Abstract
Lignocellulose represents the most abundant source of carbon in the Earth. Thus, fraction technology of the biomass turns up as an emerging technology for the development of biorefineries. Saccharification and fermentation processes require the formulation of enzymatic cocktails or the development of microorganisms (naturally or genetically modified) with the appropriate toolbox to produce a cost‐effective fermentation technology. Therefore, the search for microorganisms capable of developing effective cellulose hydrolysis represents one of the main challenges in this era. Schizophyllum commune is an edible agarical with a great capability to secrete a myriad of hydrolytic enzymes such as xylanases and endoglucanases that are expressed in a high range of substrates. In addition, a large number of protein‐coding genes for glycoside hydrolases, oxidoreductases like laccases (Lacs; EC 1.10.3.2), as well as some sequences encoding for lytic polysaccharide monooxygenases (LPMOs) and expansins‐like proteins demonstrate the potential of this fungus to be applied in different biotechnological process. In this review, we focus on the enzymatic toolbox of S. commune at the genetic, transcriptomic, and proteomic level, as well as the requirements to be employed for fermentable sugars production in biorefineries. At the end the trend of its use in patent registration is also reviewed.
Keywords: biorefinery, biotechnology, lignocellulolytic enzymes, lignocellulose, Schizophyllum commune
1. INTRODUCTION
In the past decade, the amount of research related to lignocellulosic ethanol (second generation ethanol) has increased extensively in the scientific community. Several bacteria and fungi species have been studied in terms of the intra and extracellular enzymatic complexes involved in the deconstruction of the polymeric components that make up the lignocellulosic biomass. On the other hand, every year a novel or modified pretreatment technology becomes available with the aim of improving yields during the saccharification of the lignocellulosic materials. However, we are still far away from producing economically competitive lignocellulosic bioethanol (Mohanram, Amat, Choudhary, Arora, & Nain, 2013), largely because the lack of microbial enzymatic cocktails that break down the recalcitrant lignocellulosic biomass in an efficient manner (Gupta, 2016). Since the amount of plant biomass has been estimated to be of 180 billions of tons only above the ground and near 40 millions tons in the ocean (Chen, 2014), the exploitation of these materials for production of biofuels and value‐added products is a great alternative to reduce the fossil fuels dependence that as a society we have.
This review focuses on the unexploited and enormous biotechnological potential of the basidiomycete fungus Schizophyllum commune for the production of novel enzymes that could boost the biofuel and biomass derived product research. Also, this work summarizes the research that has been conducted in the last two decades and that supports the use of S. commune as a current aspirant for white, green and gray biotechnology applications.
2. GENERAL ASPECTS OF SCHIZOPHYLLUM COMMUNE
Schizophyllum commune is an agarical mushroom‐forming fungus, able to complete its life cycle in about 10 days and is one of the most commonly found fungi, whose distribution covers all continents with the exception of Antarctica (Ohm, de Jong, Lugones, et al., 2010). Schizophyllum commune has been successfully genetically modified and used as molecular tool for studying cell wall biogenesis (Wessels, 1986), hyphal fusion and development (Ahmad & Miles, 1970; Van Wetter, Schuren, Schuurs, & Wessels, 1996), mating type (Kothe, 1999; Yang, Shen, Park, Novotny, & Ullrich, 1995), heterologous expression of genes (Schuren & Wessels, 1998), gene deletions (De Jong, Ohm, De Bekker, Wösten, & Lugones, 2010; Ohm, de Jong, Berends, et al., 2010), among others. Although it has been detected causing illness in animals and humans, its lifestyle is mainly saprobic by causing white rot. Actually, it has been reported that at least 150 genera of woody plants are substrates for S. commune, but it also colonizes softwood and grass silage (Ohm, de Jong, Lugones, et al., 2010). This feature is one of the most interesting points in a biotechnological sense about this fungus, since it allows S. commune to colonize a vast diversity of lignocellulosic substrates, expanding the range of possibilities and biotechnological products (e.g., enzymes (phytase, lipase, holocellulase, etc.) (Arboleda Valencia et al., 2011; Salmon et al., 2012; Singh, Singh, Kumar, & Thakur, 2015), bioethanol (Horisawa, Ando, Ariga, & Sakuma, 2015), biosurfactants (Wessels, de Vries, Asgeirsdottir, & Springer, 1991), industrial cleaning‐in‐place (CiP) agents (Boyce & Walsh, 2012), polysaccharides (Singh, Kumar, & Thakur, 2017), polymers (Jayakumar, Kanth, Chandrasekaran, Raghava Rao, & Nair, 2010), etc.) that can be obtained with this microbe (Figure 1). As a matter of fact, S. commune has the potential to degrade all components of the lignocellulosic biomass, since its genome contain 240 gene candidates for glycoside hydrolases (89 account for plant polysaccharides degradation, see Figure 2), 75 for glycosyl transferases, 16 for polysaccharide lyases, 17 for expansin‐related proteins, 30 for carbohydrate esterases, and 16 for lignin‐degrading oxidoreductases (Ohm, de Jong, Lugones, et al., 2010). This extensive repertoire of plant cell wall degrading and modifying enzymes makes S. commune an outstanding candidate for studies regarding the mechanism by which this fungus degrades biomass in order to exploit its potential and improve the efficiency of industrial processes such as the lignocellulosic ethanol production, bioconversion of agricultural by‐products or biodegradation of xenobiotics and pollutants (Table 1).
Figure 1.
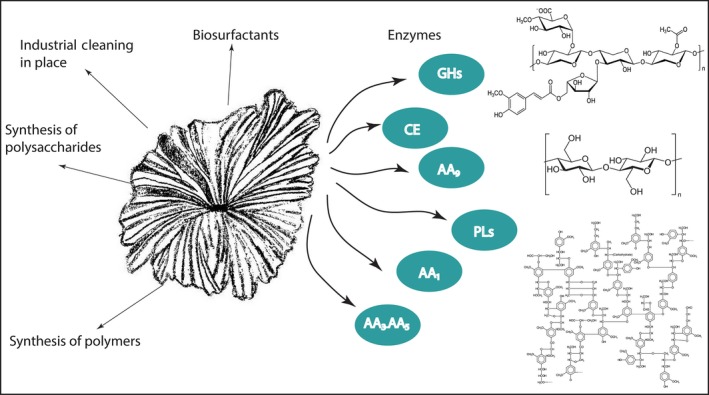
Biotechnological applications of Schyzophyllum commune. Glycoside hydrolase (GH), carbohydrate esterase (CE), glycosyltransferase (GT), polysaccharide lyase (PL), lytic polysaccharide monooxygenase (AA9), laccase (AA1), peroxide‐producing enzymes (AA3, and AA5)
Figure 2.
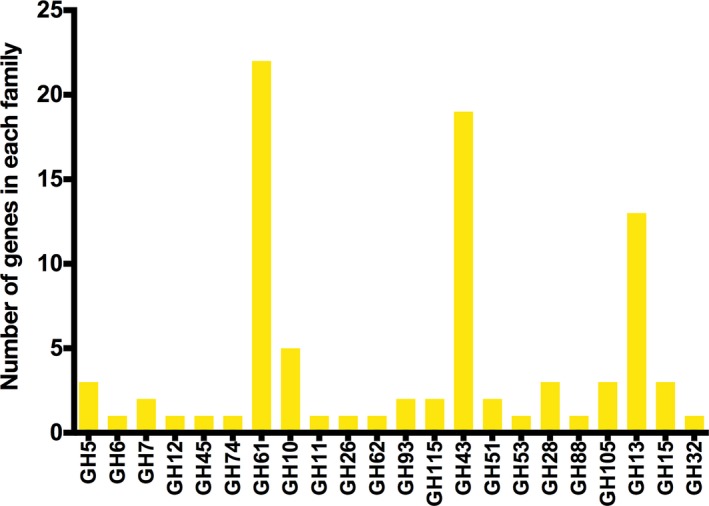
Glycoside hydrolase (GH) genes present in the genome of Schizophyllum commune. Only those involved in plant cell wall deconstruction were considered
Table 1.
