Abstract
Background
The mu-opioid antagonist, naltrexone (NTX), is a FDA-approved treatment for alcohol use disorder (AUD); however, the data on whether it differentially affects males vs. females are mixed. NTX increases hypothalamic-pituitary-adrenal (HPA) axis activity that associates with subjective responses to alcohol and craving in individuals with AUD. The present study tested for sex differences in the ability of NTX to decrease appetitive and consummatory behaviors in rats in operant alcohol self-administration. Because the opioid system and HPA axis are sexually dimorphic, we examined NTX’s effect on adrenocorticotropic hormone (ACTH) and corticosterone (CORT) levels.
Methods
Male and female Sprague-Dawley rats (n’s=6–8) were trained to lever press for alcohol (10% v/v) under a fixed-ratio 2 schedule of reinforcement. NTX doses (0, 0.1–10 mg/kg) were assessed in tests conducted under a progressive ratio schedule of reinforcement. Separate groups of alcohol and water drinking rats (n’s=8) were used to assess NTX’s (10mg/kg) effects on HPA axis hormones.
Results
NTX decreased consummatory behaviors for alcohol in a dose-related manner, but not appetitive behaviors in males. In females, NTX decreased appetitive behaviors for alcohol in a dose-dependent manner, but only decreased consummatory behaviors at the highest (10 mg/kg) NTX dose. NTX increased ACTH levels in alcohol drinking females in diestrus, but not in other groups. However, NTX increased CORT levels for longer durations in alcohol drinking males relative to alcohol drinking females in diestrus.
Conclusions
Our findings suggest that NTX selectively reduces consummatory behaviors for alcohol in males and appetitive behaviors in females, while also showing differential sex effects on HPA hormones.
Keywords: naltrexone, alcohol, operant self-administration, sex differences, HPA axis
1.0 Introduction
According to the 2015 National Survey on Drug Use and Health, 15 million adults had a diagnosis of alcohol use disorder (AUD) with more than 5 million of these cases occurring in females (SAMHSA, 2015). Although AUD is twice as likely to be diagnosed in males, females are more susceptible to negative health consequences associated with alcohol consumption. For example, females who consume alcohol have an increased risk of cirrhosis of the liver and hepatitis (Loft et al., 1987), breast cancer (Smith-Warner et al., 1998, Hamajima et al., 2002), cardiovascular disease (Urbano-Marquez et al., 1995), and brain damage (Mann et al., 2005). Increased susceptibility to these conditions may be due to underlying sex differences in alcohol metabolism. For instance, females have greater blood alcohol levels after drinking equivalent amounts of alcohol (Baraona et al., 2001, Frezza et al., 1990) and less alcohol metabolizing enzymes compared to males (Baraona et al., 2001). Given the detrimental effect of AUD in females, it is essential to have treatments that are effective in this vulnerable population.
Naltrexone (NTX) is a mu opioid antagonist that has shown modest efficacy in treating AUD. In human laboratory studies, NTX reduces alcohol’s positive subjective effects (Ray and Hutchison, 2007, Drobes et al., 2004, Ray et al., 2009) and exacerbates negative subjective effects (King et al., 1997, Ray et al., 2009), thereby contributing to attenuation of alcohol self-administration. NTX prevents alcohol-induced dopamine release in the ventral tegmental area and nucleus accumbens (Koob and Le Moal, 2008, Kreek, 1996), key brain regions involved in reward processes. In addition, NTX’s efficacy in treating AUD may also be due to its effects on dopamine-independent mechanisms. Acute alcohol consumption stimulates the hypothalamic-pituitary-adrenal (HPA) axis (Jenkins and Connolly, 1968), while chronic alcohol intake leads to allostatic changes that contribute to blunted HPA activity in several HPA functional tests (Wand and Dobs, 1991, Vescovi et al., 1997, Sorocco et al., 2006) and in response to alcohol consumption (Adinoff et al., 1990, Inder et al., 1995). Indeed, individuals with AUD often suffer from neuroendocrine tolerance (Adinoff et al., 1998, Adinoff et al., 2005), a condition that associates with an increased risk of early relapse in humans (Junghanns et al., 2003, Junghanns et al., 2005) and doubles alcohol consumption in both continuous- and limited-access two bottle choice paradigms in mice (Olive et al., 2003). NTX stimulates the HPA axis in individuals with AUD by preventing beta endorphin-induced inhibition of corticotrophin releasing factor in the paraventricular nucleus of the hypothalamus (Zhou and Kreek, 2014). NTX-induced stimulation of HPA activity negatively associates with alcohol craving and risk of relapse in individuals with AUD (Farren et al., 1999, Kiefer et al., 2006, O’Malley et al., 2002). Interestingly, in a sample of hazardous drinkers that do not meet clinical criteria for AUD, no association between NTX-induced elevations in cortisol levels and alcohol craving was found (Ray et al., 2009). Unfortunately, NTX’s efficacy in females with AUD has not been thoroughly explored. Additionally, there is a sparse literature examining NTX’s effects on HPA activity in alcohol drinking females.
The few studies examining NTX’s efficacy in females with AUD have revealed inconsistent findings (Agabio et al., 2016, Canidate et al., 2017). For example, in humans, studies have found decreases, increases, and no change in NTX’s efficacy in males compared to females (Garbutt et al., 2005, Kiefer et al., 2005, Greenfield et al., 2010, Baros et al., 2008, Pettinati et al., 2008). There are several differences across clinical studies. For instance, there are variations in drinking outcomes measured, duration of NTX treatment, and drinking history of participants, among others. Furthermore, males and females are often collapsed together in statistical analyses examining the relationship between NTX and the HPA axis in alcohol drinking humans. Thus, it remains unclear whether naltrexone stimulates HPA axis activity in females with AUD, and if so, whether cortisol associates with appetitive and/or consummatory behaviors for alcohol in females. Animal studies are beneficial to address these questions in that the environment can be strictly controlled and variables of interest can be selectively manipulated and precisely measured. Although many animal studies have supported NTX’s efficacy at reducing alcohol consumption, few have included females, especially in studies using operant self-administration procedures. To our knowledge, no study has examined sex differences in NTX’s effect on operant alcohol self-administration using an outbred rat strain. Lack of such information is concerning given evidence that the opioid system (Zubieta et al., 1999, Zubieta et al., 2002) and HPA axis (Kudielka and Kirschbaum, 2005, Kitay, 1963, Kitay, 1961) are sexually dimorphic. In addition, males and females of outbred rat strains differ in appetitive and consummatory responses for alcohol in operant procedures (Nieto and Kosten, 2017, Bertholomey et al., 2016). Appetitive and consummatory behaviors characterize distinct stages of the addiction cycle (Koob et al., 2009) and are regulated by separate neurobiological processes (Slawecki and Roth, 2003, Sharpe and Samson, 2001). In addition, appetitive behaviors can interrupt consumption (Breland and Breland, 1961); thus, it would be useful to determine the efficacy of AUD pharmacotherapies on both behaviors within the same operant session (Kosten and Meisch, 2013). Therefore, the purpose of this study is to examine sex differences in NTX effects on appetitive and consummatory behaviors during operant alcohol self-administration and HPA axis activity in alcohol drinking Sprague-Dawley rats.
2.0 Materials and Methods
2.1 Animals
Adult (postnatal day 90–100) male (400–500 g) and female (200–250 g) Sprague-Dawley rats (Charles River, Wilmington, MA) were used in this study. Rats were single-housed in amber polysulfone cages and kept in a temperature- and humidity-controlled vivarium maintained on a 12:12 light/dark cycle (lights on at 7:00 AM). Rats were single-housed to more accurately measure alcohol consumption during overnight drinking in the dark (described below). Additionally, females used in Experiment 1 were free-cycling, while females in diestrus were used in Experiment 2. Animals were given ad libitum access to food and water except during fluid restriction as described below. The Institutional Animal Care and Use Committee at the University of Houston approved the experimental procedures in accordance with guidelines set forth in the “Guide for the Care and Use of Laboratory Animals 8th Edition”.
2.2 Solution and drug preparations
Alcohol (ethyl alcohol, 190 Proof, USP grade, Koptec, King of Prussia, PA) was mixed with tap water to reach a concentration of 10% alcohol (v/v) solution. Naltrexone HCI (NTX; Sigma-Aldrich, St. Louis, MO) was dissolved in isotonic saline at a concentration of 1 mg/ml. NTX was administered subcutaneously (SC) at the following doses: 0 (isotonic saline), 0.1, 0.3, 1, 3, and 10 mg/kg immediately prior to the start of the test session. In the operant studies, each dose was tested twice per animal and the means of those two tests were used. Order of dose presentation was non-systematic and counterbalanced across animals.
2.3 Alcohol drinking in the dark schedule
Rats in the operant studies (Experiment 1) were subjected to a drinking in the dark (DID) schedule beginning two weeks before self-administration training and continued for the remainder of the experiment. Outbred rat strains do not readily self-administer unadulterated alcohol; thus, certain procedures have been developed to facilitate operant alcohol self-administration (Bell et al., 2017). A prior drinking history is one approach to encourage operant alcohol self-administration in outbred rats (Weiss, 2011). Thus, rats were kept on DID throughout Experiment 1 to maintain stable self-administration. Alcohol drinking rats used for the HPA axis hormone studies (Experiment 2) remained on DID schedule for 16 weeks (Table 1). DID was conducted as described previously in (Nieto and Kosten, 2017). Briefly, rats were given access to only 10% alcohol (v/v) for a 16 hr period (5pm to 9am) with water available for 1 hr during the mornings. Self-administration procedures and blood withdrawals began 3–6 hrs after the end of the DID period. Since rats advanced through the self-administration phases individually, only alcohol intake prior to NTX administration was analyzed. Alcohol intake and body weights were measured weekly throughout the entire course of the study. Alcohol intake was converted to g/kg to provide amount of alcohol consumed.
