Abstract
Wolbachia is a group of intracellular bacteria that infect a wide range of arthropods including the Asian citrus psyllid (ACP), Diaphorina citri Kuwayama. This insect is the vector of Candidatus Liberibacter asiaticus (CLas), the causal pathogen of Huanglongbing or citrus greening disease. Here, we investigated the localization pattern and infection dynamics of Wolbachia in different developmental stages of ACP. Results revealed that all developmental stages of ACP including egg, 1st–5th instar nymphs, and adults of both gender were infected with Wolbachia. FISH visualization of an ACP egg showed that Wolbachia moved from the egg stalk of newly laid eggs to a randomly distributed pattern throughout the egg prior to hatching. The infection rate varied between nymphal instars. The titers of Wolbachia in fourth and fifth instar nymphs were significantly higher than those in the first and second instar nymphs. Wolbachia were scattered in all nymphal stages, but with highest intensity in the U‐shaped bacteriome located in the abdomen of the nymph. Wolbachia was confined to two symmetrical organizations in the abdomen of newly emerged female and male adults. The potential mechanisms of Wolbachia infection dynamics are discussed.
Keywords: Asian citrus psyllid, endosymbiont, infection dynamic, localization, Wolbachia
1. INTRODUCTION
Bacterial endosymbionts are widespread microorganisms that are found in many invertebrates including insects, spiders, mites, isopod crustaceans, and filarial nematodes (O'Neill, Giordano, Colbert, Karr, & Robertson, 1992; Pietri, DeBruhl, & Sullivan, 2016; Weeks, Velten, & Stouthamer, 2003; Weinert, Araujo‐Jnr, Ahmed, & Welch, 2015; Zchori‐Fein & Perlman, 2004; Zug & Hammerstein, 2012). Obligate, primary endosymbionts such as Buchnera in aphids, Portiera in whiteflies and Uzinura diaspidicola in armored scales can provide nutrients to these insects that live on a nutritionally unbalanced diet of plant sap during their lifetime (Baumann, 2005; Gruwell, Flarhety, & Dittmar, 2012; Nakabachi et al., 2005). These symbionts are harbored in germ cells of their insect hosts and are vertically transmitted (Baumann, 2005; Weinert et al., 2015). Facultative, secondary endosymbionts are usually dispensable for survival of their hosts, but they can play important roles in manipulating host reproduction in ways that enhance vertical transmission, as well as host fitness, and host defense against thermal stress, natural enemies or pathogens (Brumin, Kontsedalov, & Ghanim, 2011; Hosokawa, Kikuchi, Shimada, & Fukatsu, 2007; Montllor, Maxmen, & Purcell, 2002; Oliver, Moran, & Hunter, 2005; Oliver, Russell, Moran, & Hunter, 2003; Oliver, Smith, & Russell, 2014). Primary symbionts are generally localized in the special cells called bacteriocytes grouped together in a bacteriome, while secondary symbionts have been reported in diverse insect tissues including the brain (Min & Benzer, 1997), salivary glands (Macaluso, Pornwiroon, Popov, & Foil, 2008) malpighian tubules (Bution, Caetano, & Zara, 2008) and hemolymph (Braquart‐Varnier et al., 2008; Fukatsu, Tsuchida, Nikoh, & Koga, 2001).
The Asian citrus psyllid (ACP), Diaphorina citri (Hemiptera: Liviidae), is a serious agricultural sap‐sucking pest in citrus‐growing regions of the world. ACP transmits Candidatus Liberibacter asiaticus (CLas) bacteria, the causal agent of Huanglongbing (HLB) also known as citrus greening disease (Grafton‐Cardwell, Stelinski, & Stansly, 2013). In addition, feeding and honeydew production of D. citri can result in reduced photosynthesis, growth of sooty mold and the death of young foliage at high population densities (Gottwald, 2010; Halbert & Manjunath, 2004). CLas is a phloem‐limited fastidious bacterium, which has not yet been cultured in vitro (Duan et al., 2009; Halbert & Manjunath, 2004). Typical symptoms of HLB include small and bitter fruits; chorotic shoots, blotchy mottle or variegated type of chlorosis, poor root growth, twig dieback and ultimately plant death (Bove, 2006; Gottwald, 2010; Yang et al., 2006).
Two distinct intracellular symbionts are harbored in the yellow and bilobed bacteriome located in the psyllid abdomen. The primary endosymbiont, Candidatus Carsonella ruddii, is located in uninucleate bacteriocytes on the surface of the bacteriome, while Candidatus Profftella armatura is found in syncytial cytoplasm at the center of the bacteriome (Nakabachi et al., 2013). Besides these primary symbionts, citrus psyllids also harbor secondary symbionts including Wolbachia and Arsenophonus (Chu, Gill, Hoffmann, & Pelz‐Stelinski, 2016; Saha et al., 2012).
Asian citrus psyllid is the primary vector of Candidatus Liberibacter asiaticus in Asia and North America (Gottwald, 2010; Halbert & Manjunath, 2004; Yang et al., 2006). There is no method of cure for HLB‐infected plants (Lopes, Frare, Yamamoto, Ayres, & Barbosa, 2007). Thus, there is an urgent need for effective means to manage the insect vector in order to reduce the incidence of this disease. Symbionts have been considered as a potential approach for control of many insect pests (Benlarbi & Ready, 2003; Mcmeniman et al., 2009; Moreira et al., 2009; Zabalou et al., 2004). Among the secondary endosymbionts, Wolbachia is the most abundant in arthropods (Weinert et al., 2015). It can induce reproductive disorders, cytoplasmic incompatibility (CI), parthenogenesis, male feminization and death; all of which warrant their manipulation as potential control agents (O'Neill et al., 1997; Werren, 1997; Werren, Baldo, & Clark, 2008) with cytoplasmic incompatibility being the most promising. This favors a particular Wolbachia strain that induces early embryonic death to egg and sperm combinations that are not both infected with the same strain. The potential use of this mechanism to control mosquitos has been explored in several studies including Xi and Dobson (2005), Kambris, Cook, Phuc, and Sinkins (2009), Moreira et al. (2009), Bian, Xu, Lu, Xie, and Xi (2010) and Walker et al. (2011). In addition, Wolbachia strains such as wMel, wAlbB have been used to suppress transmission of human pathogens in Anopheles gambiae, A. stephensi and Aedes albopictus, respectively (Bian et al., 2013; Blagrove, Arias‐Goeta, Failloux, & Sinkins, 2012; Hughes, Koga, Xue, Fukatsu, & Rasgon, 2011). It is therefore likely that endosymbionts, such as Wolbachia could be used to manipulate reproduction of ACP through cytoplasmic incompatibility and so suppress transmission of CLas to citrus plants (Hoffmann, Coy, Gibbard, & Pelz‐Stelinski, 2014). However, to achieve this goal it is essential to understand the infection biology of Wolbachia in ACP, including determining the identity of the strains, their infection level and localization patterns (Chu et al., 2016; Kruse et al., 2017).
In this study, we used PCR, qPCR, and whole‐mount fluorescence in situ hybridization (wFISH) to firstly detect the infection prevalence of Wolbachia, and secondly, determine the localization pattern of this endosymbiont in all life stages of ACP.
2. MATERIAL AND METHODS
2.1. Insects
The Asian citrus psyllid population used in this study was collected in September 2013, from healthy Murraya exotica L. (Rutaceae) plants on the campus of South China Agricultural University (SCAU, 23°09′N, 113°20′E), Guangzhou city, China. The psyllids were then reared for several generations on young M. exotica plants in a greenhouse in SCAU under ambient temperature and photoperiod before experiments were initiated.
2.2. DNA extraction from ACP
To extract the DNA, eggs, nymphs, and adults of both genders were collected from M. exotica plants, washed with 70% ethanol and then dried at room temperature. Nymphs were separated by instar based on their morphological characteristics (Tsai & Liu, 2000).