Examples of biotechnological uses of Schizophyllum commune
| Biotechnological uses | References |
|---|---|
| Lipase production | Singh, Singh, Kumar, and Thakur (2014) |
| Phytase production | Salmon et al. (2012) |
| Lipase inmobilization for fatty acids methyl esters | Singh et al. (2015) |
| Decolorization of textile dyes | Asgher, Yasmeen, and Iqbal (2013) and Bhatti, Akram, and Asgher (2008) |
| Decolorization of Azo dyes and synthetic dyes | Tang, Jia, and Zhang (2011) and Yao, Jia, Zheng, and Wang (2013) |
| Biosorption of heavy metals | Amna, Bajwa, and Javaid (2010), Gabriel, Švec, Kolihová, Tlustoš, and Száková (2016), Javaid and Bajwa (2008) |
| Biotransformation of sophorocoside | Wu et al. (2012) |
| Direct ethanol production | Horisawa et al. (2015) |
| Holocellulase production | Arboleda Valencia et al. (2011) |
| Lignocellulose degradation | Asgher et al. (2016) |
| Phenolic compounds biosorption | Kumar and Min (2011) |
| Schizophyllan production | Kumari, Survase, and Singhal (2008) |
| Polysaccharide derived antimicrobials | Jayakumar et al. (2010) |
3. PROTEINS INVOLVED IN CELLULOSE DECONSTRUCTION BY SCHIZOPHYLLUM COMMUNE
The subject of cellulose deconstruction by fungi leads us to think immediately of organisms like Trichoderma reesei, Neurospora crassa, and various Aspergillus species, considering the ascomycetes group, and mainly in Phanerochaete chrysosporium when referring to the basidiomycetes group, leaving out of study a significant group of basidiomycetes with the same or even greater potential to degrade cellulose. One of these basidiomycetes is the “split gill” fungus S. commune, whose genome sequence was published in 2010 (Ohm, de Jong, Lugones, et al., 2010). Its genome revealed that it contains 240 candidate genes belonging to glycoside hydrolases from different families, almost 80 more GH genes than those reported for P. chrysosporium.
The study of the hydrolytic machinery in organisms like S. commune is attractive mainly for the lignocellulosic biofuels industry, since the number of published works is still growing year by year in this subject, but is not limited to this area. Something remarkable is that the amount and diversity of published work related with the S. commune ′s cellulolytic system is scarce (Table 2) when compared with published work (in this area) of fungi like T. reesei, N. crassa or P. chrysosporium, despite the fact that studies such as those carried out by Arboleda Valencia et al. (2011), Lee et al. (2014), Zhu et al., (2016) have demonstrated that S. commune has an important potential in the lignocellulose bioconversion field, even exhibiting cellulolytic and xylanolytic activities comparable with those obtained using an enzymatic commercial preparation of Trichoderma longibranchiatum.
Table 2.
Cellulolytic and xylanolytic enzymes studied in S. commune
| Enzyme | Inducer substrate | References |
|---|---|---|
| β‐Glucosidase | Cellulose | Desrochers, Jurasek, and Paice (1981) |
| Endoglucanase β‐glucosidase | Thiocellobiose | Rho, Desrochers, and Jurasek (1982) |
| Xylanase | CMC | |
| Cellobiose | ||
| Xylan | ||
| Endoglucanase | Cellulose | Willick and Seligy (1985) |
| β‐Glucosidase | ||
| Xylanase | ||
| Endoglucanase | Unknown | Clarke and Adams (1987) |
| Xylanase | Avicel | Steiner, Lafferty, Gomes, and Esterbauer (1987) |
| Endoglucanase | ||
| Xylanase | Bacteria cellulose | Haltrich, Sebesta, and Steiner (1996) |
| Endoglucanase | Cellobiose | |
| Sophorose | ||
| Birchwood xylan | ||
| Acetylxylan esterase | Unknown | Biely et al. (1996) |
| Cellulase GH5 | Unknown | Clarke, Drummelsmith, and Yaguchi (1997) |
| α‐Glucuronidase | Cellulose | Tenkanen and Siika‐Aho (2000) |
| Wheat bran | ||
| Distiller′s spent grain | ||
| Xylanase | Cellulose | Kolenová, Vršanská, and Biely (2005) |
| Glucuronyl esterase | Cellulose | Špániková and Biely (2006) |
| Xylanase | Bamboo fibers | Arboleda Valencia et al. (2011) |
| Mannanase | Banana stem | |
| Polygalacturonase | Sugarcane bagasse | |
| Endoglucanase | ||
| Fpase | ||
| Avicelase | ||
| α‐Glucuronidase | Recombinant | Chong et al. (2011) |
| Xylanase | Cellulose | Tsujiyama and Ueno (2011) |
| CMCase | Rice straw | |
| β‐Glucosidase | Wood | |
| Acetylesterase | ||
| Cinnamic acid esterase | ||
| β‐Glucosidase | Cellulose | Lee et al. (2014) |
| Avicelase | Avicel | Luziatelli et al. (2014) |
| FPase | Tamarix leaves | |
| β‐Glucosidase | ||
| α‐Amylase | ||
| Expansin | Recombinant | Tovar‐Herrera et al., (2015) |
| Endoglucanase | Jerusalem artichoke stalks | Zhu et al. (2016) |
| Cellobiohydrolase | ||
| β‐Glucosidase | ||
| α‐Glucuronidase | Recombinant | McKee et al. (2016) |
| β‐Glucosidase | Cellulose | Lee et al. (2017) |
| Feruloyl esterase | Recombinant | Nieter, Kelle, Linke, and Berger (2016) |
The cellulose degradation mechanisms by ascomycetes and basidiomycetes have been revised by Glass, Schmoll, Cate, and Coradetti (2013) and Baldrian and Valásková (2008). However, although the role of the classic enzymes involved in cellulose deconstruction such as endoglucanases, cellobiohydrolases, cellobiose dehydrogenases and beta glucosidases is well documented in fungi, the role of the termed “amorphogenic proteins” or plant cell wall remodeling proteins (expansins and expansin‐related proteins) in cellulose deconstruction is not well understood in both, ascomycetes and basidiomycetes. These amorphogenic proteins cause swelling of cellulose fibers and fragmentation of cellulose aggregations at the beginning of the enzymatic hydrolysis of cellulose before any detectable amount of reducing sugars is released (Gourlay et al., 2013). From these latter proteins, the swollenin from T. reesei is the most studied, and after its discovering it was suggested as the C 1 factor of the cellulose enzymatic degradation mechanism originally proposed by Mandels and Reese (1999) and Reese, Siu, and Levinson (1950). Nevertheless, that hypothesis has been rejected by the work of Eibinger et al. (2016), who demonstrates that swollenin is not an amorphogenesis factor when acting on pure cellulose. Nonetheless, the possibility that one or more of these plant cell wall remodeling proteins may be acting as the C1 factor is yet to be proven. Indeed, the genome of S. commune contains at least 17 expansin‐related proteins, one of which has already been cloned and expressed in Pichia pastoris, showing a 23% increment in avicel hydrolysis when used as pretreatment before the addition of a cellulase mixture (Tovar‐Herrera et al., 2015). However, the study of this type of proteins is relatively new in microbes, and there is a lot of information yet to be obtained from them.
Another group of proteins with great importance and also involved in biomass deconstruction is the group of enzymes known as lytic polysaccharide monooxygenases (LPMOs) classified in auxiliary activity families 9 (AA9), 10 (AA10), 11 (AA11), and 13 (AA13) in the CAZy database (Frandsen et al., 2016; Frommhagen et al., 2015; Hemsworth, Henrissat, Davies, & Walton, 2013; Silveira & Skaf, 2016; Vaaje‐Kolstad et al., 2010). From these families, AA9 corresponds to fungal proteins involved in cellulose deconstruction (some of them are also active in hemicellulose), while AA10 belongs to a bacterial group of LMPOs active on cellulose and chitin, and AA11 and AA13 are fungal proteins active on chitin and starch, respectively. AA9 proteins have been studied in N. crassa (Tian et al., 2009), T. reesei (Tanghe et al., 2015), P. chrysosporium (Westereng et al., 2011), Chaetominium globosum (Kim et al., 2015) and Myceliophthora thermophile (Frommhagen et al., 2015), and have been reported to improve the release of glucose and oligosaccharides from avicel, regenerating amorphous cellulose and lignocellulosic substrates even at a level of 150 fold increase (Frommhagen et al., 2015).
Three recent works have reported the presence of AA9 proteins in the secretomes of S. commune when cultured in avicel (one protein) (Sornlake et al., 2017), Jerusalem artichoke stalks (nine proteins) (Zhu et al., 2016), and Leucaena leucocephala wood chips (Singh et al., 2017) (3 proteins). This fact indicates that similar to the classic hydrolytic cellulase system, the expression of AA9 proteins in fungi is dependent on the substrate. Further analyses are necessary to evaluate the biochemical features and the position of the oxidative cleavages (C1 oxidation, C4 oxidation, or both) performed by these enzymes, since understanding the action mechanism of fungal LPMOs and gaining information about the transcriptional regulation of LPMO genes in fungi and bacteria would help to decipher how microbes fully deconstruct lignocellulosic biomass.
