Welcome to the JCAD Aesthetic Complications Guidelines by The Aesthetic Complications Expert (ACE) Group. The ACE Group developed a series of evidence-based, peer-reviewed guidelines that cover complications that can occur in nonsurgical aesthetic practices. The objective of this series is to help dermatologists and other physicians performing aesthetic procedures identify and manage these potential complications. Each guideline was produced after a vast literature review by leading experts in the United Kingdom. We hope these guidelines help raise treatment standards within the medical community and ensure early diagnosis and appropriate management of complications, ultimately improving outcomes for our patients.
BACKGROUND
Hyaluronic acid (HA)-based dermal fillers are the most commonly used fillers in the aesthetics market.1 A glycosaminoglycan and a chief component of the extracellular matrix, HA is mainly responsible for maintaining hydration in the dermis. HA is a linear polysaccharide chain with the alternating monosaccharides d-glucuronic acid and N-acetyl-d-glucosamine.2
Hyaluronidases are enzymes (endoglycosidases) that can depolymerise HA, leading to its degradation3 by hydrolyzing the disaccharides at hexosaminidic β-1through β-4 linkages.4 Hyaluronidase is licensed in the United Kingdom for enhancing permeation of subcutaneous or intramuscular injections, local anaesthetics, and subcutaneous infusions, and to promote resorption of excess fluids and blood.5 There is considerable evidence for the off-label use of hyaluronidase for managing vascular compromise due to inadvertent intravascular injection or external compression,6 over-correction, asymmetry, and lumps and nodules7 caused by the injection of HA filler.
There are several sources of hyaluronidase, and they are generally divided into three subgroups: mammalian (obtained from the testes); hookworm or leech; and microbes.8 Recombinant human hyaluronidase (Hylenex®, Halozyme Therapeutics, San Diego, California) has a purity 100 times higher than some of the bovine preparations.9 There is no longterm data for this product yet, but it has been speculated to have a lower incidence of allergic reactions.
Hyaluronidase has immediate effect and a half-life of two minutes with duration of action of 24 to 48 hours.10,11 Though it has a short half-life, its effectiveness lasts longer. This might be due to the low number units required to have a clinically significant effect; thus, even when the hyaluronidase has mostly degraded, its action continues. Additionally, the initial action of hyaluronidase might break cross-links in the HA dermal filler so that it behaves like native HA in the skin, which has a half-life of 24 to 48 hours.12
OFF-LABEL USE OF HYALURONIDASE
Although hyaluronidase is not licensed for the use in correcting problems with dermal filler injections and off-label promotion is not allowed by Article 87 of Directive 2001/83/EC, the use of hyaluronidase is allowed provided the patient’s best interest and autonomy are a respected part of informed consent (as indicated by the 2009 guidelines from the United Kingdom’s Medicines and Healthcare Products Regulatory Agency [MHRA]).
INDICATIONS FOR THE USE OF HYALURONIDASE IN AESTHETIC PRACTICE
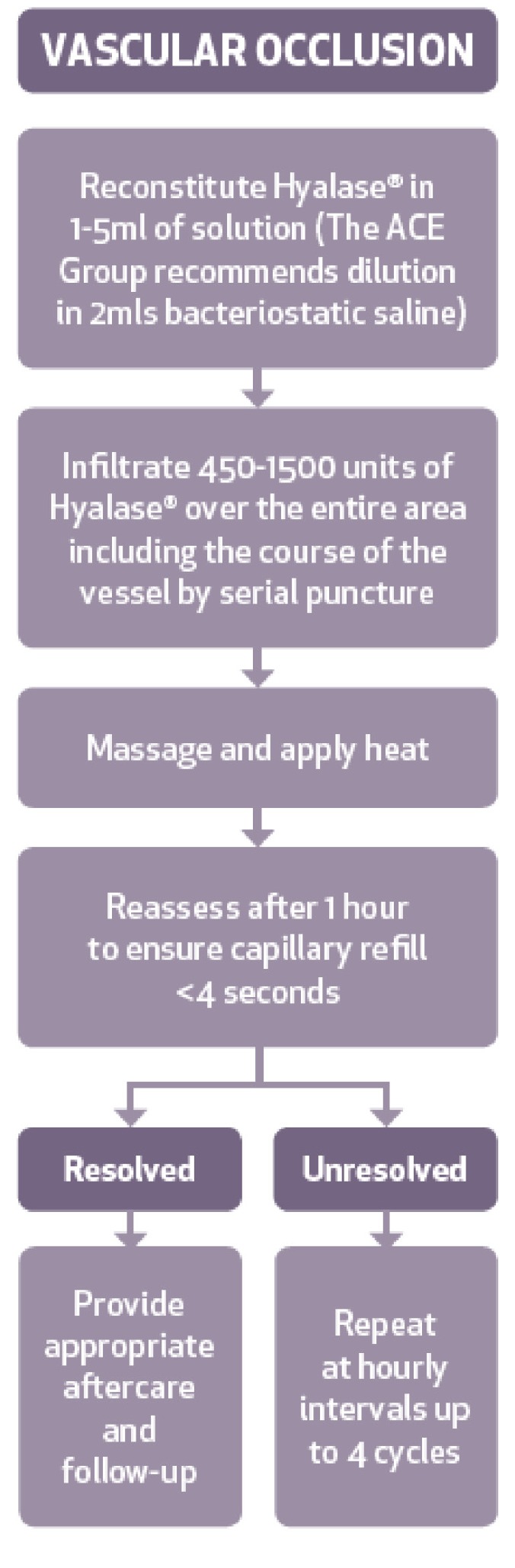
