Abstract
Purpose
There is limited information on region-specific gene expression in the human corneal stroma. In this study, we aimed to investigate the expression profile of the extracellular matrix and adhesion molecules in the normal corneal stroma using laser capture microdissection (LCM) and molecular techniques.
Methods
Frozen sections of human cornea without ocular disease were used to isolate the central and peripheral corneal stromal keratocytes by LCM. RNA was extracted from LCM-captured tissues and the RT2 Profiler PCR Arrays were used to examine the expression profile of extracellular matrix and adhesion molecules in the central and peripheral stroma. Real-time quantitative PCR was used to quantify gene expression. Proteomic and western blotting (WB) analyses were performed to confirm gene expression at protein level. Function association network was generated via the web tools String and Cytoscape.
Results
The gene expression profiling demonstrated that 35 out of the 84 extracellular matrix and adhesion molecules represented in the array were expressed in stromal keratocytes. Among them, 24 genes were not previously described in the corneal stroma. Two genes were found more abundantly expressed in the central stroma than in the periphery: TGFBI, COL6A2 (p < 0.05). ADAMTS13 was detected only in the central stroma. Proteomics and WB analysis confirmed the expression of 10 genes. Functional analysis revealed that most identified genes were presented in a core cluster that had multiple and strong associations with other genes.
Conclusion
This study identified genes not previously described in the corneal stroma, revealed regional differences in gene expression between central and peripheral stroma, and also detected some interesting candidate genes that may play important roles in corneal function. These observations serve as the foundation to further investigate the molecular and cellular mechanisms regulating the pathogenesis of regional corneal stromal disorders such as keratoconus.
Keywords: Adhesion molecules, corneal stroma, extracellular matrix, gene expression, laser capture microdissection
Introduction
The corneal stroma accounts for 90% of corneal thickness and is largely composed of extracellular matrix (ECM), embedded keratocytes and corneal nerves. In experimental models (largely chicken and rabbit), corneal stroma is shown to be hyaluronan/water-enriched ECM during the embryonic stages, but after hatch or birth it is replaced with collagen-enriched ECM.1,2 Collagen is the predominant constituent of the ECM. A variety of collagen isoforms have been identified in human corneal stroma, including collagen I, III, IV, V, VI, and XII.3–7 Among them, collagen I is the mostly abundant type and collagen V is an important regulator of collagen fibril assembly.8,9 Proteoglycans (PGs) are another important class of ECM components in the corneal stroma, consisting of a core protein covalently bound to one or more glycosaminoglycan chains and comprising 4–5% of the dry weight of the cornea. The other components of the stromal matrix include glycoproteins, salts, and some molecules such as integrins, matrix metalloproteinases (MMPs), and tissue inhibitors of metalloproteinases (TIMPs).10–12
The predominant cell type residing in the corneal stroma is the keratocyte, which is a specialized fibroblast of neural crest origin. Keratocytes play an important role in synthesizing the stromal components (such as PGs, collagen, and proteases), corneal wound healing, and maintaining corneal clarity. Under physiological conditions, keratocytes are in a quiescent state in the G0 phase of cell cycle with limited ability to self-renew. Interestingly, stem or progenitor cells for keratocytes have been recently characterized in the human corneal stroma close to the limbus.13,14 After injury, keratocytes undergo programmed cell death or become mitotically active and then attempt to repair the injured cornea by secreting the extracellular molecules needed for rebuilding corneal stroma. A number of signaling molecules that regulate the activities of keratocytes are synthesized and secreted by corneal epithelial cells such as interleukin-1α, tumor necrosis factor-α, and transforming growth factor-β (TGF-β), suggesting the interaction between the two cell types in wound healing.15,16 However, in some pathological conditions, corneal keratocytes lose the ability to respond to regulatory signaling and synthesize, and cease secretion of the ECM molecules that are required for repair, thus leading to cell death and corneal dysfunction.17
Corneal stromal structural integrity and keratocyte homeostasis are critical for normal corneal function. Though much is known about ECM change and keratocyte responses to corneal wound healing,18,19 there is limited information on ECM-related gene expression in keratocytes in the normal human cornea or regional differences in gene expression. Such baseline information is important and would serve as a reference point for gene expression studies in the human corneal stroma during wound healing and other disorders such as keratoconus (KCN) and corneal stromal dystrophies (CSD). Therefore, in this study we isolated RNAs from keratocytes in the central and peripheral stroma of normal human corneas to study the gene expression profile, with a focus on the ECM and adhesion molecules because they are the major molecules in the corneal stroma and are affected in disorders that compromise the structure of the corneal stroma. The laser capture microdissection (LCM) approach was used because the keratocytes can be isolated for gene expression analysis. The advantage of the LCM approach is that the isolated keratocytes are not to be contaminated with corneal endothelial and epithelial cells. The results showed expression of a large number of genes in stroma and identified some genes that were differentially expressed between the central and peripheral stroma, and genes that had multiple associations with other genes in the network modeling.
Materials and methods
Human cornea
Collection of corneas for the study was approved by the Institutional Review Board of Johns Hopkins University. Nine corneas were obtained from four anonymous individual donors aged 27–62 years from Tissue Banks International (TBI, Baltimore, MD, USA) and five donors aged 51–76 years from the Lions Eye Institute (Tampa, FL, USA). All eyes were phakic with no history of eye disease or previous eye surgery. The causes of death were myocardial infarction (2), traumatic death (2), stroke (1), end-stage renal disease (2), and cancer, not on chemotherapy (2). Time of death to enucleation was 3–16 h for all subjects. All these corneal buttons were dissected free from the limbus at contributing eye bank. The corneal tissues from the first four donors dying of traumatic injury and myocardial infarction were utilized for PCR array and western blotting (WB). These corneas were preserved in Optisol™ (Bausch & Lomb, Rochester, NY, USA) at the TBI eye bank. Immediately after arrival at the Wilmer Eye Institute, these four cornea buttons were trephined into the central 6 mm buttons and peripheral rings, and then bisected, halves were embedded in optimal cutting temperature (OCT) compound with central and peripheral parts intact, snap frozen in liquid nitrogen and stored at −80°C until used for cryo-sectioning. The other halves of the corneal button were stored at −80°C until processed for WB. The remaining five corneas were placed in eye bank vials and refrigerated until being received within 24–36 h and then frozen at −80°C until processed for proteomics sample preparation. The use of corneal samples is summarized in Table 1.