Table 1.
Experimental groups and testing sequence
| Experiment | n | Sex | Procedure 1 | Procedure 2 | NTX dose | Procedure 3 | Data |
|---|---|---|---|---|---|---|---|
| 1 | 8 | Female | DID (26 Weeks) | SA training | 0–10 mg/kg; SC | PR testing | Figure 2; Table 2 |
| 6 | Male | DID (26 Weeks) | SA training | 0–10 mg/kg; SC | PR testing | Figure 2; Table 2 | |
| 2 | 8 | Female | CON & DID (16 weeks) | Hormone assays | 0 & 10 mg/kg; SC | - | Figures 1, 3, & 4 |
| 8 | Male | CON & DID (16 Weeks) | Hormone assays | 0 & 10 mg/kg; SC | - | Figures 1, 3, & 4 |
NTX, Naltrexone; DID, Modified drinking in the dark; CON, Control; SA, Self-administration; SC, Subcutaneous injection; PR, Progressive ratio.
2.4 Experiment 1: NTX effects on operant alcohol self-administration
In Experiment 1, separate groups of male (n=6) and freely cycling female (n=8) rats were trained to lever-press for an alcohol solution. Baseline sex differences from these animals in appetitive and consummatory responses have been previously reported (Nieto and Kosten, 2017). All other operant data from these animals have not been previously published.
2.4.1 Self-administration apparatus
The present study used 10 standard operant chambers (Coulbourn Instruments, Allentown, PA) enclosed in sound attenuating cubicles (Coulbourn Instruments). Each chamber was equipped with two levers on either side of an access area into which a dipper (0.1 mL capacity) could protrude. The dipper rested in a small reservoir of alcohol fluid prior to activation. Head entries were tabulated using infrared sensors located in the dipper access area. Operant chambers were equipped with a house light, a dipper access light, and two sets of three-colored cue lights, one above each lever. Stimulus parameters and data tabulation were programmed using Graphic State Notation (version 4.0).
2.4.2 Self-administration training
Prior to lever training, rats were first trained to drink alcohol from the dipper for one week. Sessions started with two dipper presentations with the levers retracted. After these presentations and for the rest of these 30 min training sessions, head entries into the dipper access area triggered a dipper presentation. The length of time the dipper was presented gradually decreased from 15 sec to 3 sec, the duration used for the rest of the study. Lever press training began the following week. During these 30 min sessions the house light was illuminated and the levers were present in the chamber. Sessions began with two dipper primes and then the dipper was only activated after the active lever was pressed. Inactive lever presses had no programmed consequences.
Operant training continued until the rat achieved at least 25 active lever presses and responding was consistent (<20% variability of active lever presses over 2 days). After the animal reached stable responding on the fixed ratio 1 schedule (FR1), the response requirement to receive a dipper presentation was increased to two presses on the active lever (i.e., FR2). Once an animal showed stable responding under the FR2 schedule, test sessions were initiated.
2.4.3 Self-administration testing
Test sessions were conducted 3–6 hrs after termination of DID under a 3 hr progressive ratio (PR) schedule as described previously (Kosten, 2011, Walker and Koob, 2008, Nieto and Kosten, 2017). On this schedule of reinforcement, animals must respond for deliveries of a fluid reinforcer at higher levels for each subsequent delivery in the following steps: 1, 1, 2, 2, 3, 3, 4, 4, 5, 5, 7, 7, 9, 9, 11, 11, 13, 13, 15, 15, 18, 18, 21, 21, 24, 24, etc.
2.4.4 Appetitive and consummatory behaviors
Both appetitive and consummatory behaviors were assessed during the 3 hr PR test. Appetitive responses include: dipper approaches defined as the total number of head entries into the dipper access area whether the dipper containing alcohol was present or not, active lever approaches defined as the number of active presses, and final ratio completed. Inactive lever presses were assessed as a measure of non-specific responding. Consummatory behaviors include: reinforcers delivered defined as the number of dipper presentations into the access area and reinforcers consumed defined as the number of head entries into the dipper access area when the dipper was present.
2.5 Experiment 2: NTX effects on HPA axis activity
In Experiment 2, separate cohorts of male (n=8) and female (n=8) alcohol and water drinking rats were used for HPA axis hormone studies. At the end of the 16 week DID period, male and diestrus female rats were treated with NTX (10 mg/kg) or isotonic saline prior to blood collection for adrenocorticotropic hormone (ACTH) and corticosterone (CORT) level determination. Drug administration was counterbalanced and there was a 4–5 day interval between saline and drug administrations for all animals to account for estrous cycling in females.
2.5.1 ACTH and CORT studies
Blood was collected in EDTA-coated microcentrifuge tubes from the lateral saphenous vein of alcohol and water drinking rats immediately before (baseline) and at 15, 30, and 60 min after saline or NTX administration. After collection, blood was immediately spun down, plasma collected and stored according to manufacturer instructions. Enzyme-linked immunosorbent assays were used to measure ACTH (Phoenix Pharmaceuticals, Burlingame, CA) and CORT levels (Diagnostic System Laboratories, Webster, TX).
2.5.2 Estrous stage determination
To control for gonadal hormone fluctuation, female rodents were administered NTX in the diestrus stage of the estrous cycle. To determine estrous cycle stage, female rats were vaginally-lavaged with a cotton-tipped applicator dipped in water. Samples were withdrawn in early afternoon and placed on a microscope slide and dipped in Cresyl violet (Sigma Chemicals, St Louis, MO) and rinsed to eliminate excess stain. The cell morphology and dominant cell type (leukocyte in diestrus, nucleated epithelial in proestrus, or cornified epithelial in estrus) were identified under a microscope and the estrous stage determined. Diestrus was identified by a predominance of leukocytes. Female rats were habituated to the procedure for 5 days before NTX exposure. Although chronic alcohol consumption disrupts menstrual cycling in women (Emanuele et al., 2002), we did not observe a disruption in estrous cycling between alcohol drinking and control females. All females used in Experiment 2 displayed a 4–5 day estrous cycle. However, it is important to note that Experiment 2 was not designed to measure alcohol’s influence on estrous phases, rather diestrus females were used as a control for HPA axis hormone fluctuations that occur over the estrous cycle. As such, detailed measures of estrous cycling were not recorded. To account for estrous cycling in females, there was a 5 day interval between injections in males.
2.6 Statistical analyses
Alcohol intake (g/kg) during DID was analyzed using a two way mixed design ANOVA with Sex as the between group factor and Week as the repeated measure factor. Sex differences in average weekly alcohol intake during DID were determined using a Student’s t test. Appetitive and consummatory behaviors during self-administration were analyzed using mixed design ANOVAs with Sex as the between group factor and Dose (0–10 mg/kg) as the within factor. Given the well-known sex differences in operant responding for alcohol, we planned a priori to compare NTX doses to saline using Dunnet’s post hoc comparisons within each sex. Final ratios completed were analyzed using nonparametric Wilcoxon-Matched Pairs tests because these data are derived from an escalating exponential function. ACTH and CORT levels were analyzed using mixed design ANOVAs with Sex and Solution (alcohol vs water) as the between group factors and Treatment (saline vs 10 mg/kg NTX) and Time (Baseline-60 min) as repeated measures factors. Tukey post hoc tests were used to follow up on significant main effects. Within each sex, time points were compared to baseline using Dunnet’s post hoc comparisons. All statistical analyses were performed using SAS software 9.4 (SAS Institute, Cary, NC) with statistical significance defined as p<0.05. Data are presented as mean ± SEM; however, data for final ratio completed are presented as median and interquartile range.
3.0 Results
3.1 Alcohol drinking in the dark consumption
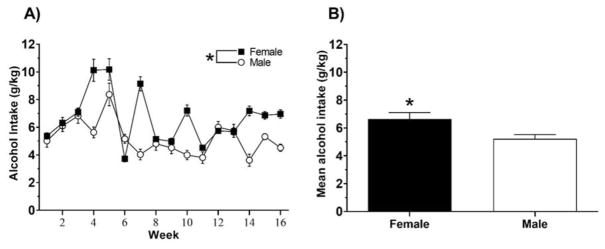
The mean weekly alcohol intake consumed (converted to g/kg) during DID for animals in Experiment 1 was previously reported in (Nieto and Kosten, 2017); however, all other data from these cohorts have not been previously published. Alcohol consumption during DID for animals in Experiment 2 is shown in Fig. 1. We averaged daily alcohol consumption into weekly blocks. A two way mixed design ANOVA yielded significant main effects of Time, F (15, 240) = 29.94, p<0.001, Sex, F (1, 16) = 11.31, p<0.01, and a significant Time x Sex interaction, F (15, 240) = 18.47, p<0.0001. Overall, females consumed between 4 and 10 g/kg daily and males consumed between 4 and 8 g/kg daily across weeks of the study (Fig. 1A). An analysis of average daily alcohol intake across all 16 weeks (Fig. 1B) revealed higher consumption in females relative to males, t (30) = 2.52, p<0.05.
Figure 1.
Alcohol intake of male and female rats during overnight drinking in the dark (DID). (A) Data are plotted as mean (± SEM) weekly alcohol consumption (g/kg) for entire length of the study. (B) Data are plotted as the mean (± SEM) across all 16 weeks of the study. An asterisk (*) represents a significant difference between males and females (p<0.05).
