Abstract
Conventional water resources in many regions are insufficient to meet the water needs of growing populations, thus reuse is gaining acceptance as a method of water supply augmentation. Recent advancements in membrane technology have allowed for the reclamation of municipal wastewater for the production of drinking water, i.e., potable reuse. Although public perception can be a challenge, potable reuse is often the least energy-intensive method of providing additional drinking water to water stressed regions. A variety of membranes have been developed that can remove water contaminants ranging from particles and pathogens to dissolved organic compounds and salts. Typically, potable reuse treatment plants use polymeric membranes for microfiltration or ultrafiltration in conjunction with reverse osmosis and, in some cases, nanofiltration. Membrane properties, including pore size, wettability, surface charge, roughness, thermal resistance, chemical stability, permeability, thickness and mechanical strength, vary between membranes and applications. Advancements in membrane technology including new membrane materials, coatings, and manufacturing methods, as well as emerging membrane processes such as membrane bioreactors, electrodialysis, and forward osmosis have been developed to improve selectivity, energy consumption, fouling resistance, and/or capital cost. The purpose of this review is to provide a comprehensive summary of the role of polymeric membranes in the treatment of wastewater to potable water quality and highlight recent advancements in separation processes. Beyond membranes themselves, this review covers the background and history of potable reuse, and commonly used potable reuse process chains, pretreatment steps, and advanced oxidation processes. Key trends in membrane technology include novel configurations, materials and fouling prevention techniques. Challenges still facing membrane-based potable reuse applications, including chemical and biological contaminant removal, membrane fouling, and public perception, are highlighted as areas in need of further research and development.
Keywords: Potable reuse, polymeric membranes, nanocomposite membranes, reverse osmosis, filtration, fouling
1. Introduction
Dwindling water supplies and growing populations have made planned potable reuse an increasingly important component of water resource management for many urban areas around the world [1–3]. Although reuse can only be a portion of a water supply portfolio due to intrinsic water losses, reuse of wastewater can augment the supply of water for agriculture, industry, and potable use, even in regions where climate change and cyclical droughts make traditional supplies unreliable. Membranes, particularly polymeric membranes, play a crucial role in the purification of municipal wastewater to potable quality, and are the core part of almost all of these systems [1, 2]. However, despite the technologies available, several challenges including membrane fouling, contaminant permeation, energy consumption, high pretreatment costs, managing treatment residuals, membrane integrity, and public perception limit widespread implementation of potable reuse [1, 3–5]. For example, as of 2010, only about 0.1% of treated municipal wastewater was directed to planned potable reuse in the U.S. [6]. The purpose of this review is to summarize recent developments for polymeric membrane that relate to potable water reuse, and also to identify areas in which future research and innovation are needed.
1.1 Reuse Terminology
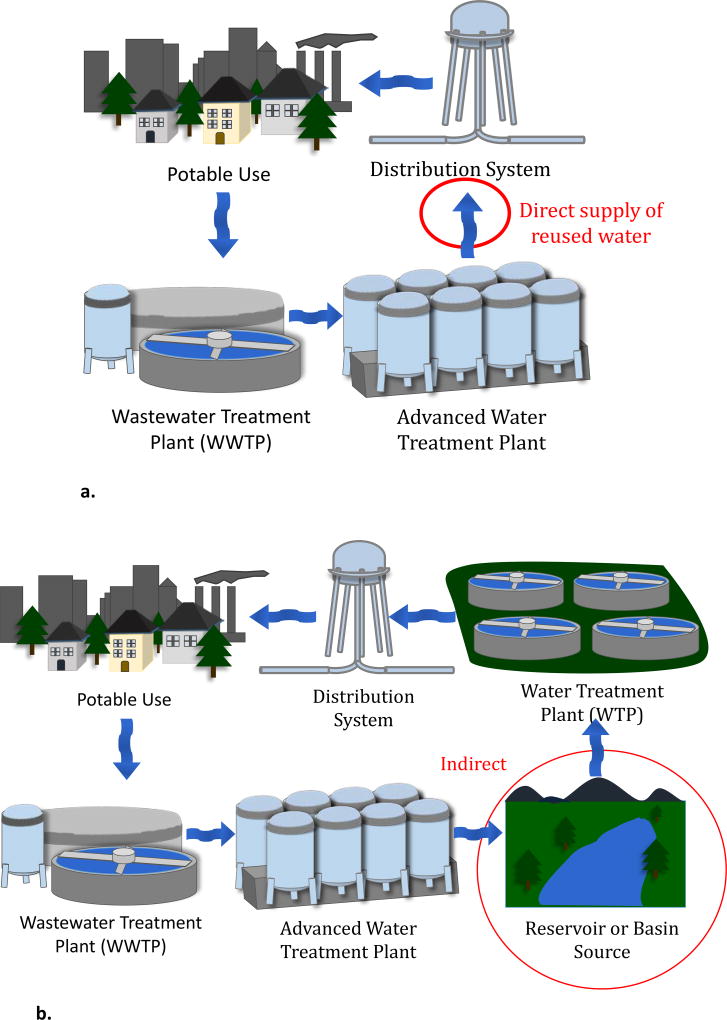
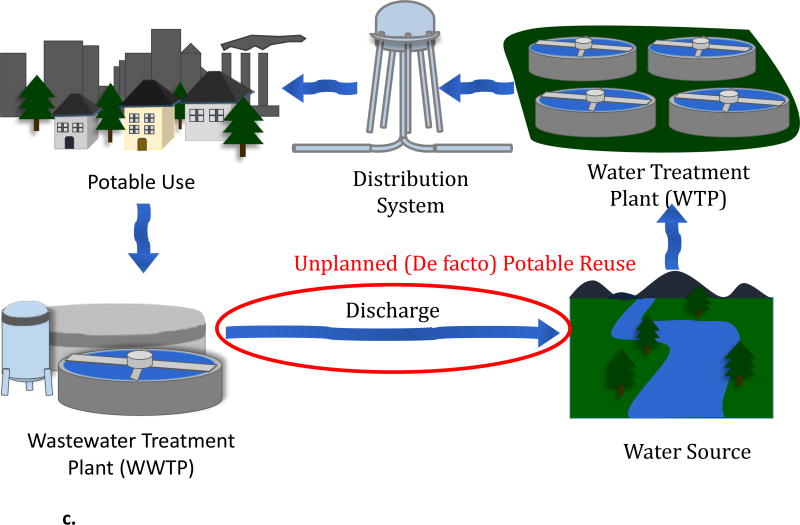
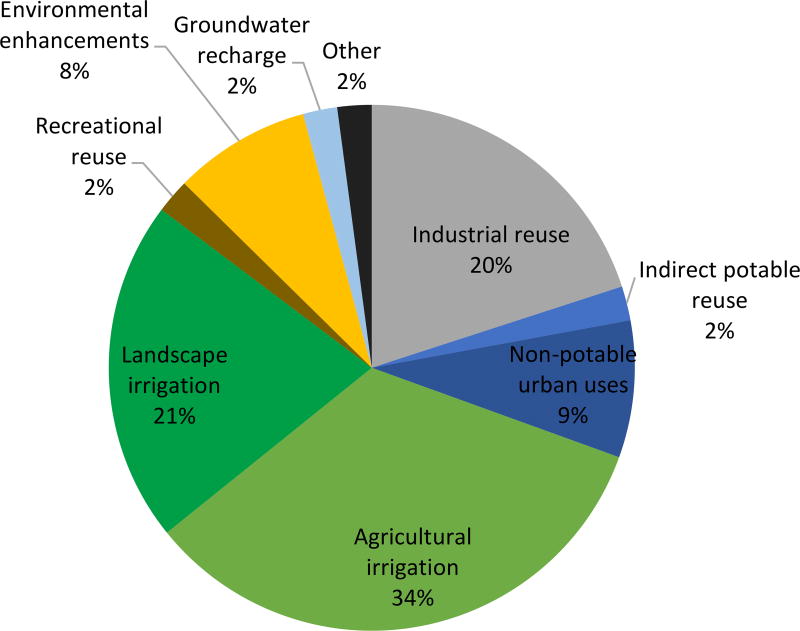
Planned potable reuse projects can be categorized as direct or indirect [3]. Direct potable reuse (DPR) is the direct addition of reclaimed wastewater to a drinking water treatment plant’s influent (referred to as “raw water augmentation”) or a drinking water distribution system (“treated drinking water augmentation”, Figure 1a). Indirect potable reuse (IPR) is the planned addition of purified wastewater (i.e., reclaimed wastewater) to an environmental buffer, namely a surface water reservoir (“reservoir water augmentation”) or groundwater aquifer (“groundwater augmentation”) [7], that is subsequently used for a drinking water supply (Figure 1b). In addition, de facto potable reuse (i.e., unplanned potable reuse) refers to the production of drinking water from wastewater-impacted water resources (Figure 1c). De facto potable reuse is common [8] and often unavoidable in major river systems such as adjacent to the Mississippi and Nile River (in the U.S. and Egypt, respectively).
Figure 1.
a. Schematic diagram of direct potable reuse (DPR). Treated water is sent directly back into the distribution system.
b. Schematic diagram of indirect potable reuse (IPR). An intermediate potable water source (bottom right, in red circle) acts as an environmental buffer and makes the process indirect.
c. Schematic diagram of de facto potable reuse (unplanned potable reuse). Note the discharge into a water source without as thorough treatment.
As one implementation of IPR, water agencies may also inject or infiltrate reclaimed water at locations in between the ocean and drinking water production wells to slow or reverse seawater intrusion into coastal aquifers. While de facto potable reuse and IPR have been practiced for some time, DPR has more recently become a technically and (to a lesser extent) socially viable reuse option in many geographies world-wide [4].
1.2 Potable Reuse History
The purification of wastewater has been examined as a means of augmenting conventional drinking water supplies for over 100 years [1]. In the 1920s, the Los Angeles Department of Water and Power constructed a wastewater purification plant to accommodate increased water demand due to rapid development and the lack of additional water supplies prior to access to Colorado River water [6]. By the 1930s, spreading basins were being used to augment groundwater with the effluent of a wastewater treatment in Southern California [1]. In 1968, the first DPR scheme was constructed in Windhoek, Namibia; in the years following, numerous IPR projects were established globally [7]. It was not until 1977 that membranes became an integral component of potable water reuse applications, when RO membranes were first used to purify wastewater at Orange County Water District’s Water Factory 21 [7].
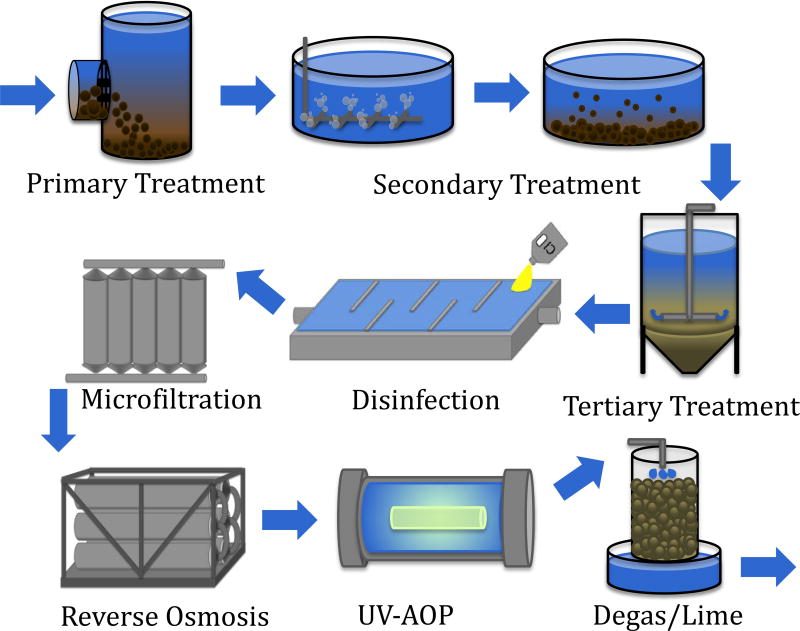
As treatment technologies for potable reuse have evolved over 50 years, there has been a gradual shift away from traditional processes, such as lime softening, toward membrane filtration [6]. As the cost and performance of membrane products have improved, polymeric membranes played an increasingly important role in potable reuse [9]. Polymeric membranes are now used to remove colloidal and dissolved materials in most potable reuse facilities. In the last 20 years, the number of IPR and DPR projects employing membrane technologies has increased significantly in the U.S., Australia, Singapore, and South Africa (Table 1). Globally, water reuse for potable and non-potable applications continues to be a critical water resource (Figure 2.), and has reached approximately 32 million m3/day [10].
Table 1.
Summary of major potable water reuse projects [11].
| Utility | Location | Membrane processes |
Additional treatment processes |
Capacity | Began operating |
Purpose |
|---|---|---|---|---|---|---|
| Orange County Water District/Orange County Sanitation District | Fountain Valley, CA, U.S. | MF-RO | Prescreening, UV-AOP, decarbonation, stabilization | 3.8E5 m3/d (100 MGD) | 2008 in current form | Seawater intrusion barrier, groundwater augmentation |
| City of Scottsdale | Scottsdale, AZ, U.S. | MF-RO | Pre-ozonation, UV disinfection, decarbonation, stabilization | 9.5E4 m3/d (25 MGD) | 1998 | Groundwater augmentation |
| West Basin Municipal Water District | Carson, CA, U.S. | MF-RO | Prescreening, pre-ozonation, UV-AOP, decarbonation, stabilization | 5.6E4 m3/d (15 MGD) | 1995 | Seawater intrusion barrier, groundwater augmentation, boiler feed water |
| Water Replenishment District of Southern California | Lakewood, CA, U.S. | MF-RO with secondary RO | UV-AOP, decarbonation, stabilization | 3E4 m3/d (8 MGD) | 2003 | Seawater intrusion barrier, groundwater augmentation |
| Los Angeles Department of Public Works | Los Angeles, CA, U.S. | MF-RO | Decarbonation, stabilization | 1.7E4 m3/d (4.5 MGD) | 2002 | Seawater intrusion barrier, groundwater augmentation |
| Singapore Public Utility Board | Singapore | MF-RO | UV disinfection, decarbonation, stabilization | >1.9E5 m3/d (>50 MGD) | 2003 | Reservoir augmentation |
| Colorado River Municipal Water District | Big Spring, TX, U.S. | MF-RO | UV-AOP | 7.6E3 m3/d (2 MGD) | 2014 | Direct potable reuse |
| Southeast Queensland Water (SEQ) | Queensland, AU | MF-RO | Pre-sedimentation, UV-AOP, stabilization | 6.4E4 m3/d (17 MGD) | 2007 | Reservoir augmentation, industrial use |
| Southeast Queensland Water (SEQ) | Queensland, AU | MF-RO | Pre-sedimentation, UV-AOP, stabilization | 7E4 m3/d (18.5 MGD) | 2008 | Reservoir augmentation, industrial use |
| Southeast Queensland Water (SEQ) | Queensland, AU | MF-RO | Pre-sedimentation, UV-AOP, stabilization | 9.8E4 m3/d (26 MGD) | 2008 | Reservoir augmentation, industrial use |
| Beaufort West Municipality | Western Cape, S.A. | UF-RO | Coagulation, media filtration, UV-AOP | 2.2E3 m3/d (0.6 MGD) | 2010 | Direct potable reuse |
| George Municipality | Western Cape, S.A. | UF | Unknown | 9.8E3 m3/d (2.6 MGD) | 2010 | Drinking water augmentation |
| Mossel Bay Municipality | Western Cape, S.A. | UF-RO | Unknown | 4.9E3 m3/d (1.3 MGD) | 2010 | Industrial water production |
| City of San Diego | San Diego, CA, U.S. | MF/UF-RO | UV-AOP, ozone | 3.8E3 m3/d (1 MGD) | 2011 | Drinking water augmentation |
| Cloudcroft Water and Wastewater Department | Cloudcroft, NM, U.S. | MBR-RO + UF | UV-AOP, GAC | 3.8E2 m3/d (0.1 MGD) | 2007 | Direct potable reuse |
Figure 2.
Planned water reuse share of market. Data from [10].
1.3 Treatment Processes
Years after the first application of reverse osmosis (RO) for wastewater purification at Orange County Water District, California, a treatment train consisting of microfiltration (MF) or ultrafiltration (UF) followed by RO and an advanced oxidation process (AOP) has emerged as an industry standard for many potable reuse applications Figure 3 [8]. The RO and AOP components are referred to as “full advanced treatment” (FAT), which is defined as the treatment of an oxidized wastewater using RO and an oxidation treatment process (e.g., UV/AOP). The use of MF/UF in front of RO is referred to as an integrated membrane system (IMS) where MF/UF acts as pretreatment to RO [7]. In Figure 3, the steps shown prior to membrane treatment help reduce membrane fouling, and the steps after (e.g., AOP) break down small neutral organic compounds that pass through the RO process. FAT and IMS are favored for many potable reuse projects due to high removal efficiencies of microbial pathogens, organic and inorganic contaminants, and other constituents relative to potable water production (e.g., particles, inorganic nitrogen, and dissolved solids; see Table 1) [11]. Post-treatment disinfection at current IPR and DPR facilities may be accomplished through the UV or UV-AOP processes. For groundwater and reservoir augmentation systems, post-treatment chlorine disinfection is generally not implemented as the water is sent to an environmental buffer. Primary disinfection for current DPR systems is typically performed downstream at the water treatment plant treating potable reuse treatment system effluent [11]. Additionally, chloramine residual is often applied before the RO membranes in order to minimize membrane biofouling, and is used instead of the stronger biocide chlorine due to polyamide RO membrane sensitivity [3]. Water stabilization (adding chemicals like calcium hydroxide) as a final step is often done to minimize distribution pipe corrosion.
Figure 3.
Schematic diagram of an industry standard potable reuse plant with MF-RO-UV-AOP, which employs low-pressure filtration (MF) followed by RO and UV advanced oxidation. To represent other conventional wastewater treatment processes, before the membrane steps, are settling tanks (primary treatment), aeration followed by settling for biological activated sludge (secondary treatment), and sand filtration (tertiary). The step UV-AOP (ultraviolet and advanced oxygenation processes) includes hydrogen peroxide or alternative oxidants, and the final step for water stabilization includes processes such as degassing and lime dosing (shown) intended to increase the water’s pH and alkalinity.
Compared to alternative unconventional water resources such as seawater desalination and water importation, potable water reuse generally requires less energy and is less costly [12, 13]. Although the FAT has become the backbone of most planned potable reuse projects and many consider it the standard for potable reuse worldwide, a number of drawbacks are associated with its implementation. Thus, alternative treatment trains may be used [14]. One such variation is to omit biologically activated carbon (BAC) from tertiary treatment plant, instead using other means for biological nutrient removal, which is often combined with ultrafiltration (UF) instead of microfiltration (MF). As another variation, biologically activated filtration may be used after RO and UV to remove byproducts of disinfection. Some trains even lack salinity control, omitting RO membranes. However, RO membranes are rarely omitted for direct potable reuse. Overall, several aims are always accomplished regardless of whether FAT or an alternative treatment train is used for potable reuse: physical removal, oxidation, and chemical inactivation [15].
Overall, the widespread use of RO membranes in potable reuse applications is due to: 1) demonstrated success in multiple installations worldwide enabling technology familiarity in a risk-averse industry responsible for protecting public health; 2) ability to handle variable input (i.e., operational reliability); 3) modularity; and 4) the very high quality of the product water, particularly with respect to pathogens, dissolved salts characterized as total dissolved solids (TDS; typically >99% removal), and wastewater-derived organic contaminants such as pharmaceuticals (removal varies, but typically >90%) [16–18]. While high pathogen removal is expected, current regulatory structures often give little or no disinfection credit for RO due to limitations in monitoring membrane integrity. Major advantages of membrane-based treatment systems over conventional processes for potable reuse include small footprints, modular designs, synergistic combinations with other treatment processes [19], fewer treatment stages and the ability to reject compounds that other processes cannot eliminate [20].
2. Membrane Technologies
Although the secondary or tertiary treated wastewater used as a source water for potable reuse applications may be of acceptable quality for environmental discharge and non-potable uses, it may still contain a wide range of undesirable constituents including [21]:
Conventional pollutants, e.g., suspended solids, colloids, nitrogen, metals, phosphorus, inorganic salts and pathogens
Unconventional pollutants, e.g., oxyhalides and refractory organics
Emerging contaminants, e.g., pharmaceuticals, plasticizers, pesticides, degradation by-products of detergents, and endocrine disrupting compounds (EDC’s).
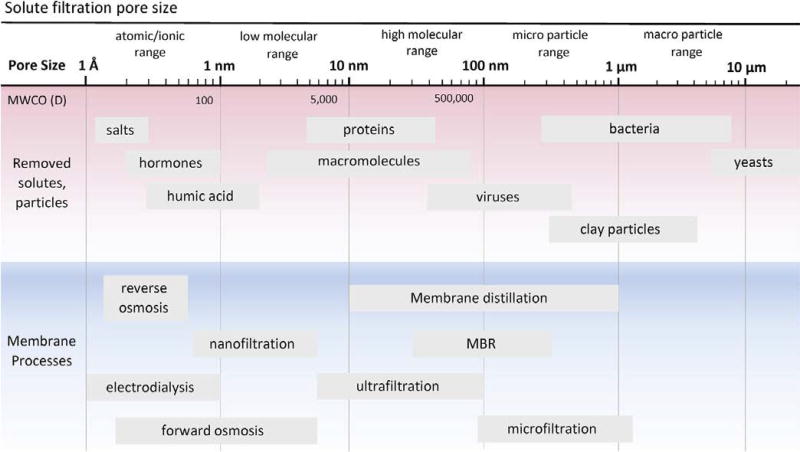
The principle of most membrane separations is the selective filtration of influent through pores of different sizes [22]. Figure 4 summarizes the separation performance of these membrane types based on the size ranges of certain common constituents found in water and the effective pore size of the membrane [23]. As the practice of potable reuse becomes more common, removal of pathogens and chemical contaminants will be an ongoing area of focus, and new treatment requirements may be on the regulatory horizon. The removal of target constituents from an aqueous solution by polymeric membrane systems can vary significantly and depends on many factors including constituent physicochemical properties, membrane type, and operational conditions [23]. The World Health Organization guidance document for potable water reuse provides recommended contaminant removal criteria [24].
Figure 4.
Membrane separation processes, pore sizes, molecular weight cut-off (MWCO) and examples of sizes of solutes and particles. (modified from [20], Copyright 2009 Reproduced with permission from Elsevier)
Membrane processes can be classified into different categories, based on different criteria including membrane configuration, type of membrane materials, driving force, separation mechanism, and size range of constituents removed. The latter is dictated by the membrane’s pore size or molecular weight cut-off (MWCO) [22]. Four main membrane types, as noted previously, are commonly used to treat wastewater to potable standards and are classified in order of decreasing pore size [22, 25]. As the pores get smaller the processes need more driving force. The technologies are often classified as low pressure (MF, UF) and high pressure (NF, RO) [22, 26].
For easier reference, a schematic diagram summarizing the most important constituents rejected by each membrane type is shown in Figure 4. For a better understanding of the role of membranes in potable water reuse, the fundamentals of the most important membrane technologies used within the context of advanced wastewater treatment are briefly discussed in the following sections.
2.1 Microfiltration and Ultrafiltration
Microfiltration (MF) and ultrafiltration (UF) are classified as low-pressure (<2 bar) processes. Separation by MF mainly occurs through sieving and, due to the relatively large pore size (approximately 0.1 to 1.0 µm), is mainly limited to the removal of suspended solids or particles, bacteria and, to lesser extent, organic colloids [16]. UF membranes also mainly operate through sieving but have a wider separation range than MF and depending on the pore size (generally between 0.01 and 0.1 µm), can remove particles, pathogens, viruses, and colloids. Potable reuse treatment (as shown in Table 1) commonly employs MF or UF for pathogen removal and as pretreatment for the NF or RO process. This pretreatment is critical to maintaining the integrity of the NF/RO system. The rejection achieved by MF and UF membranes depends on the properties of the membrane as well as the hydrodynamic conditions [27]. Additionally, disruptions in the upstream wastewater treatment processes can negatively affect the performance of a MF/UF unit and can cause significant fluctuations in MF/UF filtrate water quality [21], as well as increasing the required frequency of backwashing and chemical cleaning.