Table 1.
The usage of corneal samples in the study (a total of nine cornea buttons were used).
| Number of human corneas | Region | Usage | |
|---|---|---|---|
| 4 | Four halves | Central and peripheral stroma | LCM and PCR array |
| Three halves | Central and peripheral stroma | Protein extraction for western blotting | |
| 5 | Whole stroma | Proteomics | |
LCM, amplified antisense (a) RNA and cDNA synthesis
LCM was performed on the 20 μm cryo-sections of the central (6 mm) and peripheral corneal stroma (6–9 mm) with a LMD6000 laser capture microdissection microscope (Leica Microsystems, Deerfield, IL, USA) according to the previous published technique.20 The dissected stroma was collected in 60 μl RLT lysis buffer (Qiagen, Valencia, CA, USA) and then stored at −80°C. The cornea stroma from the four donors was processed and analyzed separately. Total RNA was isolated with RNeasy Micro kit (Qiagen, Valencia, CA, USA). RNA concentration and quality were checked with a Nanodrop 2000 (Thermo Scientific, Wilmington, DE, USA), a ratio of A260/280 between 1.8 and 2.0 were obtained in all RNA samples. RNA samples were then used for aRNA amplification and cDNA synthesis, which was conducted with the Target Amp 2-Round Aminoallyl-aRNA amplification kit (Epicentre, Wilmington, DE, USA) and SuperScript III first-strand system (Invitrogen, Carlsbad, CA, USA), respectively. After digesting the remaining RNA with RNase H, the reaction mix was diluted fivefold and used as templates in real-time PCR reactions.
Human ECM and adhesion molecule PCR array
The cDNAs from laser-dissected corneal stroma were used for the human ECM and adhesion molecule PCR array (cat #: PAHS-013A SABiosciences, Frederick, MD, USA). Eighty-four transmembrane molecules, cell–cell and cell–matrix adhesion, and ECM plus housekeeping genes (as internal controls) were included in the array that was used to screen for genes that are expressed by corneal keratocytes (a complete list of genes is available at: http://www.sabiosciences.com/rt_pcr_product/HTML/PAHS-013A.html).
The assay was performed according to the protocol described in the product manual as real-time quantitative polymerase chain reaction (QPCR). Briefly, PCR assay was carried out in 96-well PCR plates using a Bio-Rad iCycler system (Bio-Rad, Hercules, CA, USA). The 20 μl reaction mix included 10 μl of 2× SYBR Green Supermix, 20 nM of target gene primer mix, and 50 ng of cDNA template. QPCR conditions included an initial denaturing step for 3 min at 95°C, followed by 40 cycles (95°C for 15 s, 58°C for 20 s, and 72°C for 25 s). The validity of each individual PCR reaction was confirmed by melting curves, which were obtained by heating samples from 58°C to 95°C. Agarose gel analysis was performed to further confirm the amplicons. The quantification was calculated using comparable threshold cycles (CT). The data were normalized by subtracting the housekeeping gene GAPDH’s CT from the gene of interest CT (ΔCT = gene of interest CT – GAPDH CT) for each array. Then 2–ΔCT was defined as the relative expression level of individual genes. The ΔCT difference between central and peripheral stroma was designated as ΔΔCT (ΔΔCT = central stroma ΔCT – peripheral stroma ΔCT). The gene expression ratio of central stroma to peripheral stroma was calculated as 2−ΔΔCT. Only those genes detected in central and/or peripheral stromas of at least two independent corneal samples were identified as valid expressed genes and further compared between regions and analyzed. The results were averaged and expressed as mean ± SD for each gene.
Liquid chromatography/tandem mass spectrometry (LC-MS/MS) and WB
The epithelium and endothelium from the remaining five corneal buttons were removed. The epithelium was removed by scraping with a # 15 blade and alcohol. The endothelium was also removed by scraping with a blade and peeling of Descemet’s membrane. The stroma from each sample was cryogenically pulverized using a cryogenic grinder (Retsch Mixer Mill 400, Haan, Germany) and then sonicated in lysis buffer containing 6 M urea, 5% sodium dodecyl sulfate (SDS), 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 42 mM KCl, 0.1 mM ethylenediaminetetraacetic acid (EDTA), 0.1 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 1 mM dithiothreitol, and protease inhibitor cocktail (Sigma Aldrich, St. Louis, MO, USA). The samples were centrifuged, and supernatant was used for subsequent analysis. Protein concentrations were measured using the Pierce microBCA protein assay (Thermo Scientific, Rockford, IL, USA). Stromal extracts were run on 1D sodium dodecyl sulfate polyacrylamide gel (SDS PAGE) gels, stained with Coomassie blue, and cut into 10 bands. Gel bands were destained with 25% acetonitrile and 100 mM ammonium bicarbonate, dehydrated, reduced with 10 mM dithiothreitol (DTT) in 25 mM ammonium bicarbonate, alkylated with 55 mM iodoacetamide in 25 mM ammonium bicarbonate, and digested with high performance liquid chromatography (HPLC) grade of trypsin (Promega, Madison, WI, USA) overnight. Tryptic peptide samples were desalted using Pierce C18 tips and resuspended in 0.1% formic acid. Each digested gel band obtained from stroma samples was run on a 2 h linear gradient of 2–30% acetonitrile with 0.1% formic acid using an EasySpray source coupled with an Orbitrap Elite (Thermo Scientific, West Palm Beach, FL, USA) mass spectrometer. EasySpray source was run at 35°C on a 25 cm × 75 μm integrated spray tip column. Peptides were trapped at 980 bar on a 2 cm × 75 μm trapping column. The trap was a 3 μm particle and the column was a 2 μm Acclaim Pepmap C18. All individual samples were run with two technical replicates.