Vascular occlusion. The incidence of impending necrosis following dermal filler treatment has been estimated at 0.001 percent (1 in 100,000 cases).7 Vascular compromise due to HA filler injection should be treated immediately (refer to the ACE Group guidance on impending necrosis13). Normal skin should be non-discolored and warm, with a capillary refill time of 1 to 2 seconds, whereas arterial compromise will have a slow capillary refill time and dusky or blue-grey-black appearance, and venous insufficiency will have a fast capillary time and bluish discoloration.14 Signs of impending necrosis also include pain and coolness of the skin. Hyaluronidase should be administered as soon as this complication occurs (within 4 hours).4,15 There is strong evidence that tissue necrosis can be prevented or reduced in severity if treatment is administered within 48 hours.6,16 However, a small animal-based study tested this theory and found that injecting hyaluronidase at 24 hours failed to afford any benefit.17
Blindness. Blindness due to periocular embolism of HA is instant and associated with excruciating ocular pain. The retinal circulation needs to be restored within 60 to 90 minutes if the retina is to survive. Blindness is a medical emergency and the patient should be transferred immediately to the nearest hospital eye department (Refer to the ACE Group guidance on blindness18). Retrobulbar injection of hyaluronidase (150–200 units in 2–4mL of diluent) into the inferolateral orbit19 should be considered by practitioners who have appropriate experience and competence while waiting for an ambulance. Treatment of blindness is rarely successful.19
Tyndall effect. The Tyndall effect refers to the scattering of light that may be seen in some patients after injection of HA resulting in a bluish hue of the skin and most commonly seen in the sub ocular region. The problem can be resolved using hyaluronidase (Refer to ACE Group guidance on the Tyndall effect20).
Unacceptable cosmetic outcome. Overcorrection or misplacement of HA filler can be successfully treated with hyaluronidase, although this is often caused by poor injection technique or poor choice of product for a particular indication. If HA is present, then hyaluronidase is effective, and HA gel has been successfully removed 63 months post-treatment.21
Delayed onset nodules. Lumps or nodules that appear several months after the initial treatment might be amenable with hyaluronidase (Refer to ACE Group guidance on delayed onset nodules 22). It is important to remember that hyaluronidase is used to help diffuse fluids intradermally and for hypodermoclysis. To prevent potential dissemination of infection in inflammed nodules, it is important to prescribe antibiotics for one week before administering hyaluronidase.
Allergic or immunogenic reaction to the HA dermal filler. When an allergic, immunogenic, or sensitivity reaction occurs and does not settle on its own within an acceptable amount of time following a short course of antihistamines or systemic corticosteroids, removal of the filler with hyaluronidase is appropriate. If the reaction is considered moderate or severe, oral corticosteroids should be taken before hyaluronidase use to manage or prevent the potential initial worsening of symptoms due to the increase in antigens as the HA is broken down.