4. PROTEINS INVOLVED IN HEMICELLULOSE DECONSTRUCTION BY SCHIZOPHYLLUM COMMUNE
Hemicelluloses are heteropolysaccharides from the plant cell walls that constitute the second most abundant component of lignocellulosic biomass. Their complex structure is dependent on the source and mainly contains pentoses (xylose and arabinose), hexoses (glucose, galactose, and mannose) and, to a lesser extent, glucuronic and galacturonic acid. The bioconversion of hemicellulose to obtain ethanol or other value‐added products, such as chemicals and biopolymers, is a well‐researched topic. Through a pretreatment process, the hemicelluloses are degraded or broken down in the biomass, releasing fermentable sugars such as xylose, arabinose and glucose, and rendering the cellulose more accessible to cellulolytic enzymes (Lavarack, Griffin, & Rodman, 2002). Pretreatment of hemicelullose (usually chemically treated) is one of the most expensive steps of biomass processing, thus, studies to decrease the cost are of main interest from an economic point of view (Canam, Town, Iroba, Tabil, & Dumonceaux, 2013). The poor sustainability of the currently used acid/base‐catalyzed processes has highlighted the need for a more environmentally friendly and mild pretreatment of the hemicellulosic biomass, such as biological ones, that also encompasses a high efficiency (Canam et al., 2013). Another disadvantage of chemical processes is that byproducts may potentially act as microbial inhibitors during the subsequent fermentation steps (Peng, Peng, Xu, & Sun, 2012). Therefore, enzymatic pretreatment and bioconversion have arisen as a suitable alternative that could be coupled to subsequent fermentation and might enhance the industrial processing of biomass. The main drawback of enzymatic processing is that high efficiency has not been achieved to date. New enzyme cocktails that can increase the yields of fermentable products and other value‐added chemicals are currently under study (Zhu et al., 2016).
Hemicellulases are a generic family of proteins that catalyze the degradation of hemicellulosic polymers from which xylanases have been intensely researched. Xylan is the most abundant type of hemicellulose found on hardwoods and its structure is mainly (1→4)‐linked β‐d‐xylopyranosyl residues that are substituted with glucuronosyl and 4‐O‐methylglucuronosyl residues by α‐(1→2) linkages. Other substituent like acetyl, feruloyl, coumaroyl groups and α‐l‐arabinofuranose can also be of relative importance to produce the complete breakdown of hemicellulose. Generally, xylanases refer to a large group of enzymes comprising endo‐1, 4‐β‐xylanase (EC 3.2.1.8) and β‐xylosidase (EC 3.2.1.37), and several accessory enzymes with debranching activity (Peng et al., 2012). Endo‐xylanases degrade xylan at internal sites, producing xylooligosaccharides of varying length. Complementary, β‐xylosidase removes xylose residues from the end of these short oligosaccharides. Esterases are among the most studied enzymes with debranching activity on hemicellulose. Acetylxylan esterase (EC 3.1.1.72) removes the O‐acetyl of acetyl xylan, while feruloyl and coumaroyl esterases (EC 3.1.1.73) hydrolyse the phenolic compounds linked to arabinofuranoside residues. α‐l‐Arabinofuranosidase (EC 3.2.1.55) and α‐d‐glucuronidase (EC 3.2.1.139) are also responsible for the cleavage of branching structures.
The reduced capacity of S. commune to degrade the lignin components from lignocellulose has been previously reported (Floudas et al., 2015; Horisawa et al., 2015; Zhu et al., 2016) in agreement with the lack of genes encoding class II peroxidases from the AA2 family (Ohm, de Jong, Lugones, et al., 2010). Interestingly, the main enzymatic activity detected in culture supernatants from S. commune grown in lignocellulosic substrates is hemicellulolytic (Zhu et al., 2016). Nevertheless, the production of xylanase activity in this fungus is under the regulatory control of cellulosic degradation byproducts (Haltrich & Steiner, 1994). Xylan or galactomannan do not induce xylanase or mannanase activities when provided as sole carbon source. Instead, cellulose, cellobiose, lactose, and l‐sorbose induce, altogether, xylanase, cellulase, as well as mannanase activities indicating a common regulatory control in this fungus (Haltrich & Steiner, 1994).
The analysis of the genome of S. commune has shown that non‐cellulosic polysaccharide‐degrading enzymes are more abundant when compared to other model of lignocellulose decomposers (Ohm, de Jong, Lugones, et al., 2010). This fungus contains an extensive repertoire of xylan and pectin glycoside hydrolases as shown in Table 3, indicating a great potential for hemicellulose deconstruction. When compared with other basidiomycete fungi (the white‐rot Phanerochaete chrysosporium and Ceriporiopsis subvermispora and the brown‐rot Gloeophyllum trabeum), S. commune achieved the highest xylanase activity when growing on a lignocellulosic substrate (Zhu et al., 2016). Similarly, a crude enzymatic cocktail obtained from a solid‐state fermentation of S. commune was more effective than a commercial enzyme cocktail from Trichoderma longibrachiatum in terms of reducing sugar release from pretreated lignocellulosic biomass (Zhu et al., 2016). In this case, while cellulolytic activities where similar, the level of xylanases was significantly higher in the S. commune enzymatic cocktail.
Table 3.
Hemicelulose degrading glycoside hydrolases in the genome of S. commune (modified from (Ohm, de Jong, Lugones, et al., 2010))
| CAZyme family | No. Genes | Carbohydrate target | Enzyme name | No. enzymes |
|---|---|---|---|---|
| GH5 | 1 | Hemicellulose | β‐mannanase | 1 |
| GH10 | 5 | Hemicellulose | β‐1,4‐endoxylanase | 5 |
| GH11 | 1 | Hemicellulose | β‐1,4‐endoxylanase | 1 |
| GH26 | 1 | Hemicellulose | Glycosidase related | 1 |
| GH43 | 19 | Pectin + hemicellulose | Exo‐b‐1,3‐galactanase | 2 |
| α‐l‐arabinofuranosidases | 12 | |||
| Glycosidase related | 5 | |||
| GH51 | 2 | Pectin + hemicellulose | α‐l‐arabinofuranosidase | 2 |
| GH53 | 1 | Pectin + hemicellulose | Endo‐β‐1,4‐galactanase | 1 |
| GH62 | 1 | Hemicellulose | α‐l‐arabinofuranosidase | 1 |
| GH93 | 2 | Hemicellulose | Exo‐1,5‐α‐l‐arabinanase | 1 |
| Glycosidase related | 1 | |||
| GH115 | 2 | Hemicellulose | Xylan α‐1,2‐glucuronidase | 2 |
5. LIGNIN DEGRADING ENZYMES AND ALTERNATIVE BIOTECHNOLOGICAL APPLICATIONS OF SCHIZOPHYLLUM COMMUNE
In addition to the cellulases and xylanases studied in S. commune (Table 1), the lignin‐degrading enzymes of this fungus have also been evaluated for different biotechnological applications. According to the CAZy database, lignin‐degrading enzymes are grouped in some of the families with auxiliary activity. From these, S. commune produces only members of the AA1 (laccases; 2 genes), AA3 (cellobiose dehydrogenases: 1 gene; glucose oxidase: 4 genes; aryl alcohol oxidase: 1 gene; pyranose oxidase: 1 gene; alcohol oxidase: 1 gene) AA5 (glyoxal oxidase: 2 genes) and AA6 (benzoquinone reductase: 4 genes) families, lacking the production of lignin peroxidases (LiP), manganese peroxidases (MnP) and versatile peroxidases (VP), that belong to the AA2 family (Ohm, de Jong, Lugones, et al., 2010). Intriguingly, although S. commune does not produce MnP nor LiP as stated above, there are a variety of studies which mention that the Lip and MnP enzymes from S. commune are involved in the decolorization of azo and textile dyes or that the LiP and MnP from S. commune are useful enzymes for lignin removal of a variety of lignocellulosic substrates (Asgher, Wahab, Bilal, & Nasir Iqbal, 2016). It is likely that instead of MnP and LiP, the enzymes involved in the decolorization and delignification effects are members of the multi‐copper oxidases and the hydroxyl radical generation system, among others (Ohm, de Jong, Lugones, et al., 2010).
The evaluation of extracellular laccases from S. commune date to 1986 (De Vries, Kooistra, & Wessels, 1986). These enzymes are proteins with a great versatility, since they can oxidize a variety of organic and inorganic compounds, phenolic and non‐phenolic substrates, including, mono, di, polyphenols, aminophenols, and metoxyphenols (Upadhyay, Shrivastava, & Agrawal, 2016). Current laccase investigations are focused on bio‐oxidation and biotransformation processes, biosensor development, enzymatic synthesis of organic compounds, biopulping and biobleaching, textile dye transformation, removal of phenolic compounds from must and wine, waste effluent treatment, fossil fuel desulfurization, biosolubilization of coal, degradation of herbicides, food treatments, medicinal applications through the synthesis of novel compounds and delignification and biografting of lignocellulosics (Singh Arora & Kumar, 2010; Upadhyay et al., 2016). However, the search for industrial applications of S. commune ′s laccases is scarce, and is limited to dye decolorization experiments, the study of the three‐dimensional model of one of the laccases from S. commune and the activity related to delignification of lignocellulosic substrates. This lack of industrial applications of laccases from S. commune leaves open areas of studies to be exploited from various points of view.