Raw MS spectra were batch analyzed using two complementary search engines, X!Tandem and OMSSA. Data were handled using i3D (Shimadzu and Integrated Analysis, Shimadzu Scientific Instruments Inc., Columbia, MD, USA), and parameters allowed for two missed tryptic cleavages and a fragment ion mass error of 1. Spectral count was used to determine the relative abundance of proteins in each sample group. Probability scores were filtered at 90% for spectral count. Protein Prophet algorithm was used to assign protein probabilities using a false discovery rate of 0.1% for peptides and 1.0% for proteins. Proteins identified by less than two unique peptides or spectral counts were eliminated.
Corneal samples for WB were processed and proteins were extracted similarly as above. Three central half buttons and peripheral half rings were used. WB was performed to further confirm the expression of collagen type 5 alpha 1 (COL5A1). Equal amounts (20 μg) of corneal protein samples were loaded on a 4–12% gradient SDS PAGE. The proteins were then transferred into a nitrocellulose membrane. After blocking with 2% bovine serum albumin, the membranes were probed with the primary antibodies: mouse antihuman COL5A1 (1:1000, Sigma Aldrich, St. Louis, MO, USA) followed by horseradish-peroxidase-conjugated antimouse secondary antibody (1:5000; Jackson ImmunoResearch Laboratories Inc, West Grove, PA, USA). The signal was detected by enhanced chemiluminescence with SuperSignal West Pico kit (Thermo Scientific, Rockford, IL, USA).
Functional association network of ECM genes in cornea
The functional association of human genes was generated and downloaded from online STRING database program (http://string-db.org), as described by Szklarczyk et al.21 The cutoff of confidence score was 0.8, representing the probability that the functional association between two genes are larger than 0.8. Cytoscape software22 was employed to draw the functional association network of known or newly identified cornea genes.
Statistical analysis
Paired T test was used for the statistical analyses of comparisons between groups: central stroma versus peripheral stroma. p < 0.05 was designated as being statistically significant.
Results
Central and peripheral corneal stroma isolated by LCM
With LCM technique, we isolated the central and peripheral corneal stroma (Figure 1) to study the region-specific gene expression by corneal keratocytes. This approach allowed us to isolate keratocytes without contamination from the corneal endothelium and/or epithelium and divide the stroma into central and peripheral portions. The number of keratocytes captured by LCM was calculated by this equation: the area of stroma × the thickness of section × the density of keratocytes (20522 cells/mm3).23 From the four human corneal samples, we collected an average of 9891 ± 1120 keratocytes from the central area and 26404 ± 2327 from the peripheral cornea (Figure 1d). Total RNA was prepared from the laser-captured stroma for gene expression analysis (each RNA was individually processed). Only the RNAs, which were not degraded and were based on the correct amplification of housekeeping gene GAPDH, were used for further analysis of gene expression.
Figure 1.
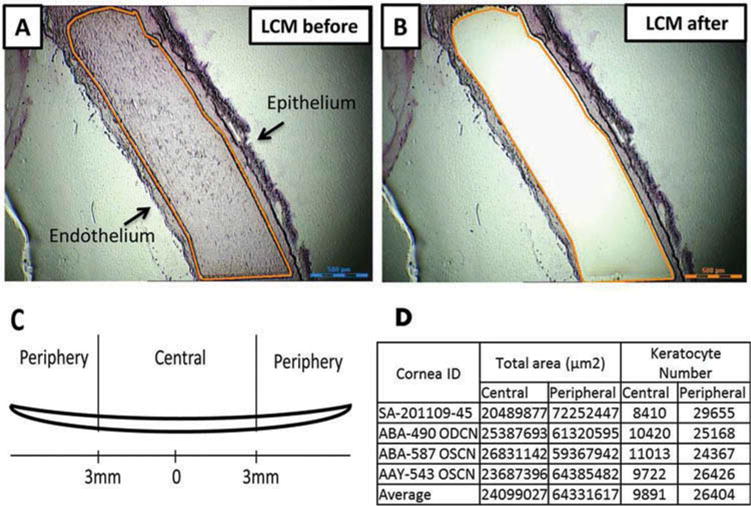
Isolation of human corneal stroma by laser capture microdissection (LCM). The 9–10 mm corneal tissues from the equator were collected and cryopreserved. Also, 20 μm sections were made for LCM. (a, b) The representative images of corneal sections that were cut before and after LCM. (c) Diagram defining central and peripheral corneal stroma. (d) The data summary of keratoctyes that were captured by laser from central and peripheral corneal stroma. Scale bar: 500 μm.
ECM gene expression in corneal keratocytes
With the human ECM and adhesion molecule RT2 PCR array, in which 84 key molecules were included, 35 ECM and adhesion molecule genes (42%) were detected from the LCM-isolated keratocytes in both central and peripheral stroma. One additional gene, ADAMTS13, was detected only in the central stroma, but not in the periphery.