In potable reuse systems, MF membranes are commonly relied on to remove most fine suspended solids (more than 99% rejection) and some colloidal material. They can also provide 3 to 6 log removal (order of magnitude reduction for every increase of 1, i.e., 99.9 to 99.9999% removal) of protozoan cysts and coliform bacteria [21, 28]. MF pores typically range between 0.1 and 1.0 µm in diameter, providing limited removal of viruses (up to 2-log), although virus disinfection credit is rarely awarded [25]. The MF filtrate in potable reuse treatment schemes will subsequently be treated by RO and UV-AOP, each of which independently provides a very high level of disinfection. However, the incentive for virus removal at the pretreatment (MF/UF) stage remains high particularly since pathogen removal regulatory credit for RO is currently limited, as described in the section 2.2.2 [25]. In contrast, MF and UF system integrity can be confirmed daily via pressure decay testing (see section 7.2), thus allowing the cyst removal disinfection credits (e.g., 4-log credit for Giardia cysts and Cryptosporidium oocysts in the California regulatory framework).
UF membranes can typically reject all suspended solids, remove organic matter, reduce BOD5 (Biological Oxygen Demand over a 5 day test) by at least 95%, and greatly reduce turbidity. In addition to the contaminants removed by MF, UF can provide up to 6-log removal of bacteria, and if the membrane modules are intact, they can completely eliminate protozoan cysts and coliform bacteria from the filtrate [21]. However, practical experience has shown that typical UF membranes operated at reuse facilities do not always perform as a complete barrier to bacterial contamination, such as indicated by positive coliform results from the literature [25]. Membrane surface defects, deterioration of membrane due to biofouling, or imperfections in the packing of membrane modules or elements are the most probable causes of bacteria permeation [25]. UF membranes also provide an improved barrier to viruses (with up to 7-log removal) compared to MF [21]. Retention of viruses is enhanced at lower transmembrane pressures. As observed in MF membranes, virus and solids aggregates can form a cake layer on the membrane surface. The presence of turbidity and/or biomass in the feed water can enhance this adsorptive removal of viruses due to additional surface area [25].
Phosphorus, nitrogen, and total organic carbon constituents in soluble and colloidal form can also be partially removed through UF (and less with MF) but the achieved rejection can vary widely, 10 to 85%, depending on the phase of the contaminants (soluble or particulate). Increased removal can be achieved if chemical coagulants are dosed into the feed water [21]. Neither MF nor UF remove dissolved constituents such as salts and organic chemicals [21, 29]. In Table 2, the rejection ranges for MF and UF membranes for tertiary effluent are tabulated. It should be noted that the actual performance of a particular installation can vary according to the system’s specifications and operating practices [26, 29].
Table 2.
Tertiary effluent water quality and rejection characteristics of microfiltration and ultrafiltration membranes [26].
| Constituent | Concentrationa | MF rejection % |
UF rejection % |
|---|---|---|---|
| TSS (mg/L) | 2–8 | 95–98 | 96–99.9 |
| BOD5 (mg/L) | < 5–20 | 75–90 | 80–90 |
| COD (mg/L) | 30–70 | 70–85 | 75–90 |
| TOC (mg/L) | 8–30 | 45–65 | 50–75 |
| NH3-N (mg/L) | 1–6 | 5–15 | 5–15 |
| NO3-N (mg/L) | 0-trace | 0–2 | 0–2 |
| TDS | 500–700 | 0–2 | 0–2 |
| Total coliform (no./100 mL) | 103–105 | 2–5b | 3–6b |
| Protozoan cysts and oocysts (no./100 mL) | 0–10 | 2–5b | > 6b |
| Viruses (PFU/100 mL) | 101–103 | 0–2b | 2–7b |
. Conventional activated sludge system with nitrification
. Log removal
In all membrane technologies, fouling prevention and mitigation can be challenging. Fouling prevention measures for MF and UF usually include regular backwashing (cleaning every ~30 minutes for large-scale applications) and chemical cleaning [20]. For cleaning, the type of chemical used depends on the membrane’s chemical tolerance, and the cleaning frequency can vary from as much as once per day to once per month in potable reuse applications depending on the membrane type and quality of wastewater treated. Polymeric UF and MF membranes are less tolerant to chemical cleaning than their inorganic (e.g., ceramic) counterparts [30].
2.1.1. Membrane Bioreactor (MBR)
A common way to utilize MF and UF membranes for wastewater treatment in various industries is to combine them into an activated sludge process termed a membrane bioreactor (MBR). In MBRs, typically the membrane is submerged inside the bioreactor and vacuum is used to permeate the treated water while solids are retained in the bioreactor. This configuration not only reduces energy consumption, but also lower the amount of membrane fouling compared to a traditional side stream configuration [31]. The membrane is usually provided as a flat-sheet or hollow fiber configuration. Typical polymeric membrane materials for MBR applications include polyvinylidene fluoride (PVDF) (which accounts for approximately 45% of polymeric MBR membranes), polyethylene (PE), polyacrylonitrile (PAN), and Polyethersulfone (PES); of which the PAN membrane is most likely the most fouling resistant due to its lower affinity with extracellular polymeric substances [32]. The nomical pore size of MBR membranes is usually between 0.03 and 0.4 µm. In addition, the versatility of PDVF membrane manufacturing makes them available in the whole range of pore sizes, while, for example, PES and PE membranes seems to be mostly available only with 0.03 and 0.2 – 0.4 µm nominal pore sizes, respectively [33]. Notably, MBR membranes often have lower integrity than UF membranes.
The MBR replaces the two-stage conventional activated sludge (CAS) process (biotreatment and clarification) with a single, integrated process. There are several advantages of MBRs compared to conventional treatment, the most important being product consistency, reduced footprint, reduced sludge production, and nearly complete suspended solids separation from the effluent [34]. MBR technology more efficiently removes a wide range of biodegradable and hydrophobic trace organics than CAS processes, as MBR systems operate at a much higher mixed liquor suspended solids (MLSS) concentration [35]. During MBR treatment, hydrophobic trace organic contaminants can be adsorbed to MLSS, increasing retention time in the bioreactor and thus enhancing removal efficiency. Furthermore, unlike a CAS treatment process, MBRs provide a definitive boundary layer that provides complete suspended solids retention. Because of these features, MBR effluent may be suitable for use as irrigation water, process water, or as a pretreatment for potable reuse applications [36].
2.2 Nanofiltration and Reverse Osmosis
Nanofiltration and reverse osmosis processes are very similar in that they are designed to remove dissolved chemical contaminants including salts. Both require high hydraulic pressures and utilize similar membrane materials. NF removes many of the same solutes as RO but to a lesser degree (see Table 3). Although NF is rarely used in potable reuse processes, treatment plants are considering it as a lower-energy alternative to RO.
Table 3.
Expected rejection values of nanofiltration and reverse osmosis membranes on tertiary effluent [37].
| Constituent | Nanofiltration rejection rate (%) |
Reverse osmosis rejection rate (%) |
|---|---|---|
| TDS | 40–60 | 90–98 |
| TOC | 90–98 | 90–98 |
| Hardness | 80–85 | 90–98 |
| NaCl | 10–50 | 90–99 |
| NaSO4 | 80–95 | 90–99 |
| CaCl2 | 10–50 | 90–99 |
| MgSO4 | 80–95 | 95–99 |
| NO3− | 80–85 | 84–96 |
| Fluoride | 10–50 | 90–98 |
| Atrazine | 85–90 | 90–96 |
| Proteinsb | log 3–5 | log 4–7 |
| Bacteriaa,b | log 3–6 | log 4–7 |
| Protozoaa,b | log >6 | log >7 |
| Virusesa,b | log 3–5 | log 4–7 |
Theoretically, all microorganisms should be removed. The presented values reflect integrity concerns.
Refers to log removal, where log 2 is 99%, log 3 is 99.9% etc.
2.2.1 Nanofiltration
NF membranes were introduced in the late 1980s as an alternative “loose” RO membrane for applications wherein some ionic solutes in the feed water are selectively and purposely passed into the permeate [22]. The pore size of NF membranes is approximately 1–5 nm, which allows passage of neutral solutes of that size or smaller as well as some passage of monovalent salts such as Cl− [22, 27]. Compared to RO membranes, NF membranes have higher water permeability and allow operation at lower pressures, thus reducing the specific energy consumption [22].
In NF, solute rejection occurs as a result of several exclusion and transport mechanisms. Solutes are excluded from the membrane through steric, dielectric, and Donnan exclusion [38] and, in some cases, by adsorption to the membrane surface [4]. Solute rejection is also a function of the relative transport resistances of solutes and water. Solute transport occurs through three mechanisms, according to the extended Nernst-Planck equation (see Ref [38]): (1) convection of solute with the flowing water, (2) diffusion down the concentration gradient across the membrane, (3) electro-migration down the potential gradient that develops across the membrane due to the unequal diffusion rates of different ions. Water transport is generally modeled using a modified Hagen–Poiseuille equation (see Refs. [4, 39]).
Typically, NF rejects more than 95% of divalent ions of the same charge as the NF membrane, whereas the rejection of monovalent ions ranges from approximately 20 to 80% [4]. For uncharged solutes, however, the rejection as a function of molecular weight is represented as a sigmoidal curve, indicating differential separation between different compounds on the basis of molecular mass [40]. Therefore, the typical NF permeate could contain molecules of size varying below and above the claimed pore size of the membrane [23]. Models of the rejection of organic micropollutants by NF membranes have been proposed by various researchers [41, 42]. As an alternative to RO, NF membranes are appealing for potable water reuse applications in which the source wastewater TDS level is relatively low (i.e., <500 mg/L) and/or where hardness (rather than monovalent salt rejection) is the primary challenge. In addition, recent research has indicated that higher recovery from NF systems can be achieved through non-thermal crystallization [43] and/or ozone pre-treatment to reduce second stage membrane fouling [44]. Recent studies have been undertaken to further demonstrate the viability of NF within a multi-barrier potable reuse treatment process [42, 45].
2.2.2 Reverse Osmosis
RO membranes consist of a homogeneous polymer layer (e.g., polyamide), which preferentially permeates water, on top of a hierarchal polymeric support material (e.g., polysulfone and polyethersulfone) [22, 46]. Pressure is applied to drive the solvent (water) through the membrane while retaining most solutes on the feed side [22]. In order to produce fresh water, the applied pressure exceeds the osmotic pressure of the feed solution [47–49]. RO is the key treatment step of FAT, the current industry standard for potable reuse [11]. The FAT train has been demonstrated to be an effective and efficient process for potable reuse, largely because RO highly purifies water through rejection of most dissolved salts and organic molecules that are common contaminants in wastewater (e.g., organics like CECs), as well as larger particulates if not already removed by upstream pre-treatment membranes (MF or UF). For example, at the Torreele IPR plant in Belgium, the RO step effectively reduces hardness, TDS, organic carbon, nitrogen, and phosphorus, and removes approximately 98% of pesticides at an energy consumption of just 0.63 kWh/m3 [50]. RO energy consumption has decreased significantly since 1970, primarily due to improvements in membrane permeability and energy recovery efficiency [47–49]. RO uses highly-selective membranes to reject salts, colloids, biological materials, and most dissolved organics [22].
With RO, salts are generally highly-rejected; commercial seawater RO membranes reject 99.5–99.8% of sodium chloride. Brackish water RO membranes commonly used for potable reuse have approximately 99.5% salt rejection, despite their high water permeability [51]. Even higher rejections are reported for divalent and multivalent ions such as sulfate and phosphate, at ranges of 99.7– 99.98% and 99.7 – 99.99%, respectively [52].
Due to the sub-nanometer scale of RO pores, RO is considered to be a complete barrier for pathogens. For example, in a pilot study of RO treatment of wastewater, neither E. coli nor viruses were detected in the RO permeate with either MBR or MF pretreatment [53]. At the Orange County Water District’s (California, USA) advanced water purification facility, currently the largest potable reuse plant in the world (IPR via groundwater recharge), twice weekly monitoring for the indicator organisms’ total coliform and E. coli and monthly monitoring for virus indicators (coliphage) in RO permeate since the plant came online in 2008 has never resulted in a detection. Nevertheless, regulatory credit in the United States for pathogen removal by RO at advanced treatment facilities for reuse is currently limited to 2-logs, based on the approximately 2-log salt removal across RO that can be continuously monitored via conductivity analyzers [27]. To obtain higher credit closer to known performance (e.g., virus log removal of 4–7 per Table 3), a permitted method/technology (e.g., fluorescent dyes) is needed for continuous or frequent demonstration of an RO system’s integrity (e.g., due to possible malfunctions, operator error, or unnoticed leakages via glue strips or permeate seals in the spiral-wound elements) [25] at greater than 2-log.
RO membranes have been found to be effective in removing high molecular weight organic constituents (such as humic and fulvic acids) [28]. The BOD and chemical oxygen demand (COD) can be reduced up to 98% and 96%, respectively, and TOC can be rejected at 96% or higher [28]. The removal of EDCs can be as high as 95 to 99% [28]. Removal of organic solutes, such as wastewater-derived pharmaceuticals, is crucial in potable reuse, but the rejection varies between solutes and membranes [33]. Urtiaga et al. [54] performed pilot-scale testing of pharmaceuticals rejection by UF and RO treatment of wastewater effluent. In this study, all 12 compounds tested had rejection values greater than 99.3%. Radjenovic et al. [55] studied rejection of pharmaceuticals in a full-scale drinking water treatment plant using RO and NF treatment of groundwater. Most compounds were rejected by 85% or more by RO, but few solutes (both neutral and charged) were rejected poorly by 30–70%. Lower removal of neutral organic solutes was observed for lower molecular weight compounds. Additionally, incomplete rejection of certain DBPs, such as nitrosamines, and some micro-pollutants, such as 1,4-dioxane, of low molecular weight (less than 1001 Da) has been observed during full and pilot scale tests of high-pressure membrane applications [28]. However, detection of the low molecular weight compounds in product water has been reported only at trace concentrations, well below health significance [28, 56].
Despite low rejection for certain organic compounds, RO generally removes most compounds to a very high degree and better than other engineered or natural alternatives. For example, Drewes et al. [57] examined the efficacy of soil-aquifer treatment (SAT), NF and RO at removing organic carbon including dissolved organic carbon (DOC), polysaccharides, and humic substances, among other types. In almost every metric, RO removed the greatest fraction, followed by NF and SAT. SAT was still moderately effective, as shown by a 77% DOC removal over 12–18-month residence time of tertiary effluent. TOC rejection by RO and NF were 94–96.4% and 91.3–94.5%, respectively. Lower-molecular weight compounds were less rejected by the RO and NF membranes. Of 36 pharmaceuticals and EDCs chosen to represent a range of contaminant types and structures, another study showed that RO removed most to below detection limits, and double-pass RO removed all but two compounds to below detection limits [58].
Although there is experimental evidence of sub-nanometer pores in the active layer of polymeric thin film composite (TFC) RO membranes [59] (see Section 3.1.3), the passage of water and rejection of dissolved matter by RO is commonly simulated with the solution–diffusion model (which neglects transport by convection; see [60]) and variations thereof (see [52]). In the solution–diffusion model, both water and solutes are considered to dissolve into the membrane and diffuse through it. The chemical potential gradients inside the membrane’s active layer depend on the feed and permeate concentrations, membrane sorption coefficients, and applied pressure. For a given membrane, the solution–diffusion model predicts that solute rejection increases with increasing flux (i.e., the amount of permeate generated per unit area of membrane surface per unit time). Other RO membrane transport models including pore-flow models are reviewed in [61]. These models are divided into pore-flow, irreversible thermodynamics, and nonporous membrane models.
Various configurations of RO modules have been developed with the intent to minimizing energy consumption and contaminant permeation. In RO plants, multi-pass design (i.e., permeate from first stage is treated in a second stage) can be used to improve rejection of potentially harmful solutes such as borate, although this is uncommon in potable reuse applications. In reuse plants, multiple stages (i.e., concentrate from one stage is treated in the following stage) are commonly used to increase recovery and improve energy efficiency. As an example of multiple RO membrane passes, Israel’s Ashkelon seawater desalination plant uses multiple passes to meet stringent water quality standards and multiple stages within the 2nd and 3rd pass to improve recovery [62]. However, multiple RO passes are uncommon in potable reuse applications. In reuse plants, multiple stages (i.e., concentrate from one stage is treated in the following stage) are commonly used to increase recovery and in some cases, can improve energy efficiency Multiple stages with inter-stage pumps improve energy efficiency by minimizing the applied pressure in the first module(s) [63].
At typical potable reuse recovery percentages (50–85%) [7], 15–50% of the feed volume is converted into concentrate that must be disposed of or reused. The RO concentrate from a reuse plant is typically disposed through surface water (e.g., ocean) discharge, recycled to the wastewater treatment plant, deep well injected, or sent to an evaporation ponds, and may require treatment beforehand [2, 19, 64]. The vast majority of large-scale RO-based potable water reuse facilities are located in coastal areas, thus concentrate disposal is most commonly achieved by discharge to the ocean. However, some regions have restrictions or prohibitions on ocean outfalls. For instance, in 2008 the Governor of Florida signed into law a requirement that wastewater utilities in southeast Florida cease to use ocean outfalls by 2025 [65]: this may require membrane and system designs for much higher recovery ratios and thus solute tolerance (fouling, etc.). Inland water utilities often have few, if any, capabilities to discharge the concentrate, which is highly enriched in salts, emerging contaminants, pathogens, and other materials rejected by the RO system. Thus, further treatment of RO concentrate may be advantageous to increase the overall water production of a water reclamation facility while at the same time minimizing the volume of concentrate that requires disposal [43].
3. Membrane Materials
Development of novel membrane materials is a major research thrust for academia, industry, and national laboratories because membrane performance is often challenged by fouling, low permeability, and high contaminant permeation relative to stringent selectivity requirements. There are unique needs for membranes made for potable reuse. Reuse applications, as opposed to industrial applications, face a diversity of contaminants of concern. Such contaminants range from microorganisms (e.g., viruses) to molecular organics (e.g., pesticides) and inorganic compounds (e.g., heavy metals). These membranes, where possible, also need to be tolerant to relatively extreme chemical cleaning processes [30]. This translates into a number of different materials that may be effectively used within potable reuse treatment trains.
3.1 Polymeric Membranes
3.1.1 Membrane Comparison
Numerous polymers are used in the creation of membranes, although a select few have emerged as leading choices for potable reuse applications. Like any other application, two main design considerations drive potable reuse membrane technologies: membrane material properties and membrane formation mechanisms. Driving factors for which material is used include pore size distribution, wetting susceptibility, porosity, mechanical strength, cost, polymer flexibility, fouling resistance, stability, durability, and chemical resistance [64]. The latter may include resistance to pH, oxidants, and chlorination (Which is of particular importance for potable reuse membranes given the high levels of dangerous microorganisms, with little tolerance for their presence) [64]. Other desirable properties that also relate to the fabrication itself include low tortuosity and surface properties that influence rejection (e.g., surface charge). Additional characteristics may influence performance as well, such as improved regeneration/fouling recovery, which results from many things including low surface roughness, poorly adhering materials, and resistance to cleaning agents [66].
Potable reuse membranes share much in common with other processes such as desalination, but have notable differences. Salt-selective potable reuse membranes gain more benefit from high permeability due to reduced concentration polarization. Such membranes also need superior solute rejection and reliability, due to the wide variety and variability of solutes in the wastewater feed, with larger toxicity concerns. Still, many reuse systems use modules and technologies borrowed from desalination and municipal water treatment.
Many reviews of membrane materials have been previously reported published, so the present discussion is intended to be concise and comparative, focusing on membrane design than individual polymers. A list of common membrane materials and properties is displayed in Table 4.
Table 4.
| Material | Acronym | Most common use |
Advantages | Disadvantages | Mechanical strength & durability |
Hydrophillicity & WCA |
pH | Chlorine resistance |
|---|---|---|---|---|---|---|---|---|
| Polysulfone | PSF | MF/UF | Good mechanical strength, chemically resistant, |
|
|
1–13* |
|
|
| Polyether sulfone | PES | MF/UF | Good thermal properties, Rigid, compaction resistant, very permeable, oxidant tolerant, narrow pore size distribution |
|
|
1–13* |
|
|
| Polyacrilonitrile | PAN | MF/UF |
|
|
||||
| Polyvinylidie ne fluoride | PVDF | MF/UF | very oxidant tolerant, chlorine resistant | Broader pore size distribution |
|
|
2–11* |
|
| Polyethylene | PE | MF/UF (uncommon) | High resistance to organic solvents, Low cost, oxidant tolerant | Poor thermal properties, Weaker fouling resistance |
|
|
|
|
| Polypropylene | PP | MF/UF (uncommon) | High resistance to organic solvents, decent mechanical strength | Weaker fouling resistance, not oxidant tolerant |
|
|
2–13 |
|
| Polyvinyl chloride | PVC | occasionally MF/UF | Poor thermal stability, not oxidant tolerant |
|
|
|||
| Cellulose acetate | CA | RO, also MF/UF | Renewable source | Poor microbial degradation resistant |
|
|
5–8.5 |
|
| Polyamide | PA | RO (TFC active layer), NF, occasionally MF/UF | Small pores, excellent rejection, selectivity | Poor acid and alkali resistant Weak, experiences compaction |
|
|
1–13** |
|
Symbols are used as follows:
 excellent,
excellent,
 good
good
 fair, and
fair, and
 poor
poor
Poor long-term stability in basic conditions
Poor long-term stability in acidic or basic conditions
With respect to membrane materials used for MF/UF in potable reuse facilities, a variety of materials are used (see Table 4) [66–68]. As an example, currently polypropylene MF membranes (Evoqua S10T submerged, outside-in configuration) are used by Orange County Water District (OCWD), which operates the largest potable reuse facility in the world. This material choice is less common today for MF compared to alternatives that have emerged (such as PVDF) since the OCWD facility came online in 2008; regardless, the original polypropylene membranes operated successfully at OCWD for nine years (past a typically assumed life of approximately seven years) until their replacement in 2016 with the same product [69]. For potable reuse applications, the industry has moved largely toward PVDF as a material of choice, which is stated to have reduced capital cost, increased membrane life, and reduced nominal pore size compared to polypropylene [70]. Like polypropylene, PVDF is amenable to the outside-in configuration (i.e., direction of flow in a hollow fiber module – see later section on module types), air scour, and backwashing, but unlike polypropylene is chlorine resistant (enabling use of this effective oxidant during monthly membrane cleaning) [30]. Within the PVDF family of hollow fiber materials, modifications in manufacturing techniques have resulted in the development of two distinct classes of materials: non-solvent induced phase separation (NIPS) and thermally induced phase separation (TIPS) fibers. While new materials with performance advantages continue to emerge, consideration of alternate MF/UF materials (products) at an existing facility can require significant capital expense for a system retrofit, because unlike the RO industry, low-pressure membrane systems have not standardized around a common platform (e.g., size, design) which would (like RO) enable a new product to be directly installed (swapped in with no system modifications). Rather, MF/UF systems are unique to each supplier and are offered in a variety of configurations in both pressurized or submerged designs [71].
RO membranes are analyzed in this review in greater detail (see next section), as they are the most crucial to potable reuse and dominate cost, energy use, and R&D interest. Membrane materials may be modified to improve performance and mixed in composite membranes.
3.1.2 Membrane Fabrication
A variety of manufacturing techniques are used to fabricate membrane. Often, these methods depend more on the membrane material than the membrane class (e.g., UF, MF, etc.), although processes vary for controlling pore size, especially for composite membranes. Phase inversion and electrospinning are respectively the most and second most common techniques to fabricate mem- brane which is mostly used for fabricating potable reuse membranes. Notable phase inversion variants include non-solvent induced phase separation (NIPS) (a dominant technique), thermal phase separation, controlled evaporation, and finally vapor induced phase inversion (VIPS).
More complex methods are used for composite membranes and/or membrane surface modifications [72]. Other techniques used in membrane fabrication or modifications include in-situ polymerization, film casting, ion assisted deposition, aerosol deposition, ion exchange, dip coating, hydrothermal synthesis, sputtering and etching, surface adsorption, layer-by-layer deposition, and spray coating [73].
Chemical methods are also used to modify polymer membrane chemistry as a whole, depending on applications. These include hydrophilization treatment with plasma, radical grafting, and chemical coupling.
For more on self-assembled polymer nanostructures for filtration membranes, readers are referred to Asatekin and Vannucci [74]. For recent reviews on reverse osmosis membrane materials and nanomaterials readers are referred to Lee at al. [51], Maleb and Ayoub [61], Giwa et al.[3], and Lau et al. [75]. For reviews on NF membrane materials, Amirilargani et al. [76], for carbon nanomaterials, Goh et al [77], and for more general nanomaterials, Santhosh et al. [78]. Additional reviews include membrane fabrication by Lalia et al. [73], NF membrane fabrication by Cheng et al. [79], RO fouling by Pandey et al. [80], and for antifouling membranes, by Kang and Cao [81] and Saqib and Aljundi [82]. Additionally, chemical cleaning for potable reuse membranes has been reviewed by Porcelli and Judd [30]. A review of electrospun membranes was recently published by Kaur et al. [83], on phase inversion membranes by Wang et al.[84], and on track etching by Chakarvarti [85].