6. PATENTS RELATED TO THE POTENTIAL OF SCHIZOPHYLLUM COMMUNE IN INDUSTRIAL APPLICATIONS
Patent databases (United States Patent Office (USPO), World Intellectual Property Organization (WIPO), European Patent Office (EPO), etc.) show an overview on technological and industrial state of the art as well as conceptual and methodological advances in the field of molecular biology and biotechnological applications (applied mycobiotechnology and myco‐remediation technology) of fungi.
In this context, there is an increase in the biotechnological significance of S. commnune in the last 15 years. Related to its genome, its enzymatic complexes and its biological versatility, more than 6,000 patent application documents and technological reports have been registered between 1995 and 2017 worldwide, which support S. commune as a biotechnologically functional microorganism, relevant in different technological, agricultural, environmental and pharmaceutical fields. At the EPO, Espacenet, more than 170 patent documents have been registered during 2000–2017, directly linked to technological and industrial applications of S. commune. The fields of innovation‐patentability‐biotechnology where the versatility of S. commune is currently being applied are summarized in Table 4.
Table 4.
Granted and applied patents related with S. commune
| Technical and industrial fields of the patents (applied for or granted) | Applicant(s) and year | References |
|---|---|---|
| Selective and oriented enzyme production and preparation | ||
| Preparation of glucoamylase | TAX ADM Agency (Japan, 1984) | Shimazaki and Sato (1984) |
| Production of bilirubin‐oxidase | Takara Shuzo Co. Ltd (Japan, 1986; 1984) | Matsui, Sato, and Nakajima (1986) and Susumu, Satou, and Takako (1984) |
| Production of cholesterol oxidase and its use in modification of natural occurring spirostanes | Toejepast Natuur Ondersoek, (Netherland, 1988); Ono Pharmaceutical Co., Ltd. (Osaka, Japan, 1977) | Kerkenar Anthonius inventor, NO voor TNO (1988) and Sugiura, Shimizu, Sugiyama, Kuratsu, and Hirata (1977) |
| Production of xyloglycan endo‐transglycosylases | Novozymes A/S (Europe, 2000) | Ilum (2000) |
| Production of cholesterol esterase | Toyobo Co. Ltd. (Japan, 1978) | Aisui, Nakagiri, and Otawara (1976) |
| Production of pantolactone hydrolase | Fuji Yakuhin Kogyo Kabushiki Kaisha (Japan, 1996) | Sakamoto, Yamada, and Shimizu (1996) |
| Production of xylanase and laccases for treatment of wood pulp and lignin decomposition | Mercian Corp. Japan Bioindustry Association Agency of Ind. Science & Technol (Japan, 2000); Clariant Finance (bvi) Limited Sandoz (Europe, 1997) | Behrendt, Blanchette, Farrell, and Iverson (1997) and Hitoshi, Watanabe, Yoshio, and Takeo (2000) |
| Production of thermostable xylanases | National Research Council of Canada (Canada, 2001) | Wing (2001) |
| Production of thermo‐resistant trehalose phosphorylase | Kureha Chem. Ind. Co. Ltd (Japan, 2004) | Eisaki, Eiichi, Yasutake, and Toshihiko (2004) |
| Multifunctional cellulases | Dyadic International (USA, 2013) Ltd. (USA); Novozymes A/S (2014). | Emalfarb et al., (2013) and Kuilderd, Wu, Li, and Zhou (2014) |
| Enzymatic complex with chlorogenic acid esterase activity and feruloyl esterase activity | Stern Enzym GmBH & Co. KG (Denmarck, 2014) | Nieter et al. (2016) |
| Obtaining and preparation of secondary metabolites and derivatives with great added value | ||
| Preparation and use of β‐glucans | Birch Stewart Kolasch & Birch (USA, 2009) | Kim, Park, and Sang‐Rin (2009) |
| Preparation of neoschizophyllan | Taito Co., Ltd. (Tokyo, Japan, 1978) & Kaken Chemical Co., Ltd. (Tokyo, Japan, 1978) | Kikumoto, Yamamoto, Komatsu, Kobayashi, and Kamasuka (1978) |
| Preparation of trehalose | Kureha Chem. Ind. Co. Ltd (Japan, 1994) | Takashi and Eisaku (1994) |
| Preparation of schizostatin | Sankyo Co. Ltd (Japan, 1995) | Yoshio, Kiyoshi, Tomoyuki, Tatsuo, and Takeshi (1995) |
| Preparation of stachyose | Infinitus (China, 2017) | Meng, Zhang, Zhou, Gao, and Duan (2017) |
| Obtention of ergothioneine | Mitsubishi Shoji Foodtech Co Ltd (Japan, 2015) | Tokumits (2015) |
| Preparation of schizophyllan | Ningbo Xinuoya Marine Biotechnology Co. Ltd (China. 2016) | Hui (2016) |
| Production of huperzine A | Univ. Fujian Traditional Chinese Medicine (China, 2014) | Yaxuan (2014) |
| Processes and prototypes | ||
| Cosmetic creams for topical use | MAX FUAKUTAA KK (Japan) | Fukada, Kobayashi, Matsuda, Kato, Toshinori, and Kojima (1993) |
| Oxidative dyeing process of keratin fibers | Casalonga Axel Bureau (Europe, 2002) | Gregory (2002) |
| Endoglucanase treatment of lignocellulosic materials and selective degradation of resin acids and triterpenes | Novozymes, A/S (USA, 2002) | Schülein et al. (2002) |
| Production of II generation biofuels from vegetable biomass via cellulolytic enzymes | IFP (France, 2009) | Margeot Antoine (2009) |
| Process for degradation of lignin and dioxin derivatives in field conditions | Idemitsu Kosan Co. Ltd (Japan, 2001) | Yuki Junishiro (2001) |
| Process for degradation of exogenous endocrine disruptors | Idemitsu Kosan Co. Ltd. (Japan, 2002) | Genshi and Takahiro (2002) |
| Process for decomposition of prions | Kondo Ryuichiro (Japan, 2005) | Ryuichiro, Yuli, and Shiro (2005) |
| Process for production of alcohol or second generation solvent | IFP Énergies Nouvelles (France, 2012) | Ropars, Aymard, Guillaume, and Menir (2012) |
| Design of immunological cancer therapies | Therapy Co. Ltd (Japan, 2000) | Akiyuni and Takashi (2000) |
| Process for selective removal of hexenuronic groups from biomass | Siika‐aho, Matti (USA, 2004) | Siika‐Aho et al. (2004) |
| Mycelial extracts formulations for potentiating the resistance of bee colonies against fungal‐viral collapse syndrome | Paul Stamets and Co. (USA, 2015, 2017) | Stamets (2015, 2017) |
| Biological saccharification method using biomass | PHYGEN Inc (Korea, 2016) | Kul et al. (2017) |
| Nutritional additives | ||
| Enhance immunity of lobster | Dingyuan County Profess. Coop. (China, 2016) | Guanghong (2016) |
| Milk cow forage | Xuzhou Jiwang Xintuo Animal Husbandry Co. Ltd. (China, 2016) | Xiume (2016) |
In the field of lignocellulosic biomass, more than 1,100 patents (altogether applied and granted) related to the use of S. commune were reported in the last two decades (Gupta, 2016), including biofuel′s production and biomass derivatives. As stated before, it is recognized that economic utilization of widely distributed lignocellulosic biomass as a feedstock for the eco‐sustainable production of biocarburants, biodiesel, molecular scaffolds, biomaterials, fuels, and chemicals with high‐added value would represent a conceptual and methodological change in the strategic utilization of natural raw materials, allowing sustainable resources to be substituted for, and compete with, petroleum‐based products.
In other research areas, S. commune has also been a subject of interest. For example, a nematicidal and bacteriostatic fumigant formulation has been prepared from an S. commune strain where the main bioactive component is β‐bisabolol. This composition is environmentally friendly and shows a very wide spectrum of action (Kaiyin, 2017). Cozen Co. Ltd reported a hot water‐extracted thrombotic dissolving enzyme (9–10 kDa) from S. commune fruiting bodies, capable of being used effectively as health supplement food or a treatment agent for thrombus‐related disease (Choi Nack Shick, 2015).
The patent database study (EPO base, 150–170 documents from 1990 to 2017) reveals that the main technological‐industrial application fields for S. commune in the last two decades were: nutritional additives for humans and animals with economic significance; agricultural biotechnology; pharmaceutical and cosmetic industry; generation of secondary metabolites with great‐added value; biotechnological application of enzyme complexes; biomass processing and bio‐refineries; and environmental issues. Some results are shown in Figure 3.
Figure 3.