The specificity of PCR amplicons is shown in Figure 2. The relative expressions of the 35 ECM genes in central and peripheral stroma, respectively, which were determined by real-time QPCR and normalized by the housekeeping gene GAPDH, are shown in Table 1. The six most abundant genes in central stroma and their relative expression were: COL6A2 (11.50 ± 10.83), SPARC (10.73 ± 14.0), COL12A1 (7.46 ± 4.82), TGFBI (7.09 ± 4.98), TIMP2 (5.79 ± 3.54), and ECM1 (3.47 ± 1.79) (Table 2). The six most abundant genes in peripheral corneal stroma and their relative expression were: ITGA6 (13.43 ± 18.58), COL12A1 (3.69 ± 3.15), COL6A2 (3.51 ± 3.43), TGFBI (3.49 ± 2.09), COL5A1 (3.04 ± 5.08), and FN1 (2.64 ± 2.5) (see Table 2).
Figure 2.
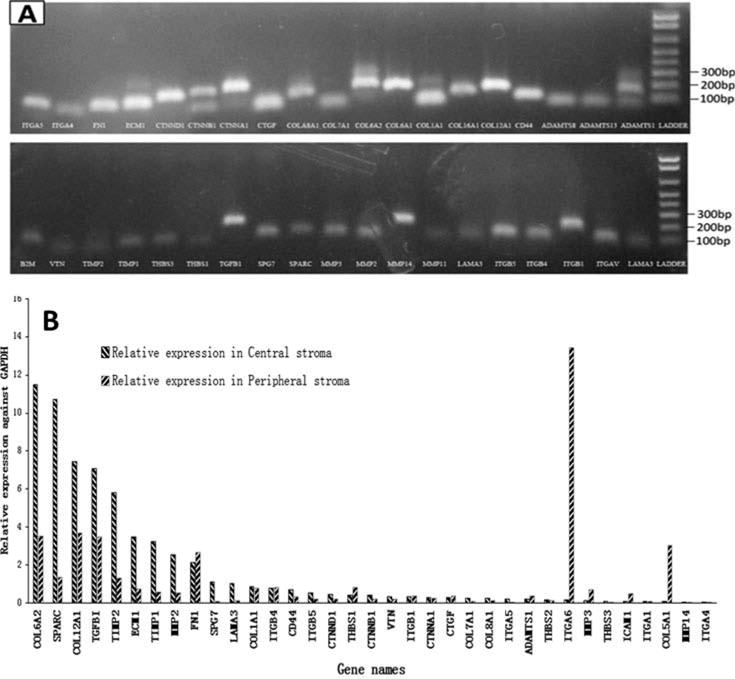
Expression of ECM genes in the corneal stroma. In total, 84 key ECM genes represented in the PCR arrays were quantified by real-time PCR. The specificity of primers was monitored with melting curves. (a) The PCR products were resolved in agarose gel and stained by ethidium bromide dye, further indicating the specificity of primers and PCR products. (b) The histogram showed the relative expression of 35 ECM genes in both central and peripheral stroma, respectively. GAPDH served as a control gene for normalization.
Table 2.
Relative expression of the 35 genes by corneal keratocytes in central and peripheral stroma.
| Gene name | Gene description | Central stroma
|
Peripheral stroma
|
||
|---|---|---|---|---|---|
| Mean CT | Mean R | Mean CT | Mean R | ||
| COL6A2 | Collagen, type VI, alpha2 | 26.44 | 11.50 | 29.63 | 3.51 |
| SPARC* | Secreted protein, acidic, cysteine-rich | 26.85 | 10.73 | 31.29 | 1.37 |
| COL12A1 | Collagen, type XII, alpha 1 | 26.08 | 7.46 | 29.38 | 3.69 |
| TGFBI* | Transforming growth factor, beta-induced, 68 kDa | 26.17 | 7.09 | 28.76 | 3.48 |
| TIMP2 | TIMP metallopeptidase inhibitor 2 | 26.22 | 5.79 | 30.07 | 1.33 |
| ECM1 | Extracellular matrix protein1 | 27.16 | 3.47 | 31.33 | 0.75 |
| TIMP1* | TIMP metallopeptidase inhibitor 1 | 27.78 | 3.24 | 32.43 | 0.56 |
| MMP2* | Matrix metallopeptidase 2 | 27.19 | 2.53 | 32.17 | 0.53 |
| FN1 | Fibronectin 1 | 29.56 | 2.11 | 29.27 | 2.64 |
| SPG7 | Spastic paraplegia 7 | 29.81 | 1.08 | 33.58 | 0.09 |
| LAMA3 | Laminin, alpha 3 | 30.90 | 1.03 | 34.56 | 0.13 |
| COL1A1* | Collagen, type I, alpha1 | 32.78 | 0.87 | 32.21 | 0.78 |
| ITGB4 | Integrin, beta 4 | 29.43 | 0.76 | 30.41 | 0.83 |
| CD44 | CD44 molecule | 30.25 | 0.70 | 31.86 | 0.35 |
| ITGB5 | Integrin, beta5 | 29.82 | 0.53 | 32.94 | 0.22 |
| CTNND1 | Catenin, delta 1 | 30.52 | 0.45 | 33.24 | 0.20 |
| THBS1* | Thrombospondin 1 | 29.90 | 0.41 | 30.69 | 0.82 |
| CTNNB1 | Catenin, beta 1, 88 kD | 31.03 | 0.40 | 32.99 | 0.21 |
| VTN | Vitronectin | 30.65 | 0.34 | 33.33 | 0.22 |
| ITGB1* | Integrin, beta 1 | 29.08 | 0.32 | 30.72 | 0.35 |
| CTNNA1 | Catenin, alpha 1, 102 kD | 30.94 | 0.30 | 31.28 | 0.25 |
| CTGF | Connective tissue growth factor | 30.48 | 0.28 | 32.08 | 0.36 |
| COL7A1 | Collagen, type VII, alpha1 | 30.00 | 0.24 | 33.75 | 0.09 |
| COL8A1 | Collagen, type VIII, alpha1 | 33.95 | 0.24 | 33.12 | 0.14 |
| ITGA5 | Integrin, alpha 5 | 33.21 | 0.20 | 33.66 | 0.05 |
| ADAMTS1 | ADAM metallopeptidase with thrombospondin type 1 | 34.39 | 0.19 | 33.36 | 0.36 |
| THBS2* | Thrombospondin 2 | 29.93 | 0.17 | 33.53 | 0.12 |
| ITGA6 | Integrin, alpha 6 | 27.78 | 0.16 | 28.55 | 13.43 |
| MMP3 | Matrix metallopeptidase 3 | 31.24 | 0.11 | 31.30 | 0.71 |
| THBS3 | Thrombospondin 3 | 31.53 | 0.10 | 34.57 | 0.03 |
| ICAM1* | Intercellular adhesion molecule 1 | 31.08 | 0.07 | 32.88 | 0.50 |
| ITGA1 | Integrin, alpha 1 | 31.28 | 0.07 | 33.73 | 0.07 |
| COL5A1* | Collagen, type V, alpha1 | 33.16 | 0.07 | 32.17 | 3.04 |
| MMP14* | Matrix metallopeptidase 14 | 33.04 | 0.06 | 33.58 | 0.04 |
| ITGA4 | Integrin, alpha 4 | 34.54 | 0.03 | 36.11 | 0.02 |
The housekeeping gene GAPDH served as control for normalization.