3.1.3 RO Membranes
RO membrane materials were reviewed in depth by Lee at al. [51] in 2011. Aspects of that review are summarized in this section, along with subsequent research. Although current potable reuse plants exclusively use thin-film composite (TFC) membranes, a range of RO membranes have historically been made from single polymers. Lee et al. [51] offer a brief history of cellulose acetate (CA) membrane development: After a symmetric CA membrane was found to have very low flux, asymmetric CA membranes were developed [51]; later, cellulose triacetate or diacetate–triacetate blends were also developed. CA has higher chlorine resistance, but it is prone to compaction (loss of permeability at high pressure) and hydrolysis in both acidic and alkaline environments, which reduces cleaning options. For CA membranes, significant acetylation (replacement of hydroxyl groups with acetyl groups using a catalyst such as H2SO4) improves salt selection but reduces permeability [86].
Other polymers including polyamide, polybenzimidazoline, and poly(piperazine-amide) have been evaluated for use in single-polymer asymmetric membranes, but none have the combination of permselectivity, compaction resistance, and chemical stability offered by the thin film composite membranes that currently dominate the RO membrane market [87].
Most state-of-the-art commercial RO membranes today are TFCs. TFC membranes consist of a semi-permeable “active” layer, typically an aromatic polyamide, of around 50–200 nm thickness supported by a microporous polymer layer (typically polysulfone) roughly 40 µm thick, which is itself supported by a non-woven polyester web of approximately 100 µm thickness [51, 59] (Figure 5). These membranes achieve salt rejection around 99.5%, and good rejection of low molecular weight organics compared to CA [51, 59, 88, 89]. Lee et al. [51, 59, 88] reviewed the history of TFC membrane development and the various combinations of polymers used. Polysulfone was identified as the optimal material for the porous support layer because of its compaction resistance and stability under acidic and alkaline conditions making it compatible with interfacial polymerization. Various polymers have been used for the formation of active layer on membrane surface, but polyamides are most common. Notable commercial TFC membranes are listed in prior studies [51]. As an example, the OCWD potable reuse facility (100 MGD) utilizes three different types of TFC RO membranes – Hydranautics ESPA2-LD (previously used ESPA2 before membranes reached end-of-life), Dow XFRLE, and CSM FLR – which were selected at different times for installation into newly-built RO units as the plant expanded over time. Regardless of the fact that RO products are all TFC, OCWD testing complete prior to any product selection has indicated that product performance varies; namely, different manufacturer’s membranes can require significantly different feed pressures (affecting operational cost) to produce the same flux with similar permeate quality on the same feed water.
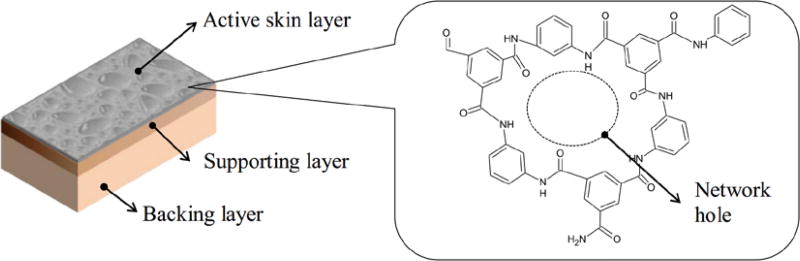
Figure 5.
Layers of typical TFC (thin film composite) RO membranes. The chemical structure of the polyamide active layer creates selectivity, from [59], Copyright 2015, reproduced with permission from Elsevier.
Modern TFC membranes are produced by interfacial polymerization, a process in which two immiscible liquids, each containing one monomeric or polymeric aromatic amine, are brought together, allowing polycondensation at the interface. Various manufacturing methods were explored before the industry settled on interfacial polymerization. Early TFC membrane manufacturing methods included float-casting, dip coating, and acid polycondensation [51].
Recent studies have elucidated various aspects of the active layer structure. Fujioka et al. [59] reviewed positron annihilation spectroscopy and found that, for various RO membranes, free-volume holes (referred to as network holes in Figure 5) in the active layer have been measured in the range of 0.20–0.29 nm. Rejection of neutral species improves as the solute increases in size with respect to the free-volume holes. The active layer also exhibits roughness on a 100 nm scale [90] (Figure 6.). SEM images of the active layer surface show villi-like structures with a thin (~20 nm) skin and 50–200 nm cavities [59]. Permeability increases with increasing roughness (linearly, for the membranes tested) [90], a phenomenon ascribed to the increased cross-sectional area for diffusion.
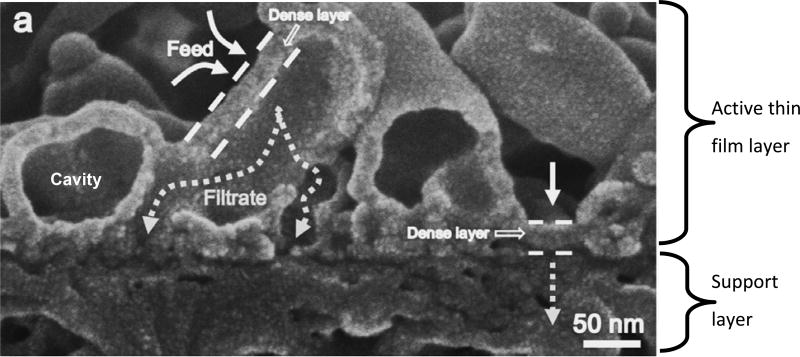
Figure 6.
SEM of a thin film composite RO membrane, showing the larger scale roughness of the ESPA2 membrane by Hydranautics/Nitto, with a typical active layer pore radius of 0.267 nm. Modified from [59, 91], Copyright 2015, reproduced with permission from Elsevier.
In contrast to RO and NF membranes, MF and UF membranes have much more porous structures, and do not have the tight relatively nonporous active layers seen in RO (Figure 7.). Because of the large particle size tolerance and high permeability, the MF/UF membranes are typically less complex than RO/NF membranes, and are thus easier and less costly to manufacture. Furthermore, their higher fluxes compared to RO membranes allows for a smaller surface area and corresponding investment, which further drives the research interest toward RO membranes over MF/UF.
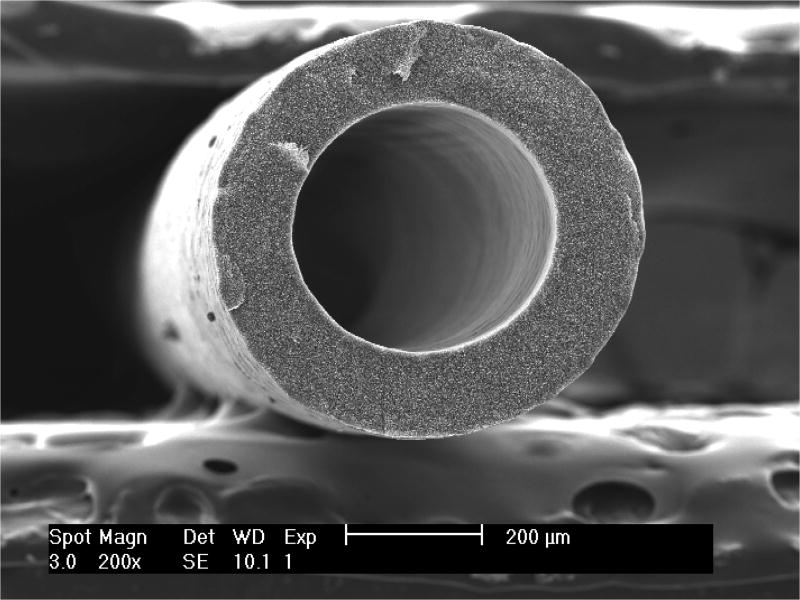
Figure 7.
SEM of polypropylene MF membrane showing a highly porous structure (Advanced Water Purification Facility membranes imaged by the Orange County Water District at University of California-Irvine Materials Characterization Center).
3.2 Nanocomposite Membranes
Introduction of nanomaterials into polymer membrane matrices has focused less on salt removal and more on niche applications. Surface coatings have produced enhancements of many physical/chemical characteristics such as water permeability, fouling resistance, selectivity, increased mechanical strength and temperature resistance. Comprehensive reviews have been recently published outlining the impacts of incorporating nanomaterials into various types of membranes [3, 92, 93]. The nanomaterials that have attracted the most attention in wastewater treatment are carbon based nanomaterials (e.g., graphene oxide (GO) and carbon nanotubes (CNTs)), titanium dioxide (TiO2), and silver nanoparticles (nAg). This section highlights areas of particular interest to the reuse industry.
Promising results have been obtained for spiral wound flat membranes modified with inorganic additives used for improving the antifouling properties including nano-sized titanium dioxide, silica, nano-sized alumina, zirconium dioxide, and lithium perchlorate as well as antimicrobial additives such as copper or silver [94]. CNTs and GO have also been actively explored in efforts to develop ultrapermeable membranes [95]. Hu and Mi [96] integrated GO into a layer-by-layer assembly for forward osmosis (FO) applications and demonstrated water permeability an order of magnitude higher than commercial polymeric membranes [97]. Computational studies have claimed that a membrane comprised of single sheet graphene would produce water flux 250 times higher than current commercial RO membranes under the same conditions [98]. Notably, in contrast to potable water reuse applications, for seawater RO, resistance to the drive for ultrapermeability has emerged because concentration polarization will sharply limit the achievable flux increase for saline feeds. [99]. However, for lower salinities, the case in most reuse applications, they find that more significant performance gains are still possible. Apart from flux, Cohen-Tanugi et al. [100] showed that the energy savings possible from high permeability reach diminishing returns rapidly as permeability rises. More significant energy savings could potentially be realized in wastewater reclamation due to its lower feed salinity.
CNTs have garnered interest for membranes because their diameters can be controlled to, in theory, allow for higher selectivity [101]. TiO2 nanoparticles have been used to impart superhydrophilicity and photocatalytic properties that help prevent biofouling and improve permeability [102]. The photocatalytic nature of TiO2 has also been utilized for degradation of natural organic matter (NOM), trace levels of pharmaceuticals and personal care products, and polishing of wastewater effluent [103]. Most research in this area focuses on finding the optimal TiO2 concentration and method of incorporation into different polymer matrices rather than scalability to commercial applications. A remaining concern is the potential degradation of the host polymer matrix by the reactive species generated at the surface of embedded nanocatalyst. Silver nanoparticles, nAg, are incorporated into membrane materials to impart biocidal properties to the membrane and prevent or reduce biofouling. Incorporation and regeneration of nAg can prove challenging and researchers have taken different approaches. For example, nAg surface functionalization [104] and in situ formation [105] were both explored for TFC polyamide RO membranes. The surface functionalization approach achieved > 95% of inactivation of surface bacteria while in situ formation led to an inactivation of more than 75% of bacteria.
A new class of hollow fiber nanocomposite membranes is emerging as a promising solution for MF and UF [106]. Hollow fiber nanocomposite membranes have several advantages, including low cost, ease of fabrication, high mechanical stability, and combination of polymeric and inorganic material properties [107]. In addition, they have been observed to efficiently disinfect and adsorb/degrade organics in potable reuse feed if incorporated during hollow fiber membrane fabrication [108]. TiO2 nanoparticles have been found to be most useful in this respect and have also been found to increase thermal resistance, permeability, hydrophilicity, porosity, and tensile strength of hollow fiber membranes [109]. Similarly, hydrophilicity, permeability, and mechanical stability of hollow fiber membranes can be enhanced by embedding zinc oxide nanoparticles (ZnO), which also increase reversible fouling which is very much essential for potable reuse applications [110]. Ag has been shown to improve antibacterial properties, fouling resistance, and mechanical stability of hollow fiber membranes [71, 111].
Apart from the properties imparted to the membrane, the scalability of the manufacturing processes and regeneration once installed are essential to ensure commercial adoption. Manufacturing scalability still remains a serious hurdle for nanocomposite membranes. At this point, very few of these emerging technologies are competitive with polymeric membranes in terms of cost [51], and while the feasibility of nanocomposite membrane technology has been demonstrated at lab-scale, commercial realization is very limited. One commercially available RO membrane that includes a propriety nano zeolite additive is manufactured by LG Chem (originally NanoH2O). Their membranes have demonstrated only slightly lower salt rejection, but 140–200% higher water permeability when compared with standard commercial thin film membranes [92].
In general, the effects of the incorporation of nanomaterials varies greatly depending upon the specific material, base membrane material, and the manner in which the nanomaterial is incorporated (e.g., during casting versus grafted onto the surface) [77, 78]. To date, the goal of many studies in early stage research is to determine the optimal loading of the nanoparticles to maximize performance measured in terms of permeability, strength, wettability, and selectivity rather than pilot studies for field applications. Additionally, nanocomposite membrane parameters and challenge tests are primarily optimized for seawater desalination, not reuse. Overall, desalination and reuse applications need research to address challenges for both membranes (e.g., low water flux, membrane fouling and regular replacement) and the reuse industry (e.g., potentially high solids loading and matrices that may contain emerging contaminants). In summary, while most nanocomposite membranes are not yet commercially available, diverse and promising ongoing research may serve the potable reuse community in terms of target contaminant removal and energy/cost savings.
3.3 Ceramic Membranes
While polymeric membranes have been the staple in the suite of membranes used in water treatment applications for all pore ranges and operating modes, ceramic membranes have recently emerged as a broad classification of materials that show significant promise for applications to potable reuse. Contrasting polymeric and ceramic membranes is important for understanding the advantages and disadvantages of both. Ceramic MF/UF membranes have demonstrated potential for pretreatment to reduce fouling of RO membranes in combination with oxidation processes (e.g. ozone, UV) to degrade pollutants reducing membrane fouling, but their cost is high relative to polymeric membranes. Where ceramic membranes are employed, they are often used in combination with polymeric membranes later in the same treatment train (e.g., polymeric RO membranes) [112]. Although the present review focuses on polymeric membranes, ceramic membranes possess unique attributes relevant to wastewater reuse that should also be described. With further research, ceramic membranes might be improved to match the performance of polymeric membranes at a more feasible cost.
Ceramic membranes have the ability to be cleaned with harsh chemicals that would damage polymeric membranes. Thus, ceramic membranes have potential for treatment of high fouling feeds such as filter backwash [113]. Ceramic membranes employed in municipal water treatment are most commonly produced from alumina. Other common active layer materials include titanium dioxide and zirconia oxide. Since the use of ceramic membranes is uncommon, few studies directly evaluate potable reuse using ceramic membranes, but there are several areas where ceramics are particularly promising.
Various ceramic membranes ranging from MF to NF in pore size have been evaluated for use in treating secondary municipal wastewater effluent [114]. Materials including α-alumina, anatase, γ-alumina, amorphous titania, and amorphous organo-silica active layers on α-alumina supports have been evaluated. The γ-alumina NF membrane was deemed the most promising membrane for treatment of wastewater treatment plant effluent based on permeate flux (6.6 LMH) and selectivity (75% of UV254 absorbing compounds and 15% of ions). Most commercially available ceramic membranes lack pore sizes small enough for virus removal, but their tolerance for higher concentration of chlorine, oxidants, and coagulants allows them in theory to achieve high degrees of virus removal. Kramer et al. [115] studied the use of ceramic UF and NF membranes for pretreatment of typical municipal sewage prior to RO. After pretreatment by 6 mm screening and 0.5 mm sieving, the ceramic filters required less frequent cleaning than polymeric membranes [116]. Kramer et al. [109] also observed that biofouling of a RO membrane treating ceramic membrane permeate was very low. This study demonstrated that ceramic membranes may be considered for raw sewage reuse, where longer cleaning intervals are desired, and/or to prevent biofouling of RO membranes. Another membrane material that warrants further study is zeolite, which has demonstrated salt rejection in both molecular dynamics simulations and, to a lesser extent, in practice [51].
The oxidative resistance of ceramic membranes may provide more flexibility in the design of treatment processes for enhanced NOM removal. Ozone threatens the integrity of many polymers (although PTFE and, to a limited extent, PVDF are exceptions), but ceramic membranes are resistant to oxidation because they are already completely oxidized. If ozone is applied before a membrane, substantially more NOM, DBP precursors, and estrogenicity is removed, and a lower fouling propensity is observed in comparison to when ozone is used after MF/UF membrane processes [117, 118]. Catalytic ozonation on the surface and inside ceramic membrane pores can also prevent fouling [119]. The limited studies of ceramic membranes in reuse applications have highlighted their ability to handle high solid loadings and resist oxidation by ozone used for pretreatment. Therefore, due to their chemical resistance, ceramic membrane technology warrants continued research attention for water reuse applications. However, in comparison to polymeric membranes, ceramic membranes are less cost competitive, generally have larger pores and less permeable.
4. Membrane Module Types
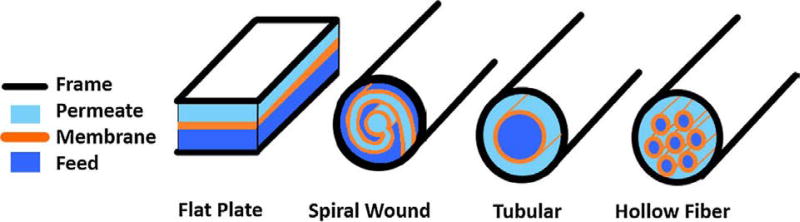
Membrane filtration technology has developed not only with respect to the membrane materials, but also how membranes are packaged in reactors and modules. Module types often place large constraints on membrane materials due to available fabrication processes. Thus, a wide range of membrane modules have been developed, suited to a variety of applications. The four conventional polymeric membrane module types are flat sheet, hollow fiber, spiral wound, and tubular (Figure 8) [120, 121]. However, flat sheet membranes and the rarer tubular modules (1–3 cm in diameter) have higher costs and lower practical packing density. These types are largely being replaced by hollow fiber or spiral wound membrane modules for water treatment and reuse applications. Some researchers have developed and tested novel configurations apart from those mentioned above. For example, one novel module configuration is a helical membrane configuration, where two pieces of membrane sheets are supported on a plastic spacer. Another is a fishbone or broom-like structured spacer, which has been examined by Liu et al. [122]. Other recent configurations, induce membrane vibrations to increase filtration rates [123, 124]. However, to date, none of these have proven to be cost competitive. Hence, the discussion below is focused on the three most commonly used polymeric membrane modules for potable water reuse applications: flat plate, hollow fiber and spiral wound. Relevant findings for each of these module configurations and challenges ahead are discussed in the following subsections.
Figure 8.
Schematic diagrams of module types for most membrane applications
Notably, flow configuration is another key distinction for membrane modules and consists of either dead-end or cross-flow (tangential flow) filtration. In dead-end configurations, all fluid passes through the membrane, perpendicular to its surface. In contrast, cross-flow systems are designed so that the flow is parallel to the membrane, and residual feed water is rejected as brine. Dead-end filtration is often used in experimental studies or when the particle loading in the feed is very low. While it has higher water recovery, dead-end filtration suffers faster fouling, requiring frequent backwashing [67, 125].
4.1 Flat Plate
Flat plate (plate and frame or flat sheet) membrane modules, also known as stacked membrane modules, are used in few water treatment applications where the feed streams to be treated contain high amount of foulants and/or have high viscosities. Modern flat plate membrane systems are built to tolerate very high pressures, in excess of 100 bar. They are used in the treatment of landfill leachates and for industrial textile wastewater reuse [126]. Even though tangential flow flat plate membranes have proved to be a popular MBR configuration for wastewater treatment (e.g., immersed flat sheet modules from Kubota®), their application is limited to advanced water treatment due to their low surface area to volume densities. Flat plate modules are predominantly used for MF and UF, with little industrial use for RO and NF. Extensive membrane fouling and low treatment efficiencies remain major challenges associated with flat sheet membrane modules in potable reuse applications [127].
To counter these challenges, recent research has focused on combining flat sheet membrane modules with embedded photo catalysts, such as TiO2, for the removal of organic matter from feed water [128, 129]. Hernandez et al. [130] showed that doping flat sheet membranes with Fe/Pd nanoparticles can enhance removal of contaminants due to an order of magnitude increase in catalytic activities on membrane surfaces. In addition, research has also shown that pretreatment of the feed water with polyaluminium chloride (PACl) and ozone can enhance the efficiency of flat sheet membranes used for potable reuse applications [131, 132]. Research in minimizing fouling of flat sheet membranes is also directed at hybrid forward osmosis (FO) systems for wastewater treatment/reuse, where FO membranes are first cast on hydrophilic glass plates and thermally annealed in water thereafter. These membranes have a highly porous sublayer sandwiched within and are termed as double-skinned membranes. They generally have less fouling propensity and mitigated internal concentration polarization (ICP) [133, 134].
4.2 Hollow Fiber
Hollow fiber (HF) membranes consist of several thousand hollow fibers with a small hollow portion called as lumen with various dimensions from of 0.5–1 mm in diameter. Due to its high surface area per volume, HF modules are mostly preferred over other configurations for large-scale operations. Generally, the hollow fiber modules used in potable reuse applications are manufactured to accommodate MF or UF membranes where they are common. However, this configuration has recently become commercially available again for RO (although it remains rare), and to some extent, for NF [135]. Challenges in making sufficiently permeable NF/RO membranes with sufficient structural strength have limited this applications, although fundamentally hollow fiber membranes have favorable mass transfer coefficients and packing densities for these processes [130]. The manufacturing of hollow fibers can be more limited than that of the flat membranes seen in flat plate or spiral wound systems, as roll-to-roll processes and coating techniques such as spray deposition or fabrication methods like polymer composites are more challenging. Due to technical limitations the technology and precision of making HF membranes and modules is still not widely explored. On the other HF membranes have higher packing density than that of other flat membranes so the separation efficiency is higher in this case for potable water reuse.
Filtration in HF membranes can operate from inside-out or vice versa, allowing for backwashing. HF membrane modules are increasingly used for wastewater potable reuse and desalination [136]. Hollow fibers are more resilient to small particulate fouling than spiral wound membranes for reuse applications, though they still benefit from pre-filtration if suspended solids are present in the feed water. Most HF membranes, even after surface modifications, are limited in their applications to pressures below 3.4 bar [70] whereas research on HF membrane with higher mechanical strength is ongoing which include inorganic additives.
Hollow fiber membranes for wastewater reuse are restricted by challenges including the trade-off relationship between permeability and rejection (related to membrane porosity), and low resistance to fouling [106]. Fouling issues are exacerbated by fiber clogging, and also by the small channels, which make high velocity cleaning difficult. Continuous efforts have been made to counter these challenges by modifying standard configurations, as in the case of spirally wound silicone rubber hollow fiber membranes and monofilament nylon [121], which display lower pressure drops and higher mass transfer rates due to combined advantages of both spirally wound and hollow fiber modules. Research has shown that inside-out hollow fiber modules, arranged in parallel configuration and operated in dead-end filtration mode, at some stage during cleaning need chlorinated water for backwashing if used for wastewater reuse [125]. Hence, tangential flow filtration is the most preferred hollow fiber membrane configuration due to reduction in fouling
Modifying membrane sometime called novel membrane surfaces is another alternative to counter the above-mentioned challenges of hollow fiber modules similar to using nanocomposite materials [71, 111]. Apart from metal nanoparticles, multi-walled carbon nanotubes (MWCNT) are increasingly being used during fabrication of hollow-fiber membranes [137] for either improved fouling resistance (carboxylated MWCNT) or increased permeabilities (hydroxylated MWCNT) [137] but the goal of high permeability and high selectivity is still a dream to fulfil.
4.3 Spiral Wound
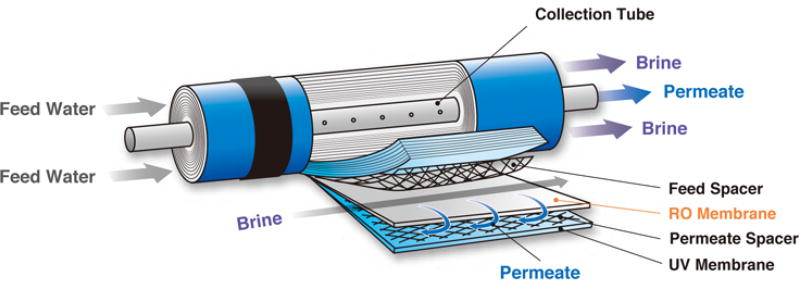
The most common membrane module used in NF/RO processes is spiral wound [120] which is also used for MF/UF. Spiral wound design success relates heavily to high packing densities and the relative ease of manufacturing the modules and the flat sheet membranes. Spiral wound modules contain a small-diameter tube tightly packed with flat sheet membranes separated by mesh spacers in the feed and permeate channels (Figure 10). This dense configuration means available surface area is higher for a filtration unit (example picture provided in Figure 11), thus overcoming the limitations of flat plate and tubular configurations. Most of these modules are composed of polymers (plastics), including the membranes, spacers, and other components, with the exception of the stainless steel pressure vessels and pipes. Spiral wound membranes have been found to be successful in not only removing traditional contaminants from feed [138, 139] but also emerging contaminants [140, 141] from wastewater sources. In the case of spiral wound RO, pretreatment is essential for water reuse applications [142]. Spiral wound membrane modules are highly sensitive to particulate fouling that can reduce process efficiency and reduce membrane lifetime (U.S.EPA, 2005). Hence, increasing fouling resistance remains the biggest challenge for spiral wound membranes.