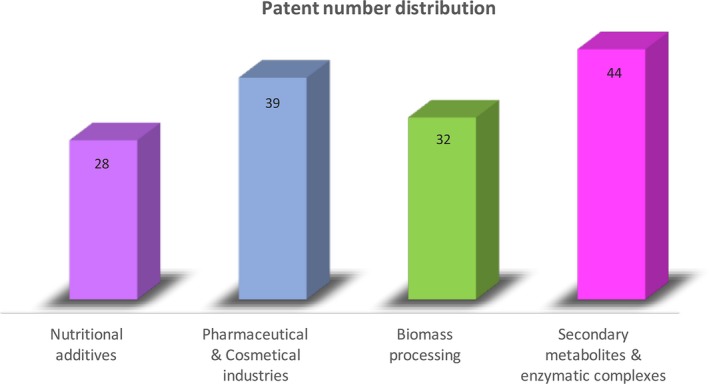
Europe Patent Office patents (1990–2017). Distribution by technological application fields
Taking this information into account, it must be highlighted that nutritional additives (nutrient feed, fermented functional beverages, forage, healthcare formulations, tonifying compositions, etc.) correspond to 20% of the overall patents reported for that period. In the case of the pharma‐cosmetic field (bioactive antibacterial‐ nematocide components, pharma compositions, extracts with selective pharmaceutical properties, anticancer and antiviral formulations, skin treatment creams, ophthalmic solutions, bio‐adhesives, anti‐oxidant and anti‐wrinkle formulations, nano‐liposomes, etc.) it corresponds to 27%. Regarding to biomass bioprocessing and related processes (bio‐pretreatment of agro‐wastes, biological saccharification, gelatin production, obtention of biofuels and bio‐derivatives at the bio‐refinery level, generation of enzymatic complexes for treatment of lignocellulosic materials and wastes, functional biofibers and bio‐oligomers, solid fermentation, pith and lignin degradation, bio‐oriented decomposition, etc.) the patents correspond to 22%, and, in the field of applied secondary metabolites, with great‐added value, and utilization of enzymatic complexes (laccases, cellulases, xylanases, esterases, oxidases, production of glucans and polysaccharides with different molecular weights, ergothioneine, schizophyllan, glucosone, xylitols, trehalose, pantolactone, retinoids, organic acids, etc.), the patents number account for 31% of the total. It is noteworthy that the observed application‐development trends will be maintained in the next 2–5 years, which supports the biotechnological versatility and applicability of this basidiomycete.
7. CONCLUSIONS
Schizophyllum commune is a fungus that has a quite complete enzymatic set that can be used for diverse areas in the biotechnological field. Its genome description as well as the recently published works and patents related to this fungus, demonstrates part of the biotechnological potential that S. commune possess. This review is the first to concentrate most of the work that has been done with S. commune in the subject of plant biomass exploitation and the enzymes involved in its degradation, with a view to its future implementation in bio‐refineries, pollutant degradation, formulation of enzymatic cocktails, bioconversion of agricultural by‐products, as an example. Additionally, S. commune is a good source for hydrolytic, non‐hydrolytic and oxidative enzymes which can help to understand the processes by which this fungus is capable of using the carbohydrates and phenolic compounds in the vast diversity of woods it can colonize, since classical genetics and genetic engineering techniques are available for S. commune.
CONFLICT OF INTEREST
Authors declare that there are no conflicts of interest.
ACKNOWLEDGMENTS
We are thankful to the National Council for Science and Technology (CONACyT) since OETH received a scholarship during the elaboration of this work. We also thank the financial support received from SEP‐PRODEP‐UAEMOR‐PITC‐381. RABG received a postdoctoral fellowship from the Quebec Government.
Tovar‐Herrera OE, Martha‐Paz AM, Pérez‐LLano Y, et al. Schizophyllum commune: An unexploited source for lignocellulose degrading enzymes. MicrobiologyOpen. 2018;7:e637 10.1002/mbo3.637
REFERENCES
- Ahmad, S. , & Miles, P. (1970). Hyphal fusions in Schizophyllum commune. Effects of environmental and chemical factors. Mycologia, 62, 1008–1017. 10.2307/3757614 [DOI] [Google Scholar]
- Aisui, S. , Nakagiri, S. , & Otawara, S. 1976. Preparation of cholesterol esterase. Japan; JPS539391 A.
- Akiyuni, Y. , & Takashi, S. (2000). Anticancer Composition. WO 01/54724 A1.
- Amna, J. , Bajwa, R. , & Javaid, A. (2010). Biosorption of heavy metals using a dead macro fungus Schizophyllum commune fries: Evaluation of equilibrium and kinetic models. The Pakistan Journal of Botany, 42(3), 2105–2118. [Google Scholar]
- Arboleda Valencia, J. W. , Valencia Jiménez, A. , Gonçalves De Siqueira, F. , Dussan Medina, K. , Restrepo Franco, G. M. , Filho, E. X. F. , … Grossi‐de‐Sa, M. F. (2011). Holocellulase activity from Schizophyllum commune grown on bamboo: A comparison with different substrates. Current Microbiology, 63(6), 581–587. 10.1007/s00284-011-0023-1 [DOI] [PubMed] [Google Scholar]
- Asgher, M. , Wahab, A. , Bilal, M. , & Nasir Iqbal, H. M. (2016). Lignocellulose degradation and production of lignin modifying enzymes by Schizophyllum commune IBL‐06 in solid‐state fermentation. Biocatalysis and Agricultural Biotechnology, 6, 195–201. [Google Scholar]
- Asgher, M. , Yasmeen, Q. , & Iqbal, H. M. N. (2013). Enhanced decolorization of Solar brilliant red 80 textile dye by an indigenous white rot fungus Schizophyllum commune IBL‐06. Saudi Journal of Biological Sciences, 20(4), 347–352. 10.1016/j.sjbs.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldrian, P. , & Valásková, V. (2008). Degradation of cellulose by basidiomycetous fungi. FEMS Microbiology Reviews, 32(3), 501–521. 10.1111/j.1574-6976.2008.00106.x [DOI] [PubMed] [Google Scholar]
- Behrendt, C. , Blanchette, R. , Farrell, R. , & Iverson, S. (1997). Method for improving the efficiency of chemical pulping processes by pretreating wood or pulpwood with white rot fungi. WO/1997/013025 A1.
- Bhatti, H. N. , Akram, N. , & Asgher, M. (2008). Optimization of culture conditions for enhanced decolorization of cibacron red FN‐2BL by Schizophyllum commune IBL‐6. Applied Biochemistry and Biotechnology, 149(3), 255–264. 10.1007/s12010-007-8123-x [DOI] [PubMed] [Google Scholar]
- Biely, P. , Côté, G. L. , Kremnický, L. , Greene, R. V. , Dupont, C. , & Kluepfel, D. (1996). Substrate specificity and mode of action of acetylxylan esterase from Streptomyces lividans . FEBS Letters, 396(2–3), 257–260. 10.1016/0014-5793(96)01080-0 [DOI] [PubMed] [Google Scholar]
- Boyce, A. , & Walsh, G. (2012). Identification of fungal proteases potentially suitable for environmentally friendly cleaning‐in‐place in the dairy industry. Chemosphere, 88(2), 211–218. 10.1016/j.chemosphere.2012.03.022 [DOI] [PubMed] [Google Scholar]
- Canam, T. , Town, J. , Iroba, K. , Tabil, L. , & Dumonceaux, T . (2013). Sustainable degradation of lignocellulosic biomass ‐ techniques, applications and commercialization In Chandel A. K. & da Silva S. S. (Eds.), Sustainable degradation of lignocellulosic biomass ‐ techniques, applications and commercialization (pp. 207–247). New York: Intech. [Google Scholar]
- Chen, H . (2014). Biotechnology of lignocellulose. Theory and practice In Biotechnology of lignocellulose theory and practice (p. 510). 1st ed. Beijing: Springer Netherlands; 10.1007/978-94-007-6898-7 [DOI] [Google Scholar]
- Choi Nack Shick, K. Y. H . (2015). Thrombotic dissolving enzyme extracted from Schizophyllum commune. Europe; KR20150053332 A.