STORAGE AND RECONSTITUTION
It is recommended that hyaluronidase be stored at cool temperatures (2–8˚C, 35–46˚F) to maintain the quality of the product over a long period of time. If stored at room temperature (25˚C, 77˚F), the stability is only guaranteed for 12 months. Once the ampoule is opened, hyaluronidase should be used immediately and any unused contents discarded (Hyalase® SPC).
Hyaluronidase may be reconstituted with either saline or water for injection (Hyalase SPC). Saline is less painful on injection and is recommended for this reason. Although unlicensed for this purpose, bacteriostatic saline is often preferred for its additional anaesthetic properties. Although local anaesthetics may be used to reconstitute the product, as the enzymatic action of hyaluronidase can be affected by pH7, caution should be applied to the choice of diluent. There is little evidence to support the addition of local anaesthetic agents to hyaluronidase,18 and when combined, may lead to widespread, increased systemic absorption of anaesthetic and potential complications.
The volume of diluent used will depend on the indication and surface area to be treated and a range of 1 to 10mL has been evidenced in clinical practice and published papers. Larger volumes of dilution are recommended when smaller amounts of Hyalase are required to allow more precise dosing. Smaller volumes should be used in the case of vascular occlusion or when large volumes of dissolution are required to allow a higher concentration of Hyalase in a smaller area. Once the volume of diluent has been chosen, add 1mL of diluent to the opened ampoule of Hyalase and ensure the powder is fully dissolved by drawing up and expelling the syringe a couple of times. Aspirate the 1mL of saline with the reconstituted Hyalase, adding this to the remaining diluent. Agitate the solution to ensure the Hyalase is mixed throughout the whole volume. The reconstituted solution can now be drawn up in a syringe and injected where needed. The formula for the number of units to be injected can be found in Figure 1.
FIGURE 1.

Formula
DOSAGES OF HYALURONIDASE
Hyaluronidase may degrade the body’s natural HA in preference to foreign HA filler that has been injected and specifically cross-linked to prevent its natural breakdown.14 The dosage required is dependent on several factors relating to the HA filler; whether it is particulate or non-particulate, the amount of cross-linking, and the concentration of HA.23 Different HA fillers have differing physical properties that influence their degradation by hyaluronidase in a time- and dose-dependent manner. A study by Rao et al24 demonstrated Restylane® (Galderma Laboratories, Lausanne, Switzerland) dissipated most and Belotero® (Merz Pharmaceuticals, Raleigh, North Carolina) least.25 However, a more recent study has shown that Belotero was the fastest to dissolve and Juvederm Voluma® (Allergan, Dublin, Ireland) and Restylane® Lyft were the slowest, with the authors concluding that a high concentration of HA, larger particle size, and increased cross-linking increases the durability of the filler.17
The literature offers examples of widely divergent doses; however, it is recommended to inject as much hyaluronidase as required to obtain the desired effect rather than following an absolute dosage.14
Dosages for all indications except vascular occlusion. Although the amount injected should be titrated to clinical effect,14 Table 1 offers a guide to actual dosages used in published articles.
TABLE 1.
Aesthetic Complications Group Emergency Kit
A consensus opinion in the literature states five units of hyaluronidase is needed to break down 0.1mL of 20mg/mL HA,10 although there is quite a range. In one instance, Woodward et al25 recommend 30 units to dissolve 0.1mL. A further study showed no statistical difference between the use of 20 or 40 units of hyaluronidase in degrading 0.2mL (4 to 6mg of HA) of various fillers.23
Treatment results may be assessed from 48 hours4 and may be repeated at intervals of 48 hours or longer. The degree of further treatment will depend upon indication, risks versus benefits, side effects from treatment, and patient and practitioner satisfaction.