DNA extractions were conducted by two methods. In the first method, individual psyllids were first washed with double distilled water to remove all alcohol. The sample containing either one individual of each nymphal instar, a male or female adult, or 10 eggs together as one unit due to their small size was homogenized in 2μl STE (10 mmol/L Tris‐HCl, pH 8.0, 25 mmol/L NaCl, 25 mmol/L EDTA, 1% SDS, proteinase K 200 mg/ml) in a 0.5 ml microcentrifuge tube. The mixture for each sample was finally complemented with 15 μl STE in the 0.5 ml microcentrifuge tube. The homogenate was incubated at 56°C for 2–3 hr and then placed in 95°C water for 10 min to inactivate the proteinase K. After incubation, the samples were centrifuged for a short time and then used for PCR detection of Wolbachia.
In the second method, total DNA was extracted from groups of 40–50 ACP eggs, 1–2 instar nymphs or 10–20 individuals of 3–5 instar nymphs, male/female adults for qPCR using the TIANamp genomic DNA kit (TIANGEN Biotech, Beijing, China) with minor modifications for preparation of DNA from animal tissues. To assess DNA integrity, each sample was separated by electrophoresis on a 1% agarose gel (1%, 0.05μl/ml GoldView, TRIS‐EDTA‐Buffer) at 5 V/cm, and visualized on a UV transilluminator and then photographed via the gel imager. Additionally, quality and quantity of total DNA was measured on a NanoDrop 2,000 spectrophotometer to ensure uniformity among all samples for qPCR (Dossi, Da Silva, & Consoli, 2014; Tiwari, Gondhalekar, Mann, Scharf, & Stelinski, 2011).
2.3. PCR detection of Wolbachia in ACP
PCR detection of Wolbachia was conducted in a 25 μl reaction volume containing: 16 μl of double distilled water, 6 μl of 2xHiFiTaq PCR starMix Genstar, Beijing, China, 1 μl of each primer solution (20 μmol/L each), and 1 μl of DNA template of each ACP sample (egg, 1st–5th instar nymph, male or female adult). The primers were wsp, 81F: 5′‐TGGTCCAATAAGTGATGA AGAAAC‐3′, 691R: 5′‐AAAAATT AAACGCTACTCCA‐3′, which are specific to the Wolbachia endosymbiont (Braig, Zhou, Dobson, & O'Neill, 1998). The PCR procedure was: pre‐denatured at 94°C for 3 min, followed by 35 cycles at 94°C for 35 s, 55°C for 30 s, 72°C for 30 s, and a final extension at 72°C for 10 min. PCR amplified products were visualized on a 1% agarose gel containing GoldView colorant. When bands with the expected size were visible in the gels, 20 μl PCR products were sent for sequencing.
As mentioned above, ten eggs together as a unit, one individual of 1st–5th instar nymph, or one adult of each gender were treated as one replicate. In total 30 replicates were tested (10 replicates in one repeat ×3) in each experiment. Each PCR reaction included a positive (primary endosymbiont, Carsonella) and negative (ddH2O) control to verify DNA quality.
2.4. Quantification of Wolbachia titer in different life stages of ACP
Wolbachia was quantified by the SYBR Premix Ex Taq in the CFX‐96 Real‐Time PCR system (Bio‐Rad). The primers for qPCR were the wsp gene specific for Wolbachia: wsp‐F: 5′‐TGGTCCAATA AGTGATGAAGAAAC‐3′, wsp‐R: 5′‐AAAAATTAAACGCTACTCCA‐3′ (Ghanim & Kontsedalov, 2009). One β‐actin gene from ACP itself was used as an internal standard for data normalization. The primers of β‐actin were F: 5′‐CCCTGGACTTTGA ACAGGAA‐3′, β‐actin R: 5′‐CTCGTGGATACCGC AAGATT‐3′ (Tiwari et al., 2011). The qPCR reaction was a 25 μl volume containing: 12.5 μl of SYBR Premix Ex Taq (TIANGEN Biotech, Beijing, China), 9.5 μl of RNase‐free water, 0.5 μl of each primer solution (10 μmol/L each), and 2 μl of DNA template for each ACP sample. The qPCR procedure was initiated with 5‐min activation at 95°C followed by 40 cycles of 10 s at 95°C, 30 s at 60°C, and 60 s at 72°C. Again, ten eggs as a unit, one individual of 1st–5th instar nymph, or one male/female adult were detected as one replicate. In total four replicates for each developmental stage were repeated in this qPCR analysis.
2.5. Distribution of Wolbachia in different life stages of ACP
Eggs, and nymphs of each instar stage along with newly eclosed adults of ACP were collected from healthy M. exotica shoots with a camel‐hair brush. Fluorescence in situ hybridization (FISH) analysis of different psyllids stages and gender was performed as described by Gottlieb et al. (2006) with the probe W2‐Cy3 (5′‐Cy3‐CTTCTGTGAGTACCGTCATTATC‐3′) in order to detect Wolbachia. The samples were whole mounted, stained, and observed using an inverted fluorescence microscope (Nikon Eclipse Ti‐U). For each sample, at least 50 specimens were examined to confirm the results. Wolbachia infected ACPs (from the Wolbachia positive population) with no FISH probe were used as a control to confirm the specificity of Wolbachia detection.
2.6. Statistical analysis
Differences among nymphal stages and between male and female adult ACP in incidence and titer of Wolbachia were analyzed using one‐way ANOVA (SPSS 17.0 software, SPSS Inc., Chicago, IL, USA). Fisher's protected Duncan test was used for mean separation contingent on a significant treatment F value.
3. RESULTS
3.1. PCR detection of Wolbachia in ACP
Wolbachia wsp specific DNA was detected by PCR in all life stages of ACP including egg, nymphs, and adults (Figure 1). However, the infection rates of Wolbachia varied somewhat among different stages: 90.0 ± 5.8% in eggs, 90%–100% among 1st–5th instar nymphs, 96.7% ± 3.3% in adult females and 100% in adult males (N = 30). However, these differences were not significantly different (Table 1).
Figure 1.
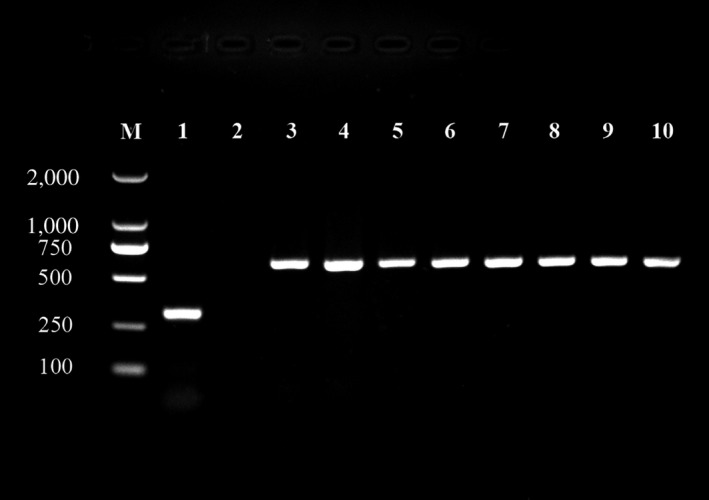
Wolbachia detection in different developmental stages of Asian citrus psyllid using PCR. Lane M: DL2,000 marker; Lane (1–10): positive control Carsonella, ddH2O negative control, male adult, female adult, fifth instar nymph, fourth instar nymph, third instar nymph, second instar nymph, first instar nymph, egg
Table 1.