3.2 Appetitive responses
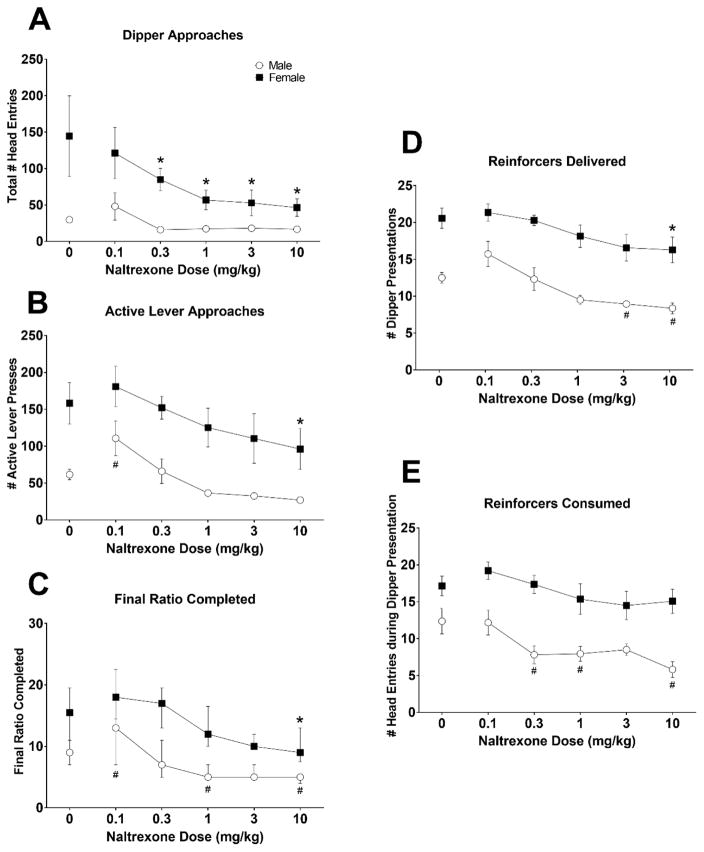
The effects of NTX on dipper approaches for alcohol are shown in Fig. 2A. There was a significant main effect for Dose, F (5, 80) = 7.87, p<0.0001, Sex, F (1, 16) = 9.91, p=0.0062, and a significant Dose x Sex interaction, F (5, 80) = 3.24, p=0.0103. Post hoc analysis revealed that NTX (0.3–10 mg/kg) decreased dipper approaches for alcohol in females, but not in males (p’s<0.05).
Figure 2.
The effects of naltrexone on appetitive and consummatory behaviors in male and female alcohol responding animals. Mean (± S.E.M.) total head entries (A) and active lever presses (B), reinforcers delivered (D), and reinforcers consumed (E) are presented by naltrexone dose for males (open circles) and females (filled squares). Final ratios completed (C) are presented as median (interquartile range) * Dose effect significantly different from vehicle in females, p<0.05.; # Dose effect significantly different from vehicle in males, p<0.05.
The effects of NTX on active lever presses for alcohol are shown in Fig. 2B. There was a significant main effect of Dose, F (5, 80) = 9.09, p<0.0001, and Sex, F (1, 16) = 19.76, p=0.0004 indicating that females emitted greater active lever presses for alcohol compared to males. Post hoc comparisons revealed that within females, the 10 mg/kg dose of NTX decreased active lever presses compared to saline, p<0.05. Within males, the 0.1 mg/kg dose of NTX increased active lever presses compared to saline, p<0.05. The effects of NTX on final ratio completed in male and female rats responding for alcohol are shown in Figure 2C. The 10 mg/kg dose of NTX significantly decreased final ratios completed in females compared to saline (p<0.05), while the 1 (p<0.01), and 10 (p<0.01) mg/kg doses significantly decreased final ratios completed in males compared to the saline dose. Males also showed increased final ratios completed at the 0.1 mg/kg NTX dose (p<0.05).
3.3 Consummatory responses
The effects of NTX on number of alcohol reinforcers delivered are shown in Fig. 2D. There was a significant main effect of Dose, F (5, 80) = 11.32, p<0.0001, and Sex, F (1, 16) = 43.09, p<0.0001 indicating that females received significantly more alcohol reinforcers delivered compared to males. Post hoc comparisons revealed that within females the 10 mg/kg dose significantly decreased alcohol reinforcers delivered compared to saline, p<0.05. Within males, the 3 mg/kg (p<0.05) and 10 mg/kg (p<0.01) doses significantly decreased alcohol reinforcers delivered compared to saline.
The effects of NTX on number of alcohol reinforcers consumed are shown in Fig. 2E. There was a significant main effect of Dose, F (5, 80) = 5.43, p=0.0002, and Sex, F (1, 16) = 31.36, p<0.0001 indicating that females consumed more reinforcers compared to males. Post hoc comparisons revealed that within males the 0.3, 1, and 10 mg/kg doses decreased alcohol reinforcers consumed compared to saline, all p’s<0.05. There were no significant effects of NTX on alcohol reinforcers consumed in females (p’s>0.10).
3.4 ACTH levels
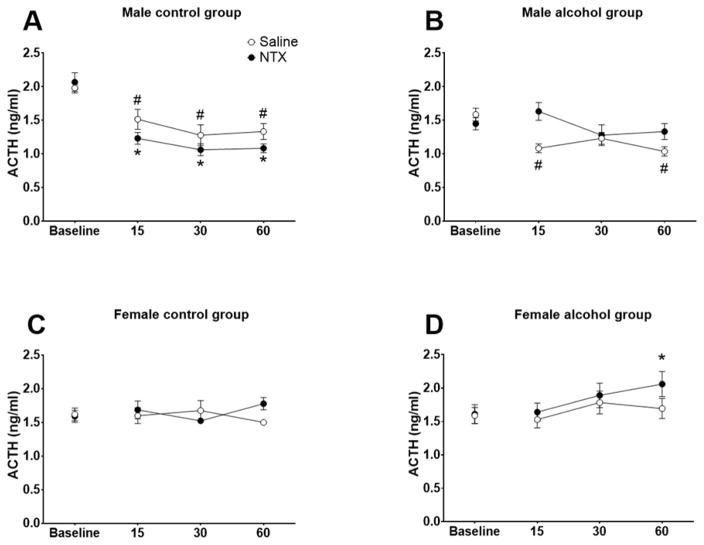
The effects of NTX on ACTH levels in water- and alcohol-drinking rats are shown in Fig. 3. There were significant main effects of Sex, F (1, 32) = 15.71, p<0.001 and Time, F (3, 96) = 5.41, p<0.01, and significant interactions of Treatment x Solution, F (3, 96) = 7.90, p<0.01, Time x Sex, F (3, 96) = 13.60, p<0.001, Treatment x Time, F (3, 96) = 4.25, p<0.01, Treatment x Time x Solution, F (3, 96) = 3.93, p<0.05, and Sex x Time x Treatment x Solution, F (3, 96) = 4.56, p<0.01. Given the significant main effect of Sex and the complex interactions, male and female ACTH data were analyzed separately. For males, there was a significant main effect of Time, F (3, 42) = 16.23, p<0.0001, Solution x Treatment interaction, F (1, 14) = 16.28, p=0.0012, Solution x Time interaction, F (3, 42) = 4.21, p=0.0109, and a Solution x Dose x Time interaction, F (3, 42) = 10.77, p<0.001. Overall, NTX (vs. saline) prevented decreases in ACTH levels in alcohol drinking males (p=0.0402), however, this effect did not reach statistical significance in control males. At baseline, ACTH levels were higher in control males relative to other groups. Dunnett’s post hoc comparisons revealed that ACTH levels decreased across time points after NTX and saline administration in control males (all p’s<0.05) compared to baseline (Fig 3A). In alcohol drinking males (Fig 3B), ACTH levels remained at baseline levels after NTX injection while showing a decline 15 and 60 min post-saline injection relative to baseline. For females, there was a significant Treatment x Time interaction, F (3, 54) = 4.63, p=0.0059. Dunnet’s post hoc comparisons revealed no statistically significant differences in control females after saline or NTX administration (Fig 3C) relative to their respective baselines. NTX increased ACTH levels in alcohol drinking females 60 min post injection (p<0.05) (Fig 3D), with no significant differences seen after saline treatment.
Figure 3.
The effects of naltrexone (NTX; 10 mg/kg) on adrencocorticotropic hormone (ACTH) levels in control and alcohol drinking rats. Mean (± S.E.M.) ACTH levels are presented for control (A) and alcohol drinking (B) males and control (C) and alcohol drinking females (D) administered saline (open circles) or NTX (closed circles). * Time effect significantly different from baseline for NTX-administered animals, p<0.05; # Time effect significantly different from baseline for saline-administered animals, p<0.05.
3.5 CORT levels
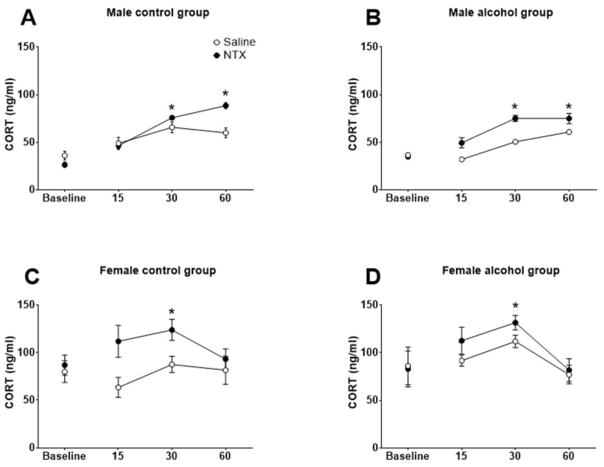
The effects of NTX on CORT levels in water- and alcohol-drinking rats are shown in Fig. 4. There were significant main effects of Sex, F (1, 32) = 58.82, p<0.0001, Treatment, F (1, 32) = 26.02, p<0.0001, and Time, F (3, 96) = 12.26, p<0.0001, and significant interactions of Time x Sex, F (3, 96) = 6.59, p<0.001, and Dose x Time, F (3, 96) = 3.96, p<0.05. Similar to the ACTH data, male and female CORT data were analyzed separately. In males, there were significant main effects of Treatment, F (1, 14) = 34.65, p<0.0001, and Time, F (3, 42) = 60.15, p<0.0001, and significant interactions of Treatment x Time, F (3, 42) = 13.43, p<0.0001, and Solution x Treatment x Time, F (3, 42) = 4.24, p=0.0027. Overall, NTX significantly elevated CORT levels in water- and alcohol-drinking male rats as a function of time. Dunnet’s post hoc comparisons revealed that within water drinking males (Fig 4A), NTX significantly increased CORT levels 30 and 60 min post NTX injection (p’s<0.05) compared to baseline, whereas saline did not significantly alter CORT levels. In alcohol drinking males (Fig 4B), NTX significantly increased CORT levels 30 and 60 after injection compared to baseline (p’s<0.05) with no effect seen after saline. In diestrus females, there was a significant main effect of Treatment, F (1, 18) = 10.09, p=0.0052, and Time, (3, 54) = 4.02, p=0.0119, and a significant Treatment x Time interaction, F (3, 54) = 2.88, p=0.0443. Overall, NTX significantly elevated CORT levels in water-and alcohol-drinking diestrus female rats as a function of time. Specifically, NTX increased CORT levels 30 min post-injection in water drinking females (p<0.05) (Fig 4C) with no effect seen after saline. In alcohol drinking females (Fig 4D), NTX increased CORT levels 30 min after injection relative to baseline (p<0.05) with no effect seen after saline.