Dosages for vascular occlusion. In the event of a suspected vascular obstruction, a high dose pulsed protocol should be adopted.31 Large volume of hyaluronidase (450–1500 units) should be infiltrated over the entire area including the course of the vessel.4,14,32 Perivascular hyaluronidase will permeate vascular walls.4,33 Massage the area to promote diffusion and mechanical breakdown. Observe and reassess capillary refill after 60 minutes; if there is still vascular compromise, repeat treatment at hourly intervals for up to four cycles.34 The patient should be kept under observation in clinic for any adverse reactions and provided with written aftercare and advice. When anaphylaxis occurs, it is usually within minutes, but there have been cases where there has been a delayed onset. All patients should be given appropriate aftercare advice, warned about the symptoms of an allergic or anaphylactic response, and instructed to seek appropriate medical attention. Daily follow up should occur until there is satisfactory resolution.
Vascular occlusion is often immediate; however, the Aesthetic Complications Expert group have found many reported cases when the symptoms of ischaemia start several hours or even days later. This may be due to the dermal filler being intravascular but trapped at a bifurcation or branch point, only to dislodge at a later point to cause an occlusion.33 Alternatively, if the venous return is compromised by secondary swelling following injection of hydrophilic dermal filler, this can cause increased pressure in the arterial tree and a reduction in tissue perfusion.
INTRADERMAL PATCH TESTING
A test patch should be performed except when the indication is for vascular compromise and a delay could result in further harm to the patient.35 An intradermal injection of 4 to 8 units of hyaluronidase in the forearm and observing the results after 30 minutes has been advocated.36 However, it is recommended that a higher test dose of 20 units of hyaluronidase is used, as a positive reaction at lower doses might not be recognised.37 A positive reaction is identified by a weal and itching observed at the injection site, minor inflammation, and erythema.
DRUG INTERACTIONS
The most common interactions occur with furosemide, benzodiazepines, phenytoin, dopamine, and α-adrenergic agonists, so it is important to obtain a medical history. Although interactions are not particularly significant, it is best to avoid them if possible. Several drugs act as antagonists to hyaluronidase, including anti-inflammatory drugs (such as ibuprofen, aspirin, diclofenac), anti-histamines, mast cell stabilisers, Vitamin C, flavonoids, and anti-oxidants.3 Higher doses or repeated treatments may be required with concomitant use of these medicines.32 Where possible, patients should be advised to stop taking non-prescribed medication in advance of treatment.
ADMINISTRATION
Prior to injection, the area should be inspected, palpated, and marked out if needed. The area should be cleansed and disinfected using an appropriate skin solution and the procedure should be carried out using an aseptic technique. A 27G or 30G needle with an appropriate length to treat the depth of the area should be used. Administration should be accurate and limited to the affected area. Depth may be difficult to assess on palpation; therefore injections should cover the upper and lower borders of the product that has been injected.
Nodules, and product that has been injected into the superficial dermis should be injected directly, injections should be placed immediately into and below the product.38 [[Not sure what the previous sentence is trying to convey]] For vascular compromise, serial puncture should be used to inject hyaluronidase along the course of the vessel and cover the affected area.4 The needle should be perpendicular to the skin and several injections are often necessary.
During and after the procedure, the treated area should be massaged vigorously to optimise the result and aid mechanical breakdown. Due to the spreading effect of hyaluronidase, treatment should not be performed in an area where botulinum toxin treatment has been performed within the last 48 hours or on an area of infected skin, unless there is a vascular occlusion and the risks outweigh the benefits.
FOLLOW-UP
Results are often seen almost immediately; although for denser, more cross-linked products, it may take 48 hours for the effects to be seen. Consent should be obtained for the practitioner to inform the patient’s General Practitioner (See Appendix 1 for an example of a patient consent form). A review appointment should be offered at this point, along with further treatment if needed.
Following administration of hyaluronidase, the patient should be observed for 60 minutes to ensure no adverse reactions occur. Aftercare instructions should be given. In the event of any delayed reaction to the treatment, the patient should be seen at the earliest opportunity.
COMPLICATIONS
Bruising and swelling post-treatment are common.15,39 The most serious complication following the administration of hyaluronidase is an allergic reaction. Depending on the area treated, different allergic responses have been described. Local reactions are by far the most common, and according to clinical studies, occur at a rate of 0.05 to 0.69 percent.3 However, these figures are likely to be a little lower due to under reporting. Signs include edema, erythema, pain, and itching. Urticaria and angioedema have been reported in less than 0.1 percent of cases.40 Anaphylaxis has occurred with the use of hyaluronidase when high doses have been administered and with intravenous administration (refer to Aesthetic Complications Expert Group Anaphylaxis guidance). Type I (IgE mediated) and Type IV (mediated by T-cells) hypersensitivity reactions have occurred after hyaluronidase treatment. Following the use of hyaluronidase, the patient should be observed for 60 minutes in a clinical environment and given appropriate aftercare information (Appendix 2).