The infection rates of Wolbachia in different developmental stages and genders of Asian citrus psyllid
| Stages | Total individuals | Positive individuals | Wolbachia infection rate (%) |
|---|---|---|---|
| Egga | 30 | 27 | 90.00 ± 5.77ab |
| 1st instar | 30 | 27 | 90.00 ± 5.77a |
| 2nd instar | 30 | 30 | 100.00 ± 0a |
| 3rd instar | 30 | 29 | 96.67 ± 3.33a |
| 4th instar | 30 | 29 | 96.67 ± 3.33a |
| 5th instar | 30 | 28 | 93.33 ± 3.33a |
| Male adult | 30 | 30 | 100.00 ± 0a |
| Female adult | 30 | 29 | 96.67 ± 3.33a |
Each individual sample contained 10 eggs.
the same letter in one volume means no significant differences between each other at p < 0.05 (Duncan test).
3.2. Quantification of Wolbachia titer in different stages of ACP
Taking the psyllid actin gene as the baseline, the titer of Wolbachia increased with successive nymphal instars (Figure 2), for example, Wolbachia titers in the 4th–5th instar nymphs were significantly higher than those in the 1st–3rd instar nymphs (F = 45.37, p < .0001). The Wolbachia titer of 5th instar ACP nymph was even higher than that of the ACP male and female adults, but no significant differences were found between the nymph and adults. One interesting finding was that, the titer of Wolbachia in ACP eggs was higher than that of the first instar nymph. The titer of Wolbachia did not differ significantly between adult genders but was relatively higher in males than in females (F = 0.51, p = .5007, Figure 3).
Figure 2.
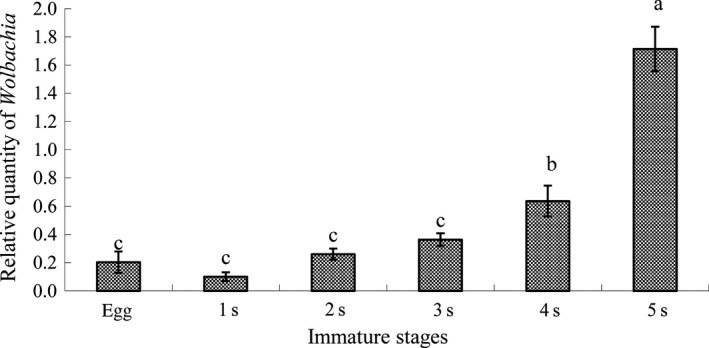
Relative quantity (mean ± SE) of Wolbachia in egg and nymphal instars of Asian citrus psyllid calculated using the method of 2−ΔΔct. Columns with the same letter represent means with no significant difference at p < .05
Figure 3.
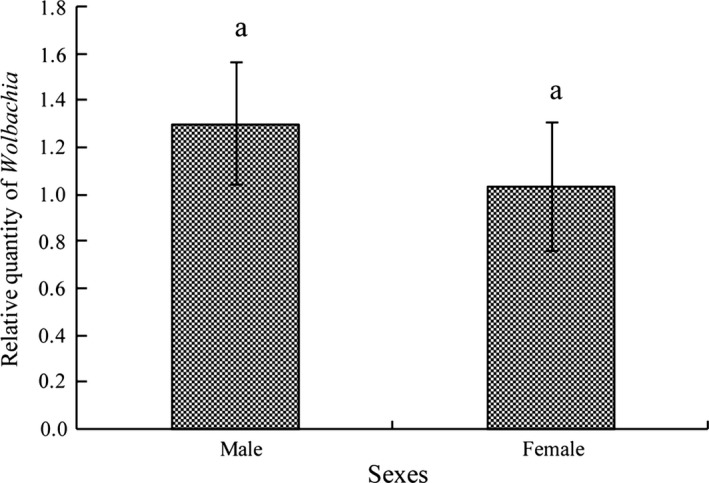
Relative quantity (mean ± SE) of Wolbachia in male and female adults of Asian citrus psyllid calculated using the method of 2−ΔΔct. No significant difference between gender
3.3. Distribution of Wolbachia in different life stages of ACP using Fluorescence in situ hybridization
Distribution of Wolbachia varied over the course of egg development. Wolbachia was most concentrated in the bacteriome at the basal pedicel end of newly laid eggs, although a more diffuse concentration could also be seen around the apex (Figure 4a and b). Later on, Wolbachia gradually spread out from the two poles to give a more uniform distribution (Figure 4c and f). In older eggs, Wolbachia were more random in distribution (Figure 4g and h). Incidence of Wolbachia over all egg specimens was 90.9% (40/44) as determined by FISH visualization detection.
Figure 4.
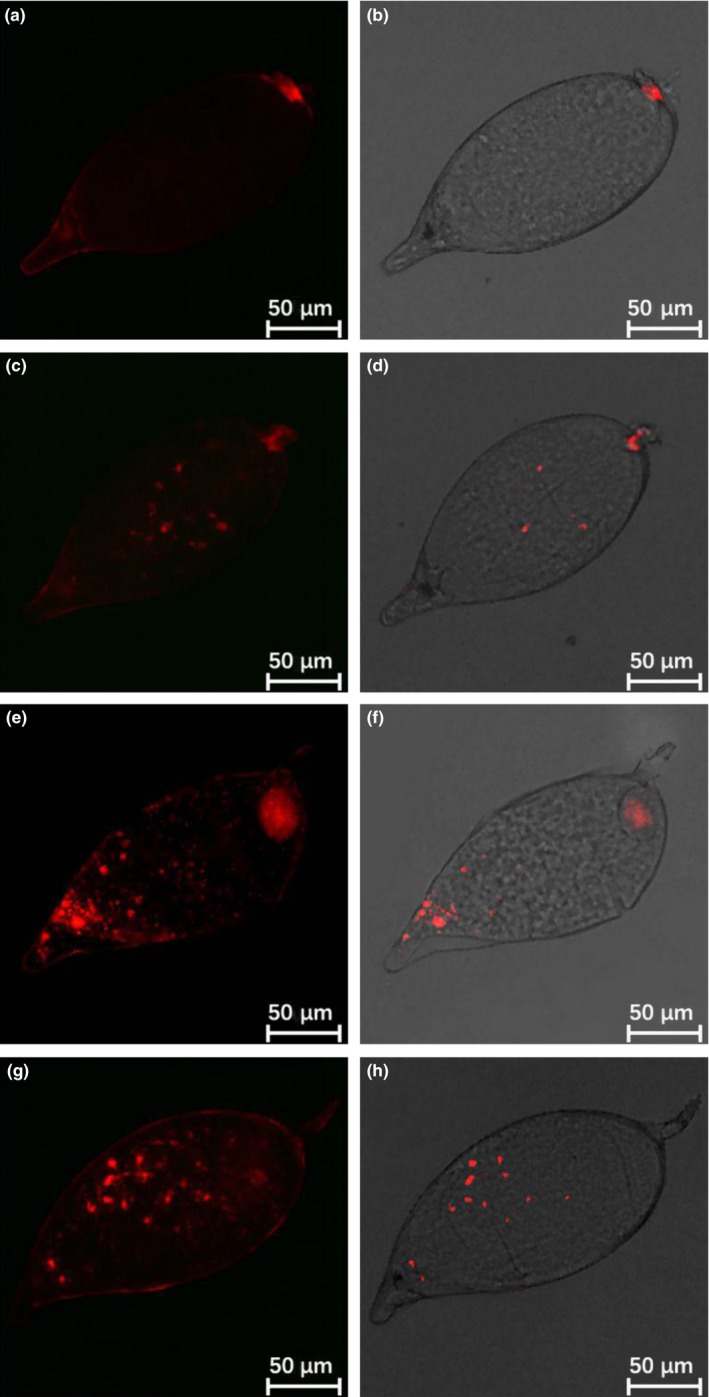
FISH visualization of Wolbachia during egg stage of Asian citrus psyllid. (a and b) 0–1 day old eggs; (c and d) 1–2 day old eggs; (e and f) 2–3 day old eggs; (g and h) 3–4 day old eggs. Left panels: fluorescence in dark field; right panels: fluorescence in bright field
Wolbachia localized primarily in the abdomen of ACP nymphs. The FISH signal could be detected throughout the nymph, but at highest intensity in the U‐shaped bacteriome in the nymphal abdomen (Figures 5 and 6). Incidence of Wolbachia infection over all nymphs examined by FISH was 78.6% (55/70). Incidence in adults was similar to nymphs at 76.2% (16/21). The symbionts occupied two symmetrical organizations in the adult abdomen thought to be the group of bacteriomes (Figure 7).