Figure 4.
The effects of naltrexone (NTX; 10 mg/kg) on corticosterone (CORT) levels in control and alcohol drinking rats. Mean (± S.E.M.) CORT levels are presented for control (A) and alcohol drinking (B) males and control (C) and alcohol drinking diestrus females (D) administered vehicle (open circles) or NTX (filled circles). * Time effect significantly different from baseline for NTX-administered animals, p<0.05.
4.0 Discussion
The results from the present study showed that NTX had dose-related and sex-dependent effects on appetitive and consummatory responses for alcohol under a PR schedule. In alcohol responding rats, NTX more readily decreased appetitive behaviors in female rats, while a reduction in consummatory behaviors was more readily achieved in male rats. Additionally, NTX (10 mg/kg) significantly increased ACTH levels in alcohol drinking females in diestrus, but not in water drinking females or males. Interestingly, alcohol drinking males showed more prolonged NTX-induced increases in CORT levels compared to diestrus females. The drinking in the dark (DID) data confirmed our previous finding that female rats, compared to males, display higher levels of alcohol consumption. To our knowledge, this is the first study that examined sex-dependent effects of NTX on operant alcohol self-administration behaviors and HPA activity in an outbred rat strain. Further, the operant methods employed in the current study divided self-administration behavior into appetitive and consummatory responses. These procedures can be used to examine the efficacy of existing and potential treatment agents for AUDs.
Numerous studies, primarily focusing on males, employing various species, and different procedures demonstrate that NTX reduces alcohol consumption and attenuates conditioned effects that likely promote alcohol drinking. In humans with AUD, NTX reduces alcohol drinking, craving, and increases time to relapse. In addition, NTX reduces alcohol drinking in rodents and non-human primates (Altshuler et al., 1980, Gardell et al., 1996, Ji et al., 2008, Kornet et al., 1991, Myers et al., 1986, Reid et al., 1996, Volpicelli et al., 1986) and suppresses operant self-administration of alcohol in rats (Bienkowski et al., 1999, Czachowski and Delory, 2009, Hay et al., 2013, Ji et al., 2008, Lê et al., 1999, Sabino et al., 2006, Walker and Koob, 2008), similar to the findings in the present study. In addition to these effects on consumption, NTX attenuates reinstatement of extinguished operant responding for alcohol induced by discriminative stimuli or by conditioned cues or contexts (Burattini et al., 2006, Ciccocioppo et al., 2002, Dayas et al., 2007, Katner et al., 1999, Marinelli et al., 2007, Pickering and Liljequist, 2003). Alcohol-induced reinstatement is also reduced (Bienkowski et al., 1999, Hay et al., 2013, Lê et al., 1999). Thus, across studies and procedures, primarily using male animals, NTX attenuates both appetitive and consummatory responses to alcohol. The results of the present study show that both response types can be assessed in the same session and that the efficacy of NTX differs by sex and response measure.
NTX reduced operant alcohol self-administration behaviors in a sex-dependent manner. We found that NTX more readily reduced appetitive behaviors in females and consummatory behaviors in males. In females, a wide range of NTX doses (0.3–10 mg/kg) decreased total number of head entries into the dipper area (dipper approaches), while the highest dose (10 mg/kg) decreased active lever presses and final ratios completed. Although NTX (1 and 3 mg/kg) also decreased final ratios completed in males, the lowest dose (0.1 mg/kg) of NTX increased active lever presses for alcohol that may be due, at least in part, to NTX’s effect as a mu opioid agonist at low doses (Leonard et al., 2017). At ultra-low doses, NTX enhances both morphine analgesia and reward (conditioned place preference) and blocks or reverses morphine tolerance in male rats (Powell et al., 2002, Shen and Crain, 1997). We speculate that we did not observe the same enhancement of active lever presses after the lowest NTX dose in female rats because responses to opioid agonists or opioid-activating stimuli occur in a sex-dependent manner. For instance, male rats display greater sensitivity to the analgesic effects of morphine than females (Cicero et al., 1996, Baamonde et al., 1989). Similarly, men show greater mu opioid activation to painful stimuli in thalamic and limbic structures, while females show lower basal activation during pain in the nucleus accumbens (Zubieta et al., 2002). In rats, mu opioid agonists selectively increase alcohol, but not water consumption, and alcohol responding when administered systemically or infused directly into brain structures associated with reward (Richard and Fields, 2016, Sabino et al., 2007, Zhang and Kelley, 2002). In addition, NTX reduced alcohol reinforcers delivered in both females (10 mg/kg) and males (3 and 10 mg/kg). Males showed dose-related NTX-induced reductions in alcohol reinforcers consumed, while no significant NTX effects were seen in females. In our study, NTX was delivered via subcutaneous injection, a route of administration that enhances NTX’s potency compared to an intraperitoneal injection (Williams and Broadbridge, 2009), immediately prior to operant testing. The half-life of subcutaneously administered NTX is 4.6 hrs (Yoburn et al., 1986) with decreases in alcohol responding occurring ~12 min post-injection (Williams and Broadbridge, 2009). Thus, it is likely that NTX-induced decreases in appetitive and consummatory behaviors observed in our study occurred earlier in the 3 hr operant session rather than later.
There is limited evidence supporting NTX’s efficacy in attenuating alcohol consumption in females with AUD. These studies have used varying criteria as indicators of alcohol consumption. A few clinical studies have shown that NTX decreases alcohol quantity drunk (e.g., drinks per day) (Kranzler et al., 2009, Pettinati et al., 2008), drinking frequency (Greenfield et al., 2010), and time to relapse in females (Kiefer et al., 2005, Greenfield et al., 2010); however, other studies have found no effect of NTX (O’Malley et al., 2007, Hernandez-Avila et al., 2006), with some even favoring placebo on some measures (Pettinati et al., 2008, Garbutt et al., 2005). Our finding that NTX did not alter alcohol reinforcers consumed, coupled with the mixed human data, may indicate that NTX has minimal effects on alcohol consumption in females. Some studies in alcohol-preferring rats have included females when assessing NTX’s effect on alcohol self-administration. NTX (1–10 mg/kg via subcutaneous injection 30 min prior to operant session) decreased alcohol lever presses in alcohol-preferring females during a FR5 maintenance session (Dhaher et al., 2012), while a lower NTX dose (1 mg/kg via intraperitoneal injection 30 min prior to start of session) decreased alcohol deliveries during a PR session with no sex differences observed (Moore and Lynch, 2015). In our study, NTX (0.3–10 mg/kg) decreased appetitive measures in alcohol responding females corroborating and extending results from Dhaher and colleagues. However, in contrast to Moore and Lynch, only the highest dose of NTX (10 mg/kg) decreased alcohol reinforcers delivered and had no effect on alcohol reinforcers consumed. Thus, female rats in our study were more sensitive to NTX-induced reductions in appetitive measures while NTX had minimal effects on consummatory behaviors. Considering variations across experimental procedures, the potential differences between rat strains highlights the need to further examine the efficacy of pharmacotherapies for AUD in outbred rat strains that better parallel genetically diverse populations, such as humans.
Few studies have directly examined sex differences in NTX’s effect on alcohol craving. In clinical studies, women with AUD display greater craving reductions during the first week of NTX treatment compared to men (Herbeck et al., 2016). The concept of drug craving is difficult to operationalize even in humans; therefore, preclinical models that mimic drug craving are lacking. In operant self-administration, procedures that employ reinstating behavior after its extinction may reflect the preoccupation-anticipation phase because it is generally tested in the absence of alcohol reinforcement and reflects appetitive behaviors (Koob et al., 2009). Indeed, NTX does decrease numbers of active lever presses in female alcohol-preferring rats during Pavlovian spontaneous recovery and reinstatement procedures (Dhaher et al., 2012). Unfortunately, the Dhaher study did not include males, so direct sex comparisons cannot be made. Our findings that NTX more readily decreased appetitive measures in females compared to males under a PR schedule corroborate and extend findings in inbred rat strains and clinical studies.
In addition to preventing alcohol-induced dopamine release in the ventral tegmental area and nucleus accumbens, NTX’s therapeutic efficacy can also be attributed to its ability to stimulate the HPA axis. This stimulation is associated with many aspects of appetitive and consummatory behaviors for alcohol in humans. For instance, NTX-induced increases in cortisol negatively associate with alcohol consumption, craving, and relapse (Kiefer et al., 2006, Ray et al., 2009, O’Malley et al., 2002, Adinoff et al., 2005). In human laboratory studies, NTX-induced cortisol also positively associates with alcohol’s negative effects (i.e. sedation, subjective intoxication) and negatively associates with alcohol’s positive subjective effects (Ray et al., 2009). It is important to note that NTX does not always result in HPA axis stimulation in individuals with AUD (Ooteman et al., 2007, McCaul et al., 2001). Although a few clinical studies have included females with AUD, the small sample sizes have not allowed for analysis of sex-dependent effects of NTX on HPA axis activation.