A history of allergic reaction to wasp or bee stings represents an increased risk of allergic reaction to hyaluronidase and should be considered as a relative contraindication, as the venom of stinging insects might contain hyaluronidase and this mechanism might be the source of sensitization in affected individuals.14,41,42 Unless there is a past medical history of allergic reaction or anaphylaxis to hyaluronidase or insect bites, previous history of allergy seems unrelated for the administration of hyaluronidase and it can be safely performed.43
HYALURONIDASE EXPERT GROUP: Martyn King, MD; Emma Davies, RN, NIP; Sharon King, RN, NIP; Cormac Convery, MD; Lee Walker, MD.
HYALURONIDASE CONSENSUS GROUP: Helena Collier, RGN, NIP; Ben Coyle, MD; Sam Robson, MD; Tamie Shoaib, Patrick Treacey, MD.
Appendix
Appendix 1: Consent for treatment with Hyalase® to dissolve hyaluronic acid dermal fillers
Hyaluronic acid (HA) fillers are sterile gels consisting of non-animal stabilised hyaluronic acid for injection into the skin to correct facial lines, wrinkles and folds, for lip enhancement and for shaping facial contours.
Occasionally these fillers need to be dissolved when the aesthetic treatment has not produced the desired outcome or there is a possibility of vascular occlusion or impending necrosis (tissue death) which could lead to compromise of healthy tissue.
Hyalase® (hyaluronidase 1500 units) has an off-license use in aesthetic medicine and except in the case of emergency administration requires the patient to undergo a skin patch test at least twenty minutes prior to the procedure being undertaken. The skin patch test is carried out by injecting Hyalase® into the subcutaneous tissue of the forearm and observed for signs of reaction (i.e. hives or wheals). If a positive patch test result is observed, treatment with Hyalase® cannot be carried out. Erythema or redness and slight vasodilation may be expected.
Hyalase® is an enzyme which breaks down hyaluronic acid fillers, but it can also break down naturally occurring hyaluronic acid present in the body, the results can be unpredictable and the effect dramatic. I understand that there will be loss of volume and there can be some skin laxity which in itself may not provide a good aesthetic result. Although some of the effects can be immediate, I understand that it can take up to 14 days for the final results to be seen and the treatment may need to be repeated.
Hyalase® administration can result in anaphylaxis (a severe allergic reaction which in itself is life threatening and requires immediate medical attention) and I understand this and have been given full counselling and the opportunity to discuss the treatment with Hyalase®, conservative treatment options or leaving the dermal filler to break down naturally which may take several months dependent on the type of filler used and the area treated.
The use of and the indications for the administration of Hyalase® have been explained to me by my practitioner and I have had the opportunity to have all questions answered to my satisfaction. After the treatment some other common injection-related reactions might occur. These reactions include redness, swelling, pain, itching, bruising and tenderness at the injection site. They have generally been described as mild to moderate and typically resolve spontaneously a few days after injection. Bruising may occasionally be more significant.
I acknowledge that I will have to remain at the clinic for ____ minutes after the procedure so that I can be observed by the medical staff and that I may need to return to the clinic ____ days/weeks after treatment to assess if further Hyalase® is to be administered.
I have answered the questions regarding my medical history to the best of my knowledge. I have also received the aftercare information and its contents have been explained to me and I will follow the advice given.
I consent to being treated with Hyalase®
Name Date
Signature Practitioner
Appendix 2: Hyalase® (Hyaluronidase) Injection Aftercare
Keep this aftercare leaflet safe and present it to the treating physician in the event of an adverse reaction
Hyalase® is an enzyme which breaks down hyaluronic acid fillers, but it can also break down naturally occurring hyaluronic acid present in the body. The results can be unpredictable and the effect dramatic with possible loss of volume and some skin laxity. Although some of the effects can be immediate, it can take up to 2 weeks for the final results to be seen and the procedure may need to be repeated.