Figure 10.
Spiral wound RO module design, Copyright 2016, reproduced with permission from Aquanext [143].
Figure 11.
Spiral wound RO membranes installed in Groundwater Replenishment System (GWRS) Advanced Water Purification Facility (Fountain Valley, California), taken at the Orange County Water District.
Ongoing research, through the last decade, focused on the optimization or complete removal of the feed spacer mesh from the spiral wound feed channels [144, 145]. Spacers in the feed can create dead regions that promote scaling, fouling, and particulate deposition. Successful spacer research has implement combinations of 3D printing with numerical modelling and experimental testing for reducing biofilms and improving flux recovery in RO and NF [146]. Limited lab-scale improvements are implemented however, because improved fabrication of complex geometries of these polymeric spacers (e.g. through 3D printing) remains expensive. Another area for innovation is higher-pressure tolerant modules composed of plastics, which can be cheaper, more chemically resistant, and less likely to induce salt nucleation than the typical stainless steel vessels and pipes.
The other significant breakthrough in this domain has been in surface modifications [147]. In addition, current research is focused on optimization of cleaning parameters for spiral wound membranes. To control biofouling and increase efficiency of the treatment, periodic cleaning of high-pressure RO/NF membranes is conducted as an effective operational strategy [148].
5. Alternative Membrane Technologies
5.1 Electrodialysis/Electrodialysis Reversal
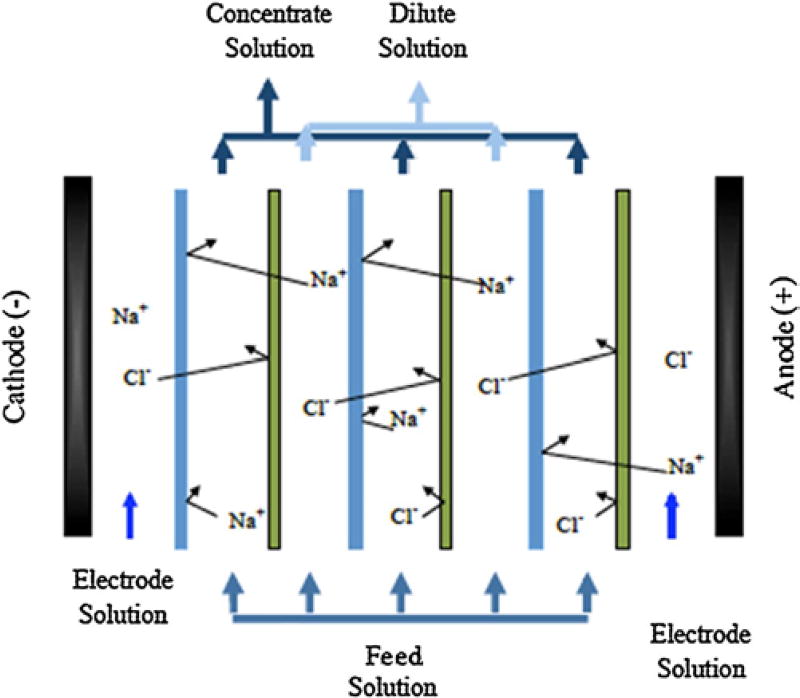
ED is a membrane-based desalination process in which ions transport through ion exchange membranes under the influence of an applied electrical field [149]. EDR was proposed by Meyer and Strauss in 1940, but its application at industrial scales started much later [150, 151]. ED was introduced and used in industrial applications before RO [150]. A schematic of an ED stack is shown in Figure 12. In EDR, the basic ED process is enhanced by periodically changing the direction of ion transport by reversing the polarity of the electrodes. This periodic change in polarity is done in order to prevent scaling and fouling problems and increase process efficiency [152, 153]. Additionally, when the polarity is reversed, automatic valves switch the flows of the dilute and concentrate streams through the cells [154].
Figure 12.
Schematic drawing of an electrodialysis stack. An applied voltage causes ions to move between electrodes, and membranes that either block negative or positive ions cause the ions to be trapped in concentrate channels.
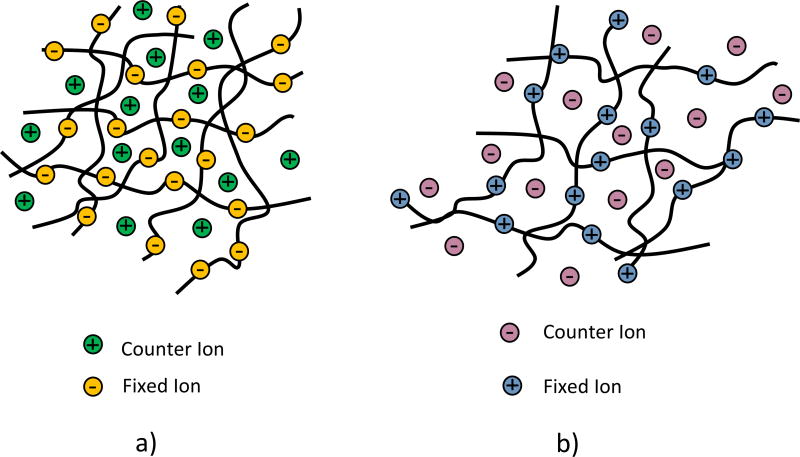
The ion exchange membranes used in ED/EDR processes can be considered as ion exchange resins in the polymeric matrix, and they are categorized into cation exchange membranes (CEMs) and anion exchange membranes (AEMs), as depicted in Figure 13. These ion exchange membranes have low electrical resistance, high pH stability, resistance to scaling, and fouling, robust structure for washing, cleaning, and long lifetime [155]. CEMs, which are negatively charged ion exchange membranes, can only pass cations. Most commercialized CEMs are composed of sulfonated cross-linked polystyrene that has a large number of sulphonate groups. In water, this ionic group is ionized to mobile H+ counter-ions and functional groups of –SO3− fixed in the structure [150]. AEMs, which are positively charged ion exchange membranes, only permeate anions. These ion exchange membranes typically have fixed quaternary ammonium groups, –NH4+ or quaternary –N-R3 in the polymeric structure [150].
Figure 13.
Schematic diagram of the structure of a) a cation exchange membrane and b) an anion exchange membrane.
Ion exchange membrane characteristics are especially important in the ED/EDR process, and these characteristics can be controlled to some extent in the manufacturing process. Ion exchange membranes are characterized by their properties of electrical resistance, ion exchange capacity, water content, ion transport number, solute permeability coefficient, electro-osmotic coefficient, water permeation coefficient, swelling ratio, and mechanical strength [156].
The ion exchange membrane performance can be significantly affected by its physico-chemical characteristics. For instance, the electrical resistance of IEMs can be changed by changing fixed charge groups or membrane charge density, strongly affect ion selectivity through interactions with different counter-ions [157]. Increasing ion exchange capacity (IEC), as another key membrane characteristic, can result in higher membrane conductivity, improved counter-ion pathways [158, 159], which results in higher swelling in the IEM and less effective Donnan exclusion, and consequently less counter-ion permselectivity [158]. Although larger water content in ion exchange membranes can elevate ion passage and conductivity of the membrane, it results in lower permselectivity for counter-ions [160].
5.1.1 Application of ED/EDR in Wastewater Reclamation
While other membrane-based water treatment processes, such as UF and RO, have been used for potable water reuse, ED/EDR processes are not very common in municipal wastewater reclamation since EDR can remove only ionized species. It thus lacks of removal of organic matter, taste, and odor. A facility that currently utilizes this technology for salinity removal with agricultural and industrial reuse is North City Water Reclamation Plant in southern California [6]. The application of ED/EDR in wastewater reuse has recently attracted attention [161]; however, only feasibility studies for applying ED/EDR in indirect potable water reuse are underway in the US and Japan. While lacking in potable applications, EDR has been widely used for desalination of industrial wastewater and concentration in pharmaceutical processes. Hence, only sparse data are available on the use of ED/EDR for water reclamation. One potential application of EDR for potable water reuse could be for higher water recovery via salts removal when coupled to RO/NF for the organics removal.
Rodrigues et al. [162] (2008) demonstrated the feasibility of an integrated photoelectrochemical oxidation (PEO) and EDR system for the removal of ionic and organic species from tannery effluents. The integrated PEO process improved the performance of the EDR system by reducing the membrane fouling potential. It has been observed that the removal of the ionic species was considerably higher which is around >> 98.5% [162]. ED systems are typically used to desalinate mainly brackish feed water at lower salinities [7].
The airport wastewater treatment plant (WWTP) in the City of San Francisco has also used EDR for irrigation purposes [163]. There, sand filters and activated carbon is use for the pretreatment of wastewater with a salinity of 2,600 ppm [163]. At the North City plant in San Diego, EDR systems are used to reduce the salinity of a portion of reclaimed water [7].
Notably, ED/EDR has a unique advantage for agricultural water, as it preferentially can remove the most harmful salts (e.g. Cl−). An EDR pilot treating municipal wastewater for agricultural applications decreased the TDS of influent by 71% from 1104 ppm to 328 ppm [164]. Additionally, ED pilot systems of wastewater have been shown to meet standards for water purity for more than 6 months [165]. These systems have been very effective for heavy metal removal from wastewater as well [16, 165–167].
Although six-month comparisons between EDR and RO systems used for the desalination of reclaimed water with TDS of 500 to 750 ppm showed that an EDR process with a cartridge filter was more cost-effective than an MF/RO process [168], RO is generally preferred for wastewater reclamation with potable reuse. On the other hand, ED/EDR can be used appropriately for the salinity reduction of wastewater when the reclaimed water is used for agricultural and industrial applications. Additionally, it can have the potential to be considered for wastewater reclamation for indirect potable water reuse if it is appropriately integrated with other required technologies.
5.2 Forward Osmosis
Forward osmosis processes use concentrated solutions (e.g., with salts such as NaCl) to remove clean water from impaired sources [169, 170]. Here, water moves from the feed to draw solutions due to an osmotic pressure difference across a semi-permeable membrane (similar to RO membranes). Then the draw agent must then be separated from the product water in an additional step such as RO [170, 171]. FO is sometimes considered to be more resistant to fouling because it is a low-pressure process, but hydraulic pressure alone has been shown not to affect fouling; therefore, low pressure does not mitigate fouling in FO. To limit fouling in FO, the smooth active layer of the membrane usually faces the feed solution and the porous support layer faces the draw solution.
FO has been successfully demonstrated as pretreatment of the feed for wastewater streams for water reuse (including in the osmotic membrane bioreactor configuration and FO-RO systems [172]), as well as to pretreat seawater and brackish water for desalination, concentrate landfill leachate, and process (concentrate) foods and beverages [172–174].
Membranes used for FO are similar to NF and RO membranes in their high solute rejection and asymmetric construction. One of the leading FO membrane materials is cellulose triacetate, which provides a robust structure, low fouling propensity, and longer shelf life [175]. Some new FO membranes have a thin-film composite (TFC) structure similar to RO membranes [176]. However, the FO membrane support layer is thinner and more porous than in RO so as to reduce internal concentration polarization. In FO, internal concentration polarization lowers the concentration gradient across the selective layer of the membrane, and thus the water flux, by maintaining a lower salt concentration inside the support layer compared to the bulk draw solution [177]. Additionally, for FO, double skinned hollow fiber membranes have been synthesized and have shown a great promise in reducing fouling and increasing permeability without compromising selectivity of hollow fiber membranes [178, 179], but this approach is still not commercially used or available.
A noteworthy application of FO membranes is their use in an osmotic membrane bioreactor (OMBR) configuration together with RO (Figure 14) The OMBR is a multiple-barrier system that has been investigated for IPR and DPR applications [180–188]. The system combines activated sludge processes with the FO process, which extracts water directly from the activated sludge into the high-salinity draw solution, which is then desalinated and recovered with RO. The FO membrane in the OMBR offers the advantage of reduced fouling and greatly increased rejection of dissolved species [189], including macromolecules [190] and TOrCs [191, 192] when compared with MF or UF [189]. As dissolved organic compounds (DOC) in the MF/UF effluent can cause organic fouling on the RO membranes and provide a substrate for microbial growth, the FO barrier may limit biological fouling on RO membranes [193]. Given the high fouling potential of activated sludge, it can be beneficial to have the activated sludge contact FO membranes rather than RO because FO tends to be easier to clean and has longer shelf life. Furthermore, the high rejection of dissolved organic and inorganic species by the FO membrane in the OMBR system (Figure 14) followed by RO for draw solution separation results in a higher RO permeate quality when compared with the conventional MF or UF followed by RO systems [194, 195]. However, FO-RO systems have higher energy consumption than direct RO systems [196, 197] when desalinating a given the same feed, so an overall system assessment should be undertaken into account when comparing FO and RO treatment options.
Figure 14.
Schematic drawing of an OMBR system comprising a bioreactor containing submerged FO membranes and an RO unit that re-concentrates the draw solution and produces water.
In another water reuse configuration, FO can be combined with seawater RO (SWRO) to create an integrated water reuse/desalination solution [190] (see Figure 15) [190]. As high water quality diffuses through the FO membrane from the impaired stream to the seawater, the impaired water becomes concentrated while the seawater draw solution becomes diluted. The SWRO membranes are protected from fouling with wastewater constituents and high quality water can be recovered for potable reuse by the RO process. The RO feed water salinity is significantly reduced, so the RO stage itself may be operated at lower pressure and energy consumption than would be required to produce the same volume of fresh water from unblended seawater [172]. However, the overall system’s energy consumption may be greater than would be needed to recover water from the wastewater using MF-RO, because the salinity of the wastewater is so much lower than that of either seawater or seawater diluted with FO permeate. Further, the FO membranes themselves are not immune to fouling [198].
Figure 15.
Schematic diagram of an FO-RO system for integrated SWRO and wastewater recovery. Water from the wastewater is extracted by osmosis through the FO process and dilutes the seawater before entering the RO process.
The FO-RO system can be operated in one of two modes: i) with the RO system at the same pressure of a standalone RO and FO applied for increased water recovery; or ii) operating at the recovery of a standalone seawater RO with a lower energy consumption and more dilute brine [199]. Both arrangements can mitigate environmental concerns associated with the discharge of concentrated brines [200].
5.3 Membrane Distillation
Membrane distillation (MD) is a thermally driven process, in contrast to the pressure driven processes usually used for potable water reuse [201, 202]. In MD, a temperature gradient across a hydrophobic porous membrane causes vapor to evaporate from the saline feed, diffuse through a membrane that is impermeable to liquid water, and condense in a cooler stream [202]. These membranes differ from MF/UF/NF/RO substantially, as they need to repel liquid water instead of preferentially passing it. Almost all MD membranes are composed of hydrophobic polymeric materials such as PVDF, PTFE, and polypropylene [203]. Hydrophilic polymers can be coated with a nano-layer of hydrophobic materials or moieties, using techniques such as chemical vapor deposition, to render them viable for MD [204, 205]. The energy needs of thermal processes like MD are relatively insensitive to the salinity [206], in contrast to the energy for processes like RO which are significantly increased by higher salinity [207]. While MD has advantages in fouling resistance and the production of very pure water (rejection >99.9%), relatively high energy consumption makes it rarely viable for potable water reuse [208].
6. Non-Membrane Processes
A major drawback to membrane processes for potable reuse application is fouling, increasing energy use and operating costs. Also, membrane processes alone are not very efficient in removing a small number of important trace-level contaminants from the permeate, such as certain pharmaceuticals and personal care products (PPCPs), endocrine disrupting chemicals (EDCs), and disinfection by-products (DBPs) as well as persistent organic pollutants (POPs) [58]. Hence, membrane technologies are often combined with other conventional non-membrane technologies, which can enhance the overall treatment efficiency and reduce operating costs, for potable water reuse applications. To maintain high permeate fluxes over long periods of time at potable reuse plants, adsorption, biological active filtration (BAF), and/or advanced oxidation processes (AOPs) are used in conjunction with membrane processes [11]. Advances and challenges in each of these processes are highlighted below.
6.1.1 Adsorption
Adsorption (largely through activated carbon) is an important step in potable reuse, where it removes small quantities of dangerous compounds (soluble organics, heavy metals, etc.) as part of tertiary treatment [209]. Classification is done by particle size, with granular activated carbon (GAC) denoting larger particles of diameter >0.1 mm, and powdered activated carbon (PAC) for smaller particles of diameter <0.074 mm [6] (Figure 16). GAC is dominantly used for potable reuse because of cost concerns, and the risk of smaller particles forming a fouling cake and irreversible pore fouling [210] on the UF/MF membranes in later steps [6, 37]. Adsorption with GAC is critical for removing compounds that cause membrane fouling. It is particularly effective in removing compounds with high and moderate hydrophobicity (e.g., steroid hormones, triclosan, bisphenol A [211]), hydrophobic NOM, and trihalomethane precursors. GAC improves removal as measured by DOC, COD, total nitrogen and phosphorous, turbidity, and other metrics. Favorable technology combinations include GAC with ultrasound (improved absorbance) and coagulation [212, 213].
Figure 16.
Granular activated carbon with their pore structure and distributions.
PAC is usually found to be superior in removing DOC and NOM in hybrid systems [210]. Hence, the challenge remains to find an innovative solution for combining PAC’s superior adsorption capacity with the convenience, affordability, and regeneration ability of GAC, for increasing effectiveness of downstream membrane processes for potable reuse.
6.1.2 Biologically Active Filtration
Biologically active filtration (BAF) is a promising membrane process for pretreatment in potable reuse, especially for dealing with wastewater with high levels of biological components. BAF combines three treatment benefits: biodegradation, micropollutants adsorption, and suspended solids filtration through the media. The microbial growth attached to the filter media (GAC) consumes the organic matter that would otherwise be responsible for membrane fouling. As shown in Figure 17, ozone is usually coupled with BAF and can together be used as a pretreatment step prior to MF/UF to reduce organic carbon. Further, depending on the finished water quality needs, ozone/BAF may eliminate the need for RO, although it does not remove salts [37]. If combined with RO directly, the ozone/BAF treatment has been shown to effectively reduce the biodegradable dissolved organic carbon (BDOC) and assimilable organic carbon (AOC) contents, and the bacterial regrowth potential, thus confirming its potential for mitigating biofouling of the RO membrane [214]. The use of bio-filtration after ozonation has also been reported to reduce the formation of DBPs during chlorine disinfection [215].
Figure 17.
Ozonation coupled with biologically active filtration (BAF)
Bio-filtration alone is not very effective, without a prior oxidation step, in removing organic contaminants if used in potable reuse treatment train. Notably, BAF (via biologically activated carbon, BAC) can improve coagulation efficiency for DOC removal [216, 217]. Overall, GAC is a preferred media for BAC due to stronger adhesion of biomass; however, other media, including anthracite, can be effective media as well [215]. The prominent challenge for incorporating BAC in a potable reuse treatment train is balancing adsorptive and biodegradation capacities, as regenerating BAC for adsorption disrupts the biofilm [216]. More research is required to understand optimum conditions (temperature, water quality, operation) for media to support all three mechanisms contributing to enhanced efficiency of BAC.
6.1.3 Advanced Oxidation Processes
Advanced oxidation processes (AOPs) are often the final step of potable reuse treatment trains and rely upon formation of highly reactive radical species, such as hydroxyl radicals (•OH), for oxidation of residual trace organics that were incompletely removed by upstream treatment units or were formed during treatment (i.e., DBPs). AOPs compliment membrane processes as they aid in removing taste, odor, and color along with more resistant chemicals, such as PPCPs, EDCs, and DBPs [218], which membranes fail to remove. As detailed in Table 5 [218], use of hydrogen peroxide coupled with ozone or UV light are the most commonly employed AOPs in potable water reuse [219]. As opposed to adsorption or BAC, AOPs form the tail end of the potable reuse treatment train and are almost always installed after membrane processes [11], to polish final water quality and because of the increased efficiencies of UV light and radical oxidants in purified water (high UV transmittance and minimal organics competition for radicals) [11].
Table 5.
Advanced oxidation process (AOP) summary
| Process | Method | Oxidant hierarchy |
Pros | Cons | Source |
|---|---|---|---|---|---|
| H2O2 + O3 | H2O2 injection, O3 generator, mixing | •OH, •O3−, HO2•, HO3•, •O2−, O3, H2O2 | effective for MTBE, well-established | Formation of bromate, nitrosamines, excess H2O2, needs ozone off-gas, O3 mass-transfer limited | [220–224] |
| H2O2 + UV | H2O2 injection, fluorescent ultraviolet bulbs, mixing | •OH, •O2−, O3, UV | effective for MTBE, UV can disinfect too | requires UV bulb cleaning, formation of THMs and HAAs, toxicity may increase | [220,225] |
| O3 + UV | O3 generator, fluorescent ultraviolet bulbs, mixing | •OH, •O3−, HO2•, HO3•, •O2−, O3, UV | reduces nitrosamines, generates more ·OH (and needs less O3) than H2O2 + UV | Formation of bromate, requires UV bulb cleaning, energy and cost intensive, needs ozone off-gas, O3 mass-transfer limited | [220,226] |
| Photocatalytic oxidation | TiO2 (usually), in recoverable slurry or integrated into membranes. | •OH, h+ (Reactive electron gap) | Higher UV wavelengths (300–380) are usable | lack of full-scale applications, fouling of photocatalyst, may need oxygen sparging, tight pH control needed, recovery if slurry used | [220] |
Ozone is itself a powerful oxidant. However, once dissolved in water, it produces radicals, including hydroxyl, superoxide, and hydroperoxyl, which can non-selectively oxidize most of the organic matter [197]. Ozone can be used alone, due to its high oxidation potential, as a polishing step for potable reuse [219, 222, 223].
UV light alone or as part of an AOP can selectively transform trace organic compounds that are sensitive to direct photolysis (e.g., nitrosamines, triclosan, acetaminophen, diclofenac, sulfamethoxazole) [58]. However, UV light alone is ineffective for broad organic compound removal since many compounds are not photosensitive. Thus, the addition of hydrogen peroxide or other oxidants, which react with UV light to generate radicals, is essential for contaminant oxidation (indirect photolysis). However, complete mineralization is likely not obtained during AOP for many constituents, resulting in transformation products including potentially undesirable ones. Overall optimization of AOP (cost and water quality performance) therefore remains a significant challenge and area of research for potable reuse treatment. Emerging AOP processes that have not been used at scale include high energy beam irradiation (e-beam), cavitation (sonication and hydrodynamic), and Fenton’s reaction [220]. Notably, better contaminant rejecting RO membranes could reduce the reliance on AOP’s, including a reduction in dosage requirements and energy use.
7. Challenges for Potable Reuse
The key challenges for potable reuse are: 1) membrane fouling and scaling, which increases project cost; 2) the incomplete removal of certain trace level dissolved compounds by advanced treatment including membranes, which may necessitate additional treatment units or, if not a public health or compliance concern, creates public perception challenges; 3) public perception of re-using treated wastewater for drinking; and 4) regulations that can be complex or entirely absent depending on the available regulatory framework in the region where the project is implemented. Each of these challenges is briefly described below. Addressing these will require research innovation and successful policy implementation.
7.1 Membrane Fouling: Overview
Much innovation in membrane and process design is driven by the need to mitigate fouling. Membrane fouling decreases membrane permeability1, raises energy requirements, alters permeate quality [227], reduces membrane life, and generally raises the cost of water production. In experimental studies conducted at constant pressure the fouling-driven decline in membrane permeability leads to declining flux. However, in full scale water recycling plants, the applied transmembrane pressure is usually increased (at increased energy cost) as the membrane permeability declines in order to achieve the plant’s target water production rate. Fouling in wastewater reuse differs from fouling in other types of desalination because of the low salinity and high organic content of the source waters and the typically high recovery of the process. In this section, the types, mechanisms, and effects of fouling are reviewed. Unique fouling issues related to the high organic and biological content and low salinity of wastewater effluents are discussed. Finally, fouling mitigation methods are reviewed. This section aggregates the findings of previous membrane fouling reviews as well as more recent primary research articles.