- Chong, S. L. , Battaglia, E. , Coutinho, P. M. , Henrissat, B. , Tenkanen, M. , & De Vries, R. P. (2011). The α‐glucuronidase Agu1 from Schizophyllum commune is a member of a novel glycoside hydrolase family (GH115). Applied Microbiology and Biotechnology, 90, 1323–1332. 10.1007/s00253-011-3157-y [DOI] [PubMed] [Google Scholar]
- Clarke, A. J. , & Adams, L. S. (1987). Irreversible inhibition of Schizophyllum commune cellulase by divalent transition metal ions. Biochimica et Biophysica Acta (BBA)‐Protein Structure and Molecular Enzymology, 916(2), 213–219. 10.1016/0167-4838(87)90111-7 [DOI] [Google Scholar]
- Clarke, A. J. , Drummelsmith, J. , & Yaguchi, M. (1997). Identification of the catalytic nucleophile in the cellulase from Schizophyllum commune and assignment of the enzyme to Family 5, subtype 5 of the glycosidases. FEBS Letters, 414(2), 359–361. [DOI] [PubMed] [Google Scholar]
- De Jong, J. F. , Ohm, R. A. , De Bekker, C. , Wösten, H. A. B. , & Lugones, L. G. (2010). Inactivation of ku80 in the mushroom‐forming fungus Schizophyllum commune increases the relative incidence of homologous recombination. FEMS Microbiology Letters, 310(1), 91–95. 10.1111/j.1574-6968.2010.02052.x [DOI] [PubMed] [Google Scholar]
- De Vries, O. M. , Kooistra, W. H. , & Wessels, J. G. H. (1986). Formation of an Extracellular Laccase by a Schizophyllum commune Dikaryon. Microbiology, 132(10), 2817–2826. 10.1099/00221287-132-10-2817 [DOI] [Google Scholar]
- Desrochers, M. , Jurasek, L. , & Paice, M. G. (1981). High Production of beta‐Glucosidase in Schizophyllum commune: Isolation of the Enzyme and Effect of the Culture Filtrate on Cellulose Hydrolysis. Applied and Environment Microbiology, 41(1), 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eibinger, M. , Sigl, K. , Sattelkow, J. , Ganner, T. , Ramoni, J. , Seiboth, B. , … Nidetzky, B. (2016). Functional characterization of the native swollenin from Trichoderma reesei: Study of its possible role as C1 factor of enzymatic lignocellulose conversion. Biotechnology for Biofuels, 9(1), 178 10.1186/s13068-016-0590-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisaki, T. , Eiichi, T. , Yasutake, S. , & Toshihiko, W. (2004). Trehalose phosphorylase and its production. Japan; JP3563104 B2.
- Emalfarb, M. , Sinitsyn, A. , Jundzil, R. , Wery, J. , Visser, J. , Joosten, R. , & Koetsier, M . (2013). Method for improving the activity of cellulose enzyme mixtures in the saccharification (lingo)cellulosic material. United States; US 2013/0280764 A1.
- Floudas, D. , Held, B. W. , Riley, R. , Nagy, L. G. , Koehler, G. , Ransdell, A. S. , … Hibbett, D. S. (2015). Evolution of novel wood decay mechanisms in Agaricales revealed by the genome sequences of Fistulina hepatica and Cylindrobasidium torrendii . Fungal Genetics and Biology, 76, 78–92. 10.1016/j.fgb.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen, K. E. H. , Simmons, T. J. , Dupree, P. , Poulsen, J.‐C. N. , Hemsworth, G. R. , Ciano, L. , … Walton, P. H. (2016). The molecular basis of polysaccharide cleavage by lytic polysaccharide monooxygenases. Nature Chemical Biology, 12(4), 298–303. 10.1038/nchembio.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommhagen, M. , Sforza, S. , Westphal, A. H. , Visser, J. , Hinz, S. W. A. , Koetsier, M. J. , … Kabel, M. A. (2015). Discovery of the combined oxidative cleavage of plant xylan and cellulose by a new fungal polysaccharide monooxygenase. Biotechnology for Biofuels, 8(1), 101 10.1186/s13068-015-0284-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada, J. , Kobayashi, S. , Matsuda, H. , Kato, J. , Toshinori, S. , & Kojima, A. (1993). Skin cosmetic containing culture mixture of suehirotake (3754/24) (Schizophyllum commune). Japan; JP05286843.
- Gabriel, J. , Švec, K. , Kolihová, D. , Tlustoš, P. , & Száková, J. (2016). Translocation of mercury from substrate to fruit bodies of Panellus stipticus, Psilocybe cubensis, Schizophyllum commune and Stropharia rugosoannulata on oat flakes. Ecotoxicology and Environmental Safety, 125, 184–189. 10.1016/j.ecoenv.2015.12.009 [DOI] [PubMed] [Google Scholar]
- Genshi, S. , & Takahiro, K. (2002). Method for decomposing exogenous endocrine disrupter. Japan; JP2002028692 A.
- Glass, N. L. , Schmoll, M. , Cate, J. H. D. , & Coradetti, S. (2013). Plant cell wall deconstruction by ascomycete fungi. Annual Review of Microbiology, 67, 477–498. 10.1146/annurev-micro-092611-150044 [DOI] [PubMed] [Google Scholar]
- Gourlay, K. , Hu, J. , Arantes, V. , Andberg, M. , Saloheimo, M. , Penttilä, M. , Saddler, J. (2013). Swollenin aids in the amorphogenesis step during the enzymatic hydrolysis of pretreated biomass. Bioresource Technology, 142, 498–503. 10.1016/j.biortech.2013.05.053 [DOI] [PubMed] [Google Scholar]
- Gregory, P . (2002). Oxidation dyeing methods using N‐acetylcysteine as a reducing agent and laccase as oxidizing agent. Europe; EP 1 165 026 B1.
- Guanghong, Z . (2016). Lobster feed capable of enhancing immunity. Europe; CN106616057 A.
- Gupta, V. K . (2016). Biofuel and biorefinery technologies In Gupta V. K. (Eds.), Biofuel and biorefinery technologies 3 (p. 347). Switzerland: Springer International Publishing. [Google Scholar]
- Haltrich, D. , Sebesta, B. , & Steiner, W . (1996). Induction of xylanase and cellulase in Schizophyllum commune In Enzymatic degradation of insoluble carbohydrates (pp. 19–305). US: American Chemical Society. [Google Scholar]
- Haltrich, D. , & Steiner, W. (1994). Formation of xylanase by Schizophyllum commune: Effect of medium components. Enyzme and Microbial Technology, 16(3), 229–235. 10.1016/0141-0229(94)90047-7 [DOI] [Google Scholar]
- Hemsworth, G. R. , Henrissat, B. , Davies, G. J. , & Walton, P. H. (2013). Discovery and characterization of a new family of lytic polysaccharide monooxygenases. Nature Chemical Biology, 10(2), 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitoshi, U. , Watanabe, J. , Yoshio, H. , & Takeo, Y. (2000). Lignin decomposition using laccase and lignin decomposition agent. Japan; JP2000064185 A.
- Horisawa, S. , Ando, H. , Ariga, O. , & Sakuma, Y. (2015). Direct ethanol production from cellulosic materials by consolidated biological processing using the wood rot fungus Schizophyllum commune . Bioresource Technology, 197, 37–41. 10.1016/j.biortech.2015.08.031 [DOI] [PubMed] [Google Scholar]
- Hui, T. J. Z . (2016). Screening and application of marine Schizophyllum commune strain. Europe; CN105886408 A.
- Ilum, N . (2000). Microbial xyloglycan endo‐ transglyco‐sylases. Europe; EP 1005536 B1.
- Javaid, A. , & Bajwa, R. (2008). Biosorption of electroplating heavy metals by some basidiomycetes. Mycopath, 6, 1–6. [Google Scholar]
- Jayakumar, G. C. , Kanth, S. V. , Chandrasekaran, B. , Raghava Rao, J. , & Nair, B. U. (2010). Preparation and antimicrobial activity of scleraldehyde from Schizophyllum commune . Carbohydrate Research, 345(15), 2213–2219. 10.1016/j.carres.2010.07.041 [DOI] [PubMed] [Google Scholar]
- Kaiyin, Y. D. W . (2017). Schizophyllum commune strain for generating volatile bacteriostatic and nematocidal active component and application thereof. Europe; CN106350459 A.
- Kerkenar Anthonius inventor, NO voor TNO . (1988). Process for preparing steroids. Europe; EP 0274147 A2.
- Kikumoto, S. , Yamamoto, O. , Komatsu, N. , Kobayashi, H. , & Kamasuka, T . (1978). Method of producing neoschizophyllan having novel pharmacological activity. United States; US4098661.
- Kim, I. J. , Nam, K. H. , Yun, E. J. , Kim, S. , Youn, H. J. , Lee, H. J. , … Kim, K. H. (2015). Optimization of synergism of a recombinant auxiliary activity 9 from Chaetomium globosum with cellulase in cellulose hydrolysis. Applied Microbiology and Biotechnology, 99(20), 8537–8547. 10.1007/s00253-015-6592-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M. S. , Park, Y. D. , & Sang‐Rin, L. E. E . (2009). Method of using beta‐glucan from Schizophyllum commune. United States; 20090023681 A1.
- Kolenová, K. , Vršanská, M. , & Biely, P. (2005). Purification and characterization of two minor endo‐β‐1,4‐xylanases of Schizophyllum commune . Enyzme and Microbial Technology, 36(7), 903–910. 10.1016/j.enzmictec.2005.01.006 [DOI] [Google Scholar]
- Kothe, E. (1999). Mating types and pheromone recognition in the Homobasidiomycete Schizophyllum commune . Fungal Genetics and Biology, 27(2–3), 146–152. 10.1006/fgbi.1999.1129 [DOI] [PubMed] [Google Scholar]
- Kuilderd, H. A. , Wu, G. , Li, H. , & Zhou, Q . (2014) Combining biopolishing & bleach clean‐up. United States; US 2014/0007357 A1.
