further neural autoantibodies being discovered in the future. Infectious encephalitis may also be underrepresented as next-generation sequencing for infectious agents was not available for this study.27,28
The higher incidence and prevalence of autoimmune encephalitis among African-Americans is consistent with our prior reports of their predisposition for autoimmune GAD65 encephalomyelitis and for a similar CNS autoimmune disease, aquaporin-4-IgG seropositive neuromyelitis optica spectrum disorder.29 This observation in autoimmune encephalitis is preliminary. Given that this population in general has less access to and receives lower quality medical care,30 further study of populations with higher proportions of African-Americans are needed.31
Distinguishing paraneoplastic/autoimmune encephalitis from infectious or other etiologies can be difficult but is aided by laboratory testing for infectious/autoimmune etiologies and the presence of clinical and radiologic clues (Figure 3). As infectious meningoencephalitis and PML (which may lack accompanying inflammation) may mimic autoimmune encephalitis these were included in the infectious category. Infection may be a trigger for CNS autoimmunity (e.g. NMDA-receptor encephalitis post HSV-1 infection) though we did not encounter such cases in this study.32,33
Strengths of our study are, firstly, that Olmsted County is an excellent population for epidemiologic study due to its almost complete population coverage.31 Secondly, the availability of the Mayo Clinic Neuroimmunology Laboratory located within this county allowed comprehensive analysis and re-analysis of samples. Limitations of our study include the lack of standardized testing for all infectious and autoimmune etiologies inherent in a population-based epidemiology study and differences in diagnostic criteria between infectious encephalitis (confirmation of pathogen) and autoimmune encephalitis (probable or definite by 2016 diagnostic criteria). When interpreting the frequency of specific autoantibodies one must be mindful of inherent variability of incidence/prevalence of rare disorders. For example, the detection rate for NMDA-receptor autoantibody in the Mayo Neuroimmunology service Laboratory (12/month) is more frequent than for AMPAR autoantibody (2/month) yet their incidence was equal in this population-based study. Nonetheless, our study provides an overview of the incidence and prevalence of autoimmune encephalitis by contemporary diagnostic criteria.
In summary, this study shows that autoimmune encephalitis represents a large proportion of encephalitis whose detection is increasing over time. More population-based studies of autoimmune encephalitis are needed to evaluate the frequency of these disorders in other populations.
Acknowledgments
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions:
Conception and design of the study: D.D. and E.P.F.
Acquisition and analysis of data: all authors.
Drafting the manuscript or figures: D.D. and E.P.F.
Study supervision: E.P.F.
Potential conflicts of interest
No authors had conflicts of interests relevant to this study.
References
- 1.Vora NM, Holman RC, Mehal JM, Steiner CA, Blanton J, Sejvar J. Burden of encephalitis-associated hospitalizations in the United States, 1998–2010. Neurology. 2014;82(5):443–451. doi: 10.1212/WNL.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 2.George BP, Schneider EB, Venkatesan A. Encephalitis hospitalization rates and inpatient mortality in the United States, 2000–2010. PLoS One. 2014;9(9):e104169. doi: 10.1371/journal.pone.0104169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granerod J, Crowcroft NS. The epidemiology of acute encephalitis. Neuropsychol Rehabil. 2007;17(4–5):406–428. doi: 10.1080/09602010600989620. [DOI] [PubMed] [Google Scholar]
- 4.Parpia AS, Li Y, Chen C, Dhar B, Crowcroft NS. Encephalitis, Ontario, Canada, 2002–2013. Emerg Infect Dis. 2016;22(3):426–432. doi: 10.3201/eid2203.151545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venkatesan A, Geocadin RG. Diagnosis and management of acute encephalitis: A practical approach. Neurol Clin Pract. 2014;4(3):206–215. doi: 10.1212/CPJ.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkatesan A, Tunkel AR, Bloch KC, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57(8):1114–1128. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKeon A, Pittock SJ. Paraneoplastic encephalomyelopathies: pathology and mechanisms. Acta Neuropathol. 2011;122(4):381–400. doi: 10.1007/s00401-011-0876-1. [DOI] [PubMed] [Google Scholar]