Hyalase® administration can result in anaphylaxis (a severe allergic reaction) which in itself is life threatening and requires immediate medical attention. Symptoms of a severe allergic reaction can include shortness of breath, wheezing, coughing, difficulty swallowing, swelling of the tongue, eyelids, lips, hoarseness of the voice, stomach pain, nausea or diarrhoea.
If you have any of the above symptoms please report to your nearest Accident and Emergency Department or call 999 for an ambulance.
After the procedure some other common injection-related reactions might occur. These reactions include redness, swelling, pain, itching, bruising and tenderness at the injection site. They have generally been described as mild to moderate and typically resolve spontaneously after a few days after injection. Bruising may occasionally be more significant.
If you have any concerns following treatment, do not hesitate to contact us on <telephone number>. If this is outside of normal hours, please leave an answerphone message and we will normally get straight back to you.
I have been treated with _____ Units of Hyaluronidase (Hyalase®) reconstituted in ____ mls of Saline / Water (delete as applicable) to dissolve a hyaluronic acid dermal filler. A skin patch test was administered to the left/right (delete as applicable) forearm. No sign of an allergic reaction was noted and the procedure undertaken. Following injection, I was monitored for 60 minutes within the clinic.
Date of procedure: Amount administered:
Area treated:
REFERENCES
- 1.De Maio M, Rzany B. Injectable Fillers in Aesthetic Medicine. Springer Berlin Heidelberg. 2006 ISBN 3-540-23941-3. [Google Scholar]
- 2.Hirsch RJ, Brody HJ, Carruthers JDA. Hyaluronidase in the office: a necessity for every dermasurgeon that injects HA. J Cosmet and Laser Ther. 2007;9:182–185. doi: 10.1080/14764170701291674. [DOI] [PubMed] [Google Scholar]
- 3.Cavallini M, Gazzola R, Metalla M, Vaienti L. The role of hyaluronidase in the rreatment of complications from HA dermal fillers. Aesthet Surg J. 2013 Nov;33(8):1167–1174. doi: 10.1177/1090820X13511970. [DOI] [PubMed] [Google Scholar]
- 4.Buhren BA, Schrumpf H, Hoff NP, et al. Hyaluronidase: from clinical applications to molecular and cellular mechanisms. Eur J Med Res. 2016;21:5. doi: 10.1186/s40001-016-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.British National Formulary, 10.3 Drugs for the treatment of soft-tissue disorders and topical pain relief, 10.3.1 Enzymes, Hyaluronidase
- 6.Kim DW, Yoon ES, Ji YH, Park SH, Lee BI, Dhong ES. Vascular complications of HA fillers and the role of hyaluronidase in management. J Plast Reconstr Aesthet Surg. 2011;64:1590–1595. doi: 10.1016/j.bjps.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 7.DeLorenzi C. Complications of injectable fillers, part I. Aesthet Surg J. 2013;33:561–75. doi: 10.1177/1090820X13484492. [DOI] [PubMed] [Google Scholar]
- 8.Meyer K. Hyaluronidases. In: Boyer PD, editor. The Enzymes. New York, NY: Academic Press; 1971. pp. 307–320. [Google Scholar]
- 9.Andre P, Levy PM. Hyaluronidase offers an efficacious treatment for inaesthetic HA overcorrection. J Cosmet Dermatol. 2007;6(4):159–162. doi: 10.1111/j.1473-2165.2007.00332.x. [DOI] [PubMed] [Google Scholar]
- 10.Quezada-Gaón N, Wortsman X. Ultrasound-guided hyaluronidase injection in cosmetic complications. J Eur Acad Dermatol Venereol. 2016;30(10):e39–e40. doi: 10.1111/jdv.13286. [DOI] [PubMed] [Google Scholar]