- Kul, L. J. , Kim, S. , Sadashiv, S. , Cha, M. , Lee, J. , & Roh, H. (2017). Biological saccharification method using biomass from heavy metal contaminated soil purifying plant. Europe; KR20160114772 A.
- Kumar, N. S. , & Min, K. (2011). Phenolic compounds biosorption onto Schizophyllum commune fungus: FTIR analysis, kinetics and adsorption isotherms modeling. Chemical Engineering Journal, 168(2), 562–571. 10.1016/j.cej.2011.01.023 [DOI] [Google Scholar]
- Kumari, M. , Survase, S. A. , & Singhal, R. S. (2008). Production of schizophyllan using Schizophyllum commune NRCM. Bioresource Technology, 99(5), 1036–1043. 10.1016/j.biortech.2007.02.029 [DOI] [PubMed] [Google Scholar]
- Lavarack, B. P. , Griffin, G. J. , & Rodman, D. (2002). The acid hydrolysis of sugarcane bagasse hemicellulose to produce xylose, arabinose, glucose and other products. Biomass and Bioenergy., 23(5), 367–380. 10.1016/S0961-9534(02)00066-1 [DOI] [Google Scholar]
- Lee, Y. M. , Lee, H. , Heo, Y. M. , Lee, H. , Hong, J.‐H. , & Kim, J.‐J. (2017). Transcriptional analysis of genes encoding β‐glucosidase of Schizophyllum commune KUC9397 under optimal conditions. Folia Microbiologica, 62(3), 191–196. 10.1007/s12223-016-0484-5 [DOI] [PubMed] [Google Scholar]
- Lee, Y. M. , Lee, H. , Kim, J. S. , Lee, J. , Ahn, B. J. , Kim, G. H. , & Kim, J. J. (2014). Optimization of medium components for β‐glucosidase production in Schizophyllum commune KUC9397 and enzymatic hydrolysis of lignocellulosic biomass. BioResources, 9(3), 4358–4368. [Google Scholar]
- Luziatelli, F. , Crognale, S. , D'Annibale, A. , Moresi, M. , Petruccioli, M. , & Ruzzi, M. (2014). Screening, isolation, and characterization of glycosyl‐hydrolase‐producing fungi from desert halophyte plants. International Microbiology, 17(1), 41–48. [DOI] [PubMed] [Google Scholar]
- Mandels, M. , & Reese, E. T. (1999). Fungal cellulases and the microbial decomposition of cellulosic fabric. Journal of Industrial Microbiology and Biotechnology, 22(5), 225–240. 10.1038/sj.jim.2900635 [DOI] [Google Scholar]
- Margeot Antoine, M. F . (2009). Method for producing alcohol in a bio‐refinery environment. WO/2009/098365 A2.
- Matsui, S. , Sato, T. T. , & Nakajima, K . (1986). Production of bilirubin oxidase. United States; US4569912.
- McKee, L. S. , Sunner, H. , Anasontzis, G. E. , Toriz, G. , Gatenholm, P. , Bulone, V. , et al. (2016). A GH115 α‐glucuronidase from Schizophyllum commune contributes to the synergistic enzymatic deconstruction of softwood glucuronoarabinoxylan. Biotechnology for Biofuels, 9(1), 2 10.1186/s13068-015-0417-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, L. , Zhang, L. , Zhou, J. , Gao, X. , & Duan, J. (2017). Method of preparing stachyose. Europe; CN 106636250 A.
- Mohanram, S. , Amat, D. , Choudhary, J. , Arora, A. , & Nain, L. (2013). Novel perspectives for evolving enzyme cocktails for lignocellulose hydrolysis in biorefineries. Sustainable Chemical Processes., 1(1), 15. 10.1186/2043-7129-1-15 [DOI] [Google Scholar]
- Nieter, A. , Kelle, S. , Linke, D. , & Berger, R. G. (2016). Feruloyl esterases from Schizophyllum commune to treat food industry side‐streams. Bioresource Technology, 220, 38–46. 10.1016/j.biortech.2016.08.045 [DOI] [PubMed] [Google Scholar]
- Ohm, R. A. , de Jong, J. F. , Berends, E. , Wang, F. , Wösten, H. A. B. , & Lugones, L. G. (2010). An efficient gene deletion procedure for the mushroom‐forming basidiomycete Schizophyllum commune . World Journal of Microbiology & Biotechnology, 26(10), 1919–1923. 10.1007/s11274-010-0356-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohm, R. A. , de Jong, J. F. , Lugones, L. G. , Aerts, A. , Kothe, E. , Stajich, J. E. , & … Wösten, H. A. (2010). Genome sequence of the model mushroom Schizophyllum commune . Nature Biotechnology, 28(9), 957–963. 10.1038/nbt.1643 [DOI] [PubMed] [Google Scholar]
- Peng, F. , Peng, P. , Xu, F. , & Sun, R. C. (2012). Fractional purification and bioconversion of hemicelluloses. Biotechnology Advances, 30(4), 879–903. 10.1016/j.biotechadv.2012.01.018 [DOI] [PubMed] [Google Scholar]
- Reese, E. T. , Siu, R. G. , & Levinson, H. S. (1950). The biological degradation of soluble cellulose derivatives and its relationship to the mechanism of cellulose hydrolysis. Journal of Bacteriology, 59(4), 485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho, D. , Desrochers, M. , & Jurasek, L. (1982). Induction of cellulase in Schizophyllum commune: Thiocellobiose as a new inducer. Journal of Bacteriology., 43, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropars, M. , Aymard, C. , Guillaume, A. , & Menir, S . (2012). Method for producing alcohols and/or solvents from paper pulps with recycling of the non‐hydrolysated plant material in a regeneration reactor. Europe; EP 2516661B1.
- Ryuichiro, K. , Yuli, T. , & Shiro, M. (2005). Method for decomposition of prion with wood decaying Basidiomycete. Japan; JP2005287322 A.
- Sakamoto, K. , Yamada, H. , & Shimizu, S. (1996). D‐Pantolactone hydrolase and production thereof. Europe; EP 0504421 B1.
- Salmon, D. N. X. , Piva, L. C. , Binati, R. L. , Rodrigues, C. , Vandenberghe, L. P. D. S. , Soccol, C. R. , & Spier, M. R. (2012). A bioprocess for the production of phytase from Schizophyllum commune: Studies of its optimization, profile of fermentation parameters, characterization and stability. Bioprocess and Biosystems Engineering, 35(7), 1067–1079. 10.1007/s00449-012-0692-6 [DOI] [PubMed] [Google Scholar]
- Schülein, M ., et al. (2002). Endoglucanases. United States; 6387690 B1.
- Schuren, F. H. J. , & Wessels, J. G. H. (1998). Expression of heterologous genes in Schizophyllum commune is often hampered by the formation of truncated transcripts. Current Genetics, 33(2), 151–156. 10.1007/s002940050321 [DOI] [PubMed] [Google Scholar]
- Shimazaki, J. , & Sato, T. (1984). Preparation of glucoamylase by Schizophyllum commune Fr. Japan; JPS59159779 A.
- Siika‐Aho, M ., et al. (2004). Method and enzymatic preparation for treatment of cellulose pulps. United States; 20040069426 A1.
- Silveira, R. L. , & Skaf, M. S. (2016). Molecular dynamics of the Bacillus subtilis expansin EXLX1: Interaction with substrates and structural basis of the lack of activity of mutants. Physical Chemistry Chemical Physics: PCCP, 18, 3510–3521. 10.1039/C5CP06674C [DOI] [PubMed] [Google Scholar]
- Singh Arora, D. , & Kumar Sharma, R. (2010). Ligninolytic fungal laccases and their biotechnological applications. Applied Biochemistry and Biotechnology, 160(6), 1760–1788. 10.1007/s12010-009-8676-y [DOI] [PubMed] [Google Scholar]
- Singh, M. K. , Kumar, M. , & Thakur, I. S. (2017). Proteomic characterization and schizophyllan production by Schizophyllum commune ISTL04 cultured on Leucaena leucocephala wood under submerged fermentation. Bioresource Technology, 236, 29–36. 10.1016/j.biortech.2017.03.170 [DOI] [PubMed] [Google Scholar]
- Singh, M. K. , Singh, J. , Kumar, M. , & Thakur, I. S. (2014). Novel lipase from basidiomycetes Schizophyllum commune ISTL04, produced by solid state fermentation of Leucaena leucocephala seeds. Journal of Molecular Catalysis. B, Enzymatic, 110, 92–99. 10.1016/j.molcatb.2014.10.010 [DOI] [Google Scholar]
- Singh, J. , Singh, M. K. , Kumar, M. , & Thakur, I. S. (2015). Immobilized lipase from Schizophyllum commune ISTL04 for the production of fatty acids methyl esters from cyanobacterial oil. Bioresource Technology, 188, 214–218. 10.1016/j.biortech.2015.01.086 [DOI] [PubMed] [Google Scholar]
- Sornlake, W. , Rattanaphanjak, P. , Champreda, V. , Eurwilaichitr, L. , Kittisenachai, S. , Roytrakul, S. , … Inoue, H. (2017). Characterization of cellulolytic enzyme system of Schizophyllum commune mutant and evaluation of its efficiency on biomass hydrolysis. Bioscience, Biotechnology, and Biochemistry, 81(7), 1289–1299. 10.1080/09168451.2017.1320937 [DOI] [PubMed] [Google Scholar]
- Špániková, S. , & Biely, P. (2006). Glucuronoyl esterase ‐ Novel carbohydrate esterase produced by Schizophyllum commune . FEBS Letters, 580(19), 4597–4601. [DOI] [PubMed] [Google Scholar]
- Stamets, P . (2015). Integrative fungal solution for protecting bees. United States; US9474776 B2.