- 11.Fang B, McKeon A, Hinson SR, et al. Autoimmune Glial Fibrillary Acidic Protein Astrocytopathy: A Novel Meningoencephalomyelitis. JAMA Neurol. 2016;73(11):1297–1307. doi: 10.1001/jamaneurol.2016.2549. [DOI] [PubMed] [Google Scholar]
- 12.Walikonis JE, Lennon VA. Radioimmunoassay for glutamic acid decarboxylase (GAD65) autoantibodies as a diagnostic aid for stiff-man syndrome and a correlate of susceptibility to type 1 diabetes mellitus. Mayo Clin Proc. 1998;73(12):1161–1166. doi: 10.4065/73.12.1161. [DOI] [PubMed] [Google Scholar]
- 13.van Sonderen A, Schreurs MW, de Bruijn MA, et al. The relevance of VGKC positivity in the absence of LGI1 and Caspr2 antibodies. Neurology. 2016;86(18):1692–1699. doi: 10.1212/WNL.0000000000002637. [DOI] [PubMed] [Google Scholar]
- 14.Flanagan EP, Hinson SR, Lennon VA, et al. Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: Analysis of 102 patients. Ann Neurol. 2017;81(2):298–309. doi: 10.1002/ana.24881. [DOI] [PubMed] [Google Scholar]
- 15.Glaser CA, Gilliam S, Schnurr D, et al. In search of encephalitis etiologies: diagnostic challenges in the California Encephalitis Project, 1998–2000. Clin Infect Dis. 2003;36(6):731–742. doi: 10.1086/367841. [DOI] [PubMed] [Google Scholar]
- 16.Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 17.Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis. 2012;54(7):899–904. doi: 10.1093/cid/cir1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beghi E, Nicolosi A, Kurland LT, Mulder DW, Hauser WA, Shuster L. Encephalitis and aseptic meningitis, Olmsted County, Minnesota, 1950–1981: I. Epidemiology. Ann Neurol. 1984;16(3):283–294. doi: 10.1002/ana.410160304. [DOI] [PubMed] [Google Scholar]
- 19.Lang K, Prüss H. Frequencies of neuronal autoantibodies in healthy controls: Estimation of disease specificity. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e386. doi: 10.1212/NXI.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017 doi: 10.1093/brain/awx276. [DOI] [PubMed] [Google Scholar]
- 21.Gadoth A, Pittock SJ, Dubey D, et al. Expanded phenotypes and outcomes among 256 LGI1/CASPR2-IgG positive patients. Ann Neurol. 2017 doi: 10.1002/ana.24979. [DOI] [PubMed] [Google Scholar]
- 22.McCracken L, Zhang J, Greene M, et al. Improving the antibody-based evaluation of autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm. 2017;4(6):e404. doi: 10.1212/NXI.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali F, Murray JA, Adams AC, Flanagan EP. Clinical Reasoning: A 54-year-old woman with dementia, myoclonus, and ataxia. Neurology. 2017;89(2):e7–e12. doi: 10.1212/WNL.0000000000004093. [DOI] [PubMed] [Google Scholar]
- 24.Irani SR, Stagg CJ, Schott JM, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain. 2013;136(Pt 10):3151–3162. doi: 10.1093/brain/awt212. [DOI] [PubMed] [Google Scholar]
- 25.Irani SR, Michell AW, Lang B, et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol. 2011;69(5):892–900. doi: 10.1002/ana.22307. [DOI] [PubMed] [Google Scholar]
- 26.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson MR, Naccache SN, Samayoa E, et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370(25):2408–2417. doi: 10.1056/NEJMoa1401268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salzberg SL, Breitwieser FP, Kumar A, et al. Next-generation sequencing in neuropathologic diagnosis of infections of the nervous system. Neurol Neuroimmunol Neuroinflamm. 2016;3(4):e251. doi: 10.1212/NXI.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pittock SJ, Yoshikawa H, Ahlskog JE, et al. Glutamic acid decarboxylase autoimmunity with brainstem, extrapyramidal, and spinal cord dysfunction. Mayo Clin Proc. 2006;81(9):1207–1214. doi: 10.4065/81.9.1207. [DOI] [PubMed] [Google Scholar]
- 30.Epstein AM, Ayanian JZ. Racial disparities in medical care. N Engl J Med. 2001;344(19):1471–1473. doi: 10.1056/NEJM200105103441911. [DOI] [PubMed] [Google Scholar]
- 31.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linnoila JJ, Binnicker MJ, Majed M, Klein CJ, McKeon A. CSF herpes virus and autoantibody profiles in the evaluation of encephalitis. Neurol Neuroimmunol Neuroinflamm. 2016;3(4):e245. doi: 10.1212/NXI.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leypoldt F, Titulaer MJ, Aguilar E, et al. Herpes simplex virus-1 encephalitis can trigger anti-NMDA receptor encephalitis: case report. Neurology. 2013;81(18):1637–1639. doi: 10.1212/WNL.0b013e3182a9f531. [DOI] [PMC free article] [PubMed] [Google Scholar]