In a review about fouling of RO membranes in wastewater reuse [80], fouling is categorized as scaling (crystallization of dissolved inorganic salts), cake formation by rejected material that accumulates on the membrane including colloids and organic macromolecules, or biofilm formation [228]. A more general review of RO membrane fouling [229] further divides cake formation into particulate fouling and organic fouling. Most source water have foulants of all types. For example, organic and silica fouling occur together in RO membranes treating MBR effluent [230]. Different types of fouling even enhance one another: for example, biofouling has been shown to enhance scaling by enhancing concentration polarization of sparingly soluble salts [231]. Additionally, membrane compaction and damage by chlorine are sometimes considered to be types of fouling [80]. Concentration polarization of rejected dissolved material is not typically considered fouling because of its reversibility, but the resulting increase in transmembrane osmotic pressure difference needs to be considered when designing plants [80]. Mechanisms of membrane performance degradation due to fouling have previously been reviewed [232, 233]. These include pore blocking, cake formation with associated hydraulic resistance and cake-enhanced osmotic pressure [234], biofilm-enhanced osmotic pressure [235], membrane compaction, and membrane degradation due to exposure to oils and chlorine.
Although fouling typically decreases membrane permeability (i.e., water flux per unit of pressure applied), the effects of fouling on solute rejection may be beneficial or detrimental. In a study of fouling with tertiary effluent and model organic and colloidal foulants on salt and N-nitrosamine rejection for RO and NF membranes, Fujioka et al. [236] found that, for most foulants, rejection of both salt and N-nitrosamines increased due to fouling. In contrast, during biofouling of NF membranes with MBR permeate, rejection of both organic matter and inorganic salts dropped (although only slightly) during a 10-day period in which the flux declined to one third of its initial value [237]. Xu et al. [238] measured the change in solute rejection of NF and RO membranes after fouling with microfiltered secondary effluent. The rejection of hydrophobic neutral solutes and ionic organic solutes increased after fouling, whereas rejection of the hydrophilic neutral primidone decreased due to fouling.
Although most of the worldwide RO capacity is devoted to seawater desalination, most fouling studies have used low ion concentrations because of the relatively lower pressures required. Low salinity tends to raise the recovery ratio of desalination processes, leading to scaling (see Section 7.1.2), but it also affects organic fouling, which depends on other aspects of the water composition, including pH, calcium concentration, and ionic strength [239]. Low feed salinity also reduces the pressure required in wastewater desalination, but low operating pressure does not mitigate organic fouling of desalination membranes
The mechanisms of permeability decline in wastewater RO differ from those in seawater RO because of their different ionic compositions and concentrations. The low osmotic pressure of municipal wastewater limits the strength of the contribution of cake-enhanced osmotic pressure (CEOP) to permeability decline [234]. In RO filtration of dead cells and extracellular polymeric substances (EPS) in 15 mM ionic strength synthetic wastewater and 0.01 µm LaCl3 solution, Herzberg et al. [240] found that foulant hydraulic resistance was much more significant than CEOP in EPS fouling; however, CEOP was more significant with dead cells. Sodium alginate, a polysaccharide produced in Pseudomonas aeruginosa biofilms [241], has been shown to produce a gel with smaller pores and thus more hydraulic resistance at lower sodium concentrations [169]. Protein fouling also depends strongly on the solution ionic composition [242]. Given the prevalence of polysaccharides in both wastewater and seawater RO fouling layers [243] and the relatively low osmotic pressure of municipal wastewater, wastewater RO flux decline is expected to be primarily driven by hydraulic resistance.
Several studies which have autopsied membranes from full- or pilot-scale plants revealed that the most important types of fouling in wastewater reuse are organic/biological and inorganic. Tang et al. [105, 198, 244] autopsied RO membranes used in a full-scale wastewater reclamation plant and compared the influent composition to the composition of the deposition on membranes in various positions in the train. On all membranes autopsied, the foulant layer was highly hydrated, with water content around 90%. By dry mass, the organic content of the fouling layer exceeded the inorganic content in all three positions, although the inorganic fraction increased in the later modules. Both organic and inorganic mass deposition decreased along the length of the membrane module, despite the increase in solution concentration. In a similar study, Khan et al. [243] performed autopsies of RO membranes in seawater and wastewater pilot plants. Organic and biological fouling dominated the lead elements in both seawater and wastewater plants. On the other hand, the end element had mostly organic/biological fouling in the seawater plant and inorganic fouling in the wastewater plant. The high level of inorganic fouling (scaling) in the end element in wastewater RO was attributed to the high concentration of sparingly soluble salts in the RO brine due to the high recovery ratio of the wastewater process. Organic compounds accumulated on the membranes were primarily proteins, lipids, and polysaccharides. The following sections discuss the roles of organic and biological fouling as well as scaling in wastewater reuse.
7.1.1 Organic and Biological Fouling
Organic fouling and biological fouling (biofouling) are major contributors to membrane fouling in wastewater reuse because of the high concentration of organic matter and microorganisms. Organic and biological fouling tend to occur in tandem because of the co-occurrence of organic compounds and microorganisms in wastewater, and because of the extracellular polymeric substances (EPS) produced by bacteria, which make up a significant fraction of biofilm volume [237]. Organic fouling and biofouling are mitigated through pretreatment, membrane cleaning, and membrane modification (particularly to enhance hydrophilicity), as discussed in Sec. 7.1.3. Material properties also improve organic fouling resistance, especially hydrophilicity. For protein antifouling, helpful properties include electroneutrality, minimization of hydrogen bonds, and containing surface groups with hydrogen bond acceptors.
Several studies have highlighted the importance of biopolymers and proteins in membrane fouling in wastewater reuse. Shon et al. [245] reviewed the constituents of effluent organic matter (EfOM) and treatment methods in wastewater reuse. EfOM is comprised of NOM, organics accumulated during domestic use, disinfection by-products, and “soluble microbial products” of biological wastewater treatment steps [245, 246]. Organic carbon is categorized by size as particulate (POC, over 0.45 µm) or dissolved (DOC). DOC is categorized as hydrophobic (e.g., humic acids), transphilic (moderately polar, e.g., sugar acids), or hydrophilic (e.g., polysaccharides). Organic compositions of wastewater effluents as well as the removal of various constituents by UF, MF, NF, and combinations of processes are given by Labs et al. [247] who studied fouling of MF and UF membranes with wastewater effluent and found that EfOM, composed of proteins, polysaccharides, and organic colloids in the range of 10–100 nm, are primarily responsible for the flux decline. Filloux et al. [248] examined the fouling potential of different classes of EfOM. This study concluded that biopolymers contribute to low-pressure membrane flux decline more than humic substances or low-molecular weight organics. Haberkamp et al. [249] tested UF membrane fouling with both real and synthetic wastewater and found that both biopolymers and proteins are important for UF fouling, but smaller organic compounds that are not rejected by the UF membrane (such as humic acids) are less problematic.
Several studies have examined biofouling of NF and RO membranes by wastewater [80]. Through a review of fouling studies, Pandey et al. [80] concluded that current pretreatment strategies are insufficient to control biofouling in wastewater reclamation. Ivnitsky et al. [237] studied biofilms formed on NF membranes treating MBR permeate and a synthetic wastewater effluent without suspended solids. Initial biofilm growth was characterized by polysaccharide accumulation on the membrane and the attachment of individual cells. Mature biofilms imaged by SEM showed layers containing both EPS and a variety of different other cells. The majority of the species identified in the biofilm were proteobacteria. These results were found to be largely in agreement with similar studies. Ivnitsky et al. attributed the similarity of the results of diverse studies using different membranes to the fact that microbes and nutrients are actively transported to the membrane and “the membrane surface is rapidly changed once the biofouling layer starts developing [so that] cell-to-cell and cell-to-EPS interactions may be more crucial to the development of the mature biofilm than their interaction with membrane itself” [237]. Farias et al. [250, 251] studied combined organic and biological fouling by measuring the flux decline and foulant accumulation of a bench-scale MBR-RO system treating primary clarifier effluent from a municipal WWTP. The relative concentrations of proteins, carbohydrates, and live and dead cells was measured with confocal laser scanning microscopy (CLSM) [250]. However, the RO flux decline results were not clearly related to the measured DOC, carbohydrate concentration, or protein concentrations in the MBR permeate [251], showing the complexity of fouling in wastewater treatment.
Biofouling also occurs in electrodialysis. One serious problem is fouling of AEMs in the presence of negatively charged colloids in the feed water, such as humates [252]. Surface charge, hydrophobicity, and solubility of foulants play a large role in the severity of fouling [150, 253].
7.1.2 Scaling
Scaling can cause rapid deterioration of membrane processes, including reduced permeability and membrane damage [80]. It is not very commonly an issue on potable water reuse due to the low salinities used, although can be an issue with certain wastewater sources. Generally, scaling occurs when specific minerals are supersaturated at the membrane surface, and are thus near or above supersaturation in the bulk feed solution [1, 229]. The primary salts of concern in potable reuse are sulfates and carbonates, such as CaSO4 and CaCO3. As these dissolved constituents [1, 229] are benign at low concentrations, operating conditions are controlled to avoid supersaturation by limiting water recovery, reducing pH through acid addition, and pretreating with lime softening or NF [3, 80]. When supersaturated operation cannot be avoided, crystallization kinetics can be slowed using antiscalants. Addition of antiscalants and acid to the RO feed is common in full-scale water recycling plants [3, 80]. The low salinity of municipal wastewater limits the risk of scaling at moderate recovery ratios [227], but substantial mineral deposits may form on the end membrane elements, where the concentration of sparingly soluble salts is highest [227, 243].
7.1.3 Fouling Mitigation Methods
Fouling mitigation methods include pretreatment, membrane modification, cleaning, and the use of fouling-resistant alternative processes. To reduce the potential for membrane fouling, wastewater effluents undergo a series of pretreatment steps before reaching MF, UF, NF, or RO membranes. Huang et al. [5] reviewed pretreatment strategies to enhance contaminant removal and reduce fouling in MF and UF. Strategies in the categories of coagulation, adsorption, preoxidation, and prefiltration are discussed in terms of physical, chemical, and biological pretreatment mechanisms. Emerging processes such as dissolved air floatation are also reviewed, and recommendations are made for future research in fouling prediction and assessment. Fan et al. [254] studied the efficacy of coagulation and anion-exchange resins (AER) on fouling of MF and UF membranes filtering municipal wastewater effluent. Zhang et al. [255] examined flux decline of RO membranes treating secondary wastewater effluent after various combinations of ozonation, BAC, and MF (here considered a pretreatment before RO); ozonation was the step with the largest impact on flux decline, but flux decline was minimized when all three pretreatment steps were used in combination. Continuous chemical addition in the form of acid and antiscalant is another strategy to reduce scaling [256], which is particularly important due to the high recovery ratios typical in wastewater reuse.
Membrane modification has been explored extensively as an avenue for preventing fouling. Anti-fouling modifications generally either resist foulant deposition, have anti-microbial properties, or facilitate cleaning. RO membrane modification strategies have been previously reviewed [81, 256, 257]. Hydrophilicity is generally sought to protect the membrane surface from biofouling [258], but membrane hydrophobicity has been shown to reduce the propensity for scaling [259]. Unfortunately, both biofouling and scaling occur in wastewater reuse [243], so the ideal surface wettability is not obvious. Modifications that control chemical interactions between foulants and carboxyl groups on the membrane surface have been shown to reduce both organic fouling and scaling. Liu et al. [260] found significant improvement in the resistance to organic fouling of a TFC FO membrane when it was modified with zwitterionic polymer brushes due to shielding of carboxyl groups on the membrane surface. Mi and Elimelech [261] found that hydroxyl-dominated cellulose acetate FO membranes resisted gypsum scaling under conditions that led to severe gypsum scaling of polyamide membranes, which have more surface carboxyl groups.
Most membrane modifications are targeted toward a specific type of foulant, and they may not have the same effects when used with real wastewater sources containing a range of foulants. Araújo et al. [258] tested hydrophilic membrane modifications (polydopamine, with and without grafted poly(ethylene glycol) polymer brushes), shown to be effective at preventing fouling with oil/water emulsions [262], as well as several feed spacer coatings and found that none were effective at preventing biofouling during filtration of un-disinfected tap water. Araújo et al. concluded that “it is doubtful that feed spacer and membrane modification, in general, may be effective for biofouling control regardless of the type of applied coating.” In reviewing several studies of fouling with wastewater effluents, Ivnitsky et al. [237] found little difference between long-term biofouling outcomes on different NF membranes. Even with single model foulants, membrane modification may not have a strong effect on fouling. As an example, Wang and Tang [242] found that foulant-foulant interactions based on the solution composition had a much stronger impact than the surface chemistry of the membrane itself on BSA fouling of UF, NF, and RO membranes. Similarly, in a study of membrane surface chemistry on alginate fouling, Wu et al. [263] found that different membrane functional groups had only a short-term effect on fouling rate, and that at long times, the ionic composition of the feed solution had a larger effect than that of the membrane surface chemistry. Membrane modification to prevent foulant adhesion is difficult because the formation of even a thin fouling layer covers the engineered surface, preventing it from controlling further fouling [257].
Fouled membranes can often be cleaned, but the range of cleaning protocols available depends on the chemical resistance of the membrane materials. By developing clean-in-place (CIP) protocols, membrane fouling can typically be managed to maintain water production levels over long times. Full scale water reuse plants routinely clean MF and UF membranes using a combination of chemicals that depends on the membrane manufacturer’s recommendations (CIP approximately monthly, with additional smaller “maintenance” cleans as often as daily) and routinely clean RO membranes every few months or so [30]. In the case of severe fouling event, more aggressive cleaning conditions may be created using, e.g., biocides or higher temperatures. For MF and UF, backwashing and air scouring are also automatically conducted in full scale plants a few times per hour to physically remove foulants [264]. Various cleaning chemicals, including several acids, a base, a surfactant, and a chelation agent have been tested for efficacy in removal of fouling from RO membranes used to treat wastewater [265]. Benign salt solutions have also been recently tested for the removal of biofouling in wastewater reclamation [266, 267]. Because biofouling is so common in wastewater reuse, development of more chlorine-tolerant membranes is desirable [266, 267].
Lastly, alternative membrane processes may be desirable for their superior fouling resistance. For example, forward osmosis (FO) has been shown to exhibit fouling that is more easily reversible by mechanical cleaning than RO, even when operated at the same initial flux [48]. In another example, the semi-batch or “closed-circuit” RO process is reported to minimize scaling compared to conventional RO due to the non-steady state composition of the RO feed disrupting the kinetics of crystallization [48, 268]. One potable reuse plant in Windhoek, Namibia reduces membrane fouling by reducing the need to remove micropollutants by controlling the sources of wastewater intake and using a complex treatment train that involves UF but not NF or RO. Several alternative processes are discussed in Section 5.
7.2 Contaminant Removal
The removal of harmful pathogens and contaminants is one of the primary goals for the design of potable reuse treatment trains. The objective of treatment is to ensure safety of potable reuse via contaminant removal, which requires a treatment train (multi-barrier) approach of which membranes are just one part. Other barriers include non-membrane treatment units such as the upstream wastewater process and related source control, post-membrane water quality “polishing” such as UV/AOP, treatment system performance monitoring, and response protocols for when issues arise [15]. In design of treatment trains for potable reuse, several criteria are key: reliability (by product water testing), redundancy (in case of failure), robustness (over a range of contaminants), and resilience (to failure) [269]).
Recommendations for pathogen log reduction are typically relatively stringent, e.g., a 12-10-9 log reduction of viruses, Cryptosporidium, and total coliform bacteria, respectively, depending on site specific regulations [1]. Recently promulgated California potable reuse regulations which have been adopted by other States, require log reductions of 12-10-10 for enteric viruses, Cryptosporidium, and Giardia, respectively [2]. In California, log reduction credits for a potable reuse treatment train depend on various factors including the unit processes implemented (at least three separate processes), pathogen surrogate removal by unit processes, process integrity monitoring methods, and in the context of IPR, residence time in the environment after treatment. For FAT systems, log pathogen removal credits are awarded separately for the MF/UF, RO, and UV-AOP systems as well as any disinfection steps [3]. For pressurized MF and UF systems, direct integrity testing (i.e., pressure decay testing) over prescribed intervals (usually once per day) is generally required to obtain up to 4-log reduction credits for viruses (for UF only), Cryptosporidium and Giardia (both MF and UF). For the pressure decay test (PDT), a MF or UF module is isolated and pressurized on one side with air at approximately 10 psi. Once the pressure has stabilized, the air is shut off and the pressure is monitored over time [4]. The PDT is considered to have resolution to detect integrity breaches less than 3 µm, which is smaller than the nominal size of Cryptosporidium and Giardia oocysts and required by the USEPA to validate low-pressure membranes based on Surface Water Treatment Rules [4]. Typically PDTs are used in conjunction with effluent turbidity measurements to semi-continuously verify membrane integrity [1]. It is worth noting that other direct integrity testing methods can be applied including challenge or marker tests. One major caveat with the PDT is that, in most cases, it cannot be applied to submerged membrane systems, which makes giving pathogen removal credits to common MBR systems challenging with regards to potable reuse applications.
An additional significant challenge for potable reuse is the presence of wastewater-derived organic contaminants, although these can largely be addressed (with some exceptions) through membrane treatment. These exceptions may be referred to as contaminants of emerging concern (CECs), denoting a lack of formal regulation (i.e., no drinking water MCLs). Challenging emerging contaminants include pharmaceuticals, ingredients from personal care or commercial products, and endocrine disrupting compounds (EDCs), as well as disinfection byproducts (DBPs) from treatmento [270, 271]. Risk assessments conducted over the last 15 years have found no adverse human health effects or significant risks from the presence of wastewater-derived organic contaminants in potable and non-potable water reuse, which if present, occur at trace levels [24, 272]. However, some uncertainty with respect to risk stems from the fact that risk assessments must focus on a subset of the potentially present compounds, requiring prioritization; new chemicals are constantly introduced into commerce and ultimately enter water supplies; effects on more sensitive populations (e.g., pregnant women, fetuses) are unclear; and potential cumulative (additive or synergistic) effects due to mixtures of CECs are not well understood [270, 272, 273] Thus, advanced treatment for water reuse is desirable to minimize the concentrations of these compounds in finished water and therefore any potential for risk (precautionary principle) and to help alleviate public concern. In fact, it is likely that water (reuse) agencies will continue to seek the water treatment technologies that allow the lowest, non-detectable levels of CECs in finished water, for as long as our understanding of risk at these low concentrations is incomplete.
To that end, it appears that one of the most effective; if not the most effective, single unit process for removal of CECs during water treatment is RO [271, 272, 274, 275] RO typically removes >90% (often >99%, depending on the compound) of organic, wastewater-derived compounds. However, RO is not a complete barrier for all wastewater-derived organics. In general, relying on only membrane-based processes is not recommend for wastewater treatment when the waters have a high content of boron, formic acid, methanol, formaldehyde, or urea because membrane-based processes are inefficient at removing these and certain other low molecular weight contaminants. Removal of these contaminants can be completed using physicochemical and biological processes in the pretreatment stages [16, 167].
A recent human health risk evaluation based on published CEC occurrence in U.S. point sources and watersheds identified that, among CECs, perfluorooctanoate (PFOA), N-nitrosodimethylamine (NDMA), 1,4-dioxane, chloroform, low molecular weight aldehydes, and certain hormones are of the most significance based on a conservative comparison of environmental concentrations with the levels potentially associated with human health risk [276] In other words, these are the compounds that occur in water sources at concentrations closest to levels of concern, in addition to DBPs in reuse waters, as noted by the NRC [6] Of these compounds, poly- and perfluorinated compounds (e.g., PFOA) and hormones are well rejected by RO, but NDMA, certain other DBPs, and 1,4-dioxane are not [277–279]. Hence, for potable reuse, treatment train designs often include the advanced oxidation process (AOP), e.g., ultraviolet light with hydrogen peroxide (UV/H2O2), after RO as a final polishing step for CECs removal. UV/AOP is effective for NDMA and 1,4-dioxane destruction [222, 226, 280]. As seen in Figure 18, the example potable reuse treatment train shown provides effective removal of most compounds in the feed. However, removal is incomplete for some compounds, even though the RO membranes. These compounds typically have small molecular weight, are polar, and are uncharged [277, 278].
Figure 18.
Chemical detection after different stages of the potable reuse AWTP treatment train, adapted from [56], Copyright 2015, reproduced with permission from the Australian Water Recycling Centre of Excellence.
Related to CECs removal, an important advantage of membrane-based technologies for water reuse is the high removal of total organic carbon (TOC). TOC is a measure of bulk organic matter present in the water, and has been a key parameter in the history of reuse (see [274]) in part due to the ease of measurement. It has been used as a surrogate for organic contaminants, i.e., as a sum parameter for potentially present organic CECs, given the difficulty of measuring CECs and the impossibility of identifying all unknown CECs in a water sample. RO can produce a product water quality with < 0.2 mg/L TOC for reuse applications. Membrane-free alternative treatment trains for potable reuse are currently unable to reach an equivalently low TOC level. This may be acceptable depending on local reuse water quality requirements
Despite the high rejection of RO membranes for most wastewater-derived organics and CECs, numerous individual compounds have been detected in the RO permeate in various studies of potable reuse, owing to the high sensitivity (low detection limit) of available chemical analysis methods. One study reported the following, summarized here with one example of each: fuel (benzyl alcohol), cosmetics (1-nonanol octanol), perfume solvents (alpha-terpineol), flame retardants (tributyl phosphate), insecticide (diethyltoluamide), detergent byproducts (4-tert-octylphenol), pharmaceutical products (L-menthol), citrizine, sulphamide, animal byproducts (cholesterol), plasticizers (dimethyl phthalate), and resins (bisphenol A or BPA, a known endocrine disruptor) [56]. This plant in particular was designed for remote locations, and thus needed minimal energy and chemical consumption [112].
Importantly, in addition to the ability to remove contaminants, in situ monitoring measuring contaminant removal is important to ensure consistent removal in real systems [281]. Such measurements, which enable direct integrity testing (DIT), are critical for demonstrating continuous membrane performance. Better monitoring parameters or equipment is another useful research area itself, for both MF/UF and RO. Such measurements are also critical for obtaining pathogen removal credits [7].
7.3 Public Perception
Public perception can be a larger challenge for potable reuse thantechnical issues. The history of a water source strongly impacts opinions: many people prefer lower quality water that comes from a “natural” source (aquifer or river) over a higher quality, advanced treated wastewater. People’s risk perceptions of recycled water are influenced by their sources of information, prior experience with the water, and other factors; generally, people are more supportive of non-potable uses (e.g., landscape and agricultural irrigation, industrial uses) over potable use [6, 7].
Public perception of the risks and benefits of water reuse is an area of active research, as well as methods for public communication that establish project understanding and support (e.g. [282, 283]). This work began in earnest in the mid-1990s in Australia, Singapore, and the United States and internationally since the early 2000s. Beyond communication and marketing strategies, recent research on social legitimacy of water reuse leverages information from the social sciences to provide recommendations for actively establishing the legitimacy of potable reuse [277, 284]. Recommendations from this work include the need to establish standards, procedures, and possibly new institutions. Public outreach is generally recommended early in the project planning phase to increase community knowledge of the water cycle and water reuse [7], since improved understanding generally increases the public’s acceptance of reuse [285]. This requires ensuring access to credible scientific information for the public, decision makers, and the local media, toward improved understanding of water supplies in the region and the costs/benefits analysis of water supply options.
Indirect potable reuse (via environmental buffers) still encounters public perception challenges, but perhaps less so than direct potable reuse. These buffers (storage in groundwater aquifers or surface water reservoirs) are characterized by retention time, attenuation of contaminants, and blending (dilution), but their benefits can be reached through engineered means [6] In a recent review, Trussell et al. describe these aesthetic concerns and new approaches for evaluating, and tracking the elimination of, “wastewater character” [274] or “wastewater identity”. While there is no standard of wastewater identity, metrics that have been proposed are organic matter concentration, color, absorbance, fluorescence, solids concentration, odor, and mineralization, with corresponding measurable parameters [274] e.g., total organic carbon (TOC), UV254 absorbance, BDOC [286], indicator compounds [274, 286, 287], etc. For the membrane designer, improving these metrics centers around rejection of specific (mostly organic) contaminants, and increases the need for more reliable and high integrity membranes [274].