- 11.Bailey SH, Fagien S, Rohrich RJ. Changing role of hyaluronidase in plastic surgery. Plast Reconstr Surg. 2014;133(2):127e–132e. doi: 10.1097/PRS.0b013e3182a4c282. [DOI] [PubMed] [Google Scholar]
- 12.Papakonstantinou E, Roth M, Karakiulakis G. HA: A key molecule in skin aging. Dermatoendocrinol. 2012;4(3):253–258. doi: 10.4161/derm.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King M. Management of necrosis. Aesthetic Complications Expert (ACE) Group guidelines. Oct, 2016. [Accessed 16 May 2018]. http://acegroup.online/wp-content/uploads/2016/01/Necrosis-v1.4.pdf
- 14.DeLorenzi C. Complications of injectable fillers, part 2: vascular complications. Aesthet Surg J. 2014;34(4):584–600. doi: 10.1177/1090820X14525035. [DOI] [PubMed] [Google Scholar]
- 15.Cavallini M, Gazzola R, Metalla M, Vaienti L. The role of hyaluronidase in the treatment of complications from HA dermal fillers. Aesthet Surg J. 2013;33(8):1167–1174. doi: 10.1177/1090820X13511970. [DOI] [PubMed] [Google Scholar]
- 16.Sun ZS, Zhu GZ, Wang HB, et al. Clinical outcomes of impending nasal skin necrosis related to nose and nasolabial fold augmentation with HA fillers. Plast Reconstr Surg. 2015;136(4):434e–441e. doi: 10.1097/PRS.0000000000001579. [DOI] [PubMed] [Google Scholar]
- 17.Kim DW, Yoon ES, Ji YH, Park SH, Lee BI, Dhong ES. Vascular complications of HA fillers and the role of hyaluronidase in management. J Plast Reconstr Aesthet Surg. 2011;64(12):1590–1595. doi: 10.1016/j.bjps.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Walker L, King M. Visual loss secondary to cosmetic filler injection. Aesthetic Complications Expert (ACE) Group guidelines. Oct, 2017. [Accessed 16 May 2018]. http://acegroup.online/wp-content/uploads/2016/01/ACE-Group-Blindness-v2.5.pdf [PMC free article] [PubMed]
- 19.17Carruthers JD, Fagien S, Rohrich RJ, Weinkle S, Carruthers A. Blindness caused by cosmetic filler injection: a review of cause and therapy. Plast Reconstr Surg. 2014;134(6):1197–1201. doi: 10.1097/PRS.0000000000000754. [DOI] [PubMed] [Google Scholar]
- 20.King M. Management of Tyndall effect. Aesthetic Complications Expert (ACE) Group guidelines. Jun, 2016. [Accessed 16 May 2018]. http://acegroup.online/wp-content/uploads/2016/01/Tyndalls-v1.2.pdf
- 21.United Kingdom Medicines and Healthcare Products Regulatory Agency. Hyaluronidase 1500 I.U. powder for solution for injection / infusion. Summary of product characteristics (SPC) Dec 12, 2014. [Accessed 16 May 2018]. http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1523594013492.pdf
- 22.Rzany B, Becker-Wegerich P, Bachmann F, Erdmann R, Wollina U. Hyaluronidase in the correction of HA-based fillers: a review and a recommendation for use. J Cosmet Dermatol. 2009;8(4):317–323. doi: 10.1111/j.1473-2165.2009.00462.x. [DOI] [PubMed] [Google Scholar]
- 23.Juhász MLW, Levin MK, Marmur ES. The kinetics of reversible HA filler injection treated with hyaluronidase. Dermatol Surg.. 2017;43:841–847. doi: 10.1097/DSS.0000000000001084. [DOI] [PubMed] [Google Scholar]
- 24.Rao V, Chi S, Woodward J. Reversing facial fillers: interactions between hyaluronidase and commercially available hyaluronic-acid based fillers. J Drugs Dermatol. 2014;13(9):1053–1056. [PubMed] [Google Scholar]
- 25.Woodward J, Khan T, Martin J. Facial filler complications. Facial Plast Surg Clin North Am. 2015;23(4):447–458. doi: 10.1016/j.fsc.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch RJ, Cohen JL, Carruthers JD. Successful management of an unusual presentation of impending necrosis following a HA injection embolus and a proposed algorithm for management with hyaluronidase. Dermatol Surg. 2007;33(3):357–360. doi: 10.1111/j.1524-4725.2007.33073.x. [DOI] [PubMed] [Google Scholar]