Figure 5.
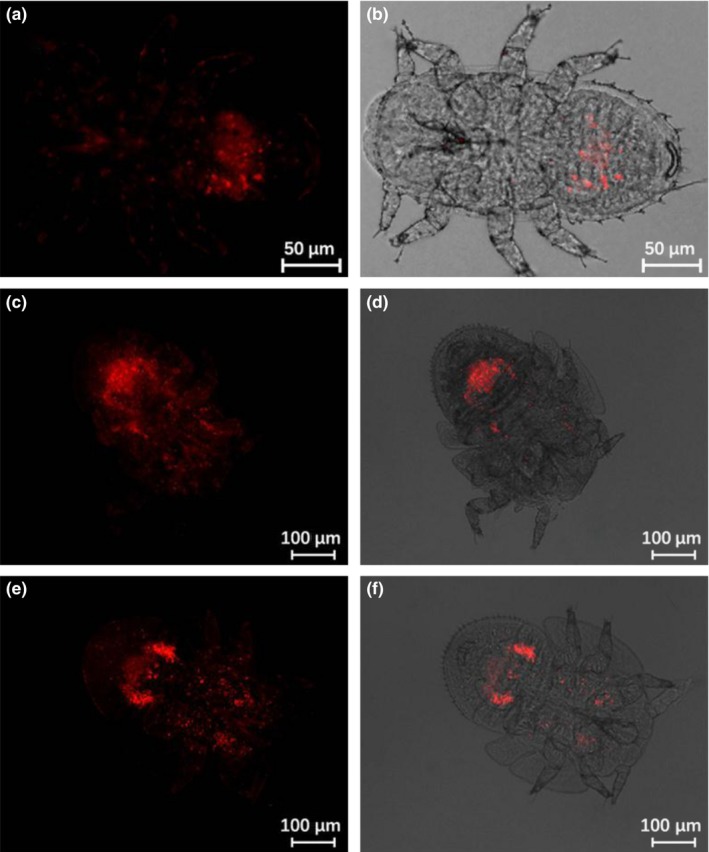
FISH visualization of Wolbachia in young‐instar nymphs of Asian citrus psyllid. (a and b) first instar nymphs; (c and d) second instar nymphs; (e and f) third instar nymphs. Left panels: fluorescence in dark field; right panels: fluorescence in bright field
Figure 6.
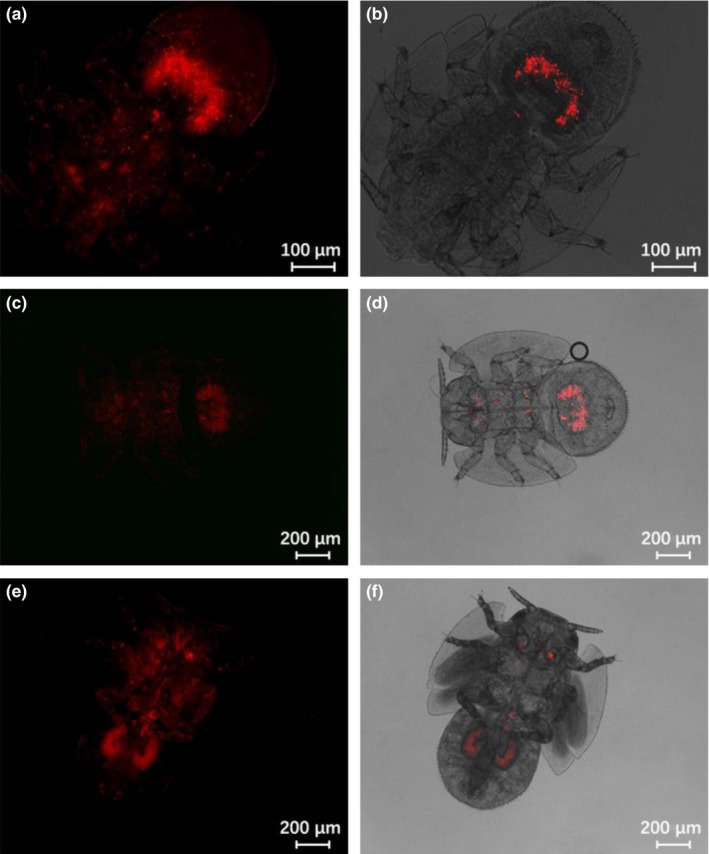
FISH visualization of Wolbachia in mature‐instar nymphs of Asian citrus psyllid. (a and b) fourth instar nymphs; (c and d) fifth instar nymphs; (e‐f) the end of fifth instar nymphs. Left panels: fluorescence in dark field; right panels: fluorescence in bright field
Figure 7.
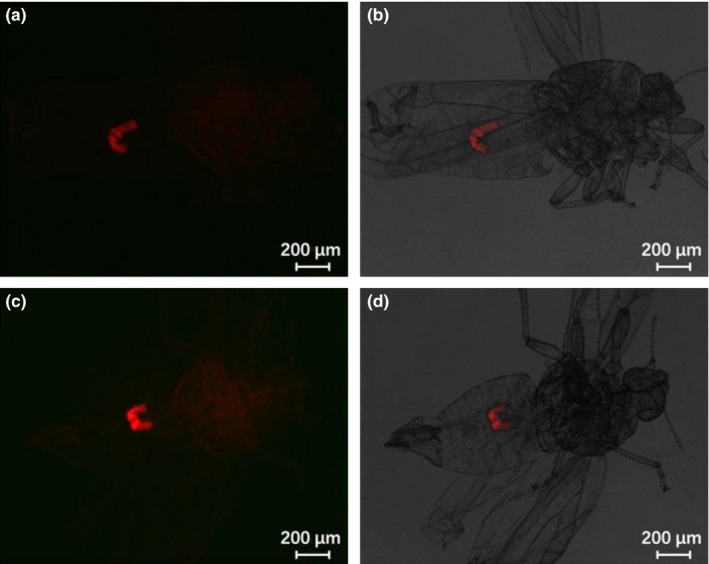
FISH visualization of Wolbachia in male and female adults of Asian citrus psyllid. (a and b) female adults; (c and d) male adults. Left panels: fluorescence in dark field; right panels: fluorescence in bright field
4. DISCUSSION
Numerous studies have revealed the biological roles of endosymbionts in the development, reproduction and defense of their insect hosts (Dale & Moran, 2006; Oliver, Degnan, Burke, & Moran, 2010; Siozios, Sapountzis, Ioannidis, & Bourtzis, 2008; Zug & Hammerstein, 2015a). Among these, Wolbachia are intracellular bacteria that infect a vast range of arthropod species, making them one of the most abundant endosymbionts in nature. The stunning evolutionary success of Wolbachia is mostly due to their reproductive parasitism but also mutualistic effects such as increased host fecundity and protection against pathogens (Zug & Hammerstein, 2015b). In the current study, detection frequencies of Wolbachia in ACP varied among different life stages and between gender from 100% in both the second instar nymphs and adult males to 90.0% in eggs and first instar nymphs. Guidolin and Consoli (2013) reported 100% incidence of Wolbachia in ACP specimens tested in Brazil. Subandiyah, Nikoh, Tsuyumu, Somowiyarjo, and Fukatsu (2000) found Wolbachia in 76.2% of D. citri adults sampled in Japan. Some differences in Wolbachia infection rates may result from geographic variation, number of ACP sampled and the methods used for detection. Furthermore, the infection of Wolbachia in ACPs was detected by three methods, normal PCR, FISH and qPCR in this study; the revealed infection rates were around 90%–100% by normal PCR, 77%–79% by FISH and 100% by qPCR, which indicated that there were certain differences among the three methods with qPCR being the most sensitive and accurate.