Recent studies have used absorbance and fluorescence to characterize the removal or transformations of organic matter during water treatment for reuse. Fluorescence measurements via a three-dimensional excitation-emission matrix spectra (EEMS) establish a fingerprint of the water (e.g., secondary effluent) that changes with increasing treatment (Figure 19) Membrane treatment via RO results in substantial loss of the wastewater character, producing fluorescence spectra for the finished water that are very similar to a “pristine” source water. Further research is needed to address limitations and understand the potential applications of this and related tools [274].
Figure 19.
Fluorescence excitation-emission matrix spectra for conventional drinking water treatment (top) compared to advanced water treatment for potable reuse (bottom), modified from [274]. TOC = total organic carbon; UV254A = UV254 absorbance; TF = total fluorescence. Reproduced with permission from the Water Environment & Reuse Foundation, Copyright 2013.
7.4 Law and Policy
Regulations and stringent facility requirements are often a larger constraint and even barrier to the implementation of potable water reuse than the technologies themselves. These rules tend to be complex and multilayered, and often place requirements on which technologies are permitted, siting, who may acquire and purchase water, specific contaminant removal measures, quality metrics, and oversight [6, 7, 274, 288]. They also may mandate certain treatment processes, such as requiring RO for potable reuse [6, 24]. From a membrane design perspective, these rules put pressure on increasing contaminant specific rejection (e.g. for Boron or NDMA), disinfection ability, reliability, and required process chains. An important milestone from such rules is improving and proving RO reliability to ensure it more realistic log removal credits [6,24, 274].
8. Conclusions
Potable water reuse is emerging as one of the fastest growing practices for combating water scarcity and one of the most energy efficient options for augmenting municipal water supplies. Membranes, especially as used in the MF-RO-UV/H2O2 treatment train (IMS), have emerged as the core potable water reuse technology. Several challenges remain, each of which points to an objective for future innovation:
Improving rejection (or destruction) of emerging contaminants
Improving membrane permeability
Improving permanence and degree of membrane hydrophilicity
Demonstrating RO membrane integrity for increase log-removal credits for pathogens
Predicting and preventing membrane fouling, developing antifouling membrane materials, and optimizing cleaning processes
Increasing membrane resistance to cleaning and antibacterial agents
Reducing the cost and environmental impact of pretreatment technologies
Reducing energy use through new membrane, module, and process designs
Improving public perception of water reuse and navigating resulting regulations
To achieve these improvements in potable reuse, polymer membrane technology will be crucial. Enhancements may center on improved membrane materials, better composite and multilayer membranes, novel coatings and modifications, and improved fabrication processes. Research aimed at enhancing understanding of fouling mechanisms, transport phenomena in membranes, and health impacts of trace contaminants will further accelerate progress in membrane development for potable reuse.
Figure 9.
SEM cross section image of hollow fiber polypropylene MF membrane (Advanced Water Purification Facility membranes imaged by the Orange County Water District at University of California-Irvine Materials Characterization Center).
Acknowledgments
This article has been subjected to the Environmental Protection Agency’s review and has been approved for publication. Note that approval does not signify that the contents necessarily reflect the views of the Agency. Mention of trade names, products, or services does not convey official EPA approval, endorsement, or recommendation. SC thanks to μ,-Perla (PON-Supported by European Commission) for supporting the work on tunable membrane development project for waste valorization. Additionally, EWT thanks the MIT Martin Fellowship for Sustainability and notes that this material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. 1122374. DMW thanks Xuan Zhang and Zhi Geng for insights contrasting membrane materials. DMW, SC, SL, HAA and JHL acknowledge partial funding by the Cooperative Agreement between the Masdar Institute of Science and Technology (currently Khalifa University), Abu Dhabi, UAE and the Massachusetts Institute of Technology (MIT), Cambridge, MA, USA, Reference no. 02/MI/MI/ CP/11/07633/GEN/G/00 This work was organized through the American Water Works Association’s Membrane Technology Research Committee.
Nomenclature
Processes
- AOP
Advanced oxidation process
- BAC
Biologically activated carbon
- BAF
Biological active filtration
- DIT
Direct Integrity Testing
- ED
Electrodialysis
- EDR
Electrodialysis reversal
- FAT
Full advanced treatment
- FO
Forward osmosis
- GAC
Granular activated carbon
- IMS
Integrated membrane system
- MBR
Membrane bioreactor
- MD
Membrane distillation
- MF
Microfiltration
- UF
Ultrafiltration
- NF
Nanofiltration
- OMBR
Osmotic membrane bioreactor
- PAC
Powdered activated carbon
- RO
Reverse osmosis
- SAT
Soil-aquifer treatment
Other terms
- AEM
Anion exchange membrane
- AOC
Assimilable organic carbon
- BOD
Biochemical oxygen demand
- BDOC
Biodegradable dissolved organic carbon
- CA
Cellulose acetate
- CEC
Contaminants of emerging concern
- CEM
Cation exchange membrane
- COD
Chemical oxygen demand
- DBP
Disinfection byproducts
- DOC
Dissolved organic carbon
- EDC
Endocrine disrupting contaminants
- EEMS
Excitation-emission matrix spectra
- EfOM
Effluent organic matter
- EPS
Extracellular polymeric substances
- ICP
Internal concentration polarization
- IPR
Indirect potable reuse
- MEC
Maximum environmental concentration
- MLD
Million liters per day
- MWCNT
Multi-walled carbon nanotubes
- NDMA
N-nitrosodimethylamine
- NOM
Natural organic matter
- NTU
Nephelometric turbidity units
- PAN
Polyacrilonitrile
- PE
Polyethylene
- PEO
Photoelectrochemical oxidation
- PES
Polyether sulfone
- PNEC
Probable no effect concentration
- POC
Particulate organic carbon
- PP
Polypropylene
- PPCP
Pharmaceuticals and personal care products
- PS
Polysulfone
- PTFE
Polytetrafluoroethylene
- PVC
Polyvinyl chloride
- PVDF
Polyvinylidene fluoride
- TDS
Total dissolved solids
- TFCs
Thin-film composites
- TOC
Total organic carbon
- TSS
Total suspended solids
- WWTP
Wastewater treatment plant
Footnotes
In experimental studies conducted at constant pressure the fouling-driven decline in membrane permeability leads to declining flux. However, in full scale water recycling plants, the applied transmembrane pressure is usually increased (at increased energy cost) as the membrane permeability declines in order to achieve the plant’s target water production rate.
References
- 1.Pierre Kwan HE. Tech. Rep. 06-10 (Phase A) California: Department of Water Resources; 2013. State of the science review of membrane fouling: Organic, inorganic, and biological; p. 250. [Google Scholar]
- 2.Sethi S, Xu P, Drewes J. When less is more. Civil Engineering Magazine Archive. 2007;77:72–75. [Google Scholar]
- 3.Giwa A, Akther N, Dufour V, Hasan SW. A critical review on recent polymeric and nano-enhanced membranes for reverse osmosis. RSC Adv. 2016;6:8134–8163. [Google Scholar]
- 4.Bolong N, Ismail A, Salim MR, Matsuura T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination. 2009;239:229–246. [Google Scholar]
- 5.Huang H, Schwab K, Jacangelo JG. Pretreatment for low pressure membranes in water treatment: A review. Environ Sci Technol. 2009;43:3011–3019. doi: 10.1021/es802473r. [DOI] [PubMed] [Google Scholar]
- 6.National Research Council (US) Water Reuse: Potential for Expanding the Nation's Water Supply Through Reuse of Municipal Wastewater. Washington DC: National Academies Press; 2012. p. 276. [Google Scholar]
- 7.Asano T, Burton F, Leverenz H, Tsuchihashi R, Tchobanoglous G. Water Reuse: Issues, technologies and applications. first. New York, NY: McGraw-Hill; 2007. p. 1570. [Google Scholar]
- 8.Rice J, Westerhoff P. Spatial and temporal variation in de facto wastewater reuse in drinking water systems across the USA. Environ Sci Technol. 2014;49:982–989. doi: 10.1021/es5048057. [DOI] [PubMed] [Google Scholar]
- 9.Childress AE, Brant JA, Rempala P, Phipps DW, Jr, Kwan P. Evaluation of membrane characterization methods. Water Research Foundation and US Environmental Protection Agency, Report #4102. 2012:179. [Google Scholar]
- 10.Lautze J, Stander E, Drechsel P, da Silva AK, Keraita B. CGIAR Research Program on Water, Land and Ecosystems (WLE) Colombo, Sri Lanka: International Water Management Institute (IWMI); 2014. Global experiences in water reuse; p. 34. [Google Scholar]
- 11.Gerrity D, Pecson B, Trussell RS, Trussell RR. Potable reuse treatment trains throughout the world. J Water Supply Res T-Aqua. 2013;62:321–338. [Google Scholar]
- 12.Warsinger DM, Tow EW, Nayar K, Masawadeh LA, Lienhard JH., V Energy efficiency of batch and semi-batch (CCRO) reverse osmosis desalination. Water Res. 2016;106:272–282. doi: 10.1016/j.watres.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 13.Plappally AK, Lienhard JH., V Costs for water supply, treatment, end-use and reclamation. Desalin Water Treat. 2013;51:200–232. [Google Scholar]
- 14.Raffin M, Germain E, Judd S. Wastewater polishing using membrane technology: a review of existing installations. Environ Technol. 2013;34:617–627. doi: 10.1080/09593330.2012.710385. [DOI] [PubMed] [Google Scholar]
- 15.Olivieri A, Crook J, Anderson M, Bull R, Drewes J, Haas C, Jakubowski W, McCarty P, Nelson K, Rose J, Sedlak D, Wade T. Expert Panel Final Report: Evaluation of the Feasibility of Developing Uniform Water Recycling Criteria for Direct Potable Reuse. National Water Research Institute; 2016. p. 420. [Google Scholar]
- 16.del Pino MP, Durham B. Wastewater reuse through dual-membrane processes: opportunities for sustainable water resources. Desalination. 1999;124:271–277. [Google Scholar]
- 17.Lozier J, Fernandez A. Using a membrane bioreactor/reverse osmosis system for indirect potable reuse. Water Science and Technology: Water Supply. 2001;1:303–313. [Google Scholar]
- 18.Luo Y, Guo W, Ngo HH, Nghiem LD, Hai FI, Zhang J, Liang S, Wang XC. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Science of the Total Environment. 2014;473:619–641. doi: 10.1016/j.scitotenv.2013.12.065. [DOI] [PubMed] [Google Scholar]
- 19.Perez. State of the art and review on the treatment technologies of water reverse osmosis concentrates. Water Res. 2012;46:267–283. doi: 10.1016/j.watres.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 20.Peter-Varbanets M, Zurbrügg C, Swartz C, Pronk W. Decentralized systems for potable water and the potential of membrane technology. Water Res. 2009;43:245–265. doi: 10.1016/j.watres.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 21.Wilf M. The Guidebook to Membrane Technology for Wastewater Reclamation. first. Hopkinton: Balaban Publishers; 2010. p. 788. [Google Scholar]
- 22.Martina Hamingerova LB, Beckmann M. Tech. Rep. Fraunhofer center for International Management and Knowledge Economy; 2010. Membrane Technologies for Water and Wastewater Treatment on the European and Indian Market; p. 37. [Google Scholar]
- 23.Van der Bruggen B, Mänttäri, Nyström M M. Drawbacks of applying nanofiltration and how to avoid them: a review. Sep Purif Technol. 2008;63:251–263. [Google Scholar]
- 24.WHO. Potable reuse: Guidance for producing safe drinking-water. Geneva: World Health Organization; 2017. p. 152. Licence: CC BY-NC-SA 30. [Google Scholar]
- 25.Wintgens T, Melin T, Schäfer A, Khan S, Muston M, Bixio D, Thoeye C. Membranes in drinking and industrial water production the role of membrane processes in municipal wastewater reclamation and reuse. Desalination. 2005;178:1–11. [Google Scholar]
- 26.Tchobanoglous G, Stensel HD, Tsuchihashi R, Burton F. Wastewater Engineering: Treatment and Resource Recovery. fifth. New York: McGraw-Hill (Metcalf & Eddy, Inc.); 2013. p. 2048. [Google Scholar]
- 27.Fane AGT, Wang R, Jia Y. In: Membrane technology: Past, present and future. Wang LK, Chen JP, Hyng Y-T, Shammas NK, editors. Totowa, NJ: Humana Press; 2011. pp. 1–45. [Google Scholar]
- 28.Rodriguez C, Van Buynder P, Lugg R, Blair P, Devine B, Cook A, Weinstein P. Indirect potable reuse: a sustainable water supply alternative. Int J Environ Res Pu. 2009;6:1174–1203. doi: 10.3390/ijerph6031174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartels C, Franks R, Andes K. Operational performance and optimization of RO wastewater treatment plants. Singapore International Water Week; Singapore: 2010. p. 12. [Google Scholar]
- 30.Porcelli N, Judd S. Chemical cleaning of potable water membranes: a review. Sep Purif Technol. 2010;71:137–143. doi: 10.1016/j.watres.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 31.Le-Clech P, Jefferson B, Judd S. A comparison of submerged and sidestream tubular membrane bioreactor configurations. Desalination. 2005;173:113–122. [Google Scholar]
- 32.Meng F, Chae SR, Drews A, Kraume M, Shin HS, Yang F. Recent advances in membrane bioreactors (mbrs): membrane fouling and membrane material. Water Res. 2009;43:1489–1512. doi: 10.1016/j.watres.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 33.Judd S. The status of industrial and municipal effluent treatment with membrane bioreactor technology. Chem Eng J. 2016;305:37–45. [Google Scholar]
- 34.Stephenson T, Brindle K, Judd S, Jefferson B. Membrane bioreactors for wastewater treatment. Stuart, Florida: IWA publishing; 2000. p. 150. [Google Scholar]
- 35.Judd S. The status of membrane bioreactor technology. Trends Biotechnol. 2008;26:109–116. doi: 10.1016/j.tibtech.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Lawrence P, Adham S, Barrott L. Ensuring water re-use projects succeed institutional and technical issues for treated wastewater re-use. Desalination. 2003;152:291–298. [Google Scholar]
- 37.Tchobanoglous G, Cotruvo J, Crook J, McDonald E, Olivieri A, Salveson A, Trussel S. Framework for direct potable reuse. Alexandria, VA: WateReuse Research Foundation; 2015. p. 211. [Google Scholar]
- 38.Roy Y, Warsinger DM, Lienhard JH., V Effect of temperature on ion transport in nanofiltration membranes: Diffusion, convection and electromigration. Desalination. 2017;420:241–257. [Google Scholar]
- 39.Bowen W, Welfoot JS. Modelling the performance of membrane nanofiltration -critical assessment and model development. Chem Eng Sci. 2002;57:1121–1137. [Google Scholar]
- 40.Bellona C, Drewes JE, Oelker G, Luna J, Filteau G, Amy G. Comparing nanofiltration and reverse osmosis for drinking water augmentation. J Am Water Works Ass. 2008;100:102–116. [Google Scholar]
- 41.Verliefde AR, Cornelissen ER, Heijman S, Verberk JQ, Amy GL, Van der Bruggen B, Van Dijk J. Construction and validation of a full-scale model for rejection of organic micropollutants by NF membranes. J Membr Sci. 2009;339:10–20. [Google Scholar]
- 42.Verliefde AR, Cornelissen E, Heijman S, Verberk J, Amy G, Van der Bruggen B, Van Dijk J. The role of electrostatic interactions on the rejection of organic solutes in aqueous solutions with nanofiltration. J Membr Sci. 2008;322:52–66. [Google Scholar]
- 43.Aghdam MA, Zraick F, Simon J, Farrell J, Snyder SA. A novel brine precipitation process for higher water recovery. Desalination. 2016;385:69–74. [Google Scholar]
- 44.Park M, Anumol T, Simon J, Zraick F, Snyder SA. Pre-ozonation for high recovery of nanofiltration (NF) membrane system: Membrane fouling reduction and trace organic compound attenuation. J Membr Sci. 2017;523:255–263. [Google Scholar]
- 45.Schimmoller L, Biggs J. Potable reuse for inland applications: Pilot testing results from a new potable reuse treatment scheme, (WRRF-13-09); 29th Annual WateReuse Symposium; Dallas, TX. 2014. p. 22. [Google Scholar]
- 46.Fernando Casado, Caneque David, Smith PJ. Market Analysis of Key Water Reuse Technologies. Innovation Demonstration for a Competitive and Innovative European Water Reuse Sector (DEMOWARE); 2015. p. 68. [Google Scholar]
- 47.Elimelech M, Phillip WA. The future of seawater desalination: energy, technology, and the environment. Science. 2011;333:712–717. doi: 10.1126/science.1200488. [DOI] [PubMed] [Google Scholar]
- 48.Lee S, Boo C, Elimelech M, Hong S. Comparison of fouling behavior in forward osmosis (FO) and reverse osmosis (RO) J Membr Sci. 2010;365:34–39. [Google Scholar]
- 49.Peñate B, García-Rodríguez L. Current trends and future prospects in the design of seawater reverse osmosis desalination technology. Desalination. 2012;284(0):1–8. [Google Scholar]
- 50.Houtte EV, Verbauwhede J. Operational experience with indirect potable reuse at the Flemish coast. Desalination. 2008;218:198–207. [Google Scholar]
- 51.Lee KP, Arnot TC, Mattia D. A review of reverse osmosis membrane materials for desalination development to date and future potential. J Membr Sci. 2011;370:1–22. [Google Scholar]
- 52.Bellona C, Drewes JE, Xu P, Amy G. Factors affecting the rejection of organic solutes during NF/RO treatment - a literature review. Water Res. 2004;38:2795–2809. doi: 10.1016/j.watres.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 53.Tam L, Tang T, Lau G, Sharma K, Chen G. A pilot study for wastewater reclamation and reuse with MBR/RO and MF/RO systems. Desalination. 2007;202:106–113. [Google Scholar]
- 54.Urtiaga A, Perez G, Ibanez R, Ortiz I. Removal of pharmaceuticals from a WWTP secondary effluent by ultrafiltration/reverse osmosis followed by electrochemical oxidation of the RO concentrate. Desalination. 2013;331:26–34. [Google Scholar]
- 55.Radjenovic J, Petrovic M, Ventura F, Barcelo D. Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res. 2008;42:3601–3610. doi: 10.1016/j.watres.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 56.Allinson G, Allinson M, Kadokami K, Shiraishi F, Nakajima D, Zhang J, Knight A, Gray S, Scales P. Demonstration of robust water recycling-Monitoring the levels of trace organic chemicals (TrOCs) Australian Water Recycling Centre of Excellence; 2015. p. 91. [Google Scholar]
- 57.Drewes JE, Reinhard M, Fox P. Comparing microfiltration-reverse osmosis and soil-aquifer treatment for indirect potable reuse of water. Water Res. 2003;37:3612–3621. doi: 10.1016/S0043-1354(03)00230-6. [DOI] [PubMed] [Google Scholar]
- 58.Snyder SA, Adham S, Redding AM, Cannon FS, DeCarolis J, Oppenheimer J, Wert EC, Yoon Y. Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination. 2007;202:156–181. [Google Scholar]
- 59.Fujioka T, Oshima N, Suzuki R, Price WE, Nghiem LD. Probing the internal structure of reverse osmosis membranes by positron annihilation spectroscopy: Gaining more insight into the transport of water and small solutes. J Membr Sci. 2015;486:106–118. [Google Scholar]
- 60.Wijmans J, Baker R. The solution-diffusion model: a review. J Membr Sci. 1995;107:1–21. [Google Scholar]
- 61.Malaeb L, Ayoub GM. Reverse osmosis technology for water treatment: State of the art review. Desalination. 2011;267:1–8. [Google Scholar]
- 62.Sauvet-Goichon B. Ashkelon desalination plant - a successful challenge. Desalination. 2007;203:75–81. [Google Scholar]
- 63.Lin S, Elimelech M. Staged reverse osmosis operation: Configurations, energy efficiency, and application potential. Desalination. 2015;366:9–14. [Google Scholar]
- 64.Van der Bruggen B, Vandecasteele C, Van Gestel T, Doyen W, Leysen R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ Prog. 2003;22:46–56. [Google Scholar]
- 65.Miami-Dade Water and Sewer Department, Ocean outfall legislation. Chapter 2008-232 Laws of Florida Wastewater Disposal/Ocean Outfalls[Section 403086 (9), Florida Statutes and Amendment CS/SB 444] 2013:100. URL: https://www.miamidade.gov/water/library/reports/ocean-outfall-legislation.pdf.