CT: threshold cycle; RE: Relative expression.
Genes previously known to be expressed in the corneal keratocytes with specific functions and involvement in stromal diseases. Other genes listed were not previously described in keratocytes. The six most abundant genes in central stroma are marked in bold; the six most abundant genes in peripheral stroma are shown in shade or kept in bold.
We compared the gene expression between the central and peripheral keratocytes isolated by LCM. Two genes (TGFBI and COL6A2) showed significantly greater expression in the central keratocytes when compared to the peripheral keratocytes (the expression ratio was 2.08 ± 0.44 for TGFBI, 3.42 ± 1.80 for COL6A2, respectively, p < 0.05). In addition, the expression ratio of central versus peripheral cornea was 8.41 ± 6.76 for TIMP2 and 5.22 ± 4.02 for ITGB5. However, this difference was not statistically significant (p = 0.07 and 0.08, respectively). We did not detect genes that showed lower expression in central than in the peripheral cornea stroma.
Protein identification
An LC-MS/MS-based proteomics analysis revealed that a total of 537 nonredundant proteins were identified in the corneal stroma. A complete list of proteins is shown in supplemental table 1. Among them, nine proteins were found and their corresponding mRNA identified by PCR array analysis: thrombospondin (THBS), collagen alpha (XII) chain (COL12A), integrin beta protein1 (ITGB1), vitronectin (VTN), collagen alpha-2 (VI) chain (COL6A2), collagen alpha-1(I) chain (COL1A1), ECM protein 1 (ECM1), TGF-β-induced (TGFBI), and fibronectin 1 (FN1). Of nine genes/proteins, COL12A, COL6A2, FN1, and ECM1 belong to the most abundant genes in central or peripheral stroma; TGFBI and COL6A2 belong to differentially expressed genes. Figure 3a shows their spectral counts (SC), peptide hits, and SC ratio relative to GAPDH. WB analysis confirmed that another protein COL5A1 was expressed both in central and peripheral cornea (Figure 3b and c).
Figure 3.
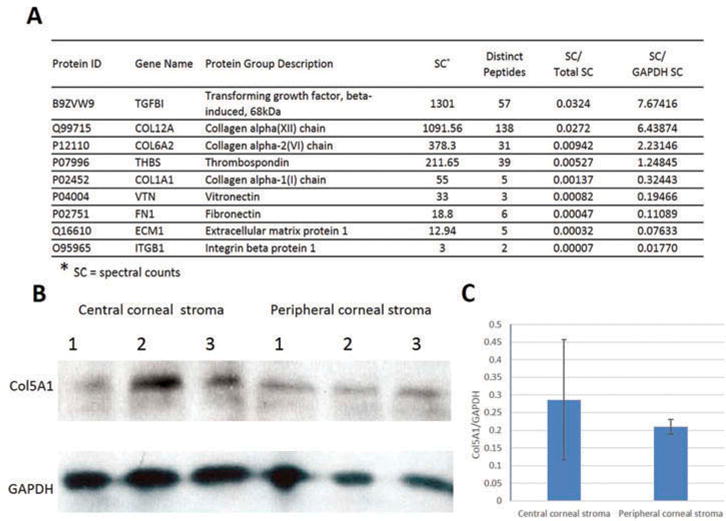
The cornea stroma genes whose protein expressions were confirmed. Corneal stroma without endothelium and epithelium were subjected to LC/MS/MS proteome analysis and western blotting. (a) Proteomics analysis revealed nine proteins matching our gene findings. Among them, four were known genes and the other five were newly identified in our study. (b) Western blotting showed the positive staining for COL5A1 with a band of about 184 kD size both in central stroma and peripheral stroma samples. GAPDH was used as loading control. (c) Densitometric analysis of the mean relative intensity (n = 3) for Col5A1 normalized by GAPDH showed no significant difference between the central and peripheral corneal stroma (p = 0.55).