We found that the infection titer of Wolbachia tended to increase with successive nymphal instars in concert with developmental time. This result agreed with a recent study of Dossi et al. (2014), which reported an increase in Wolbachia densities with development of ACP populations in Brazil. Both studies found that Wolbachia titer was greater in the late embryonic egg stage of ACP compared with the first instar nymph. We deduce that this may due to two reasons: firstly, Wolbachia is mostly maternally transmitted from female to offspring, therefore, ovaries and mature eggs usually harbor more Wolbachia than other tissues, however, after egg hatching Wolbachia probably get scattered, reducing in numbers due to spreading into newly developing tissues; secondly, it might not be able to adapt to the new immune system which starts after the stage change from egg to nymph in order to regulate the related host immunity (Douglas, Bouvaine, & Russell, 2011; Gorman, Kankanala, & Kanost, 2004; Nishikori, Morioka, Kubo, & Morioka, 2009). However, regardless of both possibilities, the causation of the mechanism of infection warrants further study. Other studies have shown that environmental changes, such as insecticide exposure, temperature, host genotype diversity and Wolbachia strain can also influence their titer (Hurst, Jiggins, & Robinson, 2001; Weeks, Reynolds, Hoffmann, & Mann, 2002).
Our finding that more Wolbachia is present in males than females is consistent with previous related work with ACP (Hoffmann et al., 2014) as well as the pattern of wAlbB infection in Aedes albopictus (Tortosa et al., 2010). In many other insect species, the titer of Wolbachia is usually higher in adult females than in males (Correa & Ballard, 2012; Mouton et al., 2004; Tortosa et al., 2010). However, the reasons for the higher titers of Wolbachia in male compared to female ACP are still not clear. Rio, Wu, Filardo, and Aksoy (2006) found that the Wolbachia density in male tsetse fly was much higher than that of female, with males also showing a broader tissue distribution of Wolbachia compared to females. They deduced that there might be a sex specific opportunist role for Wolbachia replication in males, or that there exists a density regulation that is mediated by the insect host or a symbiont in the female, whereas this regulation efficacy is lost in males. Dossi et al. (2014) supposed that the lower density of Wolbachia in older females could be a consequence of the reduced growth rate of third instar due to the process of transovarian transmission. Moreover, the difference of Wolbachia titers between ACP male and females maybe also related to their Las infection status. Fagen et al. (2012) observed a strong positive correlation between Wolbachia and CLas titers within ACP, Kolora, Powell, Hunter, Bextine, and Lauzon (2015) also found that the amount of Wolbachia in ACP was greater in insects infected with CLas, whereas Chu et al. (2016) revealed that both the densities of primary Carsonella and facultative Wolbachia were significantly higher in CLas negative ACP compared to CLas‐positive ACP Florida populations. Therefore, to reveal which gender has a higher capability to harbor and transmit CLas may assist in further understanding the complicated association among CLas, Wolbachia, different gender of ACP as well as different populations or genotypes of ACP.
In this study, we were able to pinpoint the dynamics of localization patterns of Wolbachia in ACP using the whole‐mount fluorescence in situ hybridization method. Our FISH results revealed an uneven distribution pattern of Wolbachia in most of the ACP eggs and nymphal stages. Migration of Wolbachia from the egg stalk toward the central egg region is reminiscent of displacement of Rickettsia in Bemisia tabaci eggs (Gottlieb et al., 2006). Localization of Wolbachia in different parts of the egg appears to be related to diversion of the cytoskeleton which is known to play an essential role in repartition of organelles in cells (Sicard, Dittmer, Greve, Bouchon, & Braquart‐Varnier, 2014).
In nymphs, we found the highest concentration of Wolbachia in the U‐shaped bacteriome located in the abdomen, with lower concentrations in the thorax, and occasional presence in the head. The ACP bacteriome is known to harbor three symbionts: Carsonella, Profftella (Nakabachi et al., 2013), and now Wolbachia. This result suggests a specific immune profile for Wolbachia allowing the host to maintain and control the symbiosis (Anselme, Vallier, Balmand, Fauvarque, & Heddi, 2006; Heddi et al., 2005). The distribution of Wolbachia in late fifth instar nymphs is quite similar to that in ACP adults; reflecting the transition from nymph to adult. Using FISH methodology, Kruse et al. (2017) found that Wolbachia has a widespread distribution throughout the ACP gut tissue, including the midgut, filter chamber and Malpighian tubules. They also determined that Wolbachia and CLas are capable of residing in the same ACP gut cells, but that they do not have a high degree of co‐localization within cells.
The localization of Wolbachia has also been studied in other insects. In the bedbug Cimex lectularius, Wolbachia symbiont was specifically localized in the bacteriomes and vertically transmitted via the somatic stem cell niche of germalia to oocytes. Here, it infected the incipient symbiotic organ at an early stage of the embryogenesis in adults. In the males, Wolbachia was restricted to the testis‐associated bacteriomes, whereas in the females, it was found in the bacteriomes and the ovaries (Dobson et al., 1999). In Drosophila melanogaster, Clark, Veneti, Bourtzis, and Karr (2002, 2003) determined that Wolbachia were found inside spermatocytes and spermatids or within the somatic cyst cells surrounding the germ cells, and throughout development there appeared little movement of Wolbachia between spermatids via the connecting cytoplasmic bridges. In the endosymbiont‐scale insect system, Gruwell et al. (2012) found that the endosymbiont Uzinura diaspidicola localized in all the developmental stages of armored scale insects, including embryos, eggs and adults, which is similar to the findings in this study. All of these studies indicate a close association between Wolbachia endosymbiont and its insect host development, indicating the potential to develop novel approaches for managing citrus HLB, such as prevention of CLas transmission from the endosymbiont viewpoint.
The potential of Wolbachia to control disease vectors, and interfere with the ability of mosquitos to vector malaria and dengue has been demonstrated (Bian et al., 2013; Bourtzis et al., 2014; Guidolin & Consoli, 2013). As mentioned above, Fagen et al. (2012) and Kolora et al. (2015) reported that Wolbachia has a positive association with the CLas, while Chu et al. (2016) revealed that both the densities of primary Carsonella and facultative Wolbachia were significantly higher in CLas‐negative ACP compared to CLas‐positive ACP. Whichever reflect the true infection status in the field, the interactions of Wolbachia‐CLas can be further explored as a novel strategy to potentially control HLB through artificial manipulation of insect symbionts. Moreover, our molecular phylogenetic study has indicated that the Wolbachia of ACP from South China belongs to the Con strain in the Wolbachia B supergroup. The potential strategy of using Wolbachia to reduce ACP populations in the field may be practical by releasing a male ACP population with another strain of Wolbachia (single strain strategy, to realize this work we can first eliminate the original strain of Wolbachia and infect the ACP with a new strain by artificial micro‐infection), or overlay with another strain with this Con strain (double strain strategy). Therefore, cytoplasmic incompatibility may occur when these two types of male adults mate with wild female ACP adults.
In summary, considering the potential use of Wolbachia for vector and disease management, studies on the ecological factors that affect the interactions between Wolbachia and its ACP host may be beneficial in developing novel strategies for ACP and HLB management. The current study moves toward this final goal.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
The authors thank Andrew G. S. Cuthbertson (York, UK) for his critical review on an earlier version of the manuscript.