- 66.Wilf M, Tech T. Membrane types and factors affecting membrane performance. Advanced Membrane Technologies Stanford University; 2008. p. 92. [Google Scholar]
- 67.Alspach B, Adham S, Cooke T, Delphos P, Garcia-Aleman J, Jacangelo J, Karimi A, Pressman J, Schaefer J, Sethi S. Microfiltration and ultrafiltration membranes for drinking water. J Am Water Works Ass. 2008;100:84–97. [Google Scholar]
- 68.Pearce GK. UF/MF membrane water treatment: principles and design. Bangkok: Water Treatment Academy; 2012. p. 387. [Google Scholar]
- 69.Milkovich BA, Ensley A, Dewane A history of orange county water district. 2014:85. URL: http://www.ocwd.com/about/history/
- 70.Liu F, Hashim NA, Liu Y, Abed MM, Li K. Progress in the production and modification of PVDF membranes. J Membr Sci. 2011;375:1–27. [Google Scholar]
- 71.Turken T, Sengur-Tasdemir R, Koseoglu-Imer DY, Koyuncu I. Determination of filtration performances of nanocomposite hollow fiber membranes with silver nanoparticles. Environ Eng Sci. 2015;32:656–665. [Google Scholar]
- 72.Vrouwenvelder J, Von Der Schulenburg DG, Kruithof J, Johns M, Van Loosdrecht M. Biofouling of spiral-wound nanofiltration and reverse osmosis membranes: a feed spacer problem. Water Res. 2009;43:583–594. doi: 10.1016/j.watres.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 73.Lalia BS, Kochkodan V, Hashaikeh R, Hilal N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination. 2013;326:77–95. [Google Scholar]
- 74.Asatekin A, Vannucci C. Self-assembled polymer nanostructures for liquid filtration membranes: A review. Nanosci Nanoltech Let. 2015;7:21–32. [Google Scholar]
- 75.Lau WJ, Ismail AF, Misdan N, Kassim MA. A recent progress in thin film composite membrane: a review. Desalination. 2012;287:190–199. [Google Scholar]
- 76.Amirilargani M, Sadrzadeh M, Sudhölter E, de Smet L. Surface modification methods of organic solvent nanofiltration membranes. Chem Eng J. 2016;289:562–582. [Google Scholar]
- 77.Goh K, Karahan HE, Wei L, Bae TH, Fane AG, Wang R, Chen Y. Carbon nanomaterials for advancing separation membranes: A strategic perspective. Carbon. 2016;109:694–710. [Google Scholar]
- 78.Santhosh C, Velmurugan V, Jacob G, Jeong SK, Grace AN, Bhatnagar A. Role of nanomaterials in water treatment applications: A review. Chem Eng J. 2016;306:1116–1137. [Google Scholar]
- 79.Cheng S, Oatley DL, Williams PM, Wright CJ. Positively charged nanofiltration membranes: review of current fabrication methods and introduction of a novel approach. Adv Colloid Interface Sci. 2011;164:12–20. doi: 10.1016/j.cis.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 80.Pandey SR, Jegatheesan V, Baskaran K, Shu L. Fouling in reverse osmosis (RO) membrane in water recovery from secondary effluent: a review. Rev Environ Sci Bio. 2012;11:125–145. [Google Scholar]
- 81.Kang Gd, Cao Ym. Development of antifouling reverse osmosis membranes for water treatment: a review. Water Res. 2012;46:584–600. doi: 10.1016/j.watres.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 82.Saqib J, Aljundi IH. Membrane fouling and modification using surface treatment and layer-by-layer assembly of polyelectrolytes: State-of-the-art review. J Water Proc Eng. 2016;11:68–87. [Google Scholar]
- 83.Kaur S, Sundarrajan S, Rana D, Sridhar R, Gopal R, Matsuura T, Ramakrishna S. Review: the characterization of electrospun nanofibrous liquid filtration membranes. J Mater Sci. 2014;49:6143–6159. [Google Scholar]
- 84.Wang P, Ma J, Shi F, Ma Y, Wang Z, Zhao X. Behaviors and effects of differing dimensional nanomaterials in water filtration membranes through the classical phase inversion process: a review. Ind Eng Chem Res. 2013;52:10355–10363. [Google Scholar]
- 85.Chakarvarti S. Track-etch membranes enabled nano-/microtechnology: A review. Radiat Meas. 2009;44:1085–1092. [Google Scholar]
- 86.Sarbolouki M, Ed.Sourirajan S. National Research Council of Canada. 1977. Reverse osmosis and synthetic membranes; p. 598. [Google Scholar]
- 87.Ghosh A, Bindal R, Prabhakar S, Tewari PK. Composite polyamide reverse osmosis (RO) membranes: Recent developments and future directions. BARC Newsletter. 2011;321:43–51. [Google Scholar]
- 88.Sagle A, Freeman B. Fundamentals of membranes for water treatment. The future of desalination in Texas. 2004;2:137–154. [Google Scholar]
- 89.Sanders DF, Smith ZP, Guo R, Robeson LM, McGrath JE, Paul DR, Freeman BD. Energy-efficient polymeric gas separation membranes for a sustainable future: a review. Polymer. 2013;54:4729–4761. [Google Scholar]
- 90.Hirose M, Ito H, Kamiyama Y. Effect of skin layer surface structures on the flux behaviour of RO membranes. J Membr Sci. 1996;121:209–215. [Google Scholar]
- 91.Yan H, Miao X, Xu J, Pan G, Zhang Y, Shi Y, Guo M, Liu Y. The porous structure of the fully-aromatic polyamide film in reverse osmosis membranes. J Membr Sci. 2015;475:504–510. [Google Scholar]
- 92.Baghbanzadeh M, Rana D, Lan CQ, Matsuura T. Effects of inorganic nano-additives on properties and performance of polymeric membranes in water treatment. Sep Purif Rev. 2016;45:141–167. [Google Scholar]
- 93.Qu X, Alvarez PJ, Li Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013;47:3931–3946. doi: 10.1016/j.watres.2012.09.058. [DOI] [PubMed] [Google Scholar]
- 94.Nguyen T, Roddick FA, Fan L. Biofouling of water treatment membranes: a review of the underlying causes, monitoring techniques and control measures. Membranes. 2012;2:804–840. doi: 10.3390/membranes2040804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Majumder M, Chopra N, Hinds BJ. Mass transport through carbon nanotube membranes in three different regimes: ionic diffusion and gas and liquid flow. ACS Nano. 2011;5:3867–3877. doi: 10.1021/nn200222g. [DOI] [PubMed] [Google Scholar]
- 96.Hu M, Mi B. Layer-by-layer assembly of graphene oxide membranes via electrostatic interaction. J Membr Sci. 2014;469:80–87. [Google Scholar]
- 97.Liu L, Son M, Chakraborty S, Bhattacharjee C, Choi H. Fabrication of ultra-thin polyelectrolyte/carbon nanotube membrane by spray-assisted layer-by-layer technique: characterization and its anti-protein fouling properties for water treatment. Desalin Water Treat. 2013;51:6194–6200. [Google Scholar]
- 98.Koh DY, Lively RP. Nanoporous graphene: membranes at the limit. Nat Nanotechnol. 2015;10:385–386. doi: 10.1038/nnano.2015.77. [DOI] [PubMed] [Google Scholar]
- 99.McGovern RK, Lienharv JH., V On the asymptotic flux of ultrapermeable seawater reverse osmosis membranes due to concentration polarisation. J Membr Sci. 2016;520:560–565. [Google Scholar]
- 100.Cohen-Tanugi D, McGovern RK, Dave SH, Lienhard JH, Grossman JC. Quantifying the potential of ultra-permeable membranes for water desalination. Energ Environ Sci. 2014;7:1134–1141. [Google Scholar]
- 101.Goh P, Matsuura T, Ismail A, Hilal N. Recent trends in membranes and membrane processes for desalination. Desalination. 2016;391:43–60. [Google Scholar]
- 102.Méricq JP, Mendret J, Brosillon S, Faur C. High performance PVDF-TiO2 membranes for water treatment. Chem Eng Sci. 2015;123:283–291. [Google Scholar]
- 103.Bet-moushoul E, Mansourpanah Y, Farhadi K, Tabatabaei M. TiO2 nanocomposite based polymeric membranes: a review on performance improvement for various applications in chemical engineering processes. Chem Eng J. 2016;283:29–46. [Google Scholar]
- 104.Rahaman MS, Thérien-Aubin H, Ben-Sasson M, Ober CK, Nielsen M, Elimelech M. Control of biofouling on reverse osmosis polyamide membranes modified with biocidal nanoparticles and antifouling polymer brushes. J Mater Chem B. 2014;2:1724–1732. doi: 10.1039/c3tb21681k. [DOI] [PubMed] [Google Scholar]
- 105.Ben-Sasson M, Lu X, Bar-Zeev E, Zodrow KR, Nejati S, Qi G, Giannelis EP, Elimelech M. In situ formation of silver nanoparticles on thin-film composite reverse osmosis membranes for biofouling mitigation. Water Res. 2014;62:260–270. doi: 10.1016/j.watres.2014.05.049. [DOI] [PubMed] [Google Scholar]
- 106.Yin J, Deng B. Polymer-matrix nanocomposite membranes for water treatment. J Membr Sci. 2015;479:256–275. [Google Scholar]
- 107.Chakraborty S, Loutatidou S, Palmisano G, Kujawa J, Mavukkandy MO, Al-Gharabli S, Curcio E, Arafat HA. Photocatalytic hollow fiber membranes for the degradation of pharmaceutical compounds in wastewater. J Environ Chem Eng. 2017;5:5014–5024. [Google Scholar]
- 108.Akar N, Asar B, Dizge N, Koyuncu I. Investigation of characterization and biofouling properties of pes membrane containing selenium and copper nanoparticles. J Membr Sci. 2013;437:216–226. [Google Scholar]
- 109.Razmjou A, Resosudarmo A, Holmes RL, Li H, Mansouri J, Chen V. The effect of modified TiO2 nanoparticles on the polyethersulfone ultrafiltration hollow fiber membranes. Desalination. 2012;287:271–280. [Google Scholar]
- 110.Liang S, Xiao K, Mo Y, Huang X. A novel ZnO nanoparticle blended polyvinylidene fluoride membrane for anti-irreversible fouling. J Membr Sci. 2012;394:184–192. [Google Scholar]
- 111.Koseoglu-Imer DY, Kose B, Altinbas M, Koyuncu I. The production of polysulfone (PS) membrane with silver nanoparticles (AgNP): physical properties, filtration performances, and biofouling resistances of membranes. J Membr Sci. 2013;428:620–628. [Google Scholar]
- 112.Gray S, Zhang J, Duke M, Knight A, Packer M, Northcott K, Hillis P, Dharmabalan D, Scales P. Robust water recycling plant for the Antarctic. AWA Ozwater15; Adelaide: 2015. p. 8. [Google Scholar]
- 113.Mueller U, Biwer G, Baldauf G. Ceramic membranes for water treatment. Water Sci. Technol.: Water Supply. 2010;10:987–994. [Google Scholar]
- 114.Farsi A, Jensen SH, Roslev P, Boffa V, Christensen ML. Inorganic membranes for the recovery of effluent from municipal wastewater treatment plants. Ind Eng Chem Res. 2015;54:3462–3472. [Google Scholar]
- 115.Kramer FC, Shang R, Heijman SG, Scherrenberg SM, van Lier JB, Rietveld LC. Direct water reclamation from sewage using ceramic tight ultra-and nanofiltration. Sep Purif Technol. 2015;147:329–336. [Google Scholar]
- 116.Sayed S, Tarek S, Dijkstra I, Moerman C. Optimum operation conditions of direct capillary nanofiltration for wastewater treatment. Desalination. 2007;214:215–226. [Google Scholar]
- 117.Fan X, Tao Y, Wei D, Zhang X, Lei Y, Noguchi H. Removal of organic matter and disinfection by-products precursors in a hybrid process combining ozonation with ceramic membrane ultrafiltration. Front Env Sci Eng. 2015;9:112–120. [Google Scholar]
- 118.Hu Z, Wen X, Si X. Pre-ultrafiltration or pre-ozonation for EDCs removal in a combined ultrafiltration and ozonation process. J Chem Technol Biot. 2016;91:2929–2934. [Google Scholar]
- 119.Byun S, Davies S, Alpatova A, Corneal L, Baumann M, Tarabara V, Masten S. Mn oxide coated catalytic membranes for a hybrid ozonation-membrane filtration: comparison of Ti, Fe and Mn oxide coated membranes for water quality. Water Res. 2011;45:163–170. doi: 10.1016/j.watres.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 120.Loo SL, Fane AG, Krantz WB, Lim TT. Emergency water supply: a review of potential technologies and selection criteria. Water Res. 2012;46:3125–3151. doi: 10.1016/j.watres.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 121.Crowder ML, Gooding CH. Spiral wound, hollow fiber membrane modules: A new approach to higher mass transfer efficiency. J Membr Sci. 1997;137:17–29. [Google Scholar]
- 122.Liu L, Xu X, Zhao C, Yang F. A new helical membrane module for increasing permeate flux. J Membr Sci. 2010;360:142–148. [Google Scholar]
- 123.Ding L, Jaffrin MY. Benefits of high shear rate dynamic nanofiltration and reverse osmosis: A review. Sep Sci Technol. 2014;49:1953–1967. [Google Scholar]
- 124.Jaffrin MY. Dynamic shear-enhanced membrane filtration: a review of rotating disks, rotating membranes and vibrating systems. J Membr Sci. 2008;324:7–25. [Google Scholar]
- 125.Decarolis J, Hong S, Taylor J. Fouling behavior of a pilot scale inside-out hollow fiber UF membrane during dead-end filtration of tertiary wastewater. J Membr Sci. 2001;191:165–178. [Google Scholar]
- 126.Patel TM, Roy T, Mishra A, Nath K. Reclamation of dye wastewater using a flat sheet nanofiltration membrane: Study of permeate flux and quality. J Sci Ind Res. 2012;71:437–442. [Google Scholar]
- 127.Adham S, Chiu Kp, Lehman G. Optimization of membrane treatment for direct and clarified water filtration. Vol. 91111. American Water Works Association Research Foundation; 2006. p. 200. [Google Scholar]
- 128.Damodar RA, You SJ. Performance of an integrated membrane photocatalytic reactor for the removal of reactive black 5. Sep Purif Technol. 2010;71:44–49. [Google Scholar]
- 129.Oh SJ, Kim N, Lee YT. Preparation and characterization of PVDF/TiO2 organic-inorganic composite membranes for fouling resistance improvement. J Membr Sci. 2009;345:13–20. [Google Scholar]
- 130.Herna~ndez S, Lei S, Rong W, Ormsbee L, Bhattacharyya D. Functionalization of flat sheet and hollow fiber microfiltration membranes for water applications. ACS Sustain Chem Eng. 2015;4:907–918. doi: 10.1021/acssuschemeng.5b01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kang Gd, Cao Ym. Application and modification of poly (vinylidene fluoride) (PVDF) membranes-a review. J Membr Sci. 2014;463:145–165. [Google Scholar]
- 132.Park C, Hong SW, Chung TH, Choi YS. Performance evaluation of pretreatment processes in integrated membrane system for wastewater reuse. Desalination. 2010;250:673–676. [Google Scholar]
- 133.Wang KY, Ong RC, Chung TS. Double-skinned forward osmosis membranes for reducing internal concentration polarization within the porous sublayer. Ind Eng Chem Res. 2010;49:4824–4831. [Google Scholar]
- 134.Zhang S, Wang KY, Chung TS, Chen H, Jean Y, Amy G. Well-constructed cellulose acetate membranes for forward osmosis: minimized internal concentration polarization with an ultra-thin selective layer. J Membr Sci. 2010;360:522–535. [Google Scholar]
- 135.Labban O, Liu C, Chong TH, Lienhard JH., V Fundamentals of low-pressure nanofiltration: Membrane characterization, modeling, and understanding the multi-ionic interactions in water softening. J Membr Sci. 2017;521:18–32. [Google Scholar]
- 136.Fritzmann C, Löowenberg J, Wintgens T, Melin T. State-of-the-art of reverse osmosis desalination. Desalination. 2007;216:1–76. [Google Scholar]
- 137.Liu TY, Tong Y, Liu ZH, Lin HH, Lin YK, Van der Bruggen B, Wang XL. Extracellular polymeric substances removal of dual-layer (PES/PVDF) hollow fiber UF membrane comprising multi-walled carbon nanotubes for preventing RO biofouling. Sep Purif Technol. 2015;148:57–67. [Google Scholar]
- 138.Madaeni S, Koocheki S. Application of Taguchi method in the optimization of wastewater treatment using spiral-wound reverse osmosis element. Chem Eng J. 2006;119:37–44. [Google Scholar]
- 139.Sagne C, Fargues C, Broyart B, Lameloise ML, Decloux M. Modeling permeation of volatile organic molecules through reverse osmosis spiral-wound membranes. J Membr Sci. 2009;330:40–50. [Google Scholar]
- 140.Fujioka T, Tu KL, Khan SJ, McDonald JA, Roux A, Poussade Y, Drewes JE, Nghiem LD. Rejection of small solutes by reverse osmosis membranes for water reuse applications: A pilot-scale study. Desalination. 2014;350:28–34. [Google Scholar]
- 141.Fujioka T, Khan SJ, McDonald JA, Roux A, Poussade Y, Drewes JE, Nghiem LD. Modelling the rejection of N-nitrosamines by a spiral-wound reverse osmosis system: mathematical model development and validation. J Membr Sci. 2014;454:212–219. [Google Scholar]
- 142.Pizzichini M, Russo C, Di Meo C. Purification of pulp and paper wastewater, with membrane technology, for water reuse in a closed loop. Desalination. 2005;178:351–359. [Google Scholar]
- 143.aquanext Inc. [accessed December 2016];Spiral reverse osmosis membrane. URL: http://www.aquanext-inc.com/en/product/desalination02.html.
- 144.Pervov AG, Andrianov A, Gorbunova T, Bagdasaryan A. Membrane technologies in the solution of environmental problems. Petrol Chem. 2015;55:879–886. [Google Scholar]
- 145.Pervov AG, Andrianov AP. Application of membranes to treat wastewater for its recycling and reuse: new considerations to reduce fouling and increase recovery up to 99 percent. Desalin Water Treat. 2011;35:2–9. [Google Scholar]
- 146.Siddiqui A, Farhat N, Bucs SS, Linares RV, Picioreanu C, Kruithof JC, van Loosdrecht MC, Kidwell J, Vrouwenvelder JS. Development and characterization of 3D-printed feed spacers for spiral wound membrane systems. Water Res. 2016;91:55–67. doi: 10.1016/j.watres.2015.12.052. [DOI] [PubMed] [Google Scholar]
- 147.Wibisono Y, Yandi W, Golabi M, Nugraha R, Cornelissen ER, Kemperman AJ, Ederth T, Nijmeijer K. Hydrogel-coated feed spacers in two-phase flow cleaning in spiral wound membrane elements: A novel platform for eco-friendly biofouling mitigation. Water Res. 2015;71:171–186. doi: 10.1016/j.watres.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 148.Hijnen W, Castillo C, Brouwer-Hanzens A, Harmsen D, Cornelissen E, Van der Kooij D. Quantitative assessment of the efficacy of spiral-wound membrane cleaning procedures to remove biofilms. Water res. 2012;46:6369–6381. doi: 10.1016/j.watres.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 149.Mulder M. Basic Principles of Membrane Technology. Springer; 1996. Polarisation phenomena and membrane fouling; pp. 416–464. [Google Scholar]
- 150.Bernardes AM, Rodrigues MAS, Ferreira JZ. Electrodialysis and water reuse. Lisbon: Springer; 2014. p. 150. [Google Scholar]
- 151.Strathmann H. Electrodialysis and related processes. Membrane Science and Technology. 1995;2:213–281. [Google Scholar]
- 152.Kalogirou S. Seawater Desalination Using Renewable Energy Sources. Prog Energ Combust. 2005;31:242–281. [Google Scholar]
- 153.Tanaka Y, Ehara R, Itoi S, Goto T. Ion-exchange Membrane Electrodialytic Salt Production Using Brine Discharged from a Reverse Osmosis Seawater Desalination Plant. J Membr Sci. 2003;222:71–86. [Google Scholar]
- 154.Katz WE. Desalination by ED and EDR State-of-the-Art in 1981. Desalination. 1982;42:129–139. [Google Scholar]
- 155.Valero F, Barceló A, Arbos R. Electrodialysis technology-theory and applications. In: Shorr M, editor. Desalination, Trends and Technologies. Rijeka: InTech; 2011. p. 20. [Google Scholar]
- 156.Tanaka Y. Ion Exchange Membranes: Fundamentals and Applications. First. Amsterdam: Elsevier; 2007. p. 546. [Google Scholar]
- 157.Balster J, Krupenko O, Punt I, Stamatialis DF, Wessling M. Preparation and Characterisation of Monovalent Ion Selective Cation Exchange Membranes Based on Sulphonated Poly(Ether Ether Ketone) J Membr Sci. 2005;263:137–145. [Google Scholar]
- 158.Mohammadi T, Moheb A, Sadrzadeh M, Razmi A. Separation of Copper Ions by Electrodialys Using Taguchi Experimental Design. Desalination. 2004;169:21–31. [Google Scholar]
- 159.Nemati M, Hosseini SM, Bagheripour E, Madaeni SS. Electrodialysis Heterogeneous Anion Exchange Membranes Filled with TiO2 Nanoparticles: Membranes' Fabrication and Characterization. J Membr Sci Res. 2015;1:135–140. [Google Scholar]
- 160.Geise GM, Hickner MA, Logan BE. Ionic Resistance and Permselectivity Tradeoffs in Anion Exchange Membranes. ACS Appl Mater Interfaces. 2013;5:10294–10301. doi: 10.1021/am403207w. [DOI] [PubMed] [Google Scholar]
- 161.Wiseman R. Tech. Rep. 6. Vol. 29. Water and Wastewater International; 2014. San Diego to spearhead direct potable water reuse; p. 6. [Google Scholar]
- 162.Rodrigues MAS, Amado FDR, Xavier JLN, Streit KF, Bernardes AM, Ferreira JZ. Application of photoelectrochemical-electrodialysis treatment for the recovery and reuse of water from tannery effluents. J Clean Prod. 2008;16:605–611. [Google Scholar]
- 163.Lozier JC, Bergman R. Membrane processes in water reuse. Proc. 1994 AWWA/WEF Water Reuse Symp; Dallas, TX. Citeseer; 1994. pp. 687–709. [Google Scholar]
- 164.Goodman NB, Taylor RJ, Xie Z, Gozukara Y, Clements A. A feasibility study of municipal wastewater desalination using electrodialysis reversal to provide recycled water for horticultural irrigation. Desalination. 2013;317:77–83. [Google Scholar]
- 165.Kim JO, Jeong JT, Kim SK, Kim RH, Lee YJ. Materials science forum. Vol. 510. Trans Tech Publ; 2006. Development of novel wastewater reclamation system using microfiltration with advanced new membrane material and electrodialysis; pp. 586–589. [Google Scholar]
- 166.Gherasim CV, Křivčík J, Mikulášek P. Investigation of batch electrodialysis process for removal of lead ions from aqueous solutions. Chem Eng J. 2014;256:324–334. [Google Scholar]
- 167.Escobar IC. Chapter 14 Conclusion: A Summary of Challenges Still Facing Desalination and Water Reuse. In: Escobar IC, Schäfer A, editors. Sustainable Water for the Future: Water Recycling versus Desalination. first. chap. 14. Amsterdam: Elsevier; 2010. pp. 389–397. [Google Scholar]
- 168.Adham S, Lehman G, Gillogly T, Rosenblum E, Hansen E. Tech. Rep. City of San Jose; Denver: 2009. Comparison of Advanced Treatment Methods for Partial Desalting of Tertiary Effluents; p. 84. [DOI] [Google Scholar]
- 169.Tow EW, Lienhard JH., V Quantifying osmotic membrane fouling to enable comparisons across diverse processes. J Membr Sci. 2016;511:92–107. [Google Scholar]
- 170.Cath TY, Childress AE, Elimelech M. Forward osmosis: principles, applications, and recent developments. J Membr Sci. 2006;281:70–87. [Google Scholar]
- 171.Achilli A, Cath TY, Childress AE. Selection of inorganic-based draw solutions for forward osmosis applications. J Membr Sci. 2010;364:233–241. [Google Scholar]
- 172.Cath TY, Hancock NT, Lundin CD, Hoppe-Jones C, Drewes JE. A multi-barrier osmotic dilution process for simultaneous desalination and purification of impaired water. J Membr Sci. 2010;362:417–426. [Google Scholar]
- 173.Coday BD, Xu P, Beaudry EG, Herron J, Lampi K, Hancock NT, Cath TY. The sweet spot of forward osmosis: Treatment of produced water, drilling wastewater, and other complex and difficult liquid streams. Desalination. 2014;333:23–35. [Google Scholar]
- 174.Holloway RW, Achilli A, Cath TY. The osmotic membrane bioreactor: a critical review. Environ Sci: Water Res Technol. 2015;1:581–605. [Google Scholar]
- 175.Cath TY, Elimelech M, McCutcheon JR, McGinnis RL, Achilli A, Anastasio D, Brady AR, Childress AE, Farr IV, Hancock NT, Lampi JN, Long D, Xie M, Yip NY. Standard methodology for evaluating membrane performance in osmotically driven membrane processes. Desalination. 2013;312:31–38. [Google Scholar]
- 176.Yip NY, Tiraferri A, Phillip WA, Schiffman JD, Elimelech M. High performance thin-film composite forward osmosis membrane. Environ Sci Technol. 2010;44:3812–3818. doi: 10.1021/es1002555. [DOI] [PubMed] [Google Scholar]
- 177.McCutcheon JR, Elimelech M. Influence of concentrative and dilutive internal concentration polarization on flux behavior in forward osmosis. J Membr Sci. 2006;284:237–247. [Google Scholar]
- 178.Su J, Ong RC, Wang P, Chung TS, Helmer BJ, Wit JS. Advanced FO membranes from newly synthesized cap polymer for wastewater reclamation through an integrated FO-MD hybrid system. AIChE Journal. 2013;59:1245–1254. [Google Scholar]
- 179.Su J, Chung TS, Helmer BJ, de Wit JS. Enhanced double-skinned FO membranes with inner dense layer for wastewater treatment and macromolecule recycle using sucrose as draw solute. J Membr Sci. 2012;396:92–100. [Google Scholar]
- 180.Achilli A, Cath TY, Marchand EA, Childress AE. The forward osmosis membrane bioreactor: a low fouling alternative to MBR processes. Desalination. 2009;239:10–21. [Google Scholar]
- 181.Alturki A, McDonald J, Khan SJ, Hai FI, Price WE, Nghiem LD. Performance of a novel osmotic membrane bioreactor (OMBR) system: flux stability and removal of trace organics. Bioresour Technol. 2012;113:201–206. doi: 10.1016/j.biortech.2012.01.082. [DOI] [PubMed] [Google Scholar]