Results from the present study revealed that NTX (10 mg/kg) significantly increased ACTH levels in alcohol drinking diestrus female rats but not in male rats or in water drinking animals of either sex. Furthermore, NTX increased CORT levels in water and alcohol drinking male rats for a longer duration compared to diestrus females. Previous reports indicate that opioid antagonists, including NTX, stimulate ACTH and CORT activity in drug- and alcohol-exposed animals (Guaza and Borrell, 1984, Knych and Prohaska, 1981, Almela et al., 2012, Martínez-Laorden et al., 2012, Navarro-Zaragoza et al., 2010, Budec et al., 2002). However, findings are less consistent in drug-naïve animals, with some studies showing that higher doses of opioid antagonists enhance HPA activity (Eisenberg, 1984) and others showing no effects (Retana-Márquez et al., 2009, Mellon and Bayer, 1999, Knych and Prohaska, 1981, Guaza and Borrell, 1984). Our finding that ACTH levels are less affected than CORT levels by NTX treatment is consistent with human laboratory studies showing that acute and repeated naltrexone treatments elevate basal cortisol but not ACTH levels in AUD patients (O’Malley et al., 2002) as well as in healthy males (Volavka et al., 1979). Furthermore, clinical studies have shown that NTX- induced HPA reactivity may depend on opioid receptor pharmacogenetics. For example, HPA axis activation because of opioid antagonist administration may be specific to single nucleotide polymorphisms in the mu opioid receptor particularly in Caucasian carriers and not carriers belonging to other racial/ethnic groups (Ray et al., 2012, Hernandez-Avila et al., 2003).
NTX effects on HPA reactivity may be dependent on sex. Although investigations examining the efficacy of treatments for AUD in females are sparse, NTX decreases craving (Herbeck et al., 2016) and rates of relapse (Greenfield et al., 2010, Kiefer et al., 2005) in women with AUD; however, there are less consistent findings on NTX’s ability to decrease alcohol consumption and drinking frequencies in this gender (Canidate et al., 2017). It is possible that short-term NTX-induced HPA axis stimulation in females with AUD leads to reduced appetitive behaviors for alcohol, whereas in males, naltrexone results in HPA stimulation for a longer duration leading to decreased consummatory behaviors for alcohol. Indeed, this hypothesis is supported by clinical findings mentioned above and by the preclinical data in the present study that demonstrate NTX more readily decreased appetitive measures for alcohol in female rats compared to consummatory measures across a wide range of doses. Although the highest dose of NTX (10 mg/kg) increased ACTH and CORT levels in alcohol drinking diestrus females, this dose did not alter alcohol reinforcers consumed in rats of this sex. Conversely, clinical evidence indicates that NTX reduces alcohol consumption in men with AUD, but there are mixed findings on NTX’s ability to alter alcohol craving in this gender (Ray et al., 2009, Herbeck et al., 2016). In agreement with clinical evidence, we observed that NTX more readily decreased consummatory measures in male rats compared to appetitive measures, a finding that was accompanied by more enduring increases in CORT in alcohol drinking males. Another possible mechanism responsible for the differential NTX-induced behavioral and physiological effects may be related to fluctuations in gonadal hormones. In a study of healthy individuals (no AUD diagnoses), NTX increased serum cortisol from baseline in women but not in men (Roche and King, 2015). This effect was greater in luteal phase females (intermediate estrogen levels) compared to early follicular females (low estrogen levels). Additionally, luteal phase females also reported more adverse effects in response to NTX. Thus, it is possible that NTX would have had greater effects on HPA activity and, perhaps, on alcohol consummatory behaviors in females during other phases of the estrous cycle. Future work is needed to examine these questions directly.
While this study provides evidence that NTX has sex-dependent effects on alcohol self-administration and HPA reactivity, there are some limitations. Although rats in our study were single-housed to accurately measure alcohol intake during DID, housing conditions can alter drug intake, primarily in females (Becker and Koob, 2016). For instance, isolation increases cocaine intake and breakpoints in female rats but not males (Westenbroek et al., 2013). Therefore, isolation stress may have enhanced alcohol consumption during DID and alcohol self-administration behaviors in females. However, it is unlikely that housing conditions influenced alcohol-motivated behaviors in our study because outbred rats of both sexes show greater voluntary alcohol consumption under social circumstances rather than alone (Varlinskaya et al., 2015). In addition, female outbred rats voluntarily drink more alcohol under both housing conditions compared to males (Sluyter et al., 2000, Walker et al., 2008, Vetter-O’Hagen et al., 2009, Maldonado-Devincci et al., 2010, Priddy et al., 2016). It is likely that the DID procedures used in the current study did not induce dependence in animals as evidenced by low levels of operant responding, particularly in males. It would be important to replicate and extend our findings using validated dependence-inducing procedures, such as chronic intermittent vapor exposure (Gilpin et al., 2008, Vendruscolo and Roberts, 2014). In a similar vein, an added limitation is that we did not monitor blood alcohol levels after NTX, so the consummatory behaviors observed in the operant studies may not be reflective of levels of alcohol exposure. It is also possible that alcohol intake during DID and responding during self-administration may have been influenced by thirst rather than alcohol’s reinforcing or pharmacological properties. Given that food- and fluid-restriction procedures are commonly used to encourage acquisition of alcohol and drug self-administration (Carroll and Meisch, 2011, Bell et al., 2017, Samson and Czachowski, 2003), an appropriate control for these procedures might be to assess alcohol’s efficacy as a reinforcer compared to a vehicle, such as food or water. Alcohol is chosen over natural reinforcers (water and low sucrose concentrations) when the solutions are concurrently available (Samson, 1986). Additionally, we observed that water intake during the 1 hr availability was minimal and after accounting for leakage, water intake was negligible. Thus, the reinforcing properties of alcohol, not its liquid value, likely maintained the operant behaviors observed in this study.
A wealth of human and animal studies provides evidence of sex differences in addiction. For instance, female rodents acquire drug self-administration more readily and are more sensitive to cue- and drug-induced reinstatement (Becker and Koob, 2016, Becker and Hu, 2008, Carroll, 2004, Anker and Carroll, 2010). Despite these well-known differences, female rodents are rarely used in studies exploring the efficacy of pharmacological treatments for substance use disorders, including AUD. This is unfortunate given that women have an increased risk of harmful health conditions associated with pathological alcohol consumption (Mann et al., 2005, Smith-Warner et al., 1998, Urbano-Marquez et al., 1995). Notably, the present study addresses this gap in research by providing evidence that NTX has dose-related and sex-dependent effects on operant alcohol self-administration and on HPA axis hormones in alcohol drinking rats. Based on the results of this study and others, we suggest that NTX-induced cortisol reactivity may associate with appetitive behaviors in females but with consummatory behaviors in males. Conducting preclinical studies using the methodology validated in the current study with an outbred rat strain of animals enhances the ability to determine if a potential treatment agent should be targeted to a specific-subtype of AUD or be used at distinct phases of treatment depending upon whether reducing craving or consumption is the treatment goal. Because treatment agents for AUD may affect appetitive and consummatory behaviors differentially by sex, the procedures employed in the present study show promise in providing information on the specificity of treatments by considering sex.
Highlights.
NTX dose-dependently decreased appetitive behaviors for alcohol in females.
NTX dose-dependently decreased consummatory behaviors for alcohol in males.
NTX increased CORT levels for a longer duration in males relative to females.