Ren S‐L, Li Y‐H, Ou D, et al. Localization and dynamics of Wolbachia infection in Asian citrus psyllid Diaphorina citri, the insect vector of the causal pathogens of Huanglongbing. MicrobiologyOpen. 2018;7:e561 10.1002/mbo3.561
REFERENCES
- Anselme, C. , Vallier, A. , Balmand, S. , Fauvarque, M. O. , & Heddi, A. (2006). Host PGRP gene expression and bacterial release in endosymbiosis of the weevil Sitophilus zeamais . Applied and Environmental Microbiology, 72, 6766–6772. 10.1128/AEM.00942-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann, P. (2005). Biology of bacteriocyte‐associated endosymbionts of plant sap‐sucking insects. Annual Review of Microbiology, 59, 155–189. 10.1146/annurev.micro.59.030804.121041 [DOI] [PubMed] [Google Scholar]
- Benlarbi, M. , & Ready, P. D. (2003). Host‐specific Wolbachia strains in widespread populations of Phlebotomus perniciosus and P. papatasi (Diptera: Psychodidae), and prospects for driving genes into these vectors of Leishmania. Bulletin of Entomological Research, 93, 383–391. [DOI] [PubMed] [Google Scholar]
- Bian, G. W. , Joshi, D. , Dong, Y. M. , Lu, P. , Zhou, G. L. , Pan, X. L. , … Xi, Z. Y. (2013). Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science, 340, 748–751. 10.1126/science.1236192 [DOI] [PubMed] [Google Scholar]
- Bian, G. W. , Xu, Y. , Lu, P. , Xie, Y. , & Xi, Z. Y. (2010). The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti . PLoS Pathogens, 6, e1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagrove, M. S. C. , Arias‐Goeta, C. , Failloux, A. B. , & Sinkins, S. P. (2012). Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus . Proceedings of the National Academy of Sciences of the United States of America, 109, 255–260. 10.1073/pnas.1112021108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtzis, K. , Dobson, S. L. , Xi, Z. Y. , Rasgon, J. L. , Calvitti, M. , Moreira, L. A. , … Gilles, J. R. L. (2014). Harnessing mosquito‐Wolbachia symbiosis for vector and disease control. Acta Tropica, 132, S150–S163. 10.1016/j.actatropica.2013.11.004 [DOI] [PubMed] [Google Scholar]
- Bove, J. M. (2006). Huanglongbing: A destructive, newly‐emerging, century‐old disease of citrus. Journal of Plant Pathology, 88, 7–37. [Google Scholar]
- Braig, H. R. , Zhou, W. , Dobson, S. L. , & O'Neill, S. L. (1998). Cloning and characterization of a gene encoding the major surface protein of the bacterial endosymbiont Wolbachia pipientis . Journal of Bacteriology, 180, 2373–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braquart‐Varnier, C. , Lachat, M. , Herbiniere, J. , Johnson, M. , Caubet, Y. , Bouchon, D. , & Sicard, M. (2008). Wolbachia mediate variation of host immunocompetence. PLoS ONE, 3, e3286 10.1371/journal.pone.0003286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumin, M. , Kontsedalov, S. , & Ghanim, M. (2011). Rickettsia influences thermotolerance in the whitefly Bemisia tabaci B biotype. Insect Science, 18, 57–66. 10.1111/ins.2011.18.issue-1 [DOI] [Google Scholar]
- Bution, M. L. , Caetano, F. H. , & Zara, F. J. (2008). Contribution of the Malpighian tubules for the maintenance of symbiotic microorganisms in cephalotes ants. Micron, 39, 1179–1183. 10.1016/j.micron.2008.05.003 [DOI] [PubMed] [Google Scholar]
- Chu, C. C. , Gill, T. A. , Hoffmann, M. , & Pelz‐Stelinski, K. S. (2016). Inter‐population variability of endosymbiont densities in the Asian citrus psyllid (Diaphorina citri Kuwayama). Microbial Ecology, 71, 999–1007. 10.1007/s00248-016-0733-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, M. E. , Veneti, Z. , Bourtzis, K. , & Karr, T. L. (2002). The distribution and proliferation of the intracellular bacteria Wolbachia during spermatogenesis in Drosophila . Mechanisms of Development, 111, 3–15. 10.1016/S0925-4773(01)00594-9 [DOI] [PubMed] [Google Scholar]
- Clark, M. E. , Veneti, Z. , Bourtzis, K. , & Karr, T. L. (2003). Wolbachia distribution and cytoplasmic incompatibility during sperm development: The cyst as the basic cellular unit of CI expression. Mechanisms of Development, 120, 185–198. 10.1016/S0925-4773(02)00424-0 [DOI] [PubMed] [Google Scholar]
- Correa, C. C. , & Ballard, J. W. O. (2012). Wolbachia gonadal density in female and male Drosophila vary with laboratory adaptation and respond differently to physiological and environmental challenges. Journal of Invertebrate Pathology, 111, 197–204. 10.1016/j.jip.2012.08.003 [DOI] [PubMed] [Google Scholar]
- Dale, C. , & Moran, N. A. (2006). Molecular interactions between bacterial symbionts and their hosts. Cell, 126, 453–465. 10.1016/j.cell.2006.07.014 [DOI] [PubMed] [Google Scholar]
- Dobson, S. L. , Bourtzis, K. , Braig, H. R. , Jones, B. F. , Zhou, W. , Rousset, F. , & O'Neill, S. L. (1999). Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochemistry and Molecular Biology, 29, 153–160. 10.1016/S0965-1748(98)00119-2 [DOI] [PubMed] [Google Scholar]
- Dossi, F. C. A. , Da Silva, E. P. , & Consoli, F. L. (2014). Population dynamics and growth rates of endosymbionts during Diaphorina citri (Hemiptera, Liviidae) ontogeny. Microbial Ecology, 68, 881–889. 10.1007/s00248-014-0463-9 [DOI] [PubMed] [Google Scholar]
- Douglas, A. E. , Bouvaine, S. , & Russell, R. R. (2011). How the insect immune system interacts with an obligate symbiotic bacterium. Proceedings of the Royal Society B‐Biological Sciences, 278, 333–338. 10.1098/rspb.2010.1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, Y. P. , Zhou, L. J. , Hall, D. G. , Li, W. B. , Doddapaneni, H. , Lin, H. , … Gottwald, T. (2009). Complete genome sequence of citrus huanglongbing bacterium, ‘Candidatus Liberibacter asiaticus’ obtained through metagenomics. Molecular Plant‐Microbe Interactions, 22, 1011–1020. 10.1094/MPMI-22-8-1011 [DOI] [PubMed] [Google Scholar]
- Fagen, J. R. , Giongo, A. , Brown, C. T. , Davis‐Richardson, A. G. , Gano, K. A. , & Triplett, E. W. (2012). Characterization of the relative abundance of the citrus pathogen Ca. Liberibacter asiaticus in the microbiome of its insect vector, Diaphorina citri, using high throughput 16S rRNA sequencing. The Open Microbiology Journal, 6, 29–33. 10.2174/1874285801206010029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukatsu, T. , Tsuchida, T. , Nikoh, N. , & Koga, R. (2001). Spiroplasma symbiont of the pea aphid, Acyrthosiphon pisum (Insecta: Homoptera). Applied and Environmental Microbiology, 67, 1284–1291. 10.1128/AEM.67.3.1284-1291.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanim, M. , & Kontsedalov, S. (2009). Susceptibility to insecticides in the Q biotype of Bemisia tabaci is correlated with bacterial symbiont densities. Pest Management Science, 65, 939–942. 10.1002/ps.v65:9 [DOI] [PubMed] [Google Scholar]
- Gorman, M. J. , Kankanala, P. , & Kanost, M. R. (2004). Bacterial challenge stimulates innate immune responses in extra‐embryonic tissues of tobacco hornworm eggs. Insect Molecular Biology, 13, 19–24. 10.1111/imb.2004.13.issue-1 [DOI] [PubMed] [Google Scholar]