Functional association network of ECM genes in corneal keratocytes
To demonstrate that the identified keratocyte-expressed genes play a role in cornea-specific function, we performed functional association analysis of the corneal ECM genes identified in this study and those reported in the published literature. We did a literature search using the term “human corneal stromal extracellular matrix” to obtain 259 papers (until September 10, 2015) from which we found that 80 genes were present in the corneal ECM and were associated with corneal stromal disorders (supplemental table 2). Of the 35 genes identified in our study, 11 were among those 80 reported genes, whereas the other 24 genes have not been previously reported in the corneal ECM nor involved in stromal diseases (see those genes without asterisk in Table 1). These genes may therefore represent new ECM genes that are expressed by corneal keratocytes. Additional genes such as the integrins alpha 6 and alpha 4 were identified as reported in the literature12 but not included as previously reported ones since they did not have any known function in the corneal stroma or known to be associated with corneal stromal diseases.
A total of 104 genes (80 from literatures + 35 identified in this study – 11 redundant genes = 104) were input into the online String database program to create the functional network. Ninety genes were validated for presence in the network. The results are illustrated in Figure 4. We found that 65 genes formed a core cluster in the functional association network, suggesting these genes likely play important physiological roles in the cornea. The remaining 25 genes were isolated nodes, suggesting that they may be involved in the basic universal cell processes, such as metabolism and maintenance, but not have cornea-specific roles such as transparency and light refraction. Interestingly, all the 11 known corneal genes identified in this study were found to be present in the core cluster and also have multiple connections with the other genes in the core cluster, which suggest they may be implicated in maintenance of the corneal integrity and keratocytes homeostasis (see the red nodes in Figure 4).
Figure 4.
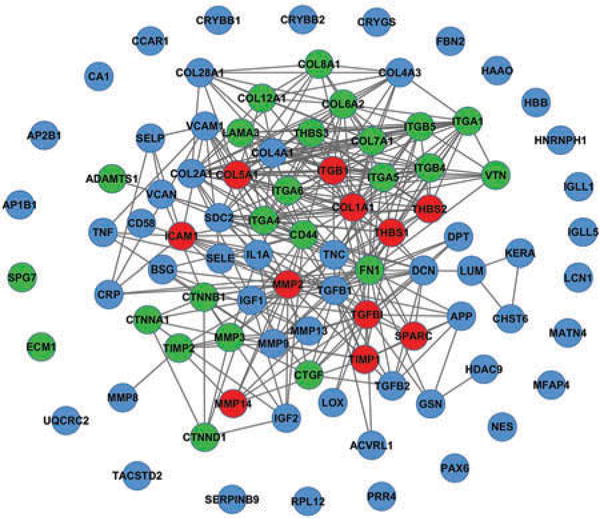
Functional association network of ECM genes in cornea. The string and Cytoscape programs were applied to model the functional association of the stroma ECM genes that were identified in the present study and from the literatures. Red nodes represent the ECM genes that were found in both literatures and our study to be expressed by stromal keratocytes and present in the core cluster. Blue nodes represent known ECM genes that were exclusively obtained from published literatures. Green nodes represent novel ECM genes that we identified in this study. Gray lines indicated that the connected gene pair may involve in common biological process (confidence score >0.8). Functional associations are extracted from STRING database. This figure is produced by Cytoscape software.
Discussion
In this study, we investigated the gene expression of ECM and adhesion molecules in the normal LCM-dissected corneal keratocytes. The LCM technique allowed isolation of the corneal stroma, where keratocytes reside, without contamination from corneal epithelium or endothelium. We further studied the LCM captured stroma from the central and peripheral cornea to compare region specific gene expression. With a focus on the ECM and adhesion molecules, quantitative expressions of ECM genes of central and peripheral keratocytes were examined. Thirty-five genes were expressed in both central and peripheral corneal stroma. Ten were further confirmed by proteomics and WB techniques. The central cornea showed differentially greater expression of two genes than the periphery as discussed below. Computational modeling analysis of novel and known corneal ECM genes permitted the functional networks of these genes to be determined, validated the quality of our expression data, and also identified 11 hub genes with multiple associations with other genes.
Several groups of genes appear to be interesting candidates that should be considered for prospective functional studies. The six most abundantly expressed genes in central stroma were COL6A2, SPARC, COL12A1, TGFBI, TIMP2, and ECM1. The other six most abundant genes in peripheral stroma were ITGA6, COL12A1, COL6A2, TGFBI, COL5A1, and FN1. There is some difference in the most abundant genes between these two corneal regions, which indicates the different locations may have different gene expression profiles. Further statistical analysis identified two genes that show significantly greater expression in the central stroma than peripheral – TGFBI, COL6A2 – and one additional gene identified only in the central stroma – ADAMTS13. ADAMTS13, a disintegrin and metalloproteinase with thrombospondin type 1 motifs, is synthesized in hepatic stellate cells and endothelial cells, and is the principal von Willebrand factor cleaving protease. It was reported to modulate angiogenesis through upregulation of vascular endothelial growth factor (VEGF) and VEGFR2.24,25 Its presence in central corneal stroma but lack in peripheral stroma deserves further validation and investigation. The regional difference in keratocyte gene expression is intriguing. Clinically, many corneal stromal disorders such as the stromal dystrophies and ectasias stromal dystrophies are regional, and the implication of these findings in regional corneal stromal disorders remains to be explored.