- 182.Zhang J, Loong WLC, Chou S, Tang C, Wang R, Fane AG. Membrane biofouling and scaling in forward osmosis membrane bioreactor. J Membr Sci. 2012;403:8–14. [Google Scholar]
- 183.Lay WC, Zhang Q, Zhang J, McDougald D, Tang C, Wang R, Liu Y, Fane AG. Study of integration of forward osmosis and biological process: membrane performance under elevated salt environment. Desalination. 2011;283:123–130. [Google Scholar]
- 184.Holloway RW, Wait AS, da Silva AF, Herron J, Schutter MD, Lampi K, Cath TY. Long-term pilot scale investigation of novel hybrid ultrafiltration-osmotic membrane bioreactors. Desalination. 2015;363:64–74. [Google Scholar]
- 185.Qiu G, Ting YP. Direct phosphorus recovery from municipal wastewater via osmotic membrane bioreactor (OMBR) for wastewater treatment. Bioresour Technol. 2014;170:221–229. doi: 10.1016/j.biortech.2014.07.103. [DOI] [PubMed] [Google Scholar]
- 186.Wang X, Yuan B, Chen Y, Li X, Ren Y. Integration of micro-filtration into osmotic membrane bioreactors to prevent salinity build-up. Bioresour Technol. 2014;167:116–123. doi: 10.1016/j.biortech.2014.05.121. [DOI] [PubMed] [Google Scholar]
- 187.Tan JM, Qiu G, Ting YP. Osmotic membrane bioreactor for municipal wastewater treatment and the effects of silver nanoparticles on system performance. J Clean Prod. 2015;88:146–151. [Google Scholar]
- 188.Cornelissen E, Harmsen D, De Korte K, Ruiken C, Qin JJ, Oo H, Wessels L. Membrane fouling and process performance of forward osmosis membranes on activated sludge. J Membr Sci. 2008;319:158–168. [Google Scholar]
- 189.Hickenbottom KL, Hancock NT, Hutchings NR, Appleton EW, Beaudry EG, Xu P, Cath TY. Forward osmosis treatment of drilling mud and fracturing wastewater from oil and gas operations. Desalination. 2013;312:60–66. [Google Scholar]
- 190.Hancock NT, Xu P, Roby MJ, Gomez JD, Cath TY. Towards direct potable reuse with forward osmosis: Technical assessment of long-term process performance at the pilot scale. J Membr Sci. 2013;445:34–46. [Google Scholar]
- 191.Alturki AA, McDonald JA, Khan SJ, Price WE, Nghiem LD, Elimelech M. Removal of trace organic contaminants by the forward osmosis process. Sep Purif Technol. 2013;103:258–266. [Google Scholar]
- 192.Cartinella JL, Cath TY, Flynn MT, Miller GC, Hunter KW, Childress AE. Removal of natural steroid hormones from wastewater using membrane contactor processes. Environ Sci Technol. 2006;40:7381–7386. doi: 10.1021/es060550i. [DOI] [PubMed] [Google Scholar]
- 193.Wu B, Kitade T, Chong TH, Uemura T, Fane AG. Impact of membrane bioreactor operating conditions on fouling behavior of reverse osmosis membranes in MBR-RO processes. Desalination. 2013;311:37–45. [Google Scholar]
- 194.Holloway RW, Regnery J, Nghiem LD, Cath TY. Removal of trace organic chemicals and performance of a novel hybrid ultrafiltration-osmotic membrane bioreactor. Environ Sci Technol. 2014;48:10859–10868. doi: 10.1021/es501051b. [DOI] [PubMed] [Google Scholar]
- 195.Mi B, Elimelech M. Organic fouling of forward osmosis membranes: fouling reversibility and cleaning without chemical reagents. J Membr Sci. 2010;348:337–345. [Google Scholar]
- 196.Lienhard JH, V, Warsinger DM, Thiel GP, Banchik LD. Abdul Latif Jameel World Water and Food Security Lab. MIT; 2016. Low Carbon Desalination: Status and Research, Development, and Demonstration Needs. [Google Scholar]
- 197.McGovern RK, Lienhard JH., V On the potential of forward osmosis to energetically outperform reverse osmosis desalination. J Membr Sci. 2014;469:245–250. [Google Scholar]
- 198.Chun Y, Zaviska F, Kim SJ, Mulcahy D, Yang E, Kim IS, Zou L. Fouling characteristics and their implications on cleaning of a FO-RO pilot process for treating brackish surface water. Desalination. 2016;394:91–100. [Google Scholar]
- 199.Hancock NT, Black ND, Cath TY. A comparative life cycle assessment of hybrid osmotic dilution desalination and established seawater desalination and wastewater reclamation processes. Water Res. 2012;46:1145–1154. doi: 10.1016/j.watres.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 200.Roberts DA, Johnston EL, Knott NA. Impacts of desalination plant discharges on the marine environment: A critical review of published studies. Water Res. 2010;44:5117–5128. doi: 10.1016/j.watres.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 201.Swaminathan J, Chung HW, Warsinger DM, Al-Marzooqi FA, Arafat AH, Lienhard JH., V Energy efficiency of permeate gap and novel conductive gap membrane distillation. J Membr Sci. 2016;502:171–178. [Google Scholar]
- 202.Warsinger DM, Swaminathan J, Lienhard JH., V Effect of module inclination angle on air gap membrane distillation; Proceedings of the 15th International Heat Transfer Conference, IHTC-15, Paper No. IHTC15-9351; Kyoto, Japan. August 2014; p. 14. [DOI] [Google Scholar]
- 203.Hilal N, Ismail AF, Wright C, Thomas R, Bilad MR, Arafat HA. Membrane Fabrication. CRC Press; 2015. PVDF membranes for membrane distillation: Controlling pore structure, porosity, hydrophobicity, and mechanical strength; pp. 249–284. [Google Scholar]
- 204.Servi AT, Kharraz J, Klee D, Notarangelo K, Eyob B, Guillen-Burrieza E, Liu A, Arafat HA, Gleason KK. A systematic study of the impact of hydrophobicity on the wetting of md membranes. J Membr Sci. 2016;520:850–859. [Google Scholar]
- 205.Guillen-Burrieza E, Servi A, Lalia BS, Arafat HA. Membrane structure and surface morphology impact on the wetting of md membranes. J Membr Sci. 2015;483:94–103. [Google Scholar]
- 206.Chung HW, Swaminathan J, Warsinger D, Lienhard JH., V Multistage vacuum membrane distillation (MSVMD) system for high salinity application. J Membr Sci. 2016;497:128–141. [Google Scholar]
- 207.Warsinger DM, Mistry KH, Nayar KG, Chung HW, Lienhard JH., V Entropy generation of desalination powered by variable temperature waste heat. Entropy. 2015;17:7530–7566. [Google Scholar]
- 208.Saffarini RB, Summers EK, Arafat HA, Lienhard JH., V Economic evaluation of stand-alone solar powered membrane distillation systems. Desalination. 2012;299:55–62. [Google Scholar]
- 209.Dialynas E, Diamadopoulos E. Integration of immersed membrane ultrafiltration with coagulation and activated carbon adsorption for advanced treatment of municipal wastewater. Desalination. 2008;230:113–127. [Google Scholar]
- 210.Wang W, Gu P, Zhang G, Wang L. Organics removal from ROC by PAC accumulative countercurrent two-stage adsorption-MF hybrid process-a laboratory-scale study. Sep Purif Technol. 2013;118:342–349. [Google Scholar]
- 211.Snyder S, Vanderford B, Drewes J, Dickenson E, Snyder E, Bruce G, Pleus RC. State of knowledge of endocrine disruptors and pharmaceuticals in drinking water. AWWA Research Foundation. 2008:268. [Google Scholar]
- 212.Kim KY, Kim HS, Kim J, Nam JW, Kim JM, Son S. A hybrid microfiltration-granular activated carbon system for water purification and wastewater reclamation/reuse. Desalination. 2009;243:132–144. [Google Scholar]
- 213.Qi L, Liu Gh, Zheng X, Li Gb. Reuse of PAC and alum sludge (RPAS) process: pretreatment to reduce membrane fouling. Desalin Water Treat. 2015;53:2421–2428. [Google Scholar]
- 214.Pramanik BK, Roddick FA, Fan L, Jeong S, Vigneswaran S. Assessment of biological activated carbon treatment to control membrane fouling in reverse osmosis of secondary effluent for reuse in irrigation. Desalination. 2015;364:90–95. [Google Scholar]
- 215.Wert EC, Neemann JJ, Rexing DJ, Zegers RE. Biofiltration for removal of BOM and residual ammonia following control of bromate formation. Water Res. 2008;42:372–378. doi: 10.1016/j.watres.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 216.Aryal A, Sathasivan A, Adhikari RA. Evidence that bac treatment enhances the doc removal by enhanced coagulation. Desalination. 2011;280:326–331. [Google Scholar]
- 217.Umar M, Roddick F, Fan L. Impact of coagulation as a pre-treatment for UVC/H2O2-biological activated carbon treatment of a municipal wastewater reverse osmosis concentrate. Water Res. 2016;88:12–19. doi: 10.1016/j.watres.2015.09.047. [DOI] [PubMed] [Google Scholar]
- 218.Esplugas S, Bila DM, Krause LGT, Dezotti M. Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J Hazard Mater. 2007;149:631–642. doi: 10.1016/j.jhazmat.2007.07.073. [DOI] [PubMed] [Google Scholar]
- 219.Von Gunten U. Ozonation of drinking water: Part i. oxidation kinetics and product formation. Water Res. 2003;37:1443–1467. doi: 10.1016/S0043-1354(02)00457-8. [DOI] [PubMed] [Google Scholar]
- 220.Melin G, Hogan T. Treatment technologies for removal of methyl tertiary butyl ether (MTBE) from drinking water. The California MTBE Research Partnership. 2000:432. [Google Scholar]
- 221.Von Gunten U. Ozonation of drinking water: Part ii. disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 2003;37:1469–1487. doi: 10.1016/S0043-1354(02)00458-X. [DOI] [PubMed] [Google Scholar]
- 222.Lee C, Schmidt C, Yoon J, Von Gunten U. Oxidation of N-nitrosodimethylamine (NDMA) precursors with ozone and chlorine dioxide: kinetics and effect on NDMA formation potential. Environ Sci Technol. 2007;41:2056–2063. doi: 10.1021/es062484q. [DOI] [PubMed] [Google Scholar]
- 223.Padhye L, Luzinova Y, Cho M, Mizaikoff B, Kim JH, Huang CH. PolyDADMAC and dimethylamine as precursors of N-nitrosodimethylamine during ozonation: reaction kinetics and mechanisms. Environ Sci Technol. 2011;45:4353–4359. doi: 10.1021/es104255e. [DOI] [PubMed] [Google Scholar]
- 224.Sgroi M, Roccaro P, Oelker GL, Snyder SA. N-nitrosodimethylamine (NDMA) formation at an indirect potable reuse facility. Water Res. 2015;70:174–183. doi: 10.1016/j.watres.2014.11.051. [DOI] [PubMed] [Google Scholar]
- 225.Rueda-Márquez JJ, Pintado-Herrera MG, Martín-Díaz ML, Acevedo-Merino A, Manzano M. Combined AOPs for potential wastewater reuse or safe discharge based on multi-barrier treatment (microfiltration-H2O2/UV-catalytic wet peroxide oxidation) Chem Eng J. 2015;270:80–90. [Google Scholar]
- 226.Pisarenko AN, Stanford BD, Yan D, Gerrity D, Snyder SA. Effects of ozone and ozone/peroxide on trace organic contaminants and NDMA in drinking water and water reuse applications. Water Res. 2012;46:316–326. doi: 10.1016/j.watres.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 227.Antony A, Subhi N, Henderson RK, Khan SJ, Stuetz RM, Le-Clech P, Chen V, Leslie G. Comparison of reverse osmosis membrane fouling profiles from Australian water recycling plants. J Membr Sci. 2012;407–408:8–16. [Google Scholar]
- 228.Hoek EM, Allred J, Knoell T, Jeong BH. Modeling the effects of fouling on full-scale reverse osmosis processes. J Membr Sci. 2008;314:33–49. [Google Scholar]
- 229.Potts DE, Ahlert RC, Wang SS. A critical review of fouling of reverse osmosis membranes. Desalination. 1981;36:235–264. [Google Scholar]
- 230.Kimura K, Okazaki S, Ohashi T, Watanabe Y. Importance of the co-presence of silica and organic matter in membrane fouling for RO filtering MBR effluent. J Membr Sci. 2016;501:60–67. [Google Scholar]
- 231.Thompson J, Lin N, Lyster E, Arbel R, Knoell T, Gilron J, et al. RO membrane mineral scaling in the presence of a biofilm. J Membr Sci. 2012;415–416:181–191. [Google Scholar]
- 232.She Q, Wang R, Fane AG, Tang CY. Membrane fouling in osmotically driven membrane processes: A review. J Membr Sci. 2016;499:201–233. [Google Scholar]
- 233.Guo W, Ngo HH, Li J. A mini-review on membrane fouling. Bioresour Technol. 2012;122:27–34. doi: 10.1016/j.biortech.2012.04.089. [DOI] [PubMed] [Google Scholar]
- 234.Hoek EMV, Elimelech M. Cake-enhanced concentration polarization: A new fouling mechanism for salt-rejecting membranes. Environ Sci Technol. 2003;37:5581–5588. doi: 10.1021/es0262636. [DOI] [PubMed] [Google Scholar]
- 235.Herzberg M, Elimelech M. Biofouling of reverse osmosis membranes: Role of biofilm-enhanced osmotic pressure. J Membr Sci. 2007;295:11–20. [Google Scholar]
- 236.Fujioka T, Khan SJ, McDonald JA, Henderson RK, Poussade Y, Drewes JE, Nghiem LD. Effects of membrane fouling on N-nitrosamine rejection by nanofiltration and reverse osmosis membranes. J Membr Sci. 2013;427:311–319. [Google Scholar]
- 237.Ivnitsky H, Katz I, Minz D, Volvovic G, Shimoni E, Kesselman E, Semiat R, Dosoretz CG. Bacterial community composition and structure of biofilms developing on nanofiltration membranes applied to wastewater treatment. Water Res. 2007;41:3924–3935. doi: 10.1016/j.watres.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 238.Xu P, Drewes JE, Kim TU, Bellona C, Amy G. Effect of membrane fouling on transport of organic contaminants in NF/RO membrane applications. J Membr Sci. 2006;279:165–175. [Google Scholar]
- 239.Kilduff JE, Mattaraj S, Belfort G. Flux decline during nanofiltration of naturally-occurring dissolved organic matter: effects of osmotic pressure, membrane permeability, and cake formation. J Membr Sci. 2004;239:39–53. [Google Scholar]
- 240.Herzberg M, Kang S, Elimelech M. Role of extracellular polymeric Substances (EPS) in biofouling of reverse osmosis membranes. Environ Sci Technol. 2009;43:4393–4398. doi: 10.1021/es900087j. [DOI] [PubMed] [Google Scholar]
- 241.Davies DG, Geesey GG. Regulation of the alginate biosynthesis gene algC in pseudomonas aeruginosa during biofilm development in continuous culture. Appl Environ Microb. 1995;61:860–867. doi: 10.1128/aem.61.3.860-867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wang YN, Tang CY. Protein fouling of nanofiltration, reverse osmosis, and ultrafiltration membranes -the role of hydrodynamic conditions, solution chemistry, and membrane properties. J Membr Sci. 2011;376:275–282. [Google Scholar]
- 243.Khan MT, Busch M, Molina VG, Emwas AH, Aubry C, Croue JP. How different is the composition of the fouling layer of wastewater reuse and seawater desalination RO membranes? Water Res. 2014;59:271–282. doi: 10.1016/j.watres.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 244.Tang F, Hu HY, Sun LJ, Sun YX, Shi N, Crittenden JC. Fouling characteristics of reverse osmosis membranes at different positions of a full-scale plant for municipal wastewater reclamation. Water Res. 2016;90:329–336. doi: 10.1016/j.watres.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 245.Shon HK, Vigneswaran S, Snyder SA. Effluent organic matter (EfOM) in wastewater: Constituents, effects, and treatment. Crit Rev Env Sci Tec. 2006;36:327–374. [Google Scholar]
- 246.Shon H, Vigneswaran S, Kim IS, Cho J, Ngo H. Effect of pretreatment on the fouling of membranes: application in biologically treated sewage effluent. J Membr Sci. 2004;234:111–120. doi: 10.1021/es040105s. [DOI] [PubMed] [Google Scholar]
- 247.Laabs CN, Amy GL, Jekel M. Understanding the size and character of fouling-causing substances from effluent organic matter (EfOM) in low-pressure membrane filtration. Environ Sci Technol. 2006;40:4495–4499. doi: 10.1021/es060070r. [DOI] [PubMed] [Google Scholar]
- 248.Filloux E, Gallard H, Croue JP. Identification of effluent organic matter fractions responsible for low-pressure membrane fouling. Water Res. 2012;46:5531–5540. doi: 10.1016/j.watres.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 249.Haberkamp J, Ernst M, Bockelmann U, Szewzyk U, Jekel M. Complexity of ultrafiltration membrane fouling caused by macromolecular dissolved organic compounds in secondary effluents. Water Res. 2008;42:3153–3161. doi: 10.1016/j.watres.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 250.Farias EL, Howe KJ, Thomson BM. Spatial and temporal evolution of organic foulant layers on reverse osmosis membranes in wastewater reuse applications. Water Res. 2014;58:102–110. doi: 10.1016/j.watres.2014.03.061. [DOI] [PubMed] [Google Scholar]
- 251.Farias EL, Howe KJ, Thomson BM. Effect of membrane bioreactor solids retention time on reverse osmosis membrane fouling for wastewater reuse. Water Res. 2014;49:53–61. doi: 10.1016/j.watres.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 252.Strathmann H. Ion-Exchange Membrane Separation Processes. First. Vol. 9. Oxford: Elsevier; 2004. p. 360. [Google Scholar]
- 253.Lyonnaise des eaux-Dumez (Firm) American Water Works Association. 1996. Water treatment membrane processes; p. 320. [Google Scholar]
- 254.Fan L, Nguyen T, Roddick FA, Harris JL. Low-pressure membrane filtration of secondary effluent in water reuse: Pre-treatment for fouling reduction. J Membr Sci. 2008;320:135–142. [Google Scholar]
- 255.Zhang J, Northcott K, Duke M, Scales P, Gray SR. Influence of pre-treatment combinations on RO membrane fouling. Desalination. 2016;393:120–126. [Google Scholar]
- 256.Geise GM, Lee HS, Miller DJ, Freeman BD, McGrath JE, Paul DR. Water purification by membranes: The role of polymer science. J Polym Sci Pol. 2010;48:1685–1718. [Google Scholar]
- 257.Mansouri J, Harrisson S, Chen V. Strategies for controlling biofouling in membrane filtration systems: challenges and opportunities. J Mater Chem. 2010;20:4567–4586. [Google Scholar]
- 258.Araújo PA, Miller DJ, Correia PB, van Loosdrecht MCM, Kruithof JC, Freeman BD, Paul DR, Vrouwenvelder JS. Impact of feed spacer and membrane modification by hydrophilic, bactericidal and biocidal coating on biofouling control. Desalination. 2012;295(Supplement C):1–10. [Google Scholar]
- 259.Warsinger DM, Servi A, Belleghem SV, Gonzalez J, Swaminathan J, Kharraz J, Chung HW, Arafat HA, Gleason KK, Lienhard JH., V Combining air recharging and membrane superhydrophobicity for fouling prevention in membrane distillation. J Membr Sci. 2016;505:241–252. [Google Scholar]
- 260.Liu C, Lee J, Small C, Ma J, Elimelech M. Comparison of organic fouling resistance of thin-film composite membranes modified by hydrophilic silica nanoparticles and zwitterionic polymer brushes. J Membr Sci. 2017;544:135–142. [Google Scholar]
- 261.Mi B, Elimelech M. Gypsum scaling and cleaning in forward osmosis: Measurements and mechanisms. Environ Sci Technol. 2010;44:2022–2028. doi: 10.1021/es903623r. [DOI] [PubMed] [Google Scholar]
- 262.McCloskey BD, Park HB, Ju H, Rowe BW, Miller DJ, Freeman BD. A bioinspired fouling-resistant surface modification for water purification membranes. J Membr Sci. 2012;413(Supplement C):82–90. [Google Scholar]
- 263.Wu J, Contreras AE, Li Q. Studying the impact of RO membrane surface functional groups on alginate fouling in seawater desalination. J Membr Sci. 2014;458:120–127. [Google Scholar]
- 264.Cornelissen E, Vrouwenvelder J, Heijman S, Viallefont X, Kooij DVD, Wessels L. Periodic air/water cleaning for control of biofouling in spiral wound membrane elements. J Membr Sci. 2007;287:94–101. [Google Scholar]
- 265.Madaeni S, Samieirad S. Chemical cleaning of reverse osmosis membrane fouled by wastewater. Desalination. 2010;257:80–86. [Google Scholar]
- 266.Qin JJ, Oo MH, Kekre KA, Liberman B. Development of novel backwash cleaning technique for reverse osmosis in reclamation of secondary effluent. J Membr Sci. 2010;346:8–14. [Google Scholar]
- 267.Bar-Zeev E, Elimelech M. Reverse osmosis biofilm dispersal by osmotic back-flushing: Cleaning via substratum perforation. Environ Sci Technol Lett. 2014;1:162–166. [Google Scholar]
- 268.Stover RL. Industrial and brackish water treatment with closed circuit reverse osmosis. Desalin Water Treat. 2013;51:1124–1130. [Google Scholar]
- 269.Mosher JJ, Vartanian GM, Tchobanoglous G. Potable reuse research compilation synthesis of findings. National Water Research Institute. 2016:224. [Google Scholar]
- 270.Rodriguez-Mozaz S, Weinberg HS. Meeting report: pharmaceuticals in water-an interdisciplinary approach to a public health challenge. Environ Health Persp. 2010:1016–1020. doi: 10.1289/ehp.0901532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Snyder SA, Westerhoff P, Yoon Y, Sedlak DL. Pharmaceuticals, personal care products, and endocrine disruptors in water: implications for the water industry. Environ Eng Sci. 2003;20:449–469. [Google Scholar]
- 272.Debroux JF, Soller JA, Plumlee MH, Kennedy LJ. Human health risk assessment of non-regulated xenobiotics in recycled water: a review. Hum Ecol Risk Assess. 2012;18:517–546. [Google Scholar]
- 273.Snyder S, Stanford B, Bruce G, Pleus R, Drewes J. Identifying hormonally active compounds, pharmaceuticals, and personal care product ingredients of health concern from potential presence in water intended for indirect potable reuse. Final Report WateReuse Research Foundation; Alexandria, VA: 2010. p. 248. [Google Scholar]
- 274.Trussell RR, Salveson A, Snyder SA, Trussell RS, Gerrity D, Pecson BM. Potable Reuse: State of the science report and equivalency criteria for treatment trains. Water Environment and Reuse Foundation. 2013:276. Report No. 11-02-2. [Google Scholar]
- 275.Snyder SA. Occurrence, treatment, and toxicological relevance of EDCs and pharmaceuticals in water. OZONE-SCI ENG. 2008;30:65–69. [Google Scholar]
- 276.Rauch-Williams T, Snyder S, Drewes J, Dickinson E. Current and Proposed Paradigms to Control CECs in the United States and Internationally. Water Research Foundation; 2016. p. 191. [Google Scholar]
- 277.Harris-Lovett SR, Binz C, Sedlak DL, Kiparsky M, Truffer B. Beyond user acceptance: a legitimacy framework for potable water reuse in California. Environ Sci Technol. 2015;49:7552–7561. doi: 10.1021/acs.est.5b00504. [DOI] [PubMed] [Google Scholar]
- 278.Plumlee MH, López-Mesas M, Heidlberger A, Ishida KP, Reinhard M. N-nitrosodimethylamine (NDMA) removal by reverse osmosis and UV treatment and analysis via lc-ms/ms. Water Res. 2008;42:347–355. doi: 10.1016/j.watres.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 279.Steinle-Darling E, Reinhard M. Nanofiltration for trace organic contaminant removal: structure, solution, and membrane fouling effects on the rejection of perfluorochemicals. Environ Sci Technol. 2008;42:5292–5297. doi: 10.1021/es703207s. [DOI] [PubMed] [Google Scholar]
- 280.Steinle-Darling E, Zedda M, Plumlee MH, Ridgway HF, Reinhard M. Evaluating the impacts of membrane type, coating, fouling, chemical properties and water chemistry on reverse osmosis rejection of seven nitrosoalklyamines, including NDMA. Water Res. 2007;41:3959–3967. doi: 10.1016/j.watres.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 281.Drewes J, Sedlak D, Snyder S. Development of indicators and surrogates for chemical contaminant removal during wastewater treatment and reclamation. Water Environment Research Foundation; 2009. p. 190. [Google Scholar]
- 282.WRF. Tech. Rep. Water Research Foundation; 2014. Risk communication about CECs; p. 4. [Google Scholar]
- 283.Millan M, Tennyson PA, Snyder SA. Model communication plans for increasing awareness and fostering acceptance of direct potable reuse. WateReuse Research Foundation; 2015. p. 260. [Google Scholar]
- 284.Binz C, Harris-Lovett S, Kiparsky M, Sedlak DL, Truffer B. The thorny road to technology legitimation: Institutional work for potable water reuse in California. Technol Forecast Soc. 2016;103:249–263. [Google Scholar]
- 285.Macpherson L, Snyder SA. Downstream: Context, understanding, acceptance: Effect of prior knowledge of unplanned potable reuse on the acceptance of planned potable reuse. WateReuse Research Foundation. 2013:234. [Google Scholar]
- 286.Panel IA. Tech. Rep. National Water Research Institute, California department of public health; 2012. BDOC as a performance measure for organics removal in groundwater recharge of recycled water; p. 53. [Google Scholar]
- 287.Anderson PD, Denslow ND, Drewes JE, Olivieri AW, Schlenk D, Scott GI, Snyder SA. Monitoring strategies for chemicals of emerging concern (CECs) in California's aquatic ecosystems-recommendations of a science advisory panel. Costa Mesa, CA: Southern California Coastal Water Research Project; 2012. p. 219. [Google Scholar]
- 288.Klosterhaus S, Maruya K, Mosher J, Sedlak M, Setty K, Vartanian GM. Managing contaminants of emerging concern in California. A Workshop to Developing Processes for Prioritizing, Monitoring, and Determining Thresholds of Concern. 2009 Apr 28–29;:52. [Google Scholar]