- Stamets, P . (2017). Integrative fungal solutions for protecting bees and overcoming colony collapse disorder (CCD). United States; 20170035820 A1.
- Steiner, W. , Lafferty, R. M. , Gomes, I. , & Esterbauer, H. (1987). Studies on a wild strain of Schizophyllum commune: Cellulase and xylanase production and formation of the extracellular polysaccharide Schizophyllan. Biotechnology and Bioengineering, 30(2), 169–178. 10.1002/(ISSN)1097-0290 [DOI] [PubMed] [Google Scholar]
- Sugiura, M. , Shimizu, H. , Sugiyama, M. , Kuratsu, T. , & Hirata, F . (1977).Process for producing cholesterol oxidase. United States; 4003794.
- Susumu, M. , Satou, M. , & Takako, N. K. (1984). Preparation of bilirubin oxidase. Japan; JPS59135886 A.
- Takashi, K. , & Eisaku, T. (1994). Production of trehalose. Japan; JPH06189779 A.
- Tang, W. , Jia, R. , & Zhang, D. (2011). Decolorization and degradation of synthetic dyes by Schizophyllum sp. F17 in a novel system. Desalination, 265(1–3), 22–27. 10.1016/j.desal.2010.07.024 [DOI] [Google Scholar]
- Tanghe, M. , Danneels, B. , Camattari, A. , Glieder, A. , Vandenberghe, I. , Devreese, B. , … Desmet, T. (2015). Recombinant Expression of Trichoderma reesei Cel61A in Pichia pastoris: Optimizing Yield and N‐terminal Processing. Molecular Biotechnology, 57(11–12), 1010–1017. 10.1007/s12033-015-9887-9 [DOI] [PubMed] [Google Scholar]
- Tenkanen, M. , & Siika‐Aho, M. (2000). An beta‐glucuronidase of Schizophyllum commune acting on polymeric xylan. Journal of Biotechnology, 78(2), 149–161. 10.1016/S0168-1656(99)00240-0 [DOI] [PubMed] [Google Scholar]
- Tian, C. , Beeson, W. T. , Iavarone, A. T. , Sun, J. , Marletta, M. A. , Cate, J. H. , & Glass, N. L. (2009). Systems analysis of plant cell wall degradation by the model filamentous fungus Neurospora crassa. Proceedings of the National Academy of Sciences, 106(52), 22157–22162. 10.1073/pnas.0906810106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumits, H. C. T. S. S. F. M . (2015). Ergothieneine‐containing composition. Europe; JP 2015116173 A.
- Tovar‐Herrera, O. E. , Batista‐García, R. A. , Sánchez‐Carbente, M. D. R. , Iracheta‐Cárdenas, M. M. , Arévalo‐Niño, K. , & Folch‐Mallol, J. L. (2015). A novel expansin protein from the white‐rot fungus Schizophyllum commune . PLoS ONE, 10(3), e0122296 10.1371/journal.pone.0122296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujiyama, S. , & Ueno, H. (2011). Production of cellulolytic enzymes containing cinnamic acid esterase from Schizophyllum commune . Journal of General and Applied Microbiology, 57(6), 309–317. 10.2323/jgam.57.309 [DOI] [PubMed] [Google Scholar]
- Upadhyay, P. , Shrivastava, R. , & Agrawal, P. K. (2016). Bioprospecting and biotechnological applications of fungal laccase. Biotechnology, 6(1), 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaaje‐Kolstad, G. , Westereng, B. , Horn, S. J. , Liu, Z. , Zhai, H. , Sorlie, M. , & Eijsink, V. G. H. (2010). An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science, 330(6001), 219–222. 10.1126/science.1192231 [DOI] [PubMed] [Google Scholar]
- Van Wetter, M. A. , Schuren, F. H. J. , Schuurs, T. A. , & Wessels, J. G. H. (1996). Targeted mutation of the SC3 hydrophobin gene of Schizophyllum commune affects formation of aerial hyphae. FEMS Microbiology Letters, 140(2–3), 265–269. 10.1111/j.1574-6968.1996.tb08347.x [DOI] [Google Scholar]
- Wessels, J. G. H. (1986). Cell wall synthesis in apical hyphal growth. International Review of Cytology, 104, 37–79. 10.1016/S0074-7696(08)61923-3 [DOI] [Google Scholar]
- Wessels, J. G. , de Vries, O. M. , Asgeirsdottir, S. A. , & Springer, J. (1991). The thn mutation of Schizophyllum commune, which suppresses formation of aerial hyphae, affects expression of the Sc3 hydrophobin gene. Journal of General Microbiology, 137(10), 2439–2445. [DOI] [PubMed] [Google Scholar]
- Westereng, B. , Ishida, T. , Vaaje‐Kolstad, G. , Wu, M. , Eijsink, V. G. H. , Igarashi, K. , … Sandgren, M. (2011). The putative endoglucanase pcGH61D from Phanerochaete chrysosporium is a metal‐dependent oxidative enzyme that cleaves cellulose. PLoS ONE, 6(11), e27807 10.1371/journal.pone.0027807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willick, G. E. , & Seligy, V. L. (1985). Multiplicity in cellulases of Schizophyllum commune Derivation partly from heterogeneity in transcription and glycosylation. European Journal of Biochemistry, 151(1), 89–96. 10.1111/j.1432-1033.1985.tb09072.x [DOI] [PubMed] [Google Scholar]
- Wing, L. (2001). Thermostable xylanases. Europe; EP 1 131 447 B1.
- Wu, J. , Yang, X. , Ge, J. , Zhang, Y. , Wu, L. , Liu, J. , et al. (2012). Biotransformation of sophoricoside in Fructus sophorae by the fungus Schizophyllum commune . Bioresource Technology, 2012(111), 496–499. [DOI] [PubMed] [Google Scholar]
- Xiume, Z . (2016). Milk cow forage. Europe; CN106071100 A.
- Yang, H. L. , Shen, G. P. , Park, D. C. , Novotny, C. P. , & Ullrich, R. C. (1995). The a‐alpha mating‐type transcripts of Schizophyllum commune . Experimental Mycology, 19(1), 16–25. 10.1006/emyc.1995.1003 [DOI] [PubMed] [Google Scholar]
- Yao, J. , Jia, R. , Zheng, L. , & Wang, B. (2013). Rapid decolorization of azo dyes by crude manganese peroxidase from Schizophyllum sp. F17 in solid‐state fermentation. Biotechnol. Bioprocess Engineering, 18(5), 868–877. 10.1007/s12257-013-0357-6 [DOI] [Google Scholar]
- Yaxuan, W. S. L. H. Z. F. Z . (2014). Phlegmariurus phlegmaria mingchegensis mycorrhizal fungi, method for production of huperzine A from the same, and application. Europe; CN 103834577 A.
- Yoshio, T. , Kiyoshi, H. , Tomoyuki, F. , Tatsuo, T. , & Takeshi, H. (1995). Schizostatin of new physiologically active substance and its production. Japan; JPH0741454 A.
- Yuki Junishiro, S. M . (2001). Mold capable of degrading dioxin, degradation of dioxin with the use of the same, method for producing composts capable of degrading dioxin and method for growing plants. Europe; EP 1074611 A1.
- Zhu, N. , Liu, J. , Yang, J. , Lin, Y. , Yang, Y. , Ji, L. , … Yuan, H. (2016). Comparative analysis of the secretomes of Schizophyllum commune and other wood‐decay basidiomycetes during solid‐state fermentation reveals its unique lignocellulose‐degrading enzyme system. Biotechnology for Biofuels, 9(1), 42 10.1186/s13068-016-0461-x [DOI] [PMC free article] [PubMed] [Google Scholar]