Acknowledgments
The authors would like to acknowledge Kevin Winoske for excellent technical assistance and Dr. Richard Meisch for valuable input on the manuscript. This work was supported by the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism (AA013476, TAK; AA026495, SJN). The authors report no financial interests or potential conflicts of interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ADINOFF B, IRANMANESH A, VELDHUIS J, FISHER L. Disturbances of the stress response: the role of the HPA axis during alcohol withdrawal and abstinence. Alcohol Health Res World. 1998;22:67–72. [PMC free article] [PubMed] [Google Scholar]
- ADINOFF B, JUNGHANNS K, KIEFER F, KRISHNAN-SARIN S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005;29:1351–5. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADINOFF B, MARTIN PR, BONE GH, ECKARDT MJ, ROEHRICH L, GEORGE DT, MOSS HB, ESKAY R, LINNOILA M, GOLD PW. Hypothalamic-pituitary-adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Arch Gen Psychiatry. 1990;47:325–30. doi: 10.1001/archpsyc.1990.01810160025004. [DOI] [PubMed] [Google Scholar]
- AGABIO R, PANI PP, PRETI A, GESSA GL, FRANCONI F. Efficacy of Medications Approved for the Treatment of Alcohol Dependence and Alcohol Withdrawal Syndrome in Female Patients: A Descriptive Review. European Addiction Research. 2016;22:1–16. doi: 10.1159/000433579. [DOI] [PubMed] [Google Scholar]
- ALMELA P, NAVARRO-ZARAGOZA J, GARCÍA-CARMONA JA, MORA L, HIDALGO J, MILANÉS MV, LAORDEN ML. Role of Corticotropin-Releasing Factor (CRF) Receptor-1 on the Catecholaminergic Response to Morphine Withdrawal in the Nucleus Accumbens (NAc) PLoS ONE. 2012;7:e47089. doi: 10.1371/journal.pone.0047089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALTSHULER HL, PHILLIPS PE, FEINHANDLER DA. Alteration of ethanol self-administration by naltrexone. Life Sci. 1980;26:679–88. doi: 10.1016/0024-3205(80)90257-x. [DOI] [PubMed] [Google Scholar]
- ANKER JJ, CARROLL ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010:107. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAAMONDE AI, HIDALGO A, ANDRES-TRELLES F. Sex-related differences in the effects of morphine and stress on visceral pain. Neuropharmacology. 1989;28:967–70. doi: 10.1016/0028-3908(89)90197-4. [DOI] [PubMed] [Google Scholar]
- BARAONA E, ABITTAN CS, DOHMEN K, MORETTI M, POZZATO G, CHAYES ZW, SCHAEFER C, LIEBER CS. Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res. 2001;25:502–7. [PubMed] [Google Scholar]
- BAROS AM, LATHAM PK, ANTON RF. Naltrexone and cognitive behavioral therapy for the treatment of alcohol dependence: do sex differences exist? Alcohol Clin Exp Res. 2008;32:771–6. doi: 10.1111/j.1530-0277.2008.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER JB, HU M. Sex differences in drug abuse. Frontiers in Neuroendocrinology. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER JB, KOOB GF. Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev. 2016;68:242–63. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELL RL, HAUSER SR, LIANG T, SARI Y, MALDONADO-DEVINCCI A, RODD ZA. Rat animal models for screening medications to treat alcohol use disorders. Neuropharmacology. 2017;122:201–243. doi: 10.1016/j.neuropharm.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTHOLOMEY ML, NAGARAJAN V, TORREGROSSA MM. Sex differences in reinstatement of alcohol seeking in response to cues and yohimbine in rats with and without a history of adolescent corticosterone exposure. Psychopharmacology (Berl) 2016;233:2277–87. doi: 10.1007/s00213-016-4278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIENKOWSKI P, KOSTOWSKI W, KOROS E. Ethanol-reinforced behaviour in the rat: effects of naltrexone. Eur J Pharmacol. 1999;374:321–7. doi: 10.1016/s0014-2999(99)00245-9. [DOI] [PubMed] [Google Scholar]
- BRELAND K, BRELAND M. The misbehavior of organisms. American Psychologist. 1961;16:681–684. [Google Scholar]
- BUDEC M, KOKO V, MILOVANOVIĆ T, BALINT-PERIĆ L, PETKOVIĆ A. Acute ethanol treatment increases level of progesterone in ovariectomized rats. Alcohol. 2002;26:173–8. doi: 10.1016/s0741-8329(02)00197-0. [DOI] [PubMed] [Google Scholar]
- BURATTINI C, GILL TM, AICARDI G, JANAK PH. The ethanol self-administration context as a reinstatement cue: acute effects of naltrexone. Neuroscience. 2006;139:877–87. doi: 10.1016/j.neuroscience.2006.01.009. [DOI] [PubMed] [Google Scholar]
- CANIDATE SS, CARNABY GD, COOK CL, COOK RL. A Systematic Review of Naltrexone for Attenuating Alcohol Consumption in Women with Alcohol Use Disorders. Alcohol Clin Exp Res. 2017;41:466–472. doi: 10.1111/acer.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARROLL ME. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004:25. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- CARROLL ME, MEISCH RA. Acquisition of Drug Self-Administration. In: OLMSTEAD MC, editor. Animal Models of Drug Addiction. Totowa, NJ: Humana Press; 2011. [Google Scholar]
- CICCOCIOPPO R, MARTIN-FARDON R, WEISS F. Effect of selective blockade of mu(1) or delta opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology. 2002;27:391–9. doi: 10.1016/S0893-133X(02)00302-0. [DOI] [PubMed] [Google Scholar]
- CICERO TJ, NOCK B, MEYER ER. Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther. 1996;279:767–73. [PubMed] [Google Scholar]
- CZACHOWSKI CL, DELORY MJ. Acamprosate and naltrexone treatment effects on ethanol and sucrose seeking and intake in ethanol-dependent and nondependent rats. Psychopharmacology (Berl) 2009;204:335–48. doi: 10.1007/s00213-009-1465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAYAS CV, LIU X, SIMMS JA, WEISS F. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol Psychiatry. 2007;61:979–89. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHAHER R, TOALSTON JE, HAUSER SR, BELL RL, MCKINZIE DL, MCBRIDE WJ, RODD ZA. Effects of naltrexone and LY255582 on ethanol maintenance, seeking, and relapse responding by alcohol-preferring (P) rats. Alcohol. 2012;46:17–27. doi: 10.1016/j.alcohol.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DROBES DJ, ANTON RF, THOMAS SE, VORONIN K. Effects of naltrexone and nalmefene on subjective response to alcohol among non-treatment-seeking alcoholics and social drinkers. Alcohol Clin Exp Res. 2004;28:1362–70. doi: 10.1097/01.alc.0000139704.88862.01. [DOI] [PubMed] [Google Scholar]
- EISENBERG RM. Effects of naltrexone on plasma corticosterone in opiate-naive rats: a central action. Life Sci. 1984;34:1185–91. doi: 10.1016/0024-3205(84)90091-2. [DOI] [PubMed] [Google Scholar]
- EMANUELE WEZEMAN, EMANUELE Alcohol’s effects on female reproductive function. Alcohol Res Health. 2002;26:274–81. [PMC free article] [PubMed] [Google Scholar]
- FARREN CK, O’MALLEY S, GREBSKI G, MANIAR S, PORTER M, KREEK MJ. Variable dose naltrexone-induced hypothalamic-pituitary-adrenal stimulation in abstinent alcoholics: a preliminary study. Alcohol Clin Exp Res. 1999;23:502–8. [PubMed] [Google Scholar]
- FREZZA M, DI PADOVA C, POZZATO G, TERPIN M, BARAONA E, LIEBER CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322:95–9. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- GARBUTT JC, KRANZLER HR, O’MALLEY SS, GASTFRIEND DR, PETTINATI HM, SILVERMAN BL, LOEWY JW, EHRICH EW, GROUP VS. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–25. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- GARDELL LR, HUBBELL CL, REID LD. Naltrexone persistently reduces rats’ intake of a palatable alcoholic beverage. Alcohol Clin Exp Res. 1996;20:584–8. doi: 10.1111/j.1530-0277.1996.tb01097.x. [DOI] [PubMed] [Google Scholar]
- GILPIN NW, RICHARDSON HN, COLE M, KOOB GF. Vapor Inhalation of Alcohol in Rats. Current protocols in neuroscience/editorial board, Jacqueline N. Crawley … [et al.] 2008;(Unit-9.29) doi: 10.1002/0471142301.ns0929s44. CHAPTER. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENFIELD SF, PETTINATI HM, O’MALLEY S, RANDALL PK, RANDALL CL. Gender differences in alcohol treatment: an analysis of outcome from the COMBINE study. Alcohol Clin Exp Res. 2010;34:1803–12. doi: 10.1111/j.1530-0277.2010.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUAZA C, BORRELL S. Effect of naloxone administration upon responses of adrenal hormones to withdrawal from ethanol. Psychopharmacology. 1984;82:181–184. doi: 10.1007/BF00427769. [DOI] [PubMed] [Google Scholar]
- HAMAJIMA N, HIROSE K, TAJIMA K, ROHAN T, CALLE EE, HEATH CW, JR, COATES RJ, LIFF JM, TALAMINI R, CHANTARAKUL N, KOETSAWANG S, RACHAWAT D, MORABIA A, SCHUMAN L, STEWART W, SZKLO M, BAIN C, SCHOFIELD F, SISKIND V, BAND P, COLDMAN AJ, GALLAGHER RP, HISLOP TG, YANG P, KOLONEL LM, NOMURA AM, HU J, JOHNSON KC, MAO Y, DE SANJOSE S, LEE N, MARCHBANKS P, ORY HW, PETERSON HB, WILSON HG, WINGO PA, EBELING K, KUNDE D, NISHAN P, HOPPER JL, COLDITZ G, GAJALANSKI V, MARTIN N, PARDTHAISONG T, SILPISORNKOSOL S, THEETRANONT C, BOOSIRI B, CHUTIVONGSE S, JIMAKORN P, VIRUTAMASEN P, WONGSRICHANALAI C, EWERTZ M, ADAMI HO, BERGKVIST L, MAGNUSSON C, PERSSON I, CHANG-CLAUDE J, PAUL C, SKEGG DC, SPEARS GF, BOYLE P, EVSTIFEEVA T, DALING JR, HUTCHINSON WB, MALONE K, NOONAN EA, STANFORD JL, THOMAS DB, WEISS NS, WHITE E, ANDRIEU N, BREMOND A, CLAVEL F, GAIRARD B, LANSAC J, PIANA L, RENAUD R, IZQUIERDO A, VILADIU P, CUEVAS HR, ONTIVEROS P, PALET A, SALAZAR SB, ARISTIZABEL N, CUADROS A, TRYGGVADOTTIR L, TULINIUS H, BACHELOT A, LE MG, PETO J, FRANCESCHI S, LUBIN F, MODAN B, RON E, WAX Y, FRIEDMAN GD, HIATT RA, LEVI F, BISHOP T, KOSMELJ K, et al. Alcohol, tobacco and breast cancer--collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87:1234–45. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAY RA, JENNINGS JH, ZITZMAN DL, HODGE CW, ROBINSON DL. Specific and nonspecific effects of naltrexone on goal-directed and habitual models of alcohol seeking and drinking. Alcohol Clin Exp Res. 2013;37:1100–10. doi: 10.1111/acer.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBECK DM, JETER KE, COUSINS SJ, ABDELMAKSOUD R, CREVECOEUR-MACPHAIL D. Gender differences in treatment and clinical characteristics among patients receiving extended release naltrexone. J Addict Dis. 2016;35:305–314. doi: 10.1080/10550887.2016.1189659. [DOI] [PubMed] [Google Scholar]