- Gottlieb, Y. , Ghanim, M. , Chiel, E. , Gerling, D. , Portnoy, V. , Steinberg, S. , … Zchori‐Fein, E. (2006). Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae). Applied and Environmental Microbiology, 72, 3646–3652. 10.1128/AEM.72.5.3646-3652.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald, T. R. (2010). Current epidemiological understanding of citrus Huanglongbing. Annual Review of Phytopathology, 48, 119–139. 10.1146/annurev-phyto-073009-114418 [DOI] [PubMed] [Google Scholar]
- Grafton‐Cardwell, E. E. , Stelinski, L. L. , & Stansly, P. A. (2013). Biology and management of Asian citrus psyllid, vector of the Huanglongbing pathogens. Annual Review of Entomology, 58, 413–432. 10.1146/annurev-ento-120811-153542 [DOI] [PubMed] [Google Scholar]
- Gruwell, M. E. , Flarhety, M. , & Dittmar, K. (2012). Distribution of the primary endosymbiont (Candidatus Uzinura Diaspidicola) within host insect from the scale insect family Diaspididae. Insects, 3, 262–269. 10.3390/insects3010262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidolin, A. S. , & Consoli, F. L. (2013). Molecular characterization of Wolbachia strains associated with the invasive Asian citrus psyllid Diaphorina citri in Brazil. Microbial Ecology, 65, 475–486. 10.1007/s00248-012-0150-7 [DOI] [PubMed] [Google Scholar]
- Halbert, S. E. , & Manjunath, K. L. (2004). Asian citrus psyllids (Sternorrhyncha:Psyllidae) and greening disease of citrus: A literature review and assessment of risk in Florida. Florida Entomologist, 87, 330–353. 10.1653/0015-4040(2004)087[0330:ACPSPA]2.0.CO;2 [DOI] [Google Scholar]
- Heddi, A. , Vallier, A. , Anselme, C. , Xin, H. , Rahbe, Y. , & Wackers, F. (2005). Molecular and cellular profiles of insect bacteriocytes: Mutualism and harm at the initial evolutionary step of symbiogenesis. Cellular Microbiology, 7, 293–305. [DOI] [PubMed] [Google Scholar]
- Hoffmann, M. , Coy, M. R. , Gibbard, H. N. K. , & Pelz‐Stelinski, K. S. (2014). Wolbachia infection density in populations of the Asian citrus psyllid (Hemiptera: Liviidae). Environmental Entomology, 43, 1215–1222. 10.1603/EN14193 [DOI] [PubMed] [Google Scholar]
- Hosokawa, T. , Kikuchi, Y. , Shimada, M. , & Fukatsu, T. (2007). Obligate symbiont involved in pest status of host insect. Proceedings of the Royal Society B‐Biological Sciences, 274, 1979–1984. 10.1098/rspb.2007.0620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, G. L. , Koga, R. , Xue, P. , Fukatsu, T. , & Rasgon, J. L. (2011). Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae . PLoS Pathogens, 7, e1002043 10.1371/journal.ppat.1002043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst, G. D. D. , Jiggins, F. M. , & Robinson, S. J. W. (2001). What causes inefficient transmission of male‐killing Wolbachia in Drosophila? Heredity, 87, 220–226. 10.1046/j.1365-2540.2001.00917.x [DOI] [PubMed] [Google Scholar]
- Kambris, Z. , Cook, P. E. , Phuc, H. K. , & Sinkins, S. P. (2009). Immune activation by life‐shortening Wolbachia and reduced filarial competence in mosquitoes. Science, 326, 134–136. 10.1126/science.1177531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolora, L. D. , Powell, C. M. , Hunter, W. , Bextine, B. , & Lauzon, C. R. (2015). Internal extracellular bacteria of Diaphorina citri Kuwayama (Hemiptera: Psyllidae), the Asian citrus psyllid. Current Microbiology, 70, 710–715. 10.1007/s00284-015-0774-1 [DOI] [PubMed] [Google Scholar]
- Kruse, A. , Fattah‐Hosseini, S. , Saha, S. , Johnson, R. , Warwick, E. , Sturgeon, K. , … Heck, M. C. (2017). Combining ‘omics and microscopy to visualize interactions between the Asian citrus psyllid vector and the Huanglongbing pathogen Candidatus Liberibacter asiaticus in the insect gut. PLoS ONE, 12, e0179531 10.1371/journal.pone.0179531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes, S. A. , Frare, G. F. , Yamamoto, P. T. , Ayres, A. J. , & Barbosa, J. C. (2007). Ineffectiveness of pruning to control citrus huanglongbing caused by Candidatus Liberibacter americanus. European Journal of Plant Pathology, 119, 463–468. 10.1007/s10658-007-9173-1 [DOI] [Google Scholar]
- Macaluso, K. R. , Pornwiroon, W. , Popov, V. L. , & Foil, L. D. (2008). Identification of Rickettsia felis in the salivary glands of cat fleas. Vector‐Borne and Zoonotic Diseases, 8, 391–396. 10.1089/vbz.2007.0218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcmeniman, C. J. , Lane, R. V. , Cass, B. N. , Fong, A. W. C. , Sidhu, M. , Wang, Y. F. , & O'neill, S. L. (2009). Stable introduction of a life‐shortening Wolbachia infection into the mosquito Aedes aegypti . Science, 323, 141–144. 10.1126/science.1165326 [DOI] [PubMed] [Google Scholar]
- Min, K. T. , & Benzer, S. (1997). Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proceedings of the National Academy of Sciences of the United States of America, 94, 10792–10796. 10.1073/pnas.94.20.10792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montllor, C. B. , Maxmen, A. , & Purcell, A. H. (2002). Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecological Entomology, 27, 189–195. 10.1046/j.1365-2311.2002.00393.x [DOI] [Google Scholar]
- Moreira, L. A. , Iturbe‐Ormaetxe, I. , Jeffery, J. A. , Lu, G. J. , Pyke, A. T. , Hedges, L. M. , … O'Neill, S. L. (2009). A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium . Cell, 139, 1268–1278. 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- Mouton, L. , Dedeine, F. , Henri, H. , Bouletreau, M. , Profizi, N. , & Vavre, F. (2004). Virulence multiple infections and regulation of symbiotic population in the Wolbachia‐Asobara tabida symbiosis. Genetics, 168, 181–189. 10.1534/genetics.104.026716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabachi, A. , Shigenobu, S. , Sakazume, N. , Shiraki, T. , Hayashizaki, Y. , Carninci, P. , … Fukatsu, T. (2005). Transcriptome analysis of the aphid bacteriocyte, the symbiotic host cell that harbors an endocellular mutualistic bacterium, Buchnera . Proceedings of the National Academy of Sciences of the United States of America, 102, 5477–5482. 10.1073/pnas.0409034102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabachi, A. , Ueoka, R. , Oshima, K. , Teta, R. , Mangoni, A. , Gurgui, M. , … Fukatsu, T. (2013). Defensive bacteriome symbiont with a drastically reduced genome. Current Biology, 23, 1478–1484. 10.1016/j.cub.2013.06.027 [DOI] [PubMed] [Google Scholar]
- Nishikori, K. , Morioka, K. , Kubo, T. , & Morioka, M. (2009). Age and morph‐dependent activation of the lysosomal system and Buchnera degradation in aphid endosymbiosis. Journal of Insect Physiology, 55, 351–357. 10.1016/j.jinsphys.2009.01.001 [DOI] [PubMed] [Google Scholar]
- Oliver, K. M. , Degnan, P. H. , Burke, G. R. , & Moran, N. A. (2010). Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annual Review of Entomology, 55, 247–266. 10.1146/annurev-ento-112408-085305 [DOI] [PubMed] [Google Scholar]