Another group of 11 genes, previously reported and also identified in our study, included MMP2, MMP14, TIMP1, COL1A1, COL5A1, ITGB1, TGFBI, SPARC, ICAM1, THBS1, and THBS2. These were expressed in ECM and present in the core cluster of functional network modeling. Based on the molecular properties of these genes and previous reports (see the references listed in supplemental table 2 for details), they are likely involved in several aspects of cornea-specific function: (1) remodeling of corneal stroma in wound healing in response to injury and other pathological changes; (2) maintenance of corneal structural integrity and transparency during development and in pathophysiological conditions such as KCN, keratoglobus, and pellucid marginal degeneration;3 and (3) involvement in the pathogenesis of corneal disorders. For example, MMP-2 and -14 are the enzymes that catalyze proteolytic degradation of ECM. TIMP-1/2 inhibits the enzymatic activities of MMPs. These opposing enzyme pairs play a role in the corneal remodeling during both development and wound healing.3,23,26 Collagen types I and V are the major fibril-forming collagens of the corneal stroma ECM,9 and transparency of the corneal stroma is accomplished by regulating collagen fibril growth and spacing. COL1A1 is the mostly predominant component in cornea stroma and is necessary for corneal transparency and stability of fibrils.1,3 COL5A1 is also thought to play a key role in the corneal thinning by regulating fibril nucleation in the corneal stroma and is associated with the pathogenesis of KCN.27,28 Our results showed a strong presence of COL5A1 transcript and the protein in both central and peripheral stroma of the normal cornea. Though higher relative expression level was noticed in the peripheral stroma than in the central stroma of our current samples, no significant difference existed between them. This may be due to the small sample size in our study. Other important genes include ITGB1, an integrin family member that plays a role in maintenance of corneal structural integrity.29 TGFBI is a gene encoding an RGD-containing protein that binds to type I, II, and IV collagens as well as a growth factor that controls matrix modulation; its mutations cause several forms of corneal dystrophies.30,31 Chakravarti and colleagues have recently demonstrated a role for TGFBI in KCN.32,33 SPARC is a marker for corneal repair.34 ICAM-1, a transmembrane glycoprotein expressed in epithelium, stroma, and endothelium, plays a key role during inflammation serving as a ligand for CD18 integrins and thereby facilitating polymorphonuclear leukocyte recruitment.35 THBS-1 and -2 are important antiangiogenic factors thought to be involved in maintaining corneal avascularity.36 THBS-1 also contributes to the transformation of keratocytes into myofibroblasts.37
Additionally, 24 out of 35 genes that were identified to be expressed by keratocytes have not been previously reported to be associated with corneal stromal diseases nor present in the corneal ECM, thereby representing a set of new corneal stromal genes (see Table 1 for gene names). The protein expression of five genes from this set of newly identified genes has been confirmed by proteomics analysis. The roles of these newly identified genes in the corneal stroma and their relationships with the other corneal genes are to be elucidated.
Our study has some limitations, one of which is the sample size. Due to the limited availability of normal human corneas, we included only four samples for PCR array in our study. Although these samples were from subjects free of ocular disease, the donor age was quite variable. It is possible that this variation in age might have had an effect on the expression of some genes, leading to biological variances. More samples are warranted for further research. Another limitation is that we performed proteomics profiling on the whole stroma without comparing the protein profiles between central and peripheral stromas, and only a small proportion of genes identified with the PCR array were confirmed by proteomics analysis. In addition, no functional research work was performed in this study.
In conclusion, our results provide insight into global and region-specific gene expression of ECM genes in normal corneal keratocytes. These observations provide foundation toward understanding of the molecular and cellular mechanisms regulating regional corneal disorders, such as KCN and CSD.
Supplementary Material
Acknowledgments
Funding
This study was supported in part by the National Eye Institute grant (R01 EY024596) and the Joint King Khaled Eye Specialist Hospital and Wilmer Eye Institute Research Grant Program.
Footnotes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/icey.
Supplementary data for this article can be accessed on the publisher’s website
Declaration of interest
The authors declare no conflict of interest and financial or business interest related to this study.
References
- 1.Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010;91:326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rada JA, Cornuet PK, Hassell JR. Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteins. Exp Eye Res. 1993;56:635–648. doi: 10.1006/exer.1993.1081. [DOI] [PubMed] [Google Scholar]
- 3.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 4.Nakayasu K, Tanaka M, Konomi H, Hayashi T. Distribution of types I, II, III, IV and V collagen in normal and keratoconus corneas. Ophthalmic Res. 1986;18:1–10. doi: 10.1159/000265406. [DOI] [PubMed] [Google Scholar]
- 5.Newsome DA, Gross J, Hassell JR. Human corneal stroma contains three distinct collagens. Invest Ophthalmol Vis Sci. 1982;22:376–381. [PubMed] [Google Scholar]
- 6.Wessel H, Anderson S, Fite D, Halvas E, Hempel J, SundarRaj N. Type XII collagen contributes to diversities in human corneal and limbal extracellular matrices. Invest Ophthalmol Vis Sci. 1997;38:2408–2422. [PubMed] [Google Scholar]
- 7.Zimmermann DR, Trüeb B, Winterhalter KH, Witmer R, Fischer RW. Type VI collagen is a major component of the human cornea. FEBS Lett. 1986;197:55–58. doi: 10.1016/0014-5793(86)80297-6. [DOI] [PubMed] [Google Scholar]