- HERNANDEZ-AVILA CA, SONG C, KUO L, TENNEN H, ARMELI S, KRANZLER HR. Targeted versus daily naltrexone: secondary analysis of effects on average daily drinking. Alcohol Clin Exp Res. 2006;30:860–5. doi: 10.1111/j.1530-0277.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- HERNANDEZ-AVILA CA, WAND G, LUO X, GELERNTER J, KRANZLER HR. Association between the cortisol response to opioid blockade and the Asn40Asp polymorphism at the mu-opioid receptor locus (OPRM1) Am J Med Genet B Neuropsychiatr Genet. 2003;118b:60–5. doi: 10.1002/ajmg.b.10054. [DOI] [PubMed] [Google Scholar]
- INDER WJ, JOYCE PR, ELLIS MJ, EVANS MJ, LIVESEY JH, DONALD RA. The effects of alcoholism on the hypothalamic-pituitary-adrenal axis: interaction with endogenous opioid peptides. Clin Endocrinol (Oxf) 1995;43:283–90. doi: 10.1111/j.1365-2265.1995.tb02033.x. [DOI] [PubMed] [Google Scholar]
- JENKINS JS, CONNOLLY J. Adrenocortical response to ethanol in man. Br Med J. 1968;2:804–5. doi: 10.1136/bmj.2.5608.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JI D, GILPIN NW, RICHARDSON HN, RIVIER CL, KOOB GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behav Pharmacol. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNGHANNS K, BACKHAUS J, TIETZ U, LANGE W, BERNZEN J, WETTERLING T, RINK L, DRIESSEN M. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol. 2003;38:189–93. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- JUNGHANNS K, TIETZ U, DIBBELT L, KUETHER M, JURTH R, EHRENTHAL D, BLANK S, BACKHAUS J. Attenuated salivary cortisol secretion under cue exposure is associated with early relapse. Alcohol Alcohol. 2005;40:80–5. doi: 10.1093/alcalc/agh107. [DOI] [PubMed] [Google Scholar]
- KATNER SN, MAGALONG JG, WEISS F. Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology. 1999;20:471–9. doi: 10.1016/S0893-133X(98)00084-0. [DOI] [PubMed] [Google Scholar]
- KIEFER F, JAHN H, OTTE C, NABER D, WIEDEMANN K. Hypothalamic-pituitary-adrenocortical axis activity: a target of pharmacological anticraving treatment? Biol Psychiatry. 2006;60:74–6. doi: 10.1016/j.biopsych.2005.11.023. [DOI] [PubMed] [Google Scholar]
- KIEFER F, JAHN H, WIEDEMANN K. A neuroendocrinological hypothesis on gender effects of naltrexone in relapse prevention treatment. Pharmacopsychiatry. 2005;38:184–6. doi: 10.1055/s-2005-871244. [DOI] [PubMed] [Google Scholar]
- KING AC, VOLPICELLI JR, GUNDUZ M, O’BRIEN CP, KREEK MJ. Naltrexone biotransformation and incidence of subjective side effects: a preliminary study. Alcohol Clin Exp Res. 1997;21:906–9. [PubMed] [Google Scholar]
- KITAY JI. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68:818–24. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- KITAY JI. PITUITARY-ADRENAL FUNCTION IN THE RAT AFTER GONADECTOMY AND GONADAL HORMONE REPLACEMENT. Endocrinology. 1963;73:253–60. doi: 10.1210/endo-73-2-253. [DOI] [PubMed] [Google Scholar]
- KNYCH ET, PROHASKA JR. Effect of chronic intoxication and naloxone on the ethanol-induced increase in plasma corticosterone. Life Sciences. 1981;28:1987–1994. doi: 10.1016/0024-3205(81)90645-7. [DOI] [PubMed] [Google Scholar]
- KOOB GF, LE MOAL M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- KOOB GF, LLOYD GK, MASON BJ. Development of pharmacotherapies for drug addiction: a Rosetta stone approach. Nat Rev Drug Discov. 2009;8:500–15. doi: 10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNET M, GOOSEN C, VAN REE JM. Effect of naltrexone on alcohol consumption during chronic alcohol drinking and after a period of imposed abstinence in free-choice drinking rhesus monkeys. Psychopharmacology (Berl) 1991;104:367–76. doi: 10.1007/BF02246038. [DOI] [PubMed] [Google Scholar]
- KOSTEN TA. Pharmacologically targeting the P2rx4 gene on maintenance and reinstatement of alcohol self-administration in rats. Pharmacol Biochem Behav. 2011;98:533–8. doi: 10.1016/j.pbb.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSTEN TA, MEISCH RA. Predicting extinction and reinstatement of alcohol and sucrose self-administration in outbred rats. Exp Clin Psychopharmacol. 2013;21:245–51. doi: 10.1037/a0031825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRANZLER HR, TENNEN H, ARMELI S, CHAN G, COVAULT J, ARIAS A, ONCKEN C. Targeted naltrexone for problem drinkers. J Clin Psychopharmacol. 2009;29:350–7. doi: 10.1097/JCP.0b013e3181ac5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREEK MJ. Opiates, opioids and addiction. Mol Psychiatry. 1996;1:232–54. [PubMed] [Google Scholar]
- KUDIELKA BM, KIRSCHBAUM C. Sex differences in HPA axis responses to stress: a review. Biological Psychology. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- LÊ AD, POULOS CX, HARDING S, WATCHUS J, JUZYTSCH W, SHAHAM Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–44. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- LEONARD JB, NAIR V, DIAZ CJ, PENOYAR JB, GOODE PA. Potential drug interaction with opioid agonist in the setting of chronic low-dose opioid antagonist use. Am J Emerg Med. 2017 doi: 10.1016/j.ajem.2017.04.012. [DOI] [PubMed] [Google Scholar]
- LOFT S, OLESEN KL, DOSSING M. Increased susceptibility to liver disease in relation to alcohol consumption in women. Scand J Gastroenterol. 1987;22:1251–6. doi: 10.3109/00365528708996472. [DOI] [PubMed] [Google Scholar]
- MALDONADO-DEVINCCI AM, ALIPOUR KK, MICHAEL LA, KIRSTEIN CL. Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in young adulthood in male and female rats. Pharmacol Biochem Behav. 2010;96:476–87. doi: 10.1016/j.pbb.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANN K, ACKERMANN K, CROISSANT B, MUNDLE G, NAKOVICS H, DIEHL A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol Clin Exp Res. 2005;29:896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- MARINELLI PW, FUNK D, JUZYTSCH W, LI Z, LE AD. Effects of opioid receptor blockade on the renewal of alcohol seeking induced by context: relationship to c-fos mRNA expression. Eur J Neurosci. 2007;26:2815–23. doi: 10.1111/j.1460-9568.2007.05898.x. [DOI] [PubMed] [Google Scholar]
- MARTÍNEZ-LAORDEN E, HURLE MA, MILANÉS MV, LAORDEN ML, ALMELA P. Morphine Withdrawal Activates Hypothalamic-Pituitary-Adrenal Axis and Heat Shock Protein 27 in the Left Ventricle: The Role of Extracellular Signal-Regulated Kinase. Journal of Pharmacology and Experimental Therapeutics. 2012;342:665. doi: 10.1124/jpet.112.193581. [DOI] [PubMed] [Google Scholar]
- MCCAUL ME, WAND GS, STAUFFER R, LEE SM, ROHDE CA. Naltrexone dampens ethanol-induced cardiovascular and hypothalamic- pituitary-adrenal axis activation. Neuropsychopharmacology. 2001;25:537–47. doi: 10.1016/S0893-133X(01)00241-X. [DOI] [PubMed] [Google Scholar]
- MELLON RD, BAYER BM. The effects of morphine, nicotine and epibatidine on lymphocyte activity and hypothalamic-pituitary-adrenal axis responses. J Pharmacol Exp Ther. 1999;288:635–42. [PubMed] [Google Scholar]
- MOORE CF, LYNCH WJ. Alcohol preferring (P) rats as a model for examining sex differences in alcohol use disorder and its treatment. Pharmacol Biochem Behav. 2015;132:1–9. doi: 10.1016/j.pbb.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS RD, BORG S, MOSSBERG R. Antagonism by naltrexone of voluntary alcohol selection in the chronically drinking macaque monkey. Alcohol. 1986;3:383–8. doi: 10.1016/0741-8329(86)90058-3. [DOI] [PubMed] [Google Scholar]
- NAVARRO-ZARAGOZA J, NÚÑEZ C, LAORDEN ML, MILANÉS MV. Effects of Corticotropin-Releasing Factor Receptor-1 Antagonists on the Brain Stress System Responses to Morphine Withdrawal. Molecular Pharmacology. 2010;77:864. doi: 10.1124/mol.109.062463. [DOI] [PubMed] [Google Scholar]
- NIETO SJ, KOSTEN TA. Female Sprague-Dawley rats display greater appetitive and consummatory responses to alcohol. Behav Brain Res. 2017;327:155–161. doi: 10.1016/j.bbr.2017.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’MALLEY SS, KRISHNAN-SARIN S, FARREN C, SINHA R, KREEK MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- O’MALLEY SS, SINHA R, GRILO CM, CAPONE C, FARREN CK, MCKEE SA, ROUNSAVILLE BJ, WU R. Naltrexone and cognitive behavioral coping skills therapy for the treatment of alcohol drinking and eating disorder features in alcohol-dependent women: a randomized controlled trial. Alcohol Clin Exp Res. 2007;31:625–34. doi: 10.1111/j.1530-0277.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- OLIVE MF, MEHMERT KK, KOENIG HN, CAMARINI R, KIM JA, NANNINI MA, OU CJ, HODGE CW. A role for corticotropin releasing factor (CRF) in ethanol consumption, sensitivity, and reward as revealed by CRF-deficient mice. Psychopharmacology (Berl) 2003;165:181–7. doi: 10.1007/s00213-002-1248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OOTEMAN W, KOETER MW, VERHEUL R, SCHIPPERS GM, VAN DEN BRINK W. The effect of naltrexone and acamprosate on cue-induced craving, autonomic nervous system and neuroendocrine reactions to alcohol-related cues in alcoholics. Eur Neuropsychopharmacol. 2007;17:558–66. doi: 10.1016/j.euroneuro.2007.02.012. [DOI] [PubMed] [Google Scholar]