- 27.Cox SE. Clinical experience with filler complications. Dermatol Surg. 2009;35(suppl 2):1661–1666. doi: 10.1111/j.1524-4725.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- 28.Menon H, Thomas M, D’silva J. Low dose of hyaluronidase to treat over correction by HA filler-a case report. J Plast Reconstr Aesthet Surg. 2010;63(4):e416–e417. doi: 10.1016/j.bjps.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Menon H, Thomas M, D’silva J. Low dose of hyaluronidase to treat over correction of HA filler-a case report. J Plast Reconstr Aesthetic Surg. 2010;63:416–417. doi: 10.1016/j.bjps.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Van Dyke S, Hays GP, Caglia AE, Caglia M. Severe acute local reactions to a HA–derived dermal filler. J Clin Aesthetic Dermatol. 2010;3(5):32–35. [PMC free article] [PubMed] [Google Scholar]
- 31.DeLorenzi C. New high dose pulsed hyaluronidase protocol for HA filler vascular adverse events. Aesthet Surg J. 2017. doi: 10.1093/asj/sjw251. [DOI] [PubMed]
- 32.Landau M. Hyaluronidase caveats in treating filler complications. Dermatol Surg. 2015;41(Suppl 1):S347–S353.2. doi: 10.1097/DSS.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 33.DeLorenzi C. Transarterial degradation of HA filler by hyaluronidase. Dermatol Surg. 2014;40(8):832–841. doi: 10.1097/DSS.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 34.Cohen JL, Biesman BS, Dayan SH, et al. Treatment of HA filler-induced impending necrosis with hyaluronidase: consensus recommendations. Aesthet Surg J. 2015;35(7):844–849. doi: 10.1093/asj/sjv018. [DOI] [PubMed] [Google Scholar]
- 35.Andre P, Fléchet ML. Angioedema after ovine hyaluronidase injection for treating HA overcorrection. J Cosmet Dermatol. 2008;7(2):136–138. doi: 10.1111/j.1473-2165.2008.00377.x. [DOI] [PubMed] [Google Scholar]
- 36.Flynn T. Hyaluronidase. Body Language, Issue 44
- 37.Vartanian JA, Frankel AS, Rubin MG. Injected hyaluronidase reduces restylane-mediated cutaneous augmentation. Arch Facial Plast Surg. 2005;7:231–237. doi: 10.1001/archfaci.7.4.231. [DOI] [PubMed] [Google Scholar]
- 38.Rzany B, Becker-Wegerich P, Bachmann F, Erdmann R, Wollina U. Hyaluronidase in the correction of HA-based fillers: a review and a recommendation for use. J Cosmet Dermatol. 2009;8(4):317–323. doi: 10.1111/j.1473-2165.2009.00462.x. [DOI] [PubMed] [Google Scholar]
- 39.Yocum RC, Kennard D, Heiner LS. Assessment and implication of the allergic sensitivity to a single dose of recombinant human hyaluronidase injection: a double-blind, placebo-controlled clinical trial. J Infus Nurs. 2007;30(5):293–299. doi: 10.1097/01.NAN.0000292572.70387.17. [DOI] [PubMed] [Google Scholar]
- 40.Dunn AL, Heavner JE, Racz G, Day M. Hyaluronidase: A review of approved formulations, indications and off-label use in chronic pain management. Expert Opin Biol Ther. 2010;10(1):127–131. doi: 10.1517/14712590903490382. [DOI] [PubMed] [Google Scholar]
- 41.Cohen BE, Bashey S, Wysong A. The use of hyaluronidase in cosmetic dermatology: a review of the literature. J Clin Investigat Dermatol. 2015;3(2):7. [Google Scholar]
- 42.Lee A, Grummer SE, Kriegel D, Marmur E. Hyaluronidase. Dermatol Surg. 2010;36(7):1071–1077. doi: 10.1111/j.1524-4725.2010.01585.x. [DOI] [PubMed] [Google Scholar]
- 43.Szepfalusi Z, Nentwich I, Dobner M, Pillwein K, Urbanek R. IgE-mediated allergic reaction to hyaluronidase in paediatric oncological patients. Eur J Pediat. 1997;156(3):199–203. doi: 10.1007/s004310050582. [DOI] [PubMed] [Google Scholar]