- 8.Birk DE. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32:223–237. doi: 10.1016/s0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 9.Birk DE, Fitch JM, Babiarz JP, Linsenmayer TF. Collagen type I and type V are present in the same fibril in the avian corneal stroma. J Cell Biol. 1988;106:999–1008. doi: 10.1083/jcb.106.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011;37:588–598. doi: 10.1016/j.jcrs.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 11.Torricelli AA, Wilson SE. Cellular and extracellular matrix modulation of corneal stromal opacity. Exp Eye Res. 2014;129:151–160. doi: 10.1016/j.exer.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stepp MA. Corneal integrins and their functions. Exp Eye Res. 2006;83:3–15. doi: 10.1016/j.exer.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Du Y, Funderburgh ML, Mann MM, SundarRaj N, Funderburgh JL. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23:1266–1275. doi: 10.1634/stemcells.2004-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funderburgh ML, Du Y, Mann MM, SundarRaj N, Funderburgh JL. PAX6 expression identifies progenitor cells for corneal keratocytes. FASEB J. 2005;19:1371–1373. doi: 10.1096/fj.04-2770fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson SE, He YG, Weng J, Li Q, McDowall AW, Vital M, et al. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996;62:325–327. doi: 10.1006/exer.1996.0038. [DOI] [PubMed] [Google Scholar]
- 16.Wilson SE, Liu JJ, Mohan RR. Stromal-epithelial interactions in the cornea. Prog Retin Eye Res. 1999;18:293–309. doi: 10.1016/s1350-9462(98)00017-2. [DOI] [PubMed] [Google Scholar]
- 17.Wilson SE, Mohan RR, Mohan RR, Ambrósio R, Jr, Hong J, Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. 2001;20:625–637. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- 18.Ha NT, Nakayasu K, Murakami A, Ishidoh K, Kanai A. Microarray analysis identified differentially expressed genes in keratocytes from keratoconus patients. Curr Eye Res. 2004;28:373–379. doi: 10.1080/02713680490502201. [DOI] [PubMed] [Google Scholar]
- 19.Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 2000;19:113–129. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 20.Wahlin KJ, Moreira EF, Huang H, Yu N, Adler R. Molecular dynamics of photoreceptor synapse formation in the developing chick retina. J Comp Neurol. 2008;506:822–837. doi: 10.1002/cne.21582. [DOI] [PubMed] [Google Scholar]
- 21.Szklarczyk D, Franceschini A, Kuhn M, Simonovic M, Roth A, Minguez P, et al. The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2011;39:D561–568. doi: 10.1093/nar/gkq973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maguen E, Zorapapel NC, Zieske JD, Ninomiya Y, Sado Y, Kenney MC, et al. Extracellular matrix and matrix metalloproteinase changes in human corneas after complicated laser-assisted in situ keratomileusis (LASIK) Cornea. 2002;21:95–100. doi: 10.1097/00003226-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Lee M, Keener J, Xiao J, Long Zheng X, Rodgers GM. ADAMTS13 and its variants promote angiogenesis via upregulation of VEGF and VEGFR2. Cell Mol Life Sci. 2015;72:349–356. doi: 10.1007/s00018-014-1667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee M, Rodansky ES, Smith JK, Rodgers GM. ADAMTS13 promotes angiogenesis and modulates VEGF-induced angiogenesis. Microvasc Res. 2012;84:109–115. doi: 10.1016/j.mvr.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Ye HQ, Maeda M, Yu FS, Azar DT. Differential expression of MT1-MMP (MMP-14) and collagenase III (MMP-13) genes in normal and wounded rat corneas. Invest Ophthalmol Vis Sci. 2000;41:2894–2899. [PubMed] [Google Scholar]
- 27.Li X, Bykhovskaya Y, Canedo AL, Haritunians T, Siscovick D, Aldave AJ, et al. Genetic association of COL5A1 variants in keratoconus patients suggests a complex connection between corneal thinning and keratoconus. Invest Ophthalmol Vis Sci. 2013;54:2696–2704. doi: 10.1167/iovs.13-11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vitart V, Bencić G, Hayward C, Skunca Herman J, Huffman J, Campbell S, et al. New loci associated with central cornea thickness include COL5A1, AKAP13 and AVGR8. Hum Mol Genet. 2010;19:4304–4311. doi: 10.1093/hmg/ddq349. [DOI] [PubMed] [Google Scholar]
- 29.Parapuram SK, Huh K, Liu S, Leask A. Integrin beta1 is necessary for the maintenance of corneal structural integrity. Invest Ophthalmol Vis Sci. 2011;52:7799–7806. doi: 10.1167/iovs.10-6945. [DOI] [PubMed] [Google Scholar]
- 30.Klintworth GK. Corneal dystrophies. Orphanet J Rare Dis. 2009;4:7–53. doi: 10.1186/1750-1172-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korvatska E, Munier FL, Djemaï A, Wang MX, Frueh B, Chiou AG, et al. Mutation hot spots in 5q31-linked corneal dystrophies. Am J Hum Genet. 1998;62:320–324. doi: 10.1086/301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engler C, Chakravarti S, Doyle J, Eberhart CG, Meng H, Stark WJ, et al. Transforming growth factor-beta signaling pathway activation in Keratoconus. Am J Ophthalmol. 2011;151:752–759. doi: 10.1016/j.ajo.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster J, Wu WH, Scott SG, Bassi M, Mohan D, Daoud Y, et al. Transforming growth factor beta and insulin signal changes in stromal fibroblasts of individual keratoconus patients. PLoS One. 2014;9:e106556. doi: 10.1371/journal.pone.0106556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berryhill BL, Kane B, Stramer BM, Fini ME, Hassell JR. Increased SPARC accumulation during corneal repair. Exp Eye Res. 2003;77:85–92. doi: 10.1016/s0014-4835(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 35.Gagen D, Laubinger S, Li Z, Petrescu MS, Brown ES, Smith CW, et al. ICAM-1 mediates surface contact between neutrophils and keratocytes following corneal epithelial abrasion in the mouse. Exp Eye Res. 2010;91:676–684. doi: 10.1016/j.exer.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cursiefen C, Masli S, Ng TF, Dana MR, Bornstein P, Lawler J, et al. Roles of thrombospondin-1 and -2 in regulating corneal and iris angiogenesis. Invest Ophthalmol Vis Sci. 2004;45:1117–1124. doi: 10.1167/iovs.03-0940. [DOI] [PubMed] [Google Scholar]
- 37.Matsuba M, Hutcheon AE, Zieske JD. Localization of thrombospondin-1 and myofibroblasts during corneal wound repair. Exp Eye Res. 2011;93:534–540. doi: 10.1016/j.exer.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