- Oliver, K. M. , Moran, N. A. , & Hunter, M. S. (2005). Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proceedings of the National Academy of Sciences of the United States of America, 102, 12795–12800. 10.1073/pnas.0506131102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, K. M. , Russell, J. A. , Moran, N. A. , & Hunter, M. S. (2003). Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proceedings of the National Academy of Sciences of the United States of America, 100, 1803–1807. 10.1073/pnas.0335320100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, K. M. , Smith, A. H. , & Russell, J. A. (2014). Defensive symbiosis in the real world ‐advancing ecological studies of heritable, protective bacteria in aphids and beyond. Functional Ecology, 28, 341–355. 10.1111/fec.2014.28.issue-2 [DOI] [Google Scholar]
- O'Neill, S. L. , Giordano, R. , Colbert, A. M. , Karr, T. L. , & Robertson, H. M. (1992). 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proceedings of the National Academy of Sciences of the United States of America, 89, 2699–2702. 10.1073/pnas.89.7.2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, S. L. , Pettigrew, M. M. , Sinkins, S. P. , Braig, H. R. , Andreadis, T. G. , & Tesh, R. B. (1997). In vitrocultivation of Wolbachia pipientis in an Aedes albopictus cell line. Insect Molecular Biology, 6, 33–39. 10.1046/j.1365-2583.1997.00157.x [DOI] [PubMed] [Google Scholar]
- Pietri, J. E. , DeBruhl, H. , & Sullivan, W. (2016). The rich somatic life of Wolbachia . MicrobiologyOpen, 5, 923–936. 10.1002/mbo3.2016.5.issue-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio, R. V. , Wu, Y. N. , Filardo, G. , & Aksoy, S. (2006). Dynamics of multiple symbiont density regulation during host development: Tsetse fly and its microbial flora. Proceedings of the Royal Society B‐Biological Sciences, 273, 805–814. 10.1098/rspb.2005.3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha, S. , Hunter, W. B. , Reese, J. , Morgan, J. K. , Marutani‐Hert, M. , Huang, H. , & Lindeberg, M. (2012). Survey of endosymbionts in the Diaphorina citri metagenome and assembly of a Wolbachia wDi draft genome. PLoS ONE, 7, e50067 10.1371/journal.pone.0050067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard, M. , Dittmer, J. , Greve, P. , Bouchon, D. , & Braquart‐Varnier, C. (2014). A host as an ecosystem: Wolbachia coping with environmental constraints. Environmental Microbiology, 16, 3583–3607. 10.1111/emi.2014.16.issue-12 [DOI] [PubMed] [Google Scholar]
- Siozios, S. , Sapountzis, P. , Ioannidis, P. , & Bourtzis, K. (2008). Wolbachia symbiosis and insect immune response. Insect Science, 15, 89–100. 10.1111/ins.2008.15.issue-1 [DOI] [Google Scholar]
- Subandiyah, S. , Nikoh, N. , Tsuyumu, S. , Somowiyarjo, S. , & Fukatsu, T. (2000). Complex endosymbiotic microbiota of the citrus psyllid Diaphorina citri (Homoptera: Psylloidea). Zoological Science, 17, 983–989. 10.2108/zsj.17.983 [DOI] [Google Scholar]
- Tiwari, S. , Gondhalekar, A. D. , Mann, R. S. , Scharf, M. E. , & Stelinski, L. L. (2011). Characterization of five CYP4 genes from Asian citrus psyllid and their expression levels in Candidatus Liberibacter asiaticus‐ infected and uninfected psyllids. Insect Molecular Biology, 20, 733–744. 10.1111/imb.2011.20.issue-6 [DOI] [PubMed] [Google Scholar]
- Tortosa, P. , Charlat, S. , Labbe, P. , Dehecq, J. S. , Barre, H. , & Weill, M. (2010). Wolbachia age‐sex‐specific density in Aedes albopictus: A host evolutionary response to cytoplasmic incompatibility? PLoS ONE, 5, e9700 10.1371/journal.pone.0009700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, J. H. , & Liu, Y. H. (2000). Biology of Diaphorina citri (Homoptera: Psyllidae) on four host plants. Journal of Economic Entomology, 93, 1721–1725. 10.1603/0022-0493-93.6.1721 [DOI] [PubMed] [Google Scholar]
- Walker, T. , Johnson, P. H. , Moreira, L. A. , Iturbe‐Ormaetxe, I. , Frentiu, F. D. , Mcmeniman, C. J. , … Hoffmann, A. A. (2011). The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature, 476, U450–U101. 10.1038/nature10355 [DOI] [PubMed] [Google Scholar]
- Weeks, A. R. , Reynolds, K. T. , Hoffmann, A. A. , & Mann, H. (2002). Wolbachia dynamics and host effects: What has (and has not) been demonstrated? Trends in Ecology & Evolution, 17, 257–262. 10.1016/S0169-5347(02)02480-1 [DOI] [Google Scholar]
- Weeks, A. R. , Velten, R. , & Stouthamer, R. (2003). Incidence of a new sex‐ratio‐distorting endosymbiotic bacterium among arthropods. Proceedings of the Royal Society B‐Biological Sciences, 270, 1857–1865. 10.1098/rspb.2003.2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert, L. A. , Araujo‐Jnr, E. V. , Ahmed, M. Z. , & Welch, J. J. (2015). The incidence of bacterial endosymbionts in terrestrial arthropods. Proceedings of the Royal Society B‐Biological Sciences, 282, 20150249 10.1098/rspb.2015.0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren, J. H. (1997). Biology of Wolbachia . Annual Review of Entomology, 42, 587–609. 10.1146/annurev.ento.42.1.587 [DOI] [PubMed] [Google Scholar]
- Werren, J. H. , Baldo, L. , & Clark, M. E. (2008). Wolbachia: Master manipulators of invertebrate biology. Nature Reviews Microbiology, 6, 741–751. 10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- Xi, Z. Y. , & Dobson, S. L. (2005). Characterization of Wolbachia transfection efficiency by using microinjection of embryonic cytoplasm and embryo homogenate. Applied and Environmental Microbiology, 71, 3199–3204. 10.1128/AEM.71.6.3199-3204.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. P. , Huang, M. D. , Beattie, G. A. C. , Xia, Y. L. , Ouyang, G. C. , & Xiong, J. J. (2006). Distribution, biology, ecology and control of the psyllid Diaphorina citri Kuwayama, a major pest of citrus: A status report for China. International Journal of Pest Management, 52, 343–352. 10.1080/09670870600872994 [DOI] [Google Scholar]
- Zabalou, S. , Riegler, M. , Theodorakopoulou, M. , Stauffer, C. , Savakis, C. , & Bourtzis, K. (2004). Wolbachia‐induced cytoplasmic incompatibility as a means for insect pest population control. Proceedings of the National Academy of Sciences of the United States of America, 101, 15042–15045. 10.1073/pnas.0403853101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zchori‐Fein, E. , & Perlman, S. J. (2004). Distribution of the bacterial symbiont Cardinium in arthropods. Molecular Ecology, 13, 2009–2016. 10.1111/mec.2004.13.issue-7 [DOI] [PubMed] [Google Scholar]
- Zug, R. , & Hammerstein, P. (2012). Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE, 7, e38544 10.1371/journal.pone.0038544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zug, R. , & Hammerstein, P. (2015a). Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biological Reviews, 90, 89–111. 10.1111/brv.2015.90.issue-1 [DOI] [PubMed] [Google Scholar]
- Zug, R. , & Hammerstein, P. (2015b). Wolbachia and the insect immune system: What reactive oxygen species can tell us about the mechanisms of Wolbachia‐host interactions. Frontiers in Microbiology, 6, 1201. [DOI] [PMC free article] [PubMed] [Google Scholar]