- PETTINATI HM, KAMPMAN KM, LYNCH KG, SUH JJ, DACKIS CA, OSLIN DW, O’BRIEN CP. Gender differences with high-dose naltrexone in patients with co-occurring cocaine and alcohol dependence. J Subst Abuse Treat. 2008;34:378–90. doi: 10.1016/j.jsat.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICKERING C, LILJEQUIST S. Cue-induced behavioural activation: a novel model of alcohol craving? Psychopharmacology (Berl) 2003;168:307–13. doi: 10.1007/s00213-003-1454-6. [DOI] [PubMed] [Google Scholar]
- POWELL KJ, ABUL-HUSN NS, JHAMANDAS A, OLMSTEAD MC, BENINGER RJ, JHAMANDAS K. Paradoxical effects of the opioid antagonist naltrexone on morphine analgesia, tolerance, and reward in rats. J Pharmacol Exp Ther. 2002;300:588–96. doi: 10.1124/jpet.300.2.588. [DOI] [PubMed] [Google Scholar]
- PRIDDY BM, CARMACK SA, THOMAS LC, VENDRUSCOLO JC, KOOB GF, VENDRUSCOLO LF. Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol Biochem Behav. 2016 doi: 10.1016/j.pbb.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAY LA, BUJARSKI S, CHIN PF, MIOTTO K. Pharmacogenetics of Naltrexone in Asian Americans: A Randomized Placebo-Controlled Laboratory Study. Neuropsychopharmacology. 2012;37:445–455. doi: 10.1038/npp.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAY LA, HUTCHISON KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64:1069–77. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- RAY LA, MACKILLOP J, LEGGIO L, MORGAN M, HUTCHISON KE. Effects of naltrexone on cortisol levels in heavy drinkers. Pharmacol Biochem Behav. 2009;91:489–94. doi: 10.1016/j.pbb.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REID LD, GARDELL LR, CHATTOPADHYAY S, HUBBELL CL. Periodic naltrexone and propensity to take alcoholic beverage. Alcohol Clin Exp Res. 1996;20:1329–34. doi: 10.1111/j.1530-0277.1996.tb01130.x. [DOI] [PubMed] [Google Scholar]
- RETANA-MÁRQUEZ S, BONILLA-JAIME H, VÁZQUEZ-PALACIOS G, MARTÍNEZ-GARCÍA R. Naltrexone effects on male sexual behavior, corticosterone, and testosterone in stressed male rats. Physiology & Behavior. 2009;96:333–342. doi: 10.1016/j.physbeh.2008.10.022. [DOI] [PubMed] [Google Scholar]
- RICHARD JM, FIELDS HL. Mu-opioid receptor activation in the medial shell of nucleus accumbens promotes alcohol consumption, self-administration and cue-induced reinstatement. Neuropharmacology. 2016;108:14–23. doi: 10.1016/j.neuropharm.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROCHE DJ, KING AC. Sex differences in acute hormonal and subjective response to naltrexone: The impact of menstrual cycle phase. Psychoneuroendocrinology. 2015;52:59–71. doi: 10.1016/j.psyneuen.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABINO V, COTTONE P, KOOB GF, STEARDO L, LEE MJ, RICE KC, ZORRILLA EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2006;189:175–86. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- SABINO V, COTTONE P, STEARDO L, SCHMIDHAMMER H, ZORRILLA EP. 14-Methoxymetopon, a highly potent mu opioid agonist, biphasically affects ethanol intake in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2007;192:537–46. doi: 10.1007/s00213-007-0746-7. [DOI] [PubMed] [Google Scholar]
- SAMHSA 2015 Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health (NSDUH) Table 5.6A—Substance Use Disorder in Past Year among Persons Aged 18 or Older, by Demographic Characteristics: Numbers in Thousands, 2014 and 2015. 2015 https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm#tab5-6a.
- SAMSON HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–42. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- SAMSON HH, CZACHOWSKI CL. International Review of Neurobiology. Academic Press; 2003. Behavioral measures of alcohol self-administration and intake control: Rodent models. [DOI] [PubMed] [Google Scholar]
- SHARPE AL, SAMSON HH. Effect of naloxone on appetitive and consummatory phases of ethanol self-administration. Alcohol Clin Exp Res. 2001;25:1006–11. [PubMed] [Google Scholar]
- SHEN KF, CRAIN SM. Ultra-low doses of naltrexone or etorphine increase morphine’s antinociceptive potency and attenuate tolerance/dependence in mice. Brain Res. 1997;757:176–90. doi: 10.1016/s0006-8993(97)00197-2. [DOI] [PubMed] [Google Scholar]
- SLAWECKI CJ, ROTH J. Neurokinin type-3 receptor stimulation impairs ethanol-associated appetitive behavior in Wistar rats. Alcohol Clin Exp Res. 2003;27:1962–70. doi: 10.1097/01.ALC.0000102412.53561.C6. [DOI] [PubMed] [Google Scholar]
- SLUYTER F, HOF M, ELLENBROEK BA, DEGEN SB, COOLS AR. Genetic, sex, and early environmental effects on the voluntary alcohol intake in Wistar rats. Pharmacol Biochem Behav. 2000;67:801–8. doi: 10.1016/s0091-3057(00)00425-1. [DOI] [PubMed] [Google Scholar]
- SMITH-WARNER SA, SPIEGELMAN D, YAUN SS, VAN DEN BRANDT PA, FOLSOM AR, GOLDBOHM RA, GRAHAM S, HOLMBERG L, HOWE GR, MARSHALL JR, MILLER AB, POTTER JD, SPEIZER FE, WILLETT WC, WOLK A, HUNTER DJ. Alcohol and breast cancer in women: a pooled analysis of cohort studies. Jama. 1998;279:535–40. doi: 10.1001/jama.279.7.535. [DOI] [PubMed] [Google Scholar]
- SOROCCO KH, LOVALLO WR, VINCENT AS, COLLINS FL. Blunted hypothalamic-pituitary-adrenocortical axis responsivity to stress in persons with a family history of alcoholism. Int J Psychophysiol. 2006;59:210–7. doi: 10.1016/j.ijpsycho.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- URBANO-MARQUEZ A, ESTRUCH R, FERNANDEZ-SOLA J, NICOLAS JM, PARE JC, RUBIN E. The greater risk of alcoholic cardiomyopathy and myopathy in women compared with men. Jama. 1995;274:149–54. doi: 10.1001/jama.1995.03530020067034. [DOI] [PubMed] [Google Scholar]
- VARLINSKAYA EI, TRUXELL EM, SPEAR LP. Ethanol intake under social circumstances or alone in Sprague-Dawley rats: Impact of age, sex, social activity and social anxiety-like behavior. Alcoholism, clinical and experimental research. 2015;39:117–125. doi: 10.1111/acer.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENDRUSCOLO LF, ROBERTS AJ. Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol. 2014;48:277–86. doi: 10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VESCOVI PP, DIGENNARO C, COIRO V. Hormonal (ACTH, cortisol, beta-endorphin, and met-enkephalin) and cardiovascular responses to hyperthermic stress in chronic alcoholics. Alcohol Clin Exp Res. 1997;21:1195–8. [PubMed] [Google Scholar]
- VETTER-O’HAGEN C, VARLINSKAYA E, SPEAR L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44:547–54. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLAVKA J, MALLYA A, BAUMAN J, PEVNICK J, CHO D, REKER D, JAMES B, DORNBUSH R. Hormonal and other effects of naltrexone in normal men. Adv Exp Med Biol. 1979;116:291–305. doi: 10.1007/978-1-4684-3503-0_17. [DOI] [PubMed] [Google Scholar]
- VOLPICELLI JR, DAVIS MA, OLGIN JE. Naltrexone blocks the post-shock increase of ethanol consumption. Life Sci. 1986;38:841–7. doi: 10.1016/0024-3205(86)90601-6. [DOI] [PubMed] [Google Scholar]
- WALKER BM, KOOB GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–52. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER BM, WALKER JL, EHLERS CL. Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol. 2008;42:83–9. doi: 10.1016/j.alcohol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAND GS, DOBS AS. Alterations in the hypothalamic-pituitary-adrenal axis in actively drinking alcoholics. J Clin Endocrinol Metab. 1991;72:1290–5. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- WESTENBROEK C, PERRY AN, BECKER JB. Pair housing differentially affects motivation to self-administer cocaine in male and female rats. Behavioural brain research. 2013;252:68–71. doi: 10.1016/j.bbr.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS KL, BROADBRIDGE CL. Potency of naltrexone to reduce ethanol self-administration in rats is greater for subcutaneous versus intraperitoneal injection. Alcohol. 2009;43:119–26. doi: 10.1016/j.alcohol.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOBURN BC, COHEN AH, INTURRISI CE. Pharmacokinetics and pharmacodynamics of subcutaneous naltrexone pellets in the rat. J Pharmacol Exp Ther. 1986;237:126–30. [PubMed] [Google Scholar]
- ZHANG M, KELLEY AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 2002;159:415–23. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]
- ZHOU Y, KREEK MJ. Alcohol: a stimulant activating brain stress responsive systems with persistent neuroadaptation. Neuropharmacology. 2014;87:51–8. doi: 10.1016/j.neuropharm.2014.05.044. [DOI] [PubMed] [Google Scholar]
- ZUBIETA JK, DANNALS RF, FROST JJ. Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am J Psychiatry. 1999;156:842–8. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]
- ZUBIETA JK, SMITH YR, BUELLER JA, XU Y, KILBOURN MR, JEWETT DM, MEYER CR, KOEPPE RA, STOHLER CS. mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22:5100–7. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]