Summary
Strict anaerobic gut microbes have been suggested as ‘next‐generation probiotics’ for treating several intestinal disorders. The development of preservation techniques is of major importance for therapeutic application. This study investigated cryopreservation (−80°C) and lyophilization survival and storage stability (4°C for 3 months) of the strict anaerobic gut microbes Bacteroides thetaiotaomicron, Faecalibacterium prausnitzii, Roseburia intestinalis, Anaerostipes caccae, Eubacterium hallii and Blautia obeum. To improve preservation survival, protectants sucrose and inulin (both 5% w/v) were added for lyophilization and were also combined with glycerol (15% v/v) for cryopreservation. Bacterial fitness, evaluated by maximum growth rate and lag phase, viability and membrane integrity were determined using a standardized growth assay and by flow cytometry as markers for preservation resistance. Lyophilization was more detrimental to viability and fitness than cryopreservation, but led to better storage stability. Adding sucrose and inulin enhanced viability and the proportion of intact cells during lyophilization of all strains. Viability of protectant‐free B. thetaiotaomicron, A. caccae and F. prausnitzii was above 50% after cryopreservation and storage and increased to above 80% if protectants were present. The addition of glycerol, sucrose and inulin strongly enhanced the viability of B. obeum, E. hallii and R. intestinalis from 0.03–2% in protectant‐free cultures to 11–37%. This is the first study that quantitatively compared the effect of cryopreservation and lyophilization and the addition of selected protectants on viability and fitness of six strict anaerobic gut microbes. Our results suggest that efficiency of protectants is process‐ and species‐specific.
Introduction
According to the WHO/FAO, probiotics are defined as ‘live microorganisms, that when administered in adequate amounts confer a health benefit on the host’. This definition implies viability as an important characteristic of probiotics for efficiency. Processing and preservation procedures that guarantee a high yield of viable cells are therefore essential for probiotic application. Recently, interest in probiotic research expanded from the classical probiotic Lactobacillus and Bifidobacterium species to a more targeted manipulation of the host gut microbiota with ‘personalized probiotic therapies’ using functional important strict anaerobic gut microbes. The administration of specific bacterial strains could address distinct differences in colonic microbiota profiles associated with intestinal diseases (Vieira et al., 2016). Strict anaerobic butyrate‐producing bacteria have been proposed as ‘next‐generation probiotics’ in the treatment of intestinal disorders (Van Immerseel et al., 2010). Butyrate, produced during bacterial fermentation, is an important short‐chain fatty acid (SCFA) providing several benefits to the host (Tan et al., 2014). Clostridia cluster IV and XIVa, including the highly abundant butyrate‐producing Faecalibacterium prausnitzii and Eubacterium rectale/Roseburia spp., harbour promising candidates for future probiotics (Louis and Flint, 2009; Hsiao et al., 2014; Miquel et al., 2015; Udayappan et al., 2016; Tamanai‐Shacoori et al., 2017). The selection of ‘next‐generation probiotics’, however, is not limited to butyrate producers. Propionate producers, such as Bacteroides, can beneficially effect the host by interacting with the immune system and by maintaining host–microbiota homoeostasis and might therefore contain species for future therapeutic administration (Wrzosek et al., 2013; El Hage et al., 2017).
The two main long‐term preservation methods for microbes are cryopreservation and lyophilization (Prakash et al., 2013). Both techniques are well established for many aerobes or facultative anaerobes (Hubalek, 2003). However, data on preservation of strict anaerobic gut microbes are limited (Staab and Ely, 1987; Khan et al., 2014). Current preservation procedures were mainly designed for culture collections when survival of only a minor proportion of cells is required (Malik, 1992). To improve survival of preserved cells, protective agents are commonly added to minimize freezing and drying injuries. In cryopreservation, glycerol is one of the most commonly used penetrating protectant; it is non‐toxic even at high concentrations (Meryman, 2007). Intracellular glycerol can stabilize cells during slow freezing by minimizing or delaying osmotic derived shrinkage off the cells to a lower temperature (Fowler and Toner, 2005). It has been suggested that glycerol can prevent damage due to increased osmotic pressure, as the presence of glycerol can reduce the excessive increase in salt concentration in fractions of unfrozen water during freezing (Lovelock, 1953). In contrast, in lyophilization glycerol is less suitable as protectants, because it can lead to an insufficiently dried and sticky product at high concentrations (Abadias et al., 2001). Sugars are another classical group of protectants used in cryopreservation and lyophilization. Disaccharides, such as maltose, sucrose and trehalose, are able to induce shrinkage of the cells by osmosis‐derived dehydration before freezing thereby reducing intracellular ice formation (Fowler and Toner, 2005). Sucrose has been frequently used for cryopreservation of microorganism (Hubalek, 2003) and improved tolerance to drying by protecting proteins from denaturation in the absence of water (Leslie et al., 1995). Inulin‐type fructans are non‐penetrating, water‐soluble protective agents that are applied in lyophilization (Hubalek, 2003). The protective action is exerted extracellularly by direct interaction and stabilization of membrane lipids under dry and cold conditions (Demel et al., 1998; Vereyken et al., 2003; Schwab et al., 2007). Fructans can serve as bulking agent and protective matrix during lyophilization (Khan et al., 2014). Combining compounds with different protective mechanisms can result in greater protection of microorganisms during freezing and drying than single‐component application, due to additive or synergic protective effects (Hubalek, 2003). We recently showed, that a combination of glycerol (15% v/v) and inulin (5% v/w) maintained viability and activity of the strict anaerobic, butyrate‐producing microbes, F. prausnitzii, Roseburia sp./E. rectale group and Eubacterium hallii in complex ‘artificial’ gut microbial communities during 3 months storage at −80°C (Bircher et al., 2017).
In this work, we investigated preservation of strict anaerobic gut microbes after freezing at −80°C and after lyophilization, and subsequent storage at 4°C for 3 months. Six strains were selected for preservation trials: Roseburia intestinalis, F. prausnitzii, E. hallii, Anaerostipes caccae, Blautia obeum and Bacteroides thetaiotaomicron are highly abundant representatives of human gut microbial butyrate and propionate producers. Bacterial fitness, evaluated by maximum growth rate and lag phase, and viability were tested during processing under strict anaerobic conditions and storage using different buffers containing non‐toxic protectants glycerol, sucrose and inulin to improve freezing and freeze‐drying resistance.
Results
Impact of protectants on fresh cultures
The effect of the protectants inulin and sucrose alone (SI, bot 5% w/v) or in combination with glycerol (GSI, 15% v/v) on viability (MPNs, percentage of intact cells) and fitness (μmax and t lag) of fresh B. thetaiotaomicron, B. obeum, R. intestinalis, E. hallii, F. prausnitzii and A. caccae cultures (t 0) was evaluated after 30 min anaerobic incubation in the corresponding protective medium, and compared to a control lacking protectants (Fig. 1).
Figure 1.
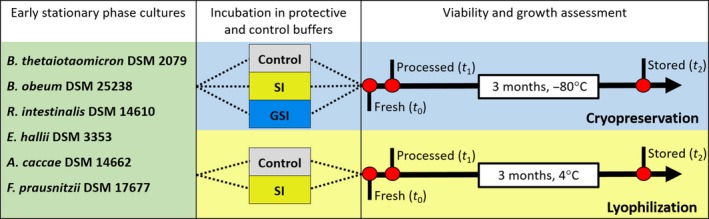
Set‐up of preservation experiments. Early stationary phase cultures were incubated for 30 min in buffers containing sucrose and inulin (SI), sucrose, inulin and glycerol (GSI) and in control buffer lacking protectants (control) before processing for preservation. Viability and growth were assessed at three different time points indicated by red dots. The first assessment was performed with fresh cultures after incubation in the protective and control buffers (t 0). The second assessment was conducted with the processed cultures immediately after freezing, respectively, lyophilization (t 1) and the third assessment with cryopreserved and lyophilized samples stored for 3 months at −80°C or 4°C, respectively (t 2).
For all tested strains, SI‐treated cultures did not differ from that of control cultures (Tables 1 and 2), except A. caccae with higher percentage of intact cells after incubation in SI than in the control (83 ± 6% and 63 ± 8%, respectively). In contrast, significant differences in viability and fitness of fresh cultures were observed when SI was combined with glycerol (GSI) for some strains. The MPN for R. intestinalis cultures was approximately 10‐fold lower with GSI than in the control (7.9 ± 0.2 and 9.0 ± 0.3 log cells ml−1, respectively), the percentage of intact cells was reduced (57 ± 5% and 104 ± 6%, respectively), and t lag was significantly increased (3.3 ± 0.1 and 1.3 ± 0.1 h, respectively). Similarly, GSI‐treated F. prausnitzii exhibited a three times longer t lag (3.6 ± 0.1 and 1.3 ± 0.1 h, respectively), a lower fraction of intact cells (65 ± 2% and 83 ± 7%, respectively) and a significantly reduced MPN (7.4 ± 0.3 and 8.0 ± 0.2 log cells ml−1, respectively) compared to the control. For B. thetaiotaomicron treated with GIS, t lag was significantly increased (1.3 ± 0.6 and 0.7 ± 0.1 h, respectively) and the percentage of intact cells was reduced (60 ± 5% and 87 ± 11%, respectively) compared to the control, but MPN was not different. GIS treatment also decreased μmax of E. hallii compared to the control (0.23 ± 0.02 and 0.36 ± 0.02 OD unit h−1, respectively).
Table 1.
Impact of protectants and cryopreservation on cell viability of fresh (t 0), processed (t 1) and stored (t 2) bacteria. Log viable cell counts ml−1 in the fresh culture, after freezing in liquid nitrogen and cryopreservation for 3 months at −80°C, were assessed with the most probable number method (MPN). Recovery rate of viable cells (in %) was calculated relative to the average viable cell counts in the fresh control (vs. control t 0) and fresh treatment culture (vs. t 0)
| Organism | Culture condition | Control | SI | GSI | |||
|---|---|---|---|---|---|---|---|
| MPN (ml−1) | Recovery vs. t0 | MPN (ml−1) | Recover vs. control t 0/t 0 | MPN (ml−1) | Recovery vs. control t 0/t 0 | ||
| B. thetaiotaomicron | Fresh (t 0) | 9.3 ± 0.2 | 9.4 ± 0.2 | 135 | 8.9 ± 0.3 | 45 | |
| Processed (t 1) | 9.2 ± 0.3 | 85 | 9.3 ± 0.2 | 114/84 | 8.8 ± 0.6 | 31/70 | |
| Stored (t 2) | 9.2 ± 0.2 | 93 | 9.3 ± 0.2 | 100/91 | 9.0 ± 0.2 | 60/134 | |
| B. obeum | Fresh (t 0) | 7.9 ± 0.4 | 7.8 ± 0.4 | 79 | 8.1 ± 0.2 | 139 | |
| Processed (t 1) | 5.7 ± 0.7b | 1 | 7.1 ± 0.5a | 15/19 | 7.7 ± 0.4a | 57/41 | |
| Stored (t 2) | 4.5 ± 0.6b | 0.03 | 6.1 ± 0.4a , b | 2/2 | 7.0 ± 0.4a | 11/8 | |
| R. intestinalis | Fresh (t 0) | 9.0 ± 0.3 | 8.9 ± 0.4 | 82 | 7.9 ± 0.2a | 9 | |
| Processed (t 1) | 8.4 ± 0.4b | 27 | 8.4 ± 0.3 | 27/33 | 8.3 ± 0.3 | 23/267 | |
| Stored (t 2) | 7.1 ± 0.2b | 1 | 7.7 ± 0.3a , b | 6/7 | 8.3 ± 0.2a | 21/247 | |
| E. hallii | Fresh (t 0) | 8.4 ± 0.2 | 8.4 ± 0.2 | 100 | 8.3 ± 0.2 | 81 | |
| Processed (t 1) | 7.6 ± 0.1b | 16 | 7.8 ± 0.2b | 25/25 | 8.1 ± 0.3a | 52/64 | |
| Stored (t 2) | 6.8 ± 0.6b | 2 | 7.1 ± 0.4b | 6/6 | 7.9 ± 0.4a | 37/45 | |
| A. caccae | Fresh (t 0) | 8.8 ± 0.3 | 8.9 ± 0.2 | 122 | 8.6 ± 0.4 | 63 | |
| Processed (t 1) | 8.4 ± 0.2b | 39 | 8.9 ± 0.2a | 104/85 | 8.7 ± 0.2 | 69/109 | |
| Stored (t 2) | 8.5 ± 0.2 | 49 | 8.8 ± 0.3 | 96/78 | 8.8 ± 0.3 | 95/152 | |
| F. prausnitzii | Fresh (t 0) | 8.0 ± 0.2 | 8.3 ± 0.3 | 181 | 7.4 ± 0.3a | 27 | |
| Processed (t 1) | 7.9 ± 0.4 | 76 | 7.8 ± 0.3b | 64/36 | 7.1 ± 0.3a | 12/46 | |
| Stored (t 2) | 7.9 ± 0.2 | 80 | 7.6 ± 0.3b | 45/25 | 7.2 ± 0.2a | 16/60 | |
Viable cell counts in samples with cryoprotectants are significantly different from the control samples within the same condition (P ˂ 0.05).
Viable cell counts after processing and after storage are significantly different from the viable cell counts of the fresh culture within the same treatment (P ˂ 0.05).
Table 2.
Impact of protectants and cryopreservation on fitness of fresh (t 0), processed (t 1) and stored (t 2) bacteria. Lag phase (t lag) and maximum growth rate (µmax) of gut microbes after freezing in liquid nitrogen and cryopreservation for 3 months at −80°C were calculated from optical density growth curves based on Baranyi's equation
| Organism | Culture condition | Control | SI | GSI | |||
|---|---|---|---|---|---|---|---|
| t lag (h) | µmax (OD*h−1) | t lag (h) | µmax (OD*h−1) | t lag (h) | µmax (OD*h−1) | ||
| B. thetaiotaomicron | Fresh (t 0) | 0.7 ± 0.1 | 0.23 ± 0.01 | 0.7 ± 0.1 | 0.22 ± 0.01a | 1.3 ± 0.6a | 0.22 ± 0.01a |
| Processed (t 1) | 1.1 ± 0.1b | 0.22 ± 0.01 | 0.8 ± 0.1b | 0.20 ± 0.01a | 1.6 ± 0.2 | 0.20 ± 0.01a | |
| Stored (t 2) | 0.8 ± 0.3 | 0.18 ± 0.04b | 0.5 ± 0.3 | 0.16 ± 0.04b | 1.2 ± 0.7 | 0.17 ± 0.04b | |
| B. obeum | Fresh (t 0) | 6.4 ± 2.2 | 0.14 ± 0.02 | 7.0 ± 2.1 | 0.14 ± 0.02 | 7.3 ± 1.1 | 0.13 ± 0.03 |
| Processed (t 1) | 15.1 ± 1.0b | 0.23 ± 0.01b | 13.7 ± 2.5b | 0.17 ± 0.05 | 13.0 ± 2.5b | 0.11 ± 0.02a | |
| Stored (t 2) | 19.7 ± 0.8b | 0.26 ± 0.03b | 13.5 ± 0.7a , b | 0.21 ± 0.03a , b | 16.1 ± 2.1a , b | 0.21 ± 0.03a , b | |
| R. intestinalis | Fresh (t 0) | 1.6 ± 0.1 | 0.29 ± 0.02 | 1.6 ± 0.1 | 0.28 ± 0.01 | 3.3 ± 0.1a | 0.26 ± 0.01 |
| Processed (t 1) | 3.2 ± 0.3b | 0.25 ± 0.01b | 3.1 ± 0.1 | 0.25 ± 0.01b | 3.1 ± 0.1 | 0.27 ± 0.00a | |
| Stored (t 2) | 9.2 ± 0.4b | 0.22 ± 0.02b | 4.7 ± 0.4a , b | 0.18 ± 0.02a , b | 4.4 ± 0.5a | 0.22 ± 0.01b | |
| E. hallii | Fresh (t 0) | 1.7 ± 0.8 | 0.36 ± 0.04 | 1.5 ± 0.0 | 0.34 ± 0.02 | 1.3 ± 0.2 | 0.23 ± 0.02a |
| Processed (t 1) | 4.1 ± 0.2 | 0.43 ± 0.09 | 3.4 ± 0.6a | 0.41 ± 0.11 | 1.9 ± 0.6a | 0.21 ± 0.04a | |
| Stored (t 2) | 8.1 ± 2.5b | 0.47 ± 0.03b | 7.3 ± 1.3a , b | 0.43 ± 0.03a , b | 5.3 ± 0.8a , b | 0.30 ± 0.02a , b | |
| A. caccae | Fresh (t 0) | 1.5 ± 0.1 | 0.20 ± 0.03 | 1.3 ± 0.2 | 0.19 ± 0.03 | 2.0 ± 0.3 | 0.21 ± 0.02 |
| Processed (t 1) | 2.4 ± 0.2b | 0.15 ± 0.03b | 1.8 ± 0.5b | 0.17 ± 0.02 | 2.2 ± 0.1a | 0.13 ± 0.02b | |
| Stored (t 2) | 3.3 ± 0.6b | 0.16 ± 0.02 | 2.2 ± 0.2a , b | 0.16 ± 0.00b | 2.1 ± 0.3a | 0.14 ± 0.01b | |
| F. prausnitzii | Fresh (t 0) | 1.3 ± 0.1 | 0.05 ± 0.00 | 1.4 ± 0.1 | 0.05 ± 0.00 | 3.6 ± 0.1a | 0.05 ± 0.00a |
| Processed (t 1) | 2.5 ± 0.2 | 0.05 ± 0.01 | 3.3 ± 0.2a | 0.06 ± 0.00 | 4.5 ± 0.3a | 0.06 ± 0.00a | |
| Stored (t 2) | 5.0 ± 1.1b | 0.02 ± 0.00b | 5.4 ± 1.4b | 0.03 ± 0.00 | 5.5 ± 1.2b | 0.03 ± 0.00a | |
Lag phase or growth rate in samples with cryoprotectants is significantly different from the control samples within the same condition (P ˂ 0.05).
Lag phase or growth rate after processing and after storage is significantly different (B) from the fresh culture within the same treatment (P ˂ 0.05).
Bacterial response to cryopreservation in the absence of protectants
The effect of cryopreservation and frozen storage at −80°C for 3 months on viability and fitness of the investigated strains was evaluated by comparing processed (t 1) and stored (t 2) control cultures lacking protectants with the fresh control culture (t 0) (Fig. 1).
R. intestinalis, E. hallii and B. obeum were strongly impacted by cryopreservation, indicated by significantly lower viable cell counts and increased t lag in the processed and stored samples compared to fresh control cultures (Table 1). R. intestinalis and E. hallii exhibited a 100‐fold lower MPN after storage (7.1 ± 0.2 and 6.8 ± 0.6 log ml−1, respectively) compared to the fresh control (9.0 ± 0.3 and 8.4 ± 0.2 log ml−1, respectively), along with a fivefold to sixfold increased t lag (Table 2). The proportion of intact E. hallii cells declined from 71 ± 9% in fresh to 3 ± 0% in stored control culture. B. obeum was the most sensitive strain towards freezing. Its MPN was strongly reduced from 7.9 ± 0.4 log ml−1 in fresh to 5.7 ± 0.7 log ml−1 after processing (Table 1), and a further decline after storage (4.5 ± 0.6 log ml−1). Consistently, t lag significantly increased from 6.4 ± 0.2 h in the fresh to 19.7 ± 0.8 h in the stored B. obeum culture. The effect of cryopreservation on μmax was species‐dependent. B. obeum and E. hallii exhibited an increase and R. intestinalis a decrease of μmax after processing and storage (0.26 ± 0.03, 0.47 ± 0.03 and 0.22 ± 0.02 OD unit h−1, respectively) compared to fresh (0.14 ± 0.03, 0.36 ± 0.04 and 0.29 ± 0.02 OD unit h−1, respectively) (Table 2).
B. thetaiotaomicron, F. prausnitzii and A. caccae were less impacted by freezing and storage as indicated by stable or little changed MPN of fresh, processed and stored cultures (Table 1). B. thetaiotaomicron was least sensitive, as the percentage of intact cells was not affected during storage (Fig. 2) although t lag was slightly but significantly increased after freezing (1.1 ± 0.1 h) compared to fresh culture (0.7 ± 0.1 h). In contrast, cryopreservation and storage reduced the fraction of intact F. prausnitzii and A. caccae cells from 83 ± 7% and 63 ± 8% in the fresh to 27 ± 4% and 10 ± 3%, respectively, in the stored samples, along with a significantly increase of t lag (Table 2). A decrease of μmax from fresh to processed and stored cultures was measured for all three strains (Table 2).
Figure 2.
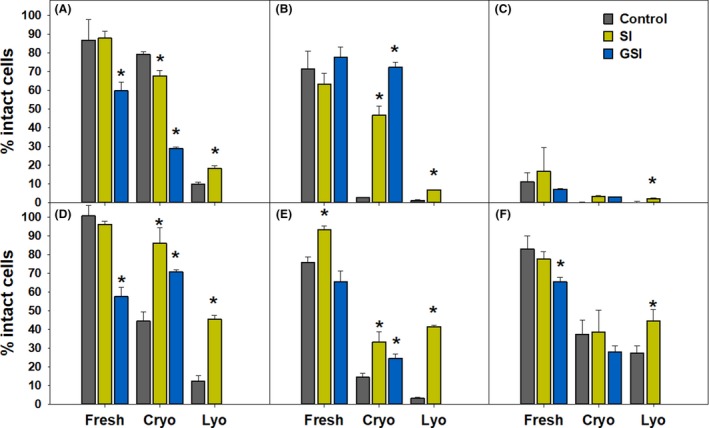
Impact of cryoprotectants on membrane integrity of cryopreserved and lyophilized strict anaerobes. Percentage of intact cells in fresh (t 0), and cryopreserved (Cryo) and lyophilized (Lyo) B. thetaiotaomicron (A), E. hallii (B), B. obeum (C), R. intestinalis (D), A. caccae (E) and F. prausnitzii (F) after 3 months storage in control (no protectant) and treated cultures (t2) (SI and GSI).
Impact of protectants on cryopreserved cultures
The protective effect of the non‐penetrating agents inulin and sucrose alone (SI) or in combination with the penetrating glycerol (GSI) on viability and fitness of cryopreserved strains was evaluated during freezing and storage at −80°C. Processed (t 1) and stored (t 2) SI‐ and GSI‐treated cultures were compared to fresh cultures (t 0) as well as processed (t 1) and stored (t 2) control samples without protectants.
SI improved viability and fitness of the stored freezing‐sensitive strains, while glycerol in the protective medium (GSI) further enhanced the protective effect (Fig. S1, Tables 1 and 2). The viable cell counts of R. intestinalis and B. obeum stored in SI (7.7 ± 0.3 and 6.1 ± 0.4 log ml−1) and GSI (8.3 ± 0.2 and 7.0 ± 0.4 log ml−1) were significantly higher than without protectants (7.1 ± 0.2 and 4.5 ± 0.6 log ml−1). Both strains exhibited similar t lag with SI and GSI which was significantly lower than in the stored control (Table 2). Viable cell counts and t lag of stored E. hallii were also increased and decreased, respectively, with GSI (7.9 ± 0.4 log ml−1 and 5.3 ± 0.8 h) compared to the control (6.8 ± 0.6 log ml−1 and 8.1 ± 2.5 h), and no effect was shown with SI. The highest fraction of intact cells was obtained with GSI (73 ± 3%), compared to SI (47 ± 5%) and the stored control (3 ± 0%). Unexpectedly, μmax of GSI‐treated E. hallii after storage (0.30 ± 0.02 OD unit h−1) was significantly lower than for the control (0.47 ± 0.03 OD unit h−1).
The positive impact of protectants was less distinct during processing than during storage. Only small differences in viability and t lag of the freezing‐sensitive strains were observed between the processed control, SI‐ and GSI‐treated samples, except for B. obeum with higher MPN in SI (7.1 ± 0.5 log ml−1) and GSI (7.7 ± 0.04 log ml−1) than the control (5.7 ± 0.7 log ml−1).
Viability and fitness of B. thetaiotaomicron, F. prausnitzii and A. caccae, which were less sensitive towards cryopreservation, were little impacted by the addition of SI and GSI. MPNs of B. thetaiotaomicron and A. caccae did not differ between fresh, processed and stored cultures, independent from the addition of protectants. MPN of F. prausnitzii remained stable after cryopreservation and storage in the GSI and control samples, but were significantly lower in SI‐treated culture after storage (7.6 ± 0.3 log ml−1) compared to the fresh SI culture (8.3 ± 0.3 log ml−1).
Bacterial response to lyophilization in the absence of protectant
The effect of lyophilization and storage at 4°C for 3 months on viability and fitness of the tested strains was evaluated by comparing processed (t 1) and stored (t 2) with fresh cultures (t 0) in the absence of protectant (control).
Lyophilization more severely affected viability and growth than freezing only. Viable cell counts decreased 100‐fold, and t lag increased up to 14‐fold after processing but remained stable during storage of all strains except F. prausnitzii and B. obeum (Tables 3 and 4). F. prausnitzii was the least sensitive strain towards lyophilization as indicated by a recovery of 14% of initial viable cells (7.1 ± 0.4 log cells ml−1) and a relative high fraction of intact cells (27 ± 4%) in the lyophilized stored control (Fig. 2). In contrast, B. obeum was most sensitive, with a large drop of viability during processing (4.3 ± 1.2 log ml−1) and during storage (2.3 ± 2.1) compared to the fresh control cultures (7.9 ± 0.4 log ml−1). For all strains except B. obeum, μmax decreased between 14% and 60% after lyophilization and storage. A significant increase of μmax from 0.14 ± 0.02 in fresh to 0.27 ± 0.00 OD unit h−1 in stored culture was measured for B. obeum.
Table 3.
Impact of protectants and lyophilization on viable cell counts of fresh (t 0), processed (t 1) and stored (t 2) bacteria. Log viable cell counts ml−1 in the fresh culture, after lyophilization and storage for 3 months at 4°C, were assessed with the most probable number method (MPN). Recovery rate of viable cells (in %) was calculated relative to the average viable cell counts in the fresh control (vs. control t 0) and to fresh treatment culture (vs. t 0)
| Organism | Culture condition | Control | SI | ||
|---|---|---|---|---|---|
| MPN (ml−1) | Recovery vs. t 0 | MPN (ml−1) | Recover vs. control t 0/t 0 | ||
| B. thetaiotaomicron | Fresh (t 0) | 9.3 ± 0.2 | 9.4 ± 0.2 | 135 | |
| Processed (t 1) | 7.0 ± 0.5 | 1 | 8.0 ± 0.7b | 6/4 | |
| Stored (t 2) | 5.4 ± 2.0b | 0.01 | 7.7 ± 0.4a , b | 3/2 | |
| B. obeum | Fresh (t 0) | 7.9 ± 0.4 | 7.8 ± 0.4 | 79 | |
| Processed (t 1) | 4.3 ± 1.2 | 0.02 | 6.6 ± 0.2a , b | 4/5 | |
| Stored (t 2) | 2.3 ± 2.1b | 0.0003 | 5.5 ± 0.3a , b | 0.3/0.4 | |
| R. intestinalis | Fresh (t 0) | 8.9 ± 0.4 | 8.9 ± 0.4 | 82 | |
| Processed (t 1) | 6.3 ± 0.3b | 0.2 | 7.8 ± 0.4a , b | 7/8 | |
| Stored (t 2) | 6.2 ± 0.2b | 0.2 | 7.6 ± 0.2a , b | 4/5 | |
| E. hallii | Fresh (t 0) | 8.4 ± 0.2 | 8.4 ± 0.2 | 100 | |
| Processed (t 1) | 4.9 ± 0.4b | 0.04 | 7.1 ± 0.1a , b | 6/6 | |
| Stored (t 2) | 5.6 ± 0.2b | 0.2 | 6.8 ± 0.2a , b | 3/3 | |
| A. caccae | Fresh (t 0) | 8.8 ± 0.3 | 8.9 ± 0.2 | 111 | |
| Processed (t 1) | 6.4 ± 0.2b | 0.4 | 8.8 ± 0.3a | 87/71 | |
| Stored (t 2) | 6.7 ± 0.5b | 1 | 8.6 ± 0.3a | 60/49 | |
| F. prausnitzii | Fresh (t 0) | 8.0 ± 0.2 | 8.2 ± 0.2 | 181 | |
| Processed (t 1) | 6.1 ± 0.3b | 1 | 7.8 ± 0.4a , b | 58/32 | |
| Stored (t 2) | 7.1 ± 0.4b | 14 | 7.8 ± 0.1a , b | 59/33 | |
Viable cell counts in samples with lyoprotectants are significantly different from the control samples within the same condition (P ˂ 0.05).
Viable cell counts after processing and after storage are significantly different from the viable cell counts of the fresh culture within the same treatment (P ˂ 0.05).
Table 4.
Impact of protectants and lyophilization on fitness of fresh (t 0) processed (t 1) and stored (t 1) bacteria. Lag phase (t lag) and maximum growth rate (µmax) were calculated from optical density growth curves based on Baranyi's equation
| Organism | Culture condition | Control | SI | ||
|---|---|---|---|---|---|
| t lag (h) | µmax (OD*h−1) | t lag (h) | µmax (OD*h−1) | ||
| B. thetaiotaomicron | Fresh (t 0) | 0.7 ± 0.1 | 0.23 ± 0.01 | 0.7 ± 0.1 | 0.22 ± 0.01a |
| Processed (t 1) | 7.5 ± 0.9b | 0.19 ± 0.01b | 3.4 ± 1.2a , b | 0.18 ± 0.01 | |
| Stored (t 2) | 10.1 ± 3.5b | 0.16 ± 0.04b | 4.1 ± 1.9a , b | 0.14 ± 0.05a | |
| B. obeum | Fresh (t 0) | 6.4 ± 2.2 | 0.14 ± 0.02 | 7.0 ± 2.1 | 0.14 ± 0.02 |
| Processed (t 1) | 25.8 ± 0.3b | 0.25 ± 0.00b | 19.3 ± 2.1a , b | 0.21 ± 0.01a , b | |
| Stored (t 2) | 25.5 ± 1.6b | 0.27 ± 0.00b | 21.5 ± 2.0a , b | 0.22 ± 0.03a , b | |
| R. intestinalis | Fresh (t 0) | 1.6 ± 0.1 | 0.29 ± 0.02 | 1.6 ± 0.1 | 0.28 ± 0.01 |
| Processed (t 1) | 9.8 ± 0.4b | 0.30 ± 0.01 | 5.6 ± 1.1a | 0.26 ± 0.01a | |
| Stored (t 2) | 11.0 ± 0.5b | 0.25 ± 0.01b | 8.5 ± 0.4a , b | 0.21 ± 0.01a , b | |
| E. hallii | Fresh (t 0) | 1.7 ± 0.7 | 0.35 ± 0.04 | 1.3 ± 0.2 | 0.30 ± 0.07 |
| Processed (t 1) | 14.8 ± 0.2b | 0.27 ± 0.05b | 8.5 ± 0.7a , b | 0.37 ± 0.02a | |
| Stored (t 2) | 13.0 ± 1.3b | 0.21 ± 0.12b | 8.6 ± 0.2a , b | 0.32 ± 0.04 | |
| A. caccae | Fresh (t 0) | 1.5 ± 0.1 | 0.20 ± 0.03 | 1.3 ± 0.2 | 0.19 ± 0.03 |
| Processed (t 1) | 8.9 ± 0.9b | 0.25 ± 0.02 | 2.7 ± 0.1a , b | 0.16 ± 0.01a , b | |
| Stored (t 2) | 7.9 ± 0.3b | 0.21 ± 0.01 | 2.6 ± 0.4a , b | 0.15 ± 0.02a | |
| F. prausnitzii | Fresh (t 0) | 1.3 ± 0.1 | 0.05 ± 0.00 | 1.4 ± 0.1 | 0.05 ± 0.00 |
| Processed (t 1) | 6.6 ± 0.4b | 0.04 ± 0.01b | 3.3 ± 0.4a , b | 0.06 ± 0.01a | |
| Stored (t 2) | 5.7 ± 2.0 | 0.02 ± 0.01b | 2.8 ± 0.7a , b | 0.03 ± 0.01a | |
Lag phase or growth rate in samples with lyoprotectants is significantly different from the control samples within the same condition (P ˂ 0.05).
Lag phase or growth rate after processing and after storage is significantly different from the fresh culture within the same treatment (P ˂ 0.05).
Impact of protectants on lyophilized cultures
The protective effect of SI was evaluated on viability and fitness of lyophilized strains after processing (t 1) and storage at 4°C (t 2), and compared to the fresh cultures (t 0) as well as to processed and stored control samples with not added protectant.
The addition of SI significantly improved viability and fitness of all lyophilized strains (Fig. S1, Tables 3 and 4), with most effect on viability recorded for A. caccae and F. prausnitzii. Viable cell counts of A. caccae remained unchanged after processing (8.8 ± 0.3 log ml−1) and after 3‐month storage (8.6 ± 0.3 log ml−1, 34.3% intact cells) compared to the fresh SI samples (8.9 ± 0.2 log ml−1, 83 ± 6% intact cells). t lag of SI‐treated A. caccae and F. prausnitzii increased approximately twofold after processing (2.7 ± 0.1 and 2.6 ± 0.4 h, respectively) but was significantly lower than for the lyophilized and stored control (8.9 ± 0.9 and 7.9 ± 0.3 h, respectively). Viable cell counts of F. prausnitzii slightly but significantly decreased from 8.2 ± 0.2 log cells ml−1 in the fresh SI samples to 7.8 ± 0.4 log cells ml−1 after lyophilization, but remained stable during storage. t lag was twofold longer after storage (2.8 ± 0.7 h) than in the fresh SI samples, however, significantly shorter than for the lyophilized control (5.7 ± 2.0 h).
B. thetaiotaomicron, R. intestinalis and E. hallii treated with SI had similar viable cell counts after storage (7.7 ± 0.4, 7.6 ± 0.2 and 6.8 ± 0.2 log cells ml−1, respectively) and improved recovery rates of 3–4% viable cells compared to the control (0.01–0.2%). SI addition also significantly shortened t lag by 33–59% after lyophilization and storage compared to the protectant‐free control (Table 4). Viability remained generally stable during storage of all lyophilized cultures with a maximal loss of 0.3 log in MPN, except for B. obeum that showed a 1 log decrease in MPN and an increased μmax after processing and storage of SI‐treated samples.
Discussion
A major challenge in the production and formulation of strict anaerobic probiotics of gut origin is maintaining viability and fitness during processing and storage. In this study, we assessed the impact of the two main preservation methods, cryopreservation and lyophilization, and of subsequent storage, respectively, on viability and fitness of six strict anaerobic gut microbes.
Impact of cryopreservation, lyophilization and storage on viability
Cryopreservation was confirmed less detrimental to sensitive bacteria than lyophilization (Heylen et al., 2012), which combines freezing and drying steps, as indicated by higher viability and shorter t lag of cryopreserved compared to lyophilized samples directly after processing in the absence of cryoprotectant. During freezing, bacterial cells are exposed to two main stresses. Mechanical stress due to intra‐ and extracellular ice crystal formation and increased osmotic pressure caused by solutes in the remaining unfrozen fraction can potentially lead to the disruption of bacterial membranes and ultimately to lethal damage (Malik, 1991; Meryman, 2007). During lyophilization, the removal of water by sublimation further increases osmotic pressure and can cause severe damage to membranes and surface proteins (Broeckx et al., 2016). However, despite lower viable cell counts in the lyophilized cultures without protectant, viability was maintained during storage at 4°C while viability of the corresponding cryopreserved cultures declined at −80°C, especially for the freezing‐sensitive B. obeum, R. intestinalis and E. hallii. Biochemical reactions occurring when free water is present in cells stored above −80°C can cause viable cell loss over time (Mazur, 1970, 1984). As a consequence, storage in electrical freezers will not guarantee indefinite viability of cryopreserved cells (Heylen et al., 2012).
Another important factor for stability of strict anaerobes is oxidative stress. The ability to tolerate oxygen differed between the tested microbes. An aerotolerance test identified R. intestinalis, F. prausnitzii and E. hallii as highly oxygen‐sensitive and A. caccae as most oxygen‐tolerant strain withstanding exposure to ambient air up to 60 min (Flint et al., 2007). Faecalibacterium prausnitzii can survive in the presence of low oxygen concentrations using an ‘extracellular electron shuttle’ over antioxidants that reduce oxygen (Khan et al., 2012). Bacteroides thetaiotaomicron also expresses defence mechanisms against oxygen by scavenging enzymes that prevent rapid formation of reactive oxygen species and facilitates recovery from oxygen exposure (Pan and Imlay, 2001; Mishra and Imlay, 2013). By adding riboflavin and cysteine HCl in the buffer formulation, we induced a reducing environment protecting bacteria from oxygen exposure during storage; nevertheless, highly sensitive strains might require complete anaerobiosis (Khan et al., 2014). Higher oxygen tolerance of A. caccae, F. prausnitzii and B. thetaiotaomicron may explain their enhanced stability during storage at −80°C in partly oxygen‐permeable screw‐cap polypropylene cryo tubes. Improved stability of the lyophilized compared to cryopreserved samples might also be partly due to the absence of oxygen during storage through vacuum‐sealed glass ampules. As suggested by Malik (1991), storage stability of strict anaerobes during cryopreservation can be improved using glass vials with oxygen‐impermeable butyl rubber septa.
Impact of protectants on viability and growth of fresh cultures
Prior preservation, fresh cultures were incubated for 30 min in protective media containing 2.0 M glycerol, 150 mM sucrose and 40 mM inulin (calculated as fructose equivalents). Solutes used as protectants can cause growth inhibition due to osmotic pressure when present in growth medium at concentrations of > 1.0 M sucrose and ≥ 1.5 M glycerol (Cebrian et al., 2014). Membranes, destabilized after exposure to osmotic pressure, are suggested to cause cell death by phase transition of membrane phospholipids in interaction with volume changes of the cells (Mille et al., 2005). Glycerol in the protective buffer already decreased survival and the proportion of intact cells of fresh B. thetaiotaomicron, R. intestinalis and F. prausnitzii cultures after 30 min incubation. The minimal inhibitory concentration of glycerol in growth medium (1.5–2.4 M) was found to be generally lower in Gram‐negative than Gram‐positive bacteria. It was also suggested that osmotolerance might be strain specific (Saegeman et al., 2008; Cebrian et al., 2014).
The presence of glycerol in the protective solution reduced μmax of E. hallii compared to the SI treatment and control without glycerol. These data may be explained by the ability of E. hallii to convert glycerol to reuterin (Engels et al., 2016a). Reuterin is a broad‐spectrum antimicrobial system, which, at physiological conditions, mainly consists of 3‐hydroxypropionaldehyde (3‐HPA), its hydrate and dimer, and acrolein (Engels et al., 2016b). Reuterin can inhibit growth of the producer strain. In the growth assessment tests, 10% (v/v) inoculation with GSI‐treated cultures transferred 20 mM glycerol to the YCFA medium. Hence, formation of reuterin could be responsible for reduced μmax of E. hallii, which is supported by the absence of an effect on μmax when inoculation with the glycerol containing culture was performed at only 1% v/v (data not shown).
Fresh cultures incubated in protective media containing sucrose (150 mM) and inulin (40 mM) were not negatively affected in terms of viability or growth. The concentration of sucrose was likely too low to induce a significant osmotic stress. Moderate osmotic stress was shown to occur at much higher concentration of sucrose (730 mM) for Lactobacillus delbrueckii (Meneghel et al., 2017). In another study, growth inhibition of Staphylococcus aureus, Listeria monocytogenes, Cronobacter sakazakii, Enterococcus faecium, Escherichia coli and Salmonella Typhimurium was observed in growth medium supplemented with sucrose concentrations ranging from 1.1 to 1.8 M (Cebrian et al., 2014).
Impact of protectants on viability and growth of preserved cultures
The addition of protectants positively influenced viability and membrane integrity of the freezing‐sensitive R. intestinalis, E. hallii and B. obeum. It has been proposed that glycerol prevents intracellular ice crystal formation at high freezing rates when bacterial cells are immersed in liquid nitrogen (Fonseca et al., 2006). The protective action of glycerol during freezing and storage also outweighed its detrimental osmotic effect observed in fresh R. intestinalis cultures, and the potential growth inhibition of reuterin produced by E. hallii. Glycerol in the protective media exhibited greater protection than sucrose and inulin alone. However, the two protectants might have acted synergistically in combination with glycerol leading to better recovery of viable cells. The observed positive impact of glycerol on R. intestinalis on viability and growth performance is in accordance with earlier findings of its protective effect on the re‐establishment of Roseburia sp./E. rectale group when cryopreserved as part of a complex artificial gut microbiota (Bircher et al., 2017).
B. thetaiotaomicron, F. prausnitzii and A. caccae were little impacted by freezing and storage. Processing conditions used in this study, characterized by a high freezing rate (immersion in liquid nitrogen) and storage temperature of −80°C, contributed to the stability of these strains, as previously reported for lactic acid‐producing starter cultures (Fonseca et al., 2001). In agreement, only limited effects were observed on viability and the proportion of intact cells when protectants were added, and the detrimental effect of glycerol on viability of F. prausnitzii was still observed after freezing and storage.
The addition of SI protected cell viability during lyophilization of all strains but had little impact on storage stability. This implies that sucrose and inulin mainly protected viability during the lyophilization process. Sucrose and inulin both interact with biological membranes and stabilize during freezing and drying (Demel et al., 1998; Vereyken et al., 2003; Schwab et al., 2007), and in agreement, the proportion of cells with integer membranes was higher if SI was present during lyophilization compared to controls.
Impact of preservation on bacterial fitness
Fitness is another important marker for preservation of bacteria, which can be evaluated by μmax and t lag as a measure of reproductive potential (Sandegren et al., 2008; Pope et al., 2010; Adkar et al., 2017). For all tested strains, t lag negatively correlated with viable cell numbers (Fig. S1). Alteration of generation times, a known reaction to stress exposure, has been reported before for frozen and stored bacterial cells (Squires and Hartsell, 1955; Lipson, 2015; Adkar et al., 2017). Indeed, μmax of B. thetaiotaomicron, R. intestinalis, A. caccae and F. prausnitzii generally decreased after preservation with only small differences between treatments and control. In contrast, cryopreserved E. hallii, and lyophilized and cryopreserved B. obeum exhibited increased μmax, particularly for the treatments characterized by low viability. Stress exposure during lyophilization and cryopreservation, which was lethal to the majority of the cells, might have selected for drying and/or freezing resistant subpopulations (Patra and Klumpp, 2013; Wang et al., 2014).
Enhanced stress resistance of bacteria can be an advantage for probiotic applications. In contrast, reduced bacterial fitness leading to slower growth may impair the ability of preserved bacteria to multiply and be metabolically active in the gastrointestinal tract. Still, further evaluation is needed to better understand the interplay between t lag, μmax and viability in terms of probiotic application success. As an example, higher viability of cryopreserved E. hallii due to the presence of glycerol might compensate for the lower μmax. We recently reported that E. hallii was little impacted by freezing within a complex artificial gut microbiota, as indicated by comparable growth of fresh and preserved microbiota in a standardized growth assay (Bircher et al., 2017). Our current data suggest that the enhanced μmax of E. hallii after cryopreservation could explain its competitiveness in batch fermentation. Furthermore, process‐impacted bacterial fitness can be a determinant for the selection of a preservation method that offers best conditions for probiotic re‐establishment in vivo. F. prausnitzii, for example, exhibited a shorter t lag but similar viable cell counts after lyophilization than after cryopreservation when SI was used as protectant, suggesting lyophilization as favourable preservation method for this strain.
Conclusion
To our best knowledge, this is the first study to date that quantitatively compared the effect of cryopreservation and lyophilization and the addition of selected protectants on viability and fitness of six strict anaerobic gut microbes. Viable cell recovery ranged from 11% to 100% after frozen and from 0.3% to 60% after dried storage, pointing towards a strong species‐dependent resistance to freezing and freeze‐drying. Membrane composition might be a determining factor as the addition of membrane‐interacting protectants sucrose and inulin improved viability of all lyophilized strain and of freezing‐sensitive strains after cryopreservation. As glycerol also differently affected strain viability and membrane integrity prior and postcryopreservation, our results suggest that selection of protectants has to be process‐ and species‐specific. Based on our results, we recommend using cryopreservation with GSI for B. obeum, R. intestinalis, E. hallii and A. caccae and SI for B. thetaiotaomicron. F. prausnitzii should be preferably preserved by lyophilization with SI.
Experimental procedures
Bacterial strains and culture conditions
B. thetaiotaomicron DSM 2079, B. obeum DSM 25238, R. intestinalis DSM 14610, E. hallii DSM 3353, A. caccae DSM 14662 and F. prausnitzii DSM 17677 were obtained from the Deutsche Sammlung für Mikroorganismen und Zellkulturen (Braunschweig, Germany). Bacterial pellets from 1 ml overnight growing cultures were snap‐frozen in 100 μl phosphate buffer (pH 6.8, 0.1 M) (PB, Table S1) supplemented with glycerol (15% v/w) (VWR International AG, Dietikon, Switzerland) and stored at −80°C (stock cultures). For each experiment, a fresh stock culture was thawed in an anaerobic chamber (10% CO2, 5% H2 and 85% N2) (Coy Laboratories, Grass Lake, Michigan, USA) and re‐suspended with 900 μl phosphate‐buffered saline (0.8% v/w, pH 6.8) (PBS, Table S2) to a final volume of 1 ml. Half a millilitre of reactivated culture was transferred to 10 ml yeast extract, casitone and fatty acid medium (YCFA) in a Hungate tube and incubated anaerobically for 10 h at 37°C to obtain a working culture. YCFA medium was prepared as described previously (Duncan et al., 2003) with slight modifications. Glucose (6 g l−1, Sigma‐Aldrich, Buchs, Switzerland) was added as sole carbon source. All components except cysteine HCl (0.01% v/w, Sigma‐Aldrich) were dissolved in deionized water, and pH was adjusted to 7.4 with 2.5 N NaOH to obtain a pH of 6.8 after autoclaving. The medium was boiled while flushing with CO2 until a colour change from blue to pink occured, caused by the addition of the indicator resazurin (0.1% of 1 mg ml−1 stock solution). Cysteine HCl was then added, and the medium was dispensed in Hungate tubes flushed with CO2 before autoclaving.
Preparation of protective buffers
All components of the PB (0.1 M, prepared in oxygen‐free distilled water) were placed in an anaerobic chamber overnight to remove traces of oxygen. Reducing agents cysteine HCl and riboflavin (Sigma‐Aldrich) were added at final concentrations of 1 g l−1 and 0.3 g l−1, respectively, to protect the bacteria from potential oxygen exposure during processing and storage (Khan et al., 2014). The pH was adjusted to 6.8 and buffers were filter‐sterilized, covered in aluminium foil as light protection and stored in an anaerobic chamber until usage.
Two protective buffers were prepared by dissolving in PB sucrose (VWR International AG) and inulin (RPN Foodtechnology AG, Sursee, Switzerland) (both 5% w/v) (SI) and adding glycerol (15% v/w) to SI (GSI). PB that only contained cysteine HCl and riboflavin served as protectant‐free control.
Cryopreservation, lyophilization and storage
Two independent preservation experiments were conducted for each bacterium. Within an experiment, fresh, processed and stored samples of each treatment were analysed in triplicates. All processing steps were either executed in an anaerobic chamber or in Hungate tubes to guarantee anoxic conditions. A working culture was generated by incubating a reactivated glycerol stock culture for 10 h at 37°C in YCFA medium. This working culture was once subcultured under the same conditions to obtain a viable and active culture for preservation trials (experimental culture). For the production of experimental cultures, 10 ml YCFA medium were inoculated at 0.2% (v/v) with a working culture of B. thetaiotaomicron, R. intestinalis, E. hallii and A. caccae or at 2% (v/v) for the slow‐growing strains F. prausnitzii and B. obeum. Cultures were incubated at 37°C for 13 to 15 h, depending on the strain, until early stationary growth phase was reached. Incubation times were assessed in preliminary growth tests in Hungate tubes at 37°C. Optical density (OD) at 600 nm was monitored during incubation (data not shown). Cells were harvested by centrifugation at 4°C for 10 min at 3000 g. The pellet was washed in 5 ml PB, centrifuged and re‐suspended in either 1 ml control, SI or GSI buffer (10‐fold concentration of the initial experimental culture). After an incubation time of 30 min at room temperature to allow penetration of glycerol, aliquots (100 μl) were snap‐frozen in liquid nitrogen and either stored at −80°C in screw‐cap polypropylene cryotubes (Bioswisstec AG, Schaffhausen, Switzerland) (control, SI and GSI cultures, Fig. 1) or dried with a manifold freeze‐dryer (VirTis BenchTop 2K, MultiTemp Scientific AG, Kloten, Switzerland). Lyophilization of the control and SI‐treated cultures was carried out in long‐stem Vacule cryogenic ampules (Sigma‐Aldrich) that were prereduced in an anaerobic chamber, plugged with sterile cotton wool, and contained blue silica gel with moisture indicator (Sigma‐Aldrich). Frozen samples in cryogenic ampules were placed on dry‐ice prior lyophilization to prevent thawing of the culture until vacuum was started. Freeze‐drying was conducted at a condenser temperature of −80°C at 80 mTorr for 6 h after which ampules were flame sealed under vacuum and stored at 4°C.
Viability assessment and growth tests were conducted under anaerobic conditions at three different time points (Fig. 1): first with the fresh culture after incubation in control and protective buffers (t 0), then with the processed culture immediately after freezing, respectively, lyophilization (t 1), and finally with the stored culture after 3 months at −80°C and 4°C for cryopreservation and lyophilization, respectively (t 2). Each experimental condition was duplicated, and triplicate samples of each treatment were analysed. Prior to the viability and growth tests, fresh cultures (t 0) were re‐suspended in 900 μl PBS immediately after incubation in the buffers. Cryopreserved cultures (t 1 and t 2) were transferred from −80°C freezer to an anaerobic chamber and thawed at room temperature before re‐suspending in 900 μl PBS buffer. Lyophilized cultures (t 1 and t 2) were rehydrated in 1 ml PBS for 1 h in an anaerobic chamber.
Measurement of viability
Viable cell counts of were assessed by the most probable number (MPN) method with a five‐replicate design (Sutton, 2010) that was adapted to 96‐well microtiter plates (Kuai et al., 2001). Before use, plates (Bioswisstec AG) were stored overnight in the anaerobic chamber to remove traces of oxygen. Samples were serially diluted tenfold, and 20 μl of each dilution was used to inoculate 5 wells, each containing 180 μl YCFA medium. Plates were incubated at 37°C for 48 h in an anaerobic chamber. Wells with visible turbidity were scored as growth positive.
Determination of membrane integrity
Membrane integrity of fresh and stored samples was determined with two fluorescence stains and subsequent flow cytometric analysis (Van Nevel et al., 2013). All dilution, staining and incubation steps were performed in an anaerobic chamber while flow cytometric analysis was conducted at ambient air. Fluorescence working solutions were prepared as follows: 10 μl SYBR Green I (SG; 10 000× concentrated) (Life Technologies, Zug, Switzerland) was diluted in 990 μl filtered dimethyl sulphoxide (DMSO) (Sigma‐Aldrich). Twenty microlitre propidium iodide (PI; 20 mM) (Life Technologies) and 10 μl SG were diluted in 970 μl DMSO. Solutions were stored at −20°C until use. Each bacterial sample was diluted to approximately 107 cells ml−1 with PBS and stained twice with SG to assess total cell counts, or with SG combined with PI to determine intact cell counts. Therefore, 30 μl diluted sample, 3 μl stain working solution and 237 μl PBS were incubated for 22 min at 37°C in the dark. PBS stained with SG and PI was used to determine background fluorescence. Prior to flow cytometric analysis, 30 μl of Flow‐Count fluorospheres (Beckman Coulter International SA, Nyon, Switzerland) was added at known concentrations to determine bacterial cell counts. Samples were analysed with a Cytomics FC 500 (Beckman Coulter International SA) equipped with an air‐cooled argon ion laser emitting 20 mW at 488 nm and a red solid state diode laser emitting 25 mW at 633 nm. Gates on green against red fluorescence plots were used to assess total and intact cell counts. The percentage of intact cells were calculated by dividing the intact cell number in the green gate of the SG and PI by the total cell number in the SG‐stained sample.
Assessment of growth performance
Growth was assessed in 96‐well microtiter plates in anaerobic conditions, as described previously (Eini et al., 2013; Geirnaert et al., 2014). Inner wells of a 96‐well plate, stored overnight in an anaerobic chamber, were filled with 180 μl YCFA medium. Wells were inoculated with 20 μl (10% v/v) of the tested culture samples. Empty wells in outer rows and columns were filled with the reducing agent of an AnaeroGen bag (Thermo Fisher Diagnostics AG, Pratteln, Switzerland) to maintain an oxygen‐free atmosphere when plates were moved outside of the anaerobic chamber. Plates were covered with a ClearSeal film (Labgene Scientific Instruments, Châtel‐Saint‐Denis, Switzerland) and the lid, which was sealed with petroleum jelly. Growth was monitored at 37°C by measuring OD at 600 nm every 30 minutes in a microplate reader (Powerwave XS). The addition of the indicator resazurin in the YCFA medium confirmed anaerobiosis in the 96‐well plates during measurements. The average OD value of six wells containing sterile YCFA medium served as blank and was subtracted from OD values of inoculated wells. To calculate maximum growth rate (μmax) and lag phase (t lag), growth curves were fitted using the DMFit 3.5 program (Institute of Food Research, Norwich, UK) based on Baranyi's equation (Baranyi and Roberts, 1994).
Statistics
Statistical analysis of viable cell counts (log10‐transformed), percentage of intact cells by flow cytometry, μmax and t lag were performed using R studio version 3.4.1 (Boston, Massachusetts, USA). Data are expressed as mean ± SD of six replicates obtained from two independent preservation experiments conducted on two different days (with three replicates each).
ANOVA tests were performed with viable cell counts, μmax and t lag of fresh, cryopreserved and lyophilized samples as dependant variables and either treatment or time point within a treatment as independent variables. Data were tested for normal distribution using the Shapiro–Wilk test, and equality of variance was assessed with the Levene test. Tukey HSD test (multiple pairwise comparison) was used to compare treatments to control (no added protectants) and preserved and stored to fresh samples. A nonparametric Kruskal–Wallis test was performed when data were not normally distributed or the assumption of equality of variance was violated. Student's t‐test was performed to compare means of viable cell counts, μmax and t lag of fresh and lyophilized control with SI‐treated samples. Data were tested for homogeneity of variance with the F‐test. Differences were considered significant for α ≤ 0.05.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Fig. S1. Correlation plots between viable cell counts and lag times (t lag).
Table S1. Composition of phosphate buffer.
Table S2. Composition of phosphate buffered saline.
Table S3. Composition of YCFA medium.
Acknowledgements
We thank Nicole Viti for experimental assistance.
Microbial Biotechnology (2018) 11(4), 721–733
Funding Information
Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Sinergia 35150); ETHIRA (39 13‐2, ETH‐Zurich).
References
- Abadias, M. , Benabarre, A. , Teixido, N. , Usall, J. , and Vinas, I. (2001) Effect of freeze drying and protectants on viability of the biocontrol yeast Candida sake . Int J Food Microbiol 65: 173–182. [DOI] [PubMed] [Google Scholar]
- Adkar, B.V. , Manhart, M. , Bhattacharyya, S. , Tian, J. , Musharbash, M. , and Shakhnovich, E.I. (2017) Optimization of lag phase shapes the evolution of a bacterial enzyme. Nat Ecol Evol 1: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranyi, J. , and Roberts, T.A. (1994) A dynamic approach to predicting bacterial growth in food. Int J Food Microbiol 23: 277–294. [DOI] [PubMed] [Google Scholar]
- Bircher, L. , Schwab, C. , Geirnaert, A. , and Lacroix, C. (2017) Cryopreservation of artificial gut microbiota produced with in vitro fermentation technology. Microb Biotechnol 11: 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeckx, G. , Vandenheuvel, D. , Claes, I.J. , Lebeer, S. , and Kiekens, F. (2016) Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. Int J Pharm 505: 303–318. [DOI] [PubMed] [Google Scholar]
- Cebrian, G. , Arroyo, C. , Manas, P. , and Condon, S. (2014) Bacterial maximum non‐inhibitory and minimum inhibitory concentrations of different water activity depressing solutes. Int J Food Microbiol 188: 67–74. [DOI] [PubMed] [Google Scholar]
- Demel, R.A. , Dorrepaal, E. , Ebskamp, M.J. , Smeekens, J.C. , and de Kruijff, B. (1998) Fructans interact strongly with model membranes. Biochim Biophys Acta 1375: 36–42. [DOI] [PubMed] [Google Scholar]
- Duncan, S.H. , Scott, K.P. , Ramsay, A.G. , Harmsen, H.J. , Welling, G.W. , Stewart, C.S. , and Flint, H.J. (2003) Effects of alternative dietary substrates on competition between human colonic bacteria in an anaerobic fermentor system. Appl Environ Microbiol 69: 1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eini, A. , Sol, A. , Coppenhagen‐Glazer, S. , Skvirsky, Y. , Zini, A. , and Bachrach, G. (2013) Oxygen deprivation affects the antimicrobial action of LL‐37 as determined by microplate real‐time kinetic measurements under anaerobic conditions. Anaerobe 22: 20–24. [DOI] [PubMed] [Google Scholar]
- El Hage, R. , Hernandez‐Sanabria, E. , and Van de Wiele, T. (2017) Emerging trends in “smart probiotics”: functional consideration for the development of novel health and industrial applications. Front Microbiol 8: 1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels, C. , Ruscheweyh, H.J. , Beerenwinkel, N. , Lacroix, C. , and Schwab, C. (2016. a) The common gut microbe Eubacterium hallii also contributes to intestinal propionate formation. Front Microbiol 7: 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels, C. , Schwab, C. , Zhang, J. , Stevens, M. , Bieri, C. , Sturla, S. , and Lacroix, C. (2016. b) Acrolein strongly contributes to antimicrobial and heterocyclic transformation activities of reuterin. Sci Rep 6: 36246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint, H.J. , Duncan, S.H. , Scott, K.P. , and Louis, P. (2007) Interactions and competition within the microbial community of the human colon: links between diet and health. Environ Microbiol 9: 1101–1111. [DOI] [PubMed] [Google Scholar]
- Fonseca, F. , Beal, C. , and Corrieu, G. (2001) Operating conditions that affect the resistance of lactic acid bacteria to freezing and frozen storage. Cryobiology 43: 189–198. [DOI] [PubMed] [Google Scholar]
- Fonseca, F. , Marin, M. , and Morris, G.J. (2006) Stabilization of frozen Lactobacillus delbrueckii subsp. bulgaricus in glycerol suspensions: freezing kinetics and storage temperature effects. Appl Environ Microbiol 72: 6474–6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, A. , and Toner, M. (2005) Cryo‐injury and biopreservation. Ann N Y Acad Sci 1066: 119–135. [DOI] [PubMed] [Google Scholar]
- Geirnaert, A. , Steyaert, A. , Eeckhaut, V. , Debruyne, B. , Arends, J.B. , Van Immerseel, F. , et al (2014) Butyricicoccus pullicaecorum, a butyrate producer with probiotic potential, is intrinsically tolerant to stomach and small intestine conditions. Anaerobe 30: 70–74. [DOI] [PubMed] [Google Scholar]
- Heylen, K. , Hoefman, S. , Vekeman, B. , Peiren, J. , and De Vos, P. (2012) Safeguarding bacterial resources promotes biotechnological innovation. Appl Microbiol Biotechnol 94: 565–574. [DOI] [PubMed] [Google Scholar]
- Hsiao, A. , Ahmed, A.M. , Subramanian, S. , Griffin, N.W. , Drewry, L.L. , Petri, W.A. Jr , et al (2014) Members of the human gut microbiota involved in recovery from Vibrio cholerae infection. Nature 515: 423–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubalek, Z. (2003) Protectants used in the cryopreservation of microorganisms. Cryobiology 46: 205–229. [DOI] [PubMed] [Google Scholar]
- Khan, M.T. , Duncan, S.H. , Stams, A.J. , van Dijl, J.M. , Flint, H.J. , and Harmsen, H.J. (2012) The gut anaerobe Faecalibacterium prausnitzii uses an extracellular electron shuttle to grow at oxic‐anoxic interphases. ISME J 6: 1578–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, M.T. , van Dijl, J.M. , and Harmsen, H.J. (2014) Antioxidants keep the potentially probiotic but highly oxygen‐sensitive human gut bacterium Faecalibacterium prausnitzii alive at ambient air. PLoS ONE 9: e96097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuai, L. , Nair, A.A. , and Polz, M.F. (2001) Rapid and simple method for the most‐probable‐number estimation of arsenic‐reducing bacteria. Appl Environ Microbiol 67: 3168–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie, S.B. , Israeli, E. , Lighthart, B. , Crowe, J.H. , and Crowe, L.M. (1995) Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl Environ Microbiol 61: 3592–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson, D.A. (2015) The complex relationship between microbial growth rate and yield and its implications for ecosystem processes. Front Microbiol 6: 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, P. , and Flint, H.J. (2009) Diversity, metabolism and microbial ecology of butyrate‐producing bacteria from the human large intestine. FEMS Microbiol Lett 294: 1–8. [DOI] [PubMed] [Google Scholar]
- Lovelock, J.E. (1953) The mechanism of the protective action of glycerol against haemolysis by freezing and thawing. Biochim Biophys Acta 11: 28–36. [DOI] [PubMed] [Google Scholar]
- Malik, K.A. (1991) Cryopreservation of bacteria with special reference to anaerobes. World J Microbiol Biotechnol 7: 629–632. [DOI] [PubMed] [Google Scholar]
- Malik, K.A. (1992) Freeze‐drying of microorganisms using a simple apparatus. World J Microbiol Biotechnol 8: 76–79. [DOI] [PubMed] [Google Scholar]
- Mazur, P. (1970) Cryobiology: the freezing of biological systems. Science 168: 939–949. [DOI] [PubMed] [Google Scholar]
- Mazur, P. (1984) Freezing of living cells: mechanisms and implications. Am J Physiol 247: C125–C142. [DOI] [PubMed] [Google Scholar]
- Meneghel, J. , Passot, S. , Dupont, S. , and Fonseca, F. (2017) Biophysical characterization of the Lactobacillus delbrueckii subsp. bulgaricus membrane during cold and osmotic stress and its relevance for cryopreservation. Appl Microbiol Biotechnol 101: 1427–1441. [DOI] [PubMed] [Google Scholar]
- Meryman, H.T. (2007) Cryopreservation of living cells: principles and practice. Transfusion (Paris) 47: 935–945. [DOI] [PubMed] [Google Scholar]
- Mille, Y. , Beney, L. , and Gervais, P. (2005) Compared tolerance to osmotic stress in various microorganisms: towards a survival prediction test. Biotechnol Bioeng 92: 479–484. [DOI] [PubMed] [Google Scholar]
- Miquel, S. , Leclerc, M. , Martin, R. , Chain, F. , Lenoir, M. , Raguideau, S. , et al (2015) Identification of metabolic signatures linked to anti‐inflammatory effects of Faecalibacterium prausnitzii . MBio 6: e00300‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, S. , and Imlay, J.A. (2013) An anaerobic bacterium, Bacteroides thetaiotaomicron, uses a consortium of enzymes to scavenge hydrogen peroxide. Mol Microbiol 90: 1356–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, N. , and Imlay, J.A. (2001) How does oxygen inhibit central metabolism in the obligate anaerobe Bacteroides thetaiotaomicron . Mol Microbiol 39: 1562–1571. [DOI] [PubMed] [Google Scholar]
- Patra, P. , and Klumpp, S. (2013) Population dynamics of bacterial persistence. PLoS ONE 8: e62814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope, C.F. , McHugh, T.D. , and Gillespie, S.H. (2010) Methods to determine fitness in bacteria. Methods Mol Biol 642: 113–121. [DOI] [PubMed] [Google Scholar]
- Prakash, O. , Nimonkar, Y. , and Shouche, Y.S. (2013) Practice and prospects of microbial preservation. FEMS Microbiol Lett 339: 1–9. [DOI] [PubMed] [Google Scholar]
- Saegeman, V.S. , Ectors, N.L. , Lismont, D. , Verduyckt, B. , and Verhaegen, J. (2008) Short‐ and long‐term bacterial inhibiting effect of high concentrations of glycerol used in the preservation of skin allografts. Burns 34: 205–211. [DOI] [PubMed] [Google Scholar]
- Sandegren, L. , Lindqvist, A. , Kahlmeter, G. , and Andersson, D.I. (2008) Nitrofurantoin resistance mechanism and fitness cost in Escherichia coli . J Antimicrob Chemother 62: 495–503. [DOI] [PubMed] [Google Scholar]
- Schwab, C. , Vogel, R. , and Ganzle, M.G. (2007) Influence of oligosaccharides on the viability and membrane properties of Lactobacillus reuteri TMW1.106 during freeze‐drying. Cryobiology 55: 108–114. [DOI] [PubMed] [Google Scholar]
- Squires, R.W. , and Hartsell, S.E. (1955) Survival and growth initiation of defrosted Escherichia coli as affected by frozen storage menstrua. Appl Microbiol 3: 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab, J.A. , and Ely, J.K. (1987) Viability of lyophilized anaerobes in two media. Cryobiology 24: 174–178. [DOI] [PubMed] [Google Scholar]
- Sutton, S. (2010) The most probable number method and its uses in enumeration, qualification, and validation. J Validat Technol 16: 35–38. [Google Scholar]
- Tamanai‐Shacoori, Z. , Smida, I. , Bousarghin, L. , Loreal, O. , Meuric, V. , Fong, S.B. , et al (2017) Roseburia spp.: a marker of health? Future Microbiol 12: 157–170. [DOI] [PubMed] [Google Scholar]
- Tan, J. , McKenzie, C. , Potamitis, M. , Thorburn, A.N. , Mackay, C.R. , and Macia, L. (2014) The role of short‐chain fatty acids in health and disease. Adv Immunol 121: 91–119. [DOI] [PubMed] [Google Scholar]
- Udayappan, S. , Manneras‐Holm, L. , Chaplin‐Scott, A. , Belzer, C. , Herrema, H. , Dallinga‐Thie, G.M. , et al (2016) Oral treatment with Eubacterium hallii improves insulin sensitivity in db/db mice. NPJ Biofilms Microbiomes 2: 16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Immerseel, F. , Ducatelle, R. , De Vos, M. , Boon, N. , Van De Wiele, T. , Verbeke, K. , et al (2010) Butyric acid‐producing anaerobic bacteria as a novel probiotic treatment approach for inflammatory bowel disease. J Med Microbiol 59: 141–143. [DOI] [PubMed] [Google Scholar]
- Van Nevel, S. , Koetzsch, S. , Weilenmann, H.U. , Boon, N. , and Hammes, F. (2013) Routine bacterial analysis with automated flow cytometry. J Microbiol Methods 94: 73–76. [DOI] [PubMed] [Google Scholar]
- Vereyken, I.J. , van Kuik, J.A. , Evers, T.H. , Rijken, P.J. , and de Kruijff, B. (2003) Structural requirements of the fructan‐lipid interaction. Biophys J 84: 3147–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira, A.T. , Fukumori, C. , and Ferreira, C.M. (2016) New insights into therapeutic strategies for gut microbiota modulation in inflammatory diseases. Clin Transl Immunol 5: e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Kang, Y. , Luo, C. , Zhao, T. , Liu, L. , Jiang, X. , et al (2014) Heteroresistance at the single‐cell level: adapting to antibiotic stress through a population‐based strategy and growth‐controlled interphenotypic coordination. MBio 5: e00942‐00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrzosek, L. , Miquel, S. , Noordine, M.L. , Bouet, S. , Joncquel Chevalier‐Curt, M. , Robert, V. , et al (2013) Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol 11: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Correlation plots between viable cell counts and lag times (t lag).
Table S1. Composition of phosphate buffer.
Table S2. Composition of phosphate buffered saline.
Table S3. Composition of YCFA medium